Introduction
Diabetes mellitus is a common metabolic disease with
increasing prevalence worldwide. According to a global study
published in 2004, the disease affected 150 million adults in 2000
and that number is expected to increase to 350 million by
2030(1). Diabetic nephropathy (DN)
affects 20-40% of all patients with diabetes mellitus and is one of
the most important microvascular complications resulting in
increased morbidity and mortality (2). DN causes irreversible proteinuria and
kidney damage and is one of the principal causes of end-stage renal
disease in this population (3). In
addition, DN is frequently accompanied by a steadily increasing
blood pressure and a slow but progressive loss of kidney function.
Once the kidney function begins to decline, it may deteriorate by
~10% per year if left untreated. Microalbuminuria is a vital marker
for the development of DN. Therefore, early DN with an albumin
excretion rate (AER) ranging between 20 and 200 µg per min (30-300
mg/24 h) is an important stage in the progression of nephropathy.
Effective treatment in this phase may reverse the albuminuria and
reduce the incidence of end-stage renal disease.
Antihypertensive drugs are able to effectively
diminish DN progression. Angiotensin-converting enzyme (ACE)
inhibitors, which belong to the class of renin-angiotensin system
(RAS) blockers, are recommended as the primary antihypertensive
drugs. In addition to inhibiting the RAS system, thereby producing
a hypotensive effect, they also decrease proteinuria, preserve the
glomerular filtration rate and limit the progression to renal
failure (4,5). However, the efficacy of other
antihypertensive agents, in particular that of calcium channel
blockers (CCBs), to confer similar effects on albuminuria has not
been clarified and the clinical importance of the selection of
different antihypertensive drugs remains elusive. The Melbourne
Diabetic Nephropathy Study Group (MDNSG) reported similar
efficacies for the ACE inhibitor perindopril and the CCB nifedipine
for preventing the development from macroalbuminuria to
microalbuminuria in patients with types 1 and 2 diabetes after
long-term treatment (6). However,
comparisons of short-term treatments with ACE inhibitors and CCB in
patients with early DN have not provided any consistent results
(5,7-9).
Therefore, the purpose of the present study was to perform a
meta-analysis of randomized controlled trials (RCTs) comparing the
efficacy of ACE inhibitors and CCBs after short- or long-term
treatments for patients with early diabetic nephropathy to
elucidate their efficacy to prevent nephropathy.
Materials and methods
Search strategy
Guidelines of the Cochrane handbook were followed
during the conduct of this study (10). A comprehensive search of the PubMed,
ScienceDirect, Embase, Cochrane Library, Chinese National Knowledge
Infrastructure, China Biomedical Literature database and Wanfang
digital periodical full-text databases from inception to June 2020
was performed. The language of publication was restricted to
Chinese and English. The following key words were used for the
literature search: ‘Angiotensin-converting enzyme inhibitors’; ‘ACE
inhibitors’; ‘calcium channel blockers’; ‘CCB’; ‘antihypertensive
drugs’; ‘nifedipine’; ‘amlodipine’; ‘lercanidipine’; ‘manidipine’;
‘enalapril’; ‘fosinopril’; ‘delapril’; perindopril’; ‘ramipril’;
‘diabetes mellitus’; ‘diabetic nephropathy’; ‘albuminuria’;
‘creatinine’; ‘kidney failure’ and ‘renal failure’. In addition,
the references of included studies and pertinent review articles
were manually searched to retrieve any additional studies.
Study selection
The following inclusion criteria were applied: i)
RCTs comparing ACE inhibitors and CCBs for the treatment of early
DN; ii) studies including adult patients of either gender with
primary type 1 or type 2 diabetes mellitus with or without
hypertension; and persistent microalbuminuria (AER between 20 and
200 µg/min or 30 and 300 mg/24 h); iii) studies providing data of
the study groups, including the baseline and follow-up period; iv)
outcomes of the study were to include AER and serum creatinine
(Scr) for short-term treatments, as well as the number of patients
with improvements in albuminuria for long-term treatments. Studies
comparing ACE inhibitors and CCBs for patients other than DN were
excluded. Non-RCTs, retrospective studies, case series, case
reports and studies not reporting relevant outcome data were also
excluded.
Data extraction and quality
evaluation
The following data were extracted independently in a
standardized manner from all eligible studies: Authors, publication
year, sample size, study duration, intervention and outcomes. Data
on AER and Scr levels were extracted for short-term treatments and
the number of patients improving (developing macroalbuminuria or
normal albuminuria) for long-term treatments.
The quality of each RCT was assessed using the
Cochrane Collaboration risk assessment tool (11). Studies were rated as having low
risk, high risk or unclear risk of bias in the following
categories: Random sequence generation, allocation concealment,
blinding of participants and personnel, blinding of outcome
assessment, incomplete outcome data and selective reporting.
Data analysis
Quantitative and qualitative analyses of the
collected data were performed. Odds ratios (OR) were calculated for
categorical variables and mean differences (MD) for continuous
variables with 95% confidence intervals (CI). Review manager
[version 5.3; 2014; Nordic Cochrane Centre (Cochrane
Collaboration)] was used for the statistical analyses.
Heterogeneity of the included studies was assessed using the
I2 test. I2 values of <50% were considered
to represent low heterogeneity and a fixed-effects model was used.
For I2 values of ≥50%, heterogeneity was considered to
be significant and a random-effects model was used. P≤0.05 was
considered to indicate statistical significance. Publication bias
was to be assessed using funnel plots if there were >10 studies
in a meta-analysis (10).
Results
Study characteristics
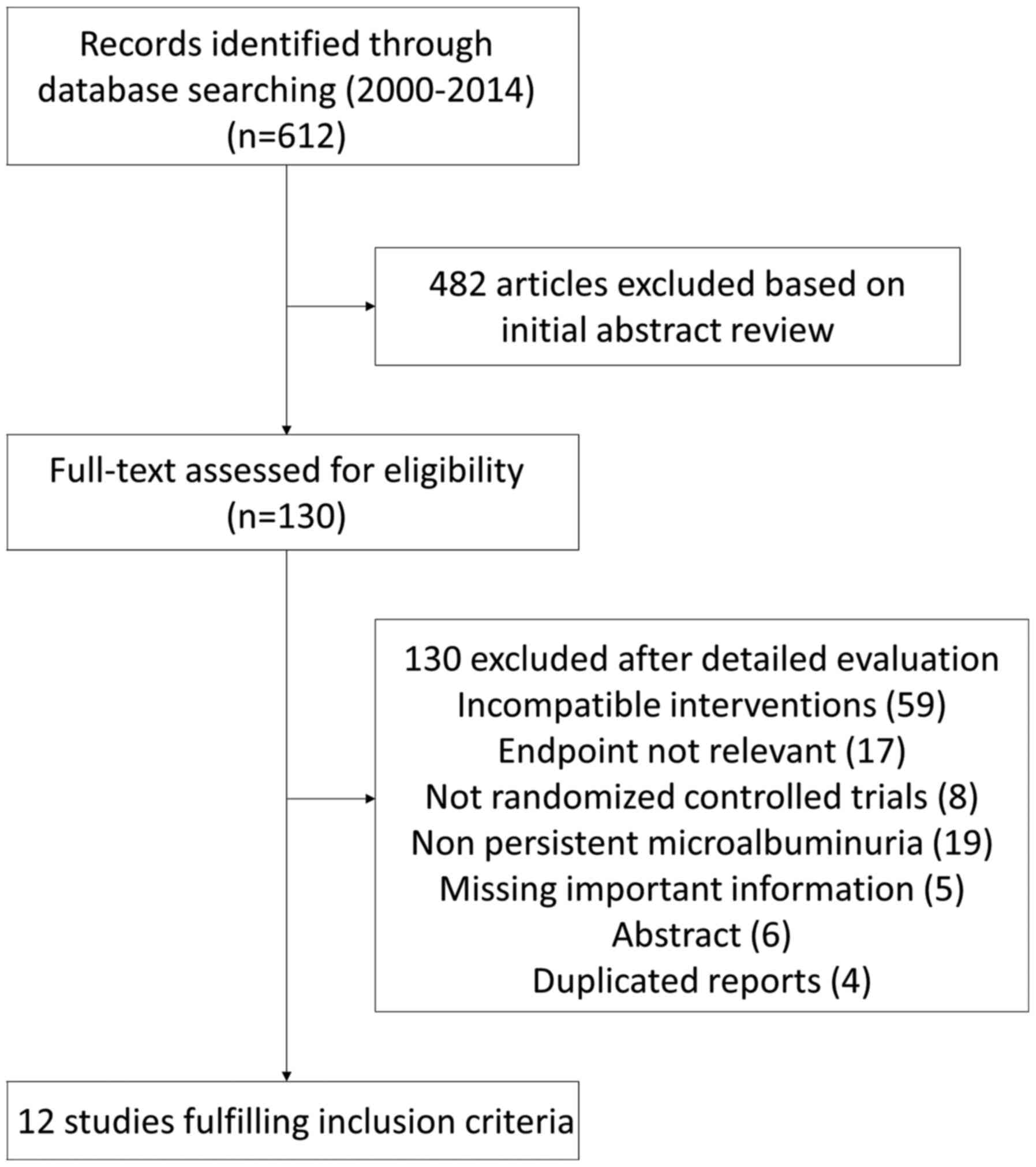
Fig. 1 presents the
preferred reporting items for systematic reviews and meta-analyses
(PRISMA) flowchart of the study. The search yielded a total of 612
citations. After serial selection and evaluation, 12 studies
(5,7-9,12-19)
were included in the meta-analysis with a total of 947 participants
from six different countries (Table
I). A total of 10 studies were in English and two in Chinese.
Treatments were classified as short-term (course of treatment,
<6 months) or long-term (≥1 year) for the meta-analysis. The
interventions in the experimental groups of the 12 studies included
ACE inhibitors (perindopril, enalapril, delapril, fosinopril,
lisinopril, ramipril) and those in the control groups included CCBs
[amlodipine, sustained-release nifedipine (tablets), manidipine,
lercanidipine].
 | Table IDetails of included studies. |
Table I
Details of included studies.
Results of the meta-analysis Effect of
short-term treatment
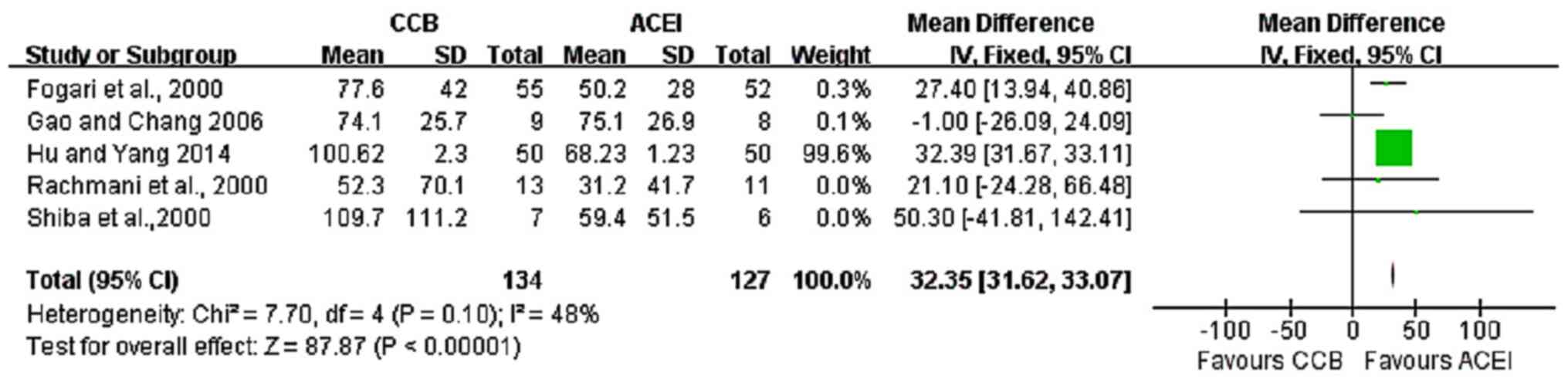
A total of six RCTs (5,9,12-15)
reported on short-term treatments with ACE inhibitors and CCBs with
a total of 338 participants. Of these, five studies (5,12-15)
reported AERs. The results indicated that ACE inhibitors were more
effective in reducing AER than CCBs (fixed-effects model analysis:
MD, 32.35; 95% CI, 31.62-33.07; P<0.00001; I2=48%;
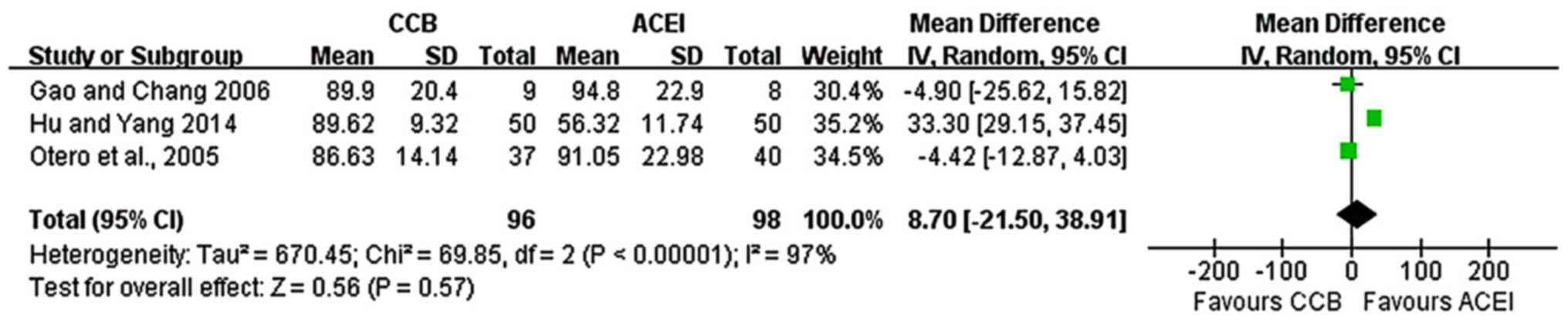
Fig. 2). A total of three studies
(5,9,15)
reported Scr values. The analysis indicated no statistically
significant difference in Scr values between the two groups
(random-effects model analysis: MD, 8.7; 95% CI, -21.5-38.91;
P=0.57; I2=97%; Fig.
3).
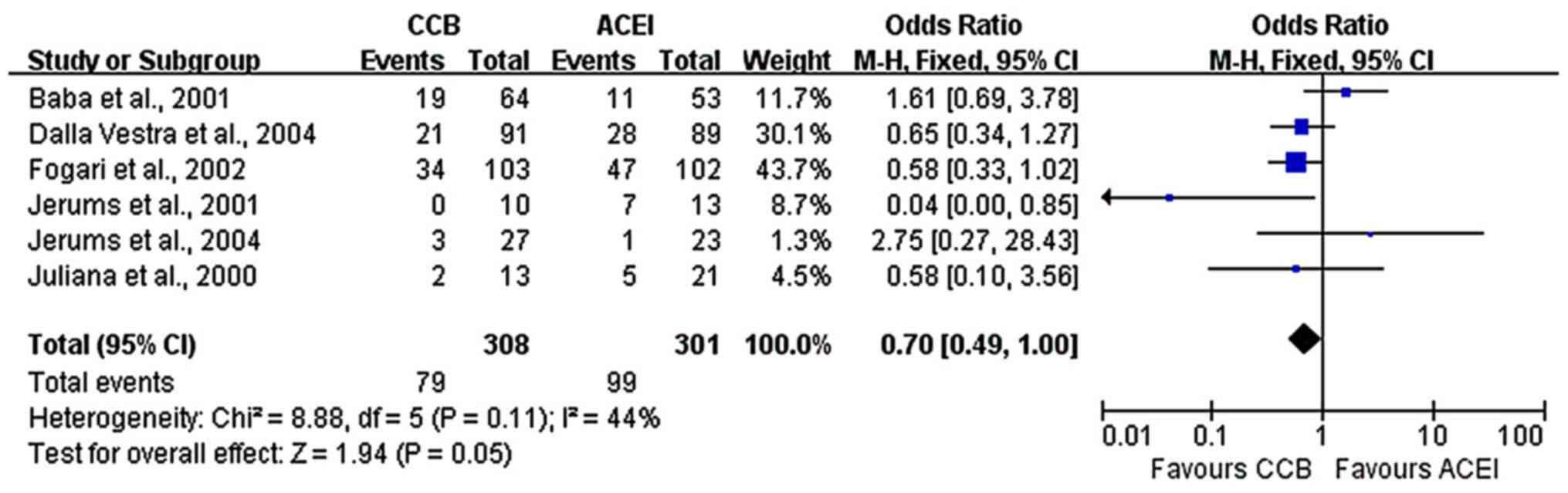
Effect of long-term treatment
A total of six studies (7,8,16-19)
with long-term treatments included 609 patients with early DN and
reported data on the progression to normoalbuminuria. The present
meta-analysis indicated a marginally significant difference between
the two groups, with better outcomes with ACE inhibitors
(fixed-effects model analysis: OR, 0.70; 95% CI, 0.49-1.00; P=0.05;
I2=44%; Fig. 4). The
P-value happened to be marginally significant, which may be
considered to point to a lack of a distinct difference between the
two groups, but the issue requires to be analyzed in further
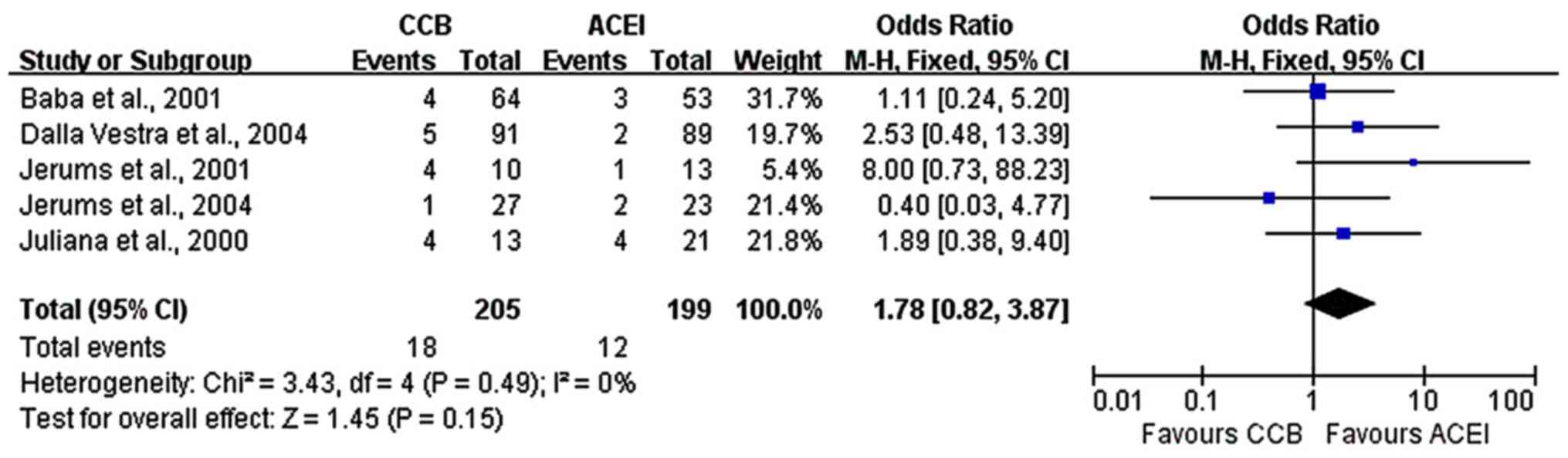
studies with larger sample sizes. Furthermore, five studies
(7,16-19)
reported data on the progression to macroalbuminuria. No difference
between ACE inhibitors and CCBs was obtained regarding the
progression from microalbuminuria to macroalbuminuria
(fixed-effects model analysis: OR, 1.78; 95% CI, 0.82-3.87; P=0.15;
I2=0%; Fig. 5).
Adverse events
Only three studies reported the number of adverse
events in both study groups (9,13,17).
While two studies (9,13) mentioned the number of patients with
adverse events, one trial reported the total number of adverse in
the entire cohort (17). Due to
this heterogeneity, no meta-analysis was performed and only a
detailed, qualitative comparison is provided in Table II. None of the studies reporting
adverse events indicated any statistically significant differences
between the two groups. In studies describing adverse events, ankle
edema was the most common side-effect with CCBs, while cough was
the most common adverse event with ACE inhibitors.
 | Table IIDetails of adverse events reported in
the included studies. |
Table II
Details of adverse events reported in
the included studies.
| | Total number of
patients with adverse events | Description of
adverse events (frequency, %) | |
|---|
| Authors (year) | CCB | ACEI | CCB | ACEI | Refs. |
|---|
| Rachmani et
al (2000) | NR | NR | NR | NR | (12) |
| Fogari et al
(2000) | 7 | 6 | Ankle edema
(5.4) | Cough (4) | (13) |
| | | | Headache (1.3) | Headache (2.7) | |
| | | | Palpitation
(1.3) | Gastric intolerance
(1.3) | |
| | | | Flushing (1.3) | | |
| Shiba et al
(2000) | NR | NR | NR | NR | (14) |
| Luque Otero et
al (2005) | 35 | 44 | Ankle edema
(11.3) | Cough (10.3) | (9) |
| | | | Hot flushes
(5.7) | | |
| | | | Mild dizziness
(3.8) | | |
| Gao and Chang
(2006) | NR | NR | NR | NR | (15) |
| Hu and Yang
(2014) | NR | NR | NR | NR | (5) |
| Chan et al
(2000) | NR | NR | NR | NR | (16) |
| Baba et al
(2001) | 33 | 34 | NR | NR | (17) |
| Jerums et al
(2001) | NR | NR | NR | NR | (19) |
| Fogari et al
(2002) | NR | NR | NR | NR | (8) |
| Dalla Vestra et
al (2004) | NR | NR | NR | NR | (18) |
| Jerums et al
(2004) | NR | NR | NR | NR | (7) |
Methodological quality
The results of the risk of bias evaluation of the
included studies are presented in Table III. The majority of studies did
not provide any information on the exact methods of randomization
and allocation concealment. Blinding was not performed in any of
the included studies. None of the trials were pre-registered. The
overall quality of the studies was deemed to be moderate.
 | Table IIIAuthors' judgment of risk of bias in
included studies. |
Table III
Authors' judgment of risk of bias in
included studies.
| Included trials
(Refs.) | Random sequence
generation | Allocation
concealment | Blinding of
participants and personnel | Blinding of outcome
assessment | Incomplete outcome
data | Selective
reporting |
|---|
| Rachmani et
al (12) | Unclear risk | Unclear risk | Low risk | High risk | Low risk | Unclear risk |
| Fogari et al
(13) | Unclear risk | Unclear risk | Low risk | Low risk | Low risk | Unclear risk |
| Shiba et al
(14) | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Unclear risk |
| Luque Otero et
al (9) | Unclear risk | Unclear risk | Low risk | Low risk | High risk | Unclear risk |
| Gao and Chang
(15) | Unclear risk | Unclear risk | Unclear risk | Low risk | Low risk | Unclear risk |
| Hu and Yang
(5) | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Low risk | Unclear risk |
| Chan et al
(16) | Unclear risk | Unclear risk | Low risk | Low risk | Unclear risk | Unclear risk |
| Baba et al
(17) | Low risk | Unclear risk | High risk | High risk | High risk | Unclear risk |
| Jerums et al
(19) | Unclear risk | Unclear risk | High risk | Low risk | High risk | Unclear risk |
| Fogari et al
(8) | Unclear risk | Unclear risk | High risk | High risk | High risk | Unclear risk |
| Dalla Vestra et
al (18) | Unclear risk | Unclear risk | Low risk | Low risk | Unclear risk | Unclear risk |
| Jerums et al
(7) | Low risk | Unclear risk | High risk | Low risk | High risk | Unclear risk |
Discussion
The blockade of RAS is essential for treating
albuminuria in patients with diabetes mellitus, as hyperactive RAS
is thought to have a pivotal role in the pathophysiology of renal
failure (20). As angiotensin
receptor blockers, ACE inhibitors are recommended as the primary
antihypertensive drugs in patients with diabetes (21). They reduce albuminuria to a greater
extent than other antihypertensive agents and are first-choice
drugs for treating patients with diabetes and early nephropathy
(22). They are also regarded as
particularly effective for limiting renal-disease progression due
to their possible kidney function benefits that are separate from
their systemic blood pressure effects. Treatment with angiotensin
receptor blockers has been associated with reduced intraglomerular
pressure, decreased filtration fraction and glomerular filtration
membrane permeability improvements that reduce urinary protein
excretion (23). However, the use
of only one class of antihypertensive agent is frequently unable to
achieve target blood pressure levels and may not be sufficient to
reduce albuminuria or proteinuria. Additional antihypertensive
drugs are indispensable to obtain target blood pressure levels and
kidney protection benefits. CCBs are another group of drugs that
not only reduce blood pressure but are also efficacious for the
management of albuminuria.
Several types of CCBs are available and have been
classified according to their biological half-life, drug delivery
systems and blocking channel types. In addition, a novel class of
dihydropyridines has been added as CCBs with sympathetic nerve
effects (24). Furthermore, at
least five subtypes based on electrophysiological and
pharmacological characteristics exist, namely the L-, N-, P/Q-, R-
and T-types (25,26). Several studies have assessed the
effects of ACE inhibitors and CCBs in reducing albuminuria when
used for patients with DN; however, the results have been
conflicting, with certain trials indicating a better
antialbuminuric effect with ACE inhibitors (16,19,27),
while others reported no differences between the two therapies with
long-term treatment (7,28-30).
The present review comparing the renal protective
effects of CCB with those of ACE inhibitors analyzed data from 12
RCTs with 6 trials reporting outcomes after short-term treatment
and another 6 studies reporting outcomes of long-term therapy. The
results of the present meta-analysis indicate that CCBs may be less
effective than ACE inhibitors after short-term treatment, but there
were no significant differences between the two groups of drugs in
terms of the progression of microalbuminuria to macroalbuminuria
and only a marginally favorable result with ACE inhibitors for
progression to normoal buminuria as the treatment time was
prolonged. The earlier renoprotective effect of ACE inhibitors has
been suggested to be independent of the antihypertensive action of
the drug (8). ACE inhibitors exert
their antiproteinuric effect by two mechanisms. They not only
reduce the efferent arteriolar resistance and subsequently the
glomerular hydraulic pressure but also have nonhemodynamic actions
such as enhancing selectivity of the glomerular barrier,
compensatory growth of residual nephrons and activation of the
renal interstitium with scar formation. These factors are thought
to contribute to the earlier renoprotective effects of ACE
inhibitors (8,13,14).
The results of the present study also indicated that
CCBs and ACE inhibitors have similar long-term effects. The
antiproteinuric effect of CCBs only with long-term treatment and a
lack of any significant short-term effects may be attributed to the
reduction of systemic blood pressure with long-term treatment and
absence of any intrinsic effects of the drug (13). Studies indicated that long-term
renoprotective actions of antihypertensive drugs are proportional
to the reduction in blood pressure for both DN and non-DN (13,31,32).
It is known that different CCB channel types reduce the production
of oxygen-free radicals that inhibit the vasoconstrictive effects
of thromboxane A2(33). By their
blocking mechanism, CCBs thereby cause significant lowering of
systemic arterial pressure by relaxing the afferent glomerular
arterioles (31). This action
results in alteration of intraglomerular pressure and AER,
depending upon the equilibrium between preglomerular vasodilation
and systemic BP reduction (31,32).
Data on adverse events were not available from all
included studies in the present review. However, of the studies
collecting these data, none reported any statistically significant
difference in the incidence of adverse events between the two
groups. Numerous studies have focused on the combination of RAS
blocking agents and CCBs to achieve a complementary effect and
reduce the incidence of side effects (34,35).
Studies have revealed that combination therapy with RAS blockade
agents and certain CCBs produces a greater reduction in AER than
either drug used as monotherapy (8,34-36).
Thus, CCBs as supplementary therapies may be a good alternative for
patients that have absolute or relative contraindications against
RAS blockers and to diminish side effects of the drug used as
monotherapy (34-36).
While the efficacy of CCBs and ACE inhibitors for
the prevention of diabetes was not one of the outcomes of the
present review, an increasing amount of research has evaluated the
effect of antihypertensive drugs on the incidence of diabetes. It
has been reported that CCBs inhibit proapoptotic β-cell
thioredoxin-interacting protein expression and thereby improve
β-cell survival and function (37).
A meta-analysis of RCTs by Noto et al (37), however, has reported that CCBs are
not significantly associated with the reduction of the incidence of
diabetes. They also reported that ACE inhibitors have the lowest
association with a reduced risk of diabetes amongst
antihypertensive drugs.
In the present meta-analysis, the CCBs used in all
trials were of the dihydropyridine class, but non-dihydropyridine
CCBs have demonstrated better reductions in urinary proteins for
patients with diabetic nephropathy (38). Furthermore, the albuminuria
reduction effects were also different when comparing different
types of CCBs (38,39). However, the CCB with the best
albuminuria/proteinuria reduction remains to be identified.
Of note, the present review had certain limitations.
There was inter-study heterogeneity amongst the included studies
with respect to sample size, choice of drug, dosage, duration of
follow-up and study outcomes. This may limit the generalization of
the present results. Furthermore, it also limited the possibility
to assess the role of different drugs and dosages on the study
outcomes. In addition, the limited number of studies analyzed along
with the relatively small sample size of certain trials may have
underestimated the true treatment effect in the present
meta-analysis. As another limitation, not all RCTs provided
adequate information on the methods of randomization and allocation
concealment. Furthermore, blinding was not performed in all trials.
Finally, a lack of rigorous methodology may have skewed the
outcomes of the trials.
In view of these limitations, there is a requirement
for further RCTs with larger sample sizes to identify the most
beneficial intervention strategy for patients with early DN. Future
studies should be high-quality, incorporating rigorous methods of
randomization, allocation concealment and blinding, and also
standardize the dose of the drugs to reduce bias in their results.
Studies should also record and compare the adverse events of both
drugs to provide high-quality comparative evidence regarding the
safety of the drugs. In addition, further studies are required
comparing the effects of different classes of CCB vs. ACE
inhibitors for the management of patients with DN.
To conclude, the present review provided up-to-date
and comprehensive level-1 evidence comparing the short- and
long-term therapeutic effects of ACE inhibitors and CCBs for
preventing the progression of nephropathy in patients with diabetes
mellitus. The results of the present study indicated that the
antiproteinuric efficacy of CCBs may be less than that of ACE
inhibitors after short-term treatments in patients with DN.
However, both types of drugs have similar efficacy in reducing the
progression of microalbuminuria to macroalbuminuria after long-term
treatment. Thus, in clinical practice, ACE inhibitors may be useful
when early antiproteinuric action is required; however, either drug
may be used for long-term action. There is a requirement for
further studies to provide robust evidence.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JLL, JRL and ML conceived and designed the study. QT
and WL collected data and performed data analysis. JLL and JRL
wrote the draft of this manuscript. ML edited the manuscript. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wild S, Roglic G, Green A, Sicree R and
King H: Global prevalence of diabetes: Estimates for the year 2000
and projections for 2030. Diabetes Care. 27:1047–1053.
2004.PubMed/NCBI View Article : Google Scholar
|
|
2
|
American Diabetes Association. Standards
of medical care in diabetes - 2011. Diabetes Care. 34 (Suppl
1):S11–S61. 2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hajhosseiny R, Khavandi K, Jivraj N,
Mashayekhi S, Goldsmith DJ and Malik RA: Have we reached the limits
for the treatment of diabetic nephropathy? Expert Opin Investig
Drugs. 23:511–522. 2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Heart Outcomes Prevention Evaluation Study
Investigators. Effects of ramipril on cardiovascular and
microvascular outcomes in people with diabetes mellitus: Results of
the HOPE study and MICRO-HOPE substudy. Lancet. 355:253–259.
2000.PubMed/NCBI
|
|
5
|
Hu R and Yang Z: Study on effect of the
combined treatment of enalapril and amlodipine in elderly diabetic
patients with hypertension. Diabetes New World. 24:6–7. 2014.(In
Chinese).
|
|
6
|
Melbourne Diabetic Nephropathy Study
Group. Comparison between perindopril and nifedipine in
hypertensive and normotensive diabetic patients with
microalbuminuria. BMJ. 302:210–216. 1991.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Jerums G, Allen TJ, Campbell DJ, Cooper
ME, Gilbert RE, Hammond JJ, O'Brien RC, Raffaele J and Tsalamandris
C: Melbourne Diabetic Nephropathy Study Group. Long-term
renoprotection by perindopril or nifedipine in non-hypertensive
patients with Type 2 diabetes and microalbuminuria. Diabet Med.
21:1192–1199. 2004.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Fogari R, Preti P, Zoppi A, Rinaldi A,
Corradi L, Pasotti C, Poletti L, Marasi G, Derosa G, Mugellini A,
et al: Effects of amlodipine fosinopril combination on
microalbuminuria in hypertensive type 2 diabetic patients. Am J
Hypertens. 15:1042–1049. 2002.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Luque Otero M and Martell Claros N: Study
Investigators Group. Manidipine versus enalapril monotherapy in
patients with hypertension and type 2 diabetes mellitus: A
multicenter, randomized, double-blind, 24-week study. Clin Ther.
27:166–173. 2005.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Higgins J and Green S: Cochrane Handbook
for Systemic Reviews of Interventions. Version 5.1. The Cochrane
Collaboration, 2011. urihttps://handbook-5-1.cochrane.orgsimplehttps://handbook-5-1.cochrane.org.
Accessed June 1, 2020.
|
|
11
|
Higgins J, Altman D and Sterne J: Cochrane
Statistical Methods Group and the Cochrane Bias Methods Group
(eds): Chapter 8: Assessing risk of bias in included studies. In:
Cochrane Handbook for Systematic Reviews of Interventions Version
5.1.0 (updated March 2011). The Cochrane Collaboration, 2011.
urihttps://handbook-5-1.cochrane.org/chapter_8/8_assessing_risk_of_bias_in_included_studies.htmsimplehttps://handbook-5-1.cochrane.org/chapter_8/8_assessing_risk_of_bias_in_included_studies.htm.
|
|
12
|
Rachmani R, Lidar M, Brosh D, Levi Z and
Ravid M: Oxidation of low-density lipoprotein in normotensive type
2 diabetic patients. Comparative effects of enalapril versus
nifedipine: A randomized cross-over over study. Diabetes Res Clin
Pract. 48:139–145. 2000.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Fogari R, Zoppi A, Corradi L, Poletti L,
Pasotti M, Fogari E and Mugellini A: Long-term effects of
amlodipine versus fosinopril on microalbuminuria in elderly
hypertensive patients with type 2 diabetes mellitus. Curr Ther Res
Clin Exp. 61:163–173. 2000.
|
|
14
|
Shiba T, Inoue M, Tada H, Hayashi Y, Okuda
Y, Fujita R, Makino F, Takahasi C, Kageyama S, Kitamura S, et al:
Delapril versus manidipine in hypertensive therapy to halt the
type-2-diabetes-mellitus-associated nephropathy. Diabetes Res Clin
Pract. 47:97–104. 2000.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Gao Z and Chang J: Effects of enalapril
combined with Levamlodipine on type 2 diabetic nephropathy. J Med
Theory Practice. 1:12–14. 2006.(In Chinese).
|
|
16
|
Chan JC, Ko GTC, Leung DHY, Cheung RC,
Cheung MY, So WY, Swaminathan R, Nicholls MG, Critchley JA and
Cockram CS: Long-term effects of angiotensin-converting enzyme
inhibition and metabolic control in hypertensive type 2 diabetic
patients. Kidney Int. 57:590–600. 2000.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Baba S: J-MIND Study Group. Nifedipine and
enalapril equally reduce the progression of nephropathy in
hypertensive type 2 diabetics. Diabetes Res Clin Pract. 54:191–201.
2001.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Dalla Vestra M, Pozza G, Mosca A, Grazioli
V, Lapolla A, Fioretto P and Crepaldi G: Effect of lercanidipine
compared with ramipril on albumin excretion rate in hypertensive
type 2 diabetic patients with microalbuminuria: DIAL study
(diabete, ipertensione, albuminuria, lercanidipina). Diabetes Nutr
Metab. 17:259–266. 2004.PubMed/NCBI
|
|
19
|
Jerums G, Allen TJ, Campbell DJ, Cooper
ME, Gilbert RE, Hammond JJ, Raffaele J and Tsalamandris C:
Long-term comparison between perindopril and nifedipine in
normotensive patients with type 1 diabetes and microalbuminuria. Am
J Kidney Dis. 37:890–899. 2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wakahara S, Konoshita T, Mizuno S,
Motomura M, Aoyama C, Makino Y, Kato N, Koni I and Miyamori I:
Synergistic expression of angiotensin-converting enzyme (ACE) and
ACE2 in human renal tissue and confounding effects of hypertension
on the ACE to ACE2 ratio. Endocrinology. 148:2453–2457.
2007.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ogihara T, Kikuchi K, Matsuoka H, Fujita
T, Higaki J, Horiuchi M, Imai Y, Imaizumi T, Ito S, Iwao H, et al:
Japanese Society of Hypertension Committee: The Japanese Society of
Hypertension Guidelines for the Management of Hypertension (JSH
2009). Hypertens Res. 32:3–107. 2009.PubMed/NCBI
|
|
22
|
American Diabetes Association. Standards
of medical care for patients with diabetes mellitus. Diabetes Care.
26 (Suppl 1):S33–S50. 2003.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Poortmans JR and Ouchinsky M: Glomerular
filtration rate and albumin excretion after maximal exercise in
aging sedentary and active men. J Gerontol A Biol Sci Med Sci.
61:1181–1185. 2006.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Takahara A: Cilnidipine: A new generation
Ca channel blocker with inhibitory action on sympathetic
neurotransmitter release. Cardiovasc Ther. 27:124–139.
2009.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Catterall WA: Structure and regulation of
voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol.
16:521–555. 2000.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ertel EA, Campbell KP, Harpold MM, Hofmann
F, Mori Y, Perez-Reyes E, Schwartz A, Snutch TP, Tanabe T,
Birnbaumer L, et al: Nomenclature of voltage-gated calcium
channels. Neuron. 25:533–535. 2000.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Agardh CD, Garcia-Puig J, Charbonnel B,
Angelkort B and Barnett AH: Greater reduction of urinary albumin
excretion in hypertensive type II diabetic patients with incipient
nephropathy by lisinopril than by nifedipine. J Hum Hypertens.
10:185–192. 1996.PubMed/NCBI
|
|
28
|
Ruggenenti P, Mosconi L, Bianchi L,
Cortesi L, Campana M, Pagani G, Mecca G and Remuzzi G: Long-term
treatment with either enalapril or nitrendipine stabilizes
albuminuria and increases glomerular filtration rate in
non-insulin-dependent diabetic patients. Am J Kidney Dis.
24:753–761. 1994.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Velussi M, Brocco E, Frigato F, Zolli M,
Muollo B, Maioli M, Carraro A, Tonolo G, Fresu P, Cernigoi AM, et
al: Effects of cilazapril and amlodipine on kidney function in
hypertensive NIDDM patients. Diabetes. 45:216–222. 1996.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Estacio RO, Jeffers BW, Gifford N and
Schrier RW: Effect of blood pressure control on diabetic
microvascular complications in patients with hypertension and type
2 diabetes. Diabetes Care. 23 (Suppl 2):B54–B64. 2000.PubMed/NCBI
|
|
31
|
Loutzenhiser R and Epstein M: Effects of
calcium antagonists on renal hemodynamics. Am J Physiol.
249:F619–F629. 1985.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Gansevoort RT, Apperloo AJ, Heeg JE, de
Jong PE and de Zeeuw D: The antiproteinuric effect of
antihypertensive agents in diabetic nephropathy. Arch Intern Med.
152:2137–2139. 1992.PubMed/NCBI
|
|
33
|
Ono T, Liu N, Kusano H, Nogaki F, Makino
T, Muso E and Sasayama S: Broad antiproliferative effects of
benidipine on cultured human mesangial cells in cell cycle phases.
Am J Nephrol. 22:581–586. 2002.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Fogari R, Zoppi A, Mugellini A, Lusardi P,
Destro M and Corradi L: Effect of benazepril plus amlodipine vs
benazepril alone on urinary albumin excretion in hypertensive
patients with type ii diabetes and microalbuminuria. Clin Drug
Investig. 13 (Suppl 1):50–55. 1997.
|
|
35
|
Shigihara T, Sato A, Hayashi K and Saruta
T: Effect of combination therapy of angiotensin-converting enzyme
inhibitor plus calcium channel blocker on urinary albumin excretion
in hypertensive microalbuminuric patients with type II diabetes.
Hypertens Res. 23:219–226. 2000.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhao J: Therapy effect observation of
Levamlodipine combined with Benazepril in the treatment of 22 cases
of type 2 diabetic nephropathy with hypertension patients. Shandong
Medicine: 53-54, 2010.
|
|
37
|
Noto H, Goto A, Tsujimoto T and Noda M:
Effect of calcium channel blockers on incidence of diabetes: A
meta-analysis. Diabetes Metab Syndr Obes. 6:257–261.
2013.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Abe H, Mita T, Yamamoto R, Komiya K,
Kawaguchi M, Sakurai Y, Shimizu T, Ohmura C, Ikeda F, Kawamori R,
et al: Comparison of effects of cilnidipine and azelnidipine on
blood pressure, heart rate and albuminuria in type 2 diabetics with
hypertension: A pilot study. J Diabetes Investig. 4:202–205.
2013.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Konoshita T, Makino Y, Kimura T, Fujii M,
Morikawa N, Wakahara S, Arakawa K, Inoki I, Nakamura H and Miyamori
I: Genomic Disease Outcome Consortium (G-DOC) Study Investigators.
A crossover comparison of urinary albumin excretion as a new
surrogate marker for cardiovascular disease among 4 types of
calcium channel blockers. Int J Cardiol. 166:448–452.
2013.PubMed/NCBI View Article : Google Scholar
|