Introduction
Glioma is the most common type of primary malignant
brain tumor in adults, constituting ~81% of all primary malignant
brain tumors (1). Standard
therapeutic regimens, including surgical resection combined with
radiotherapy and temozolomide chemotherapy, can prolong the
survival of patients with tumors characterized by methylation of
the O-6-methylguanine DNA methyltransferase promoter; however, the
median duration of survival of patients with glioblastoma
multiforme (GBM), a high-grade glioma, is <1.5 years (2). Gliomas, particularly GBM, exhibit a
high degree of angiogenesis, as determined by histological analysis
(3). Angiogenesis serves an
important role in the formation, development and recurrence of
gliomas; anti-angiogenic therapy has been considered as a novel
strategy for treating patients with gliomas (4). In addition, studies have proposed that
anti-angiogenic therapeutic regimens can improve the outcome of
patients with gliomas, yet its clinical effect remains
unsatisfactory (5). Recently,
glioma cells have been reported to exhibit vasculogenic mimicry
(VM), in which extracellular matrix (ECM)-rich, vasculogenic-like
networks form and allow glioma cells to obtain a blood supply in
the absence of endothelial cell (EC)-dependent vasculature
(6). Anti-angiogenic agents aim to
inhibit the formation of endothelium-dependent vessels but not the
development of VM, which may partially contribute to the failure of
anti-angiogenic therapy (7).
Several studies have demonstrated that certain genes, including
MMP-2, VEGF, membrane type-1 MMP (MT1-MMP), cyclooxygenase-2 and
vascular endothelial cadherin (VE-cadherin), may be involved in the
formation of VM in tumors. Agents targeting these genes have been
determined to exhibit anti-VM effects (7).
Astrocyte elevated gene-1 (AEG-1) is a downstream
gene of Ha-ras and cyclin D1, and can activate the NF-κB, PI3K/Akt
and Wnt signaling pathways, serving an important role in invasion,
metastasis, angiogenesis and chemoresistance in a variety of tumor
types, including gliomas (8,9). AEG-1
can promote the progression and invasion of gliomas through a
variety of mechanisms, including the induction of protective
autophagy (8) and suppression of
the formation of reactive oxygen species induced by hypoxia
(9). Silencing AEG-1 in glioma
cells can significantly induce tumor cell apoptosis and inhibit
tumor growth (8,10); however, whether AEG-1 participates
in VM formation in gliomas remains unknown.
The present study aimed to investigate the effects
of AEG-1 downregulation on VM formation in gliomas in vitro
and in vivo using the U87 cell line and glioma xenograft
models, respectively, to obtain further insight into the underlying
mechanisms of action.
Materials and methods
Materials
The U87 MG human glioblastoma cell line of unknown
origin (cat. no. TCHu138) and 293T cell line were purchased from
The Cell Bank of Type Culture Collection of the Chinese Academy of
Sciences. STR profiling was conducted for the authentication of the
U87 MG cell line, according to the supplier's description. A total
of 30 male, 7-9 week old, specific pathogen-free, Rowett nude rats
(Crl:NIH-Foxn1rnu), weighing 180-240 g, were purchased from Vital
River Laboratory Animal Technology Co., Ltd., and were housed in a
full-barrier rodent facility with a filtered air supply, constant
temperature (22±1˚C) and humidity(50-60%) under a 12-h light/dark
cycle. The housing environment for animals, water and animal feeds
were sterilized. All rats had free access to food and water. All
animal procedures were performed under the guidance of the Research
Ethics Committee of Xi'an Jiaotong University Health Science Center
(Xi'an, China).
Cell culture
U87 and 293T cells were cultured in DMEM (Gibco;
Thermo Fisher Scientific, Inc.) supplemented with 10% FBS (Hyclone;
GE Healthcare) in an atmosphere of 5% CO2 at 37˚C.
Design and synthesis of PCR primers
and AEG-1 small interfering RNA (siRNA)
The primers for VEGF, MMP-2, β-actin, AEG-1 and
AEG-1 siRNA were designed and synthesized by Takara Bio Inc.
(Table I).
 | Table ISequences for reverse
transcription-quantitative PCR primers, siRNAs and shRNAs. |
Table I
Sequences for reverse
transcription-quantitative PCR primers, siRNAs and shRNAs.
| Gene | Sequences |
|---|
| VEGF | Forward,
5'-TCACAGGTACAGGGATGAGGACAC-3' |
| | Reverse,
5'-CAAAGCACAGCAATGTCCTGAAG-3' |
| MMP-2 | Forward,
5'-CTCATCGCAGATGCCTGGA A-3' |
| | Reverse,
5'-TTCAGGTAATAGGCACCCTTGAAGA-3' |
| β-actin | Forward,
5'-TGGCACCCAGCACAATGAA-3' |
| | Reverse,
5'-CTAAGTCATAGTCCGCCTAGAAGCA-3' |
| AEG-1 | Forward,
5'-CACGCCATGATGGAAAGGAAGT-3' |
| | Reverse,
5'-CAGGAAATGATGCGGTTGTAAG -3' |
| AEG-1 siRNA1 | Sense,
5'-GCCGUAAUCAACCCUAUAUTT-3 ' |
| | Antisense,
5'-AUAUAGGGUUGAUUACGGCTT-3' |
| AEG-1 siRNA2 | Sense,
5'-GCUGUUCGAACACCUCAAATT-3' |
| | Antisense,
5'-UUUGAGGUGUUCGAACAGCTT-3' |
| AEG-1 siRNA3 | Sense,
5'-GCCAUCUGUAAUCUUAUCATT-3' |
| | Antisense,
5'-UGAUAAGAUUACAGAUGGCTT-3' |
| Scrambled
siRNA | Sense,
5'-GACGCATATTGACCTCACTAT-3' |
| | Antisense,
5'-ATAGTGAGGTCAATATGCGTC-3' |
| AEG-1 shRNA |
5'-GCTGTTCGAACACCTCATATTCAAGAGATATGAGGTGTTCGAACAGCTTTTTT-3' |
| Scrambled
shRNA |
5'-GACGCATATTGACCTCACATTCAAGAGATGTGAGGTCAATATGCGTCTTTTTT-3' |
siRNA transfection
U87 cells were trypsinized and seeded in 6-well
plates (Corning Inc.) at a density of 1x106 cells/well.
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) was used for the transfection of siRNA into cells
according to the manufacturer's protocols. The negative control
cells were transfected with a scrambled siRNA (Takara Bio, Inc.),
while the other two groups were transfected with AEG-1 siRNA1,
AEG-1 siRNA2 or AEG-1 siRNA3. The transfection was performed at
room temperature for 15 min. After incubation for 48 h at 37˚C, the
transfected cells were analyzed. The knockdown efficiency of siRNAs
was verified using reverse transcription-quantitative PCR (RT-qPCR)
and western blotting. The siRNA with the highest knockdown
efficiency was selected to construct the AEG-1 short hairpin
(sh)RNA lentiviral particle, while the scrambled siRNA was used to
construct the sh-control lentiviral particle.
AEG-1 shRNA lentiviral infection
The pLVX-shRNA2-puro (cat. no. VT2240), psPAX2 (cat.
no. VT1444) and pMD2.G plasmids (cat. no. VT1443) were purchased
from Hunan Keai Medical Equipment Co., Ltd (https://www.youbio.cn/product/). According to the
selected siRNA sequence, the shRNA sequence was constructed and
cloned into the pLVX-shRNA2-puro plasmid and lentiviral particles
were produced via the transduction of 293T cells with 3 µg of AEG-1
shRNA plasmid, 1.5 µg psPAX2 and 1.5 µg pMD2.G. Lentiviral
supernatants were then collected after 48 h. The construction of
sh-control lentiviral vectors was conducted as aforementioned. U87
cells were then infected with concentrated AEG-1 shRNA or
sh-control lentiviral vectors (MOI=10), and the stable transfected
cell line was selected using 1 µg/ml puromycin (Gibco; Thermo
Fisher Scientific, Inc.).
RT-qPCR
The U87 cells in each group were harvested 48 h
after infection with AEG-1 shRNA lentivirus. The cells were then
lysed and the total RNA was isolated using an RNA Fast 200 kit
(Shanghai Fastagen Biotechnology Co., Ltd.) according to the
manufacturer's instructions. RNA was reverse-transcribed using
PrimeScript™ RT Master Mix kit (Takara Bio, Inc.). The temperature
protocol for reverse transcription was 37˚C for 15 min and 85˚C for
5 sec). qPCR was performed using SYBR® Premix Ex Taq II
(Takara Bio, Inc.) on an iQ5 thermal cycler (Bio-Rad Laboratories,
Inc.). The thermocycling conditions were as follows: Initial
denaturation at 95˚C for 3 min, followed by 40 cycles of 95˚C, for
5 sec, 63˚C for 30 sec and 72˚C for 15 sec and analyzed using iQ5
software, version 2.0 (Bio-Rad Laboratories, Inc.). The data were
normalized to the expression of β-actin. Alterations in gene
expression were evaluated using the
2-∆∆Cq method (11).
Western blotting
The U87 cells in each group were harvested 48 h
after infection with AEG-1 shRNA lentivirus. The extraction of
total cellular proteins and the western blot analysis were
performed as described previously (12). Briefly, the cells were washed twice
with PBS and scraped on ice following the addition of 300 µl RIPA
buffer (Beyotime Institute of Biotechnology) with 1 mmol/l
phenylmethylsulfonyl fluoride. Protein concentration was determined
using a bicinchoninic acid protein assay kit (Beyotime Institute of
Biotechnology). The samples (40 µg) were boiled in 1X SDS-PAGE
sample loading buffer, resolved using 10% SDS-PAGE and transferred
onto polyvinylidene fluoride (PVDF) membranes (EMD Millipore). The
membranes were then blocked in TBS-0.1% Tween-20 (TBST) containing
5% non-fat dry milk at room temperature for 2 h. The membranes were
probed with primary antibodies overnight at 4˚C and incubated with
a secondary polyclonal anti-rabbit immunoglobulin G (IgG)
antibodies conjugated to horseradish peroxidase (cat. no. BA1054;
1:5,000; Boster Biological Technology;) at 37˚C for 2 h. Membranes
were developed using SuperSignal™ West Pico PLUS Chemiluminescent
Substrate (Thermo Fisher Scientific, Inc.). The primary antibodies
employed for western blotting were as follows: Rabbit anti-human
MMP-2 (dilution, 1:500; cat. no. BS1236) and rabbit anti-human
GAPDH (dilution, 1:5,000; cat. no. ap0063), which were purchased
from Bioworld Technology, Inc. Rabbit anti-human AEG-1 antibody was
purchased from ProteinTech Group, Inc. (dilution, 1:1,000; cat. no.
13860-1-AP). Rabbit anti-human VEGF antibody was purchased from
Abcam (dilution, 1:1,000; cat. no. ab53465).
In vitro VM formation assay
A Matrigel-based tube formation assay (13) was conducted to analyze VM formation
in vitro. Briefly, Matrigel (Becton, Dickinson and Company)
was maintained at 4˚C for 24 h, after which 0.3 ml of Matrigel was
evenly plated to 24-well plates (Corning Inc.) and precooled at
-20˚C for ≥10 min and then incubated for 30 min at 37˚C.
Subsequently, U87 cells (3x104 cells/well) were seeded
onto the Matrigel coated wells 48 h following transduction and
incubated for 24 h in a 5% CO2 atmosphere at 37˚C. Each
well was analyzed directly under an inverted fluorescent microscope
(magnification, x100; IX51; Olympus Corporation). VM formation was
determined by counting the number of tubes in each well. The tubes
comprised a closed cavity with a loop structure, a polygon shape or
irregular shape under a light microscope.
Animal studies
The establishment of intracranial xenograft models
was conducted as described previously (10). A total of 30 nude rats were randomly
divided into three groups (10 rats/group). Animals were
anaesthetized with intraperitoneal injection of 30 mg/kg 1%
pentobarbital sodium. The AEG-1 shRNA and negative control groups
were injected intracranially with AEG-1 shRNA or sh-control
lentivirus-infected U87 cells. The control group was injected with
normal U87 cells. All tumor-bearing rats were sacrificed 3 weeks
following tumor cell implantation. The euthanasia method was
intraperitoneal injection of 200 mg/kg pentobarbital sodium
solution. Cardiac arrest was used to identify death. The humane
endpoints included labored breathing, inability to remain upright,
impaired mobility, hunched posture for more than 48 h and no
response to external stimuli. Rats were perfused with 4%
paraformaldehyde for 1 h at 4˚C immediately after euthanasia,
before the brain and tumor samples were obtained and fixed
overnight with 4% paraformaldehyde at 4˚C. Paraffin sections were
prepared and the thickness of sections was 4 µm.
VM formation detection in xenograft
models
CD34 and Periodic Acid-Schiff (PAS) double-staining
was performed to detect VM channels. An immunohistochemical assay
kit (Boster Biological Technology) was employed for the
immunohistochemical staining of CD34. All procedures were performed
according to the manufacturer's protocols. The sections were
incubated overnight with rabbit anti-CD34 antibody (1:200; Boster
Biological Technology; cat. no. BA3414) at 4˚C. The negative
control sections were incubated with PBS instead of antibody.
Antibody localization was determined using a 3,3'-diaminobenzidine
substrate kit (Boster Biological Technology). Normal vascular
endothelium was used as a positive control.
Following immunohistochemical staining, the sections
were exposed to sodium periodate for 5 min and then washed with
distilled water three times. Subsequently, the sections were
incubated with PAS at room temperature for 15 min. All sections
were counterstained with hematoxylin at room temperature for 2 min.
VM channels were characterized as CD34-/PAS+
lumen structures. The average number of VM channels were calculated
from the analysis of five fields under the light microscope at high
magnification (x400).
Statistical analysis
Values are presented as the mean ± SD and data were
analyzed using SPSS 17.0 software (SPSS, Inc.). One-way ANOVAs were
used to compare the groups and the Least Significant Difference or
Tukey's post-hoc tests were performed to further determine
inter-group comparisons. P<0.05 was considered to indicate a
statistically significant difference.
Results
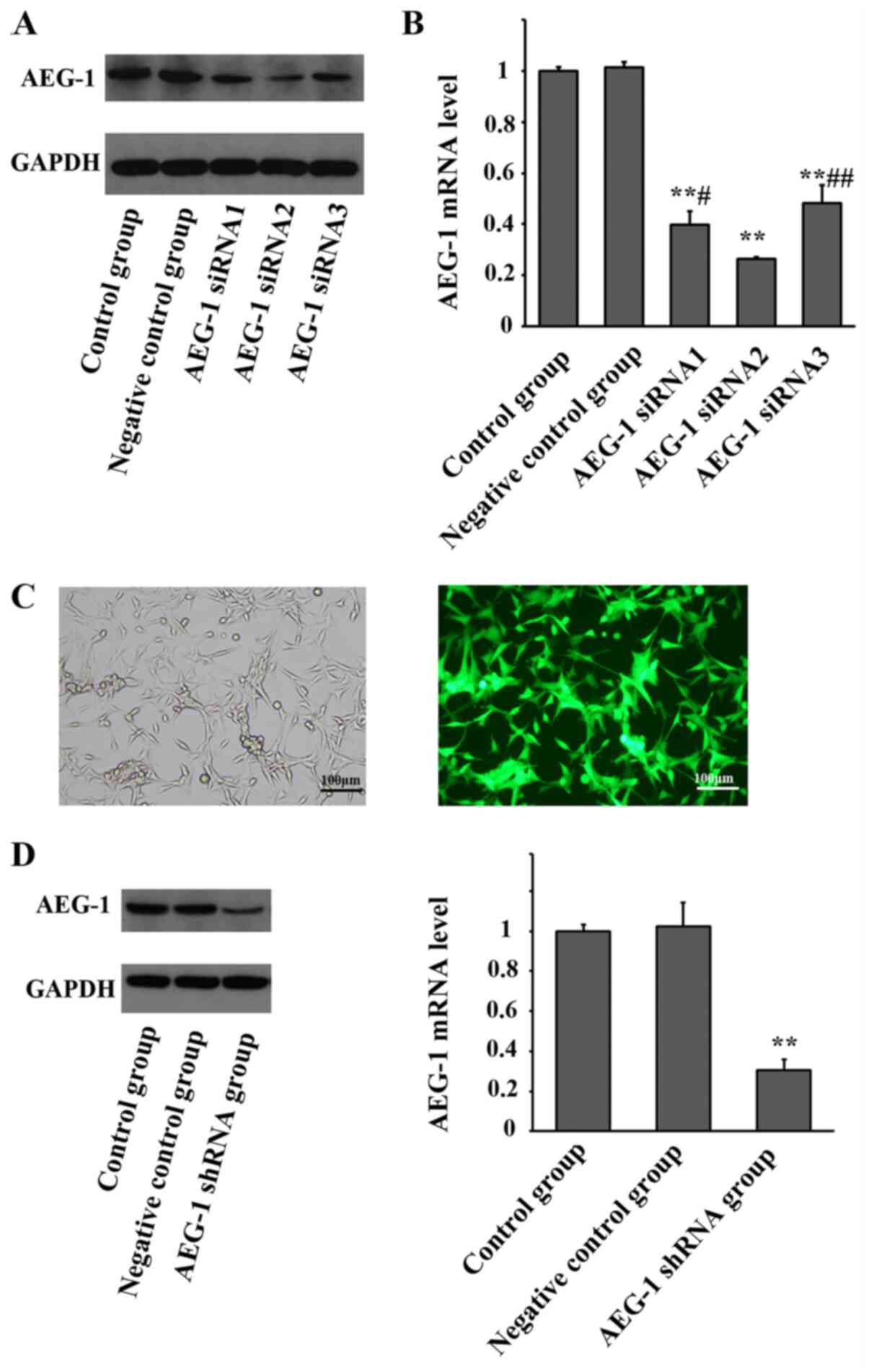
AEG-1 siRNA and AEG-1 shRNA lentivirus
inhibits AEG-1 expression in U87 glioma cells
As presented in Fig.
1, after transduction with AEG-1 siRNAs, the mRNA and protein
expression levels of AEG-1 were significantly downregulated in U87
glioma cells, compared with the control and negative control groups
(F=1080.231, P<0.001). AEG-1 siRNA2 exhibited the highest
knockdown efficiency among the three AEG-1 siRNAs (P=0.035 vs.
siRNA1; P=0.002 vs. siRNA3) and was selected to construct the AEG-1
shRNA lentiviral particle employed for subsequent experiments.
After infection with AEG-1 shRNA lentivirus, green fluorescence can
be clearly observed in the U87 glioma cells. The expression of
AEG-1 was significantly downregulated in the stably transfected U87
glioma cells, compared with the control and negative control groups
(F=85.082, P<0.001).
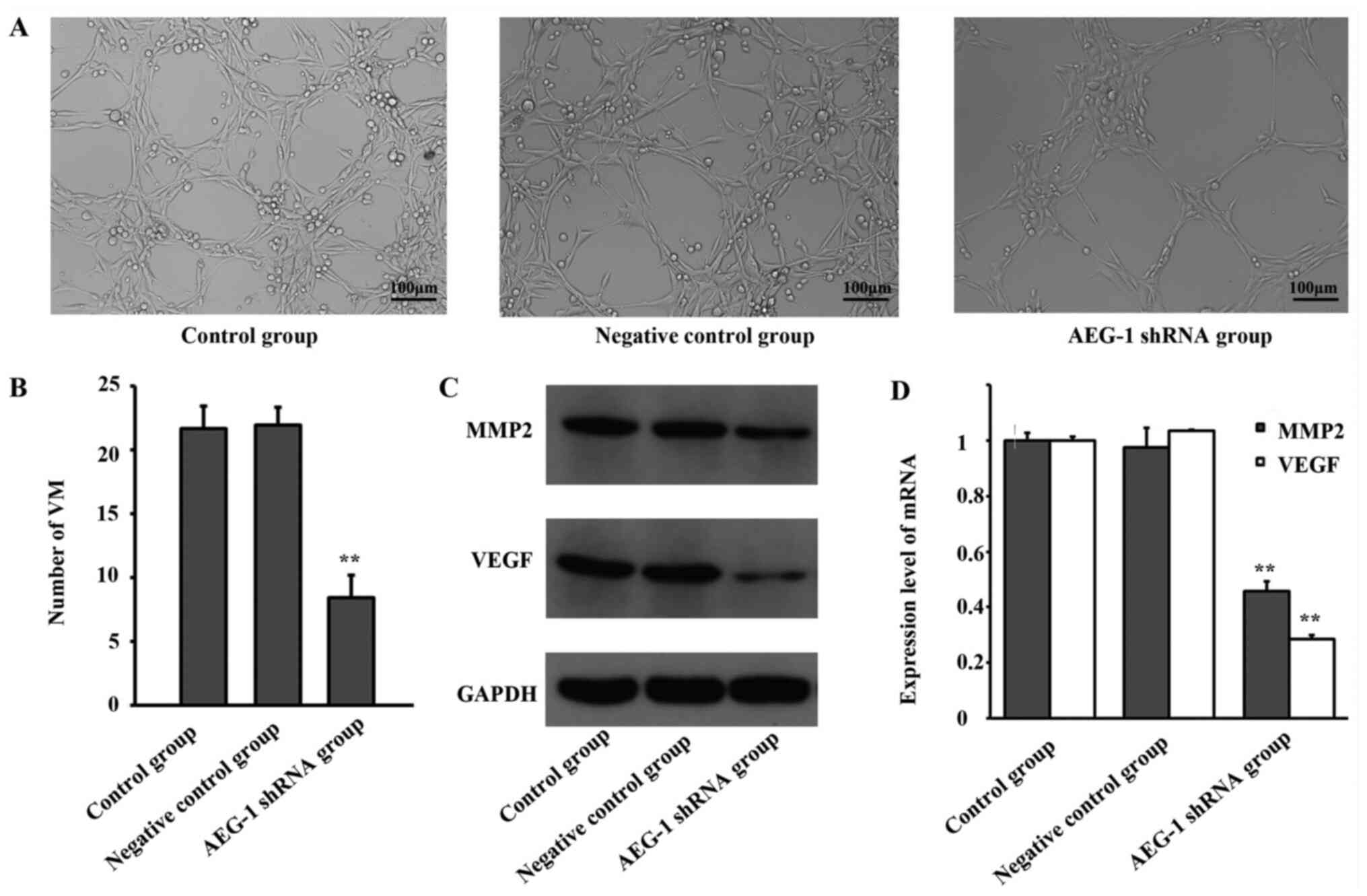
Downregulation of AEG-1 expression
inhibits VM formation of U87 glioma cells in vitro
The VM formation of U87 glioma cells was observed
in vitro following infection with AEG-1 shRNA lentivirus. As
presented in Fig. 2A and B, downregulation of AEG-1 expression
significantly inhibited the VM formation of U87 glioma cells,
compared with control groups (F=65.396, P<0.001).
Downregulation of AEG-1 expression
inhibits MMP-2 and VEGF expression in U87 glioma cells
As MMP-2 and VEGF have been reported to be involved
in VM formation in tumors (14),
the expression levels of these genes in U87 cells were detected
using RT-qPCR and western-blotting. As presented in Fig. 2C and D, downregulation of AEG-1 significantly
inhibited the expression of VEGF and MMP-2 (F=267.471, P<0.001
for VEGF mRNA; F=3671.526, P<0.001 for MMP2 mRNA).
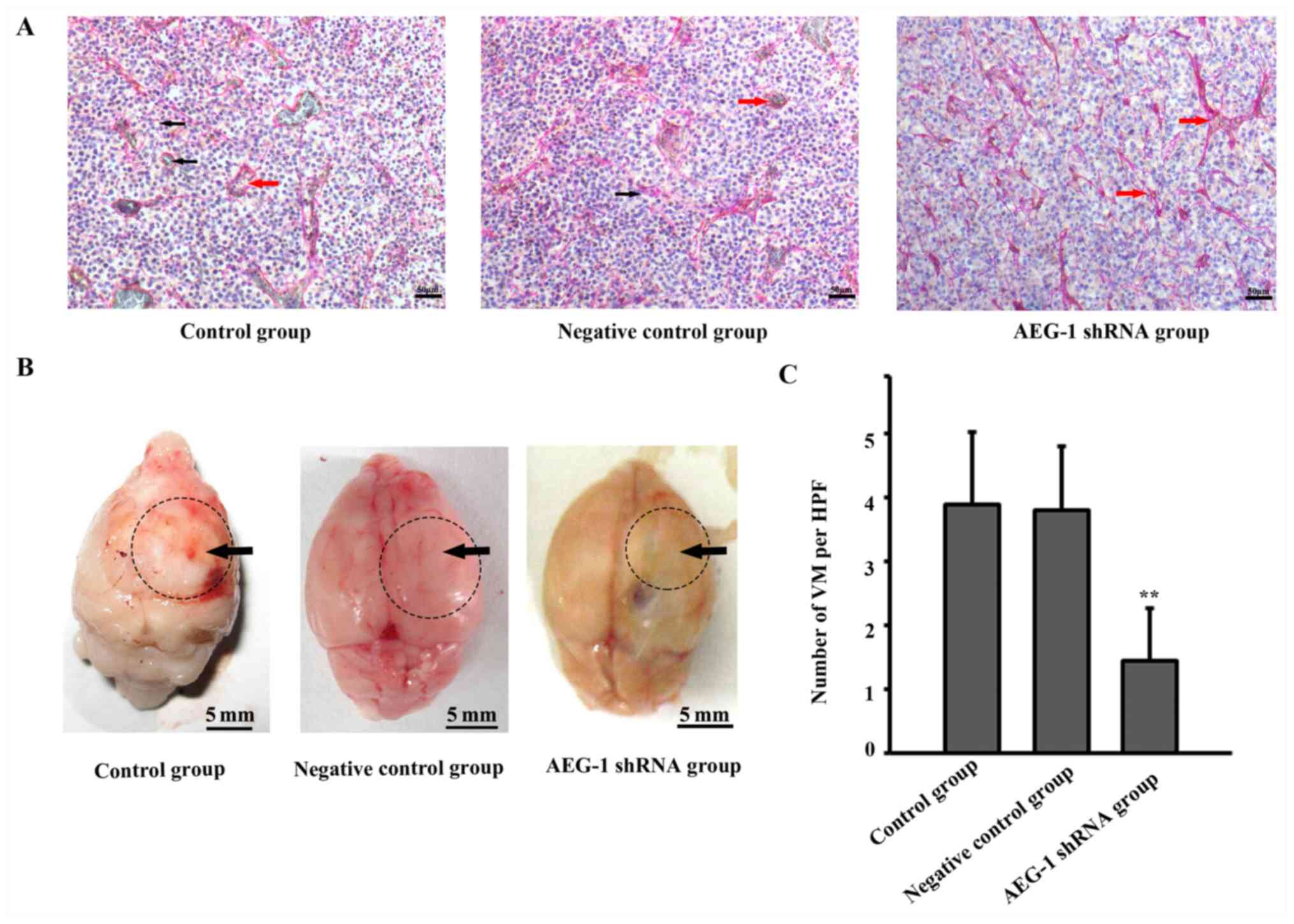
Downregulation of AEG-1 expression
inhibits VM formation in a glioma xenograft model
CD34 and PAS double-staining was used to detect VM
formation in a glioma xenograft model. As presented in Fig. 3A, the
CD34-/PAS+ lumen structures were identified
in tumors formed by normal U87 cells. Intracranial glioma
xenografts of each group were shown in Fig. 3B, black arrows represented the
tumors. In general, the tumors in AEG-1 shRNA group were smaller
compared with those in the other two groups. The number of VM
channels was significantly decreased in tumors formed by AEG-1
shRNA lentivirus-infected U87 cells (F=33.402, P<0.01).
Discussion
Anti-angiogenic therapy inhibits angiogenesis,
limiting the supply of oxygen and nutrients to tumor cells,
inhibiting tumor growth (15). The
effects of antiangiogenic drugs are unsatisfactory due to therapy
resistance (16). Current
antiangiogenic strategies mainly target the blood vessels formed by
ECs; however, tumors rely on not only EC-based vessels, but the
vasculogenic networks formed by tumor cells for nutrients (15). This type of structure is reported as
VM, whereby the function of ECs is mimicked by tumor cells to form
vasculogenic networks (6). VM has
been observed in a variety of human tumors, such as gliomas, and is
usually associated with a poor patient prognosis (7). Tumors exhibiting a high degree of VM
appear to be more malignant, with an increased tendency for
invasion and metastasis (17).
Various molecular mechanisms and signaling pathways have been
associated with VM formation and agents targeting these pathways
have specific anti-tumor effects (7).
Among all the related molecules, MMP-2 appears to be
crucial for the formation of VM. MMP-2 is associated with VM
formation in several types of human tumors, including sebaceous
carcinoma (18), intracranial
hemangiopericytoma (19) and breast
cancer (20). The mechanisms of
action underlying the effects of MMP-2 on promoting the development
of VM mainly involve the remodeling of the ECM (21). MMP-2 activation leads to the
cleavage of the laminin-5γ2 chain into fragments, which are
deposited in the ECM, contributing to ECM plasticity; cellular
migration and invasion; and VM formation (22). The focal adhesion
kinase-ERK1/2(23) and PI3K/Akt
signaling pathways (24) have been
reported to regulate the expression and activation of MMP-2 during
VM formation. The regulation of MMP-2 expression can notably affect
VM formation (20,25); thus, the anti-VM strategy aimed at
MMP-2 may be effective. Certain studies have suggested that the
inhibition of MMP-2 expression can significantly suppress VM
formation (26,27).
Several studies have demonstrated that VEGF is of
great importance in the formation of VM. VEGF can upregulate the
expression of VE-cadherin, ephrin-type A receptor 2 and MMPs,
contributing to ECM remodeling and the development of VM (28). The ERK1/2(25) and PI3K/Akt signaling pathways
(29) have been reported to serve
an essential role in VM formation induced by VEGF. Downregulation
of VEGF has been reported to significantly inhibit VM formation
(29,30).
AEG-1 is considered as an oncogene in the induction
of autophagy, invasion, metastasis, angiogenesis and drug
resistance (8); however, the
association between AEG-1 and VM formation remains unknown. The
results of the present study demonstrated that AEG-1 may serve a
crucial role for the formation of VM in glioma. The inhibition of
AEG-1 expression by siRNA significantly suppressed VM formation of
gliomas in vitro and in vivo. Additionally, the
expression levels of VEGF and MMP-2 were significantly
downregulated following the inhibition of AEG-1 expression. As VEGF
and MMP-2 are two important inducers of VM (14), the mechanisms of VM inhibition
through AEG-1 downregulation may partly be associated with the
regulation of the two genes. However, whether the mechanism of
action which underlies the regulation of VEGF and MMP-2 expression
by AEG-1 in the development of VM in glioma requires further
investigation. It has been suggested that AEG-1 may regulate the
expression of VEGF and MMP-2 through the NF-κB pathway (31,32).
In conclusion, in the present study, downregulation
of AEG-1 expression was determined to significantly inhibit the
development of VM in glioma in vitro and in vivo.
These observations may be associated with the regulation of VEGF
and MMP-2 expression; however, the underlying mechanism of action
require further research. However, there were limitations in the
present study. Because this is a preliminary study investigating
the relationship between AEG-1 and VM formation in glioma, only one
representative glioma cell line was used to demonstrate the
hypothesis. More glioma cell lines and primary patient-derived
cells will be used in further studies to verify the present
results. Furthermore, the present study doesn't include any
clinical data. Increasing the number study of clinical cases;
investigating the relationship between AEG-1 expression, VM
formation and prognosis; and analysing its clinical significance
should be performed in the further studies
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural
Science Foundation of Shaanxi Province (grant no. 2017JQ8037) and
the National Natural Science Foundation of China (grant no.
81572485).
Availability of data and materials
Not applicable.
Authors' contributions
CL performed animal studies and was a major
contributor in writing the manuscript. JS performed molecular
biology studies. LY performed statistical analysis. SG made
substantial contributions to the design of the present study. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
This research has been approved by the Research
Ethics Committee of Xi'an Jiaotong University Health Science Center
(approval no. 2018048).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ostrom QT, Cioffi G, Gittleman H, Patil N,
Waite K, Kruchko C and Barnholtz-Sloan JS: CBTRUS Statistical
Report: Primary Brain and Other Central Nervous System Tumors
Diagnosed in the United States in 2012-2016. Neuro-oncol. 21 (Suppl
5):v1–v100. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Stupp R, Taillibert S, Kanner AA, Kesari
S, Steinberg DM, Toms SA, Taylor LP, Lieberman F, Silvani A, Fink
KL, et al: Maintenance therapy with tumor-treating fields plus
temozolomide vs temozolomide alone for glioblastoma: a randomized
clinical trial. JAMA. 314:2535–2543. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Komori T: The 2016 WHO Classification of
Tumours of the Central Nervous System: The Major Points of
Revision. Neurol Med Chir (Tokyo). 57:301–311. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ameratunga M, Pavlakis N, Wheeler H, Grant
R, Simes J and Khasraw M: Anti-angiogenic therapy for high-grade
glioma. Cochrane Database Syst Rev. 11(CD008218)2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Blumenthal DT, Kanner AA, Aizenstein O,
Cagnano E, Greenberg A, Hershkovitz D, Ram Z and Bokstein F:
Surgery for Recurrent High-Grade Glioma After Treatment with
Bevacizumab. World Neurosurg. 110:e727–e737. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
El Hallani S, Boisselier B, Peglion F,
Rousseau A, Colin C, Idbaih A, Marie Y, Mokhtari K, Thomas JL,
Eichmann A, et al: A new alternative mechanism in glioblastoma
vascularization: Tubular vasculogenic mimicry. Brain. 133:973–982.
2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chen YS and Chen ZP: Vasculogenic mimicry:
A novel target for glioma therapy. Chin J Cancer. 33:74–79.
2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zou M, Zhu W, Wang L, Shi L, Gao R, Ou Y,
Chen X, Wang Z, Jiang A, Liu K, et al: AEG-1/MTDH-activated
autophagy enhances human malignant glioma susceptibility to
TGF-β1-triggered epithelial-mesenchymal transition. Oncotarget.
7:13122–13138. 2016.
|
|
9
|
Noch E, Bookland M and Khalili K:
Astrocyte-elevated gene-1 (AEG-1) induction by hypoxia and glucose
deprivation in glioblastoma. Cancer Biol Ther. 11:32–39.
2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Yang Y, Wu J, Guan H, Cai J, Fang L, Li J
and Li M: MiR-136 promotes apoptosis of glioma cells by targeting
AEG-1 and Bcl-2. FEBS Lett. 586:3608–3612. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Liang C, Guo S and Yang L: Effects of all
trans retinoic acid on VEGF and HIF 1α expression in glioma cells
under normoxia and hypoxia and its anti angiogenic effect in an
intracerebral glioma model. Mol Med Rep. 10:2713–2719.
2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Francescone RA, Faibish M and Shao R: A
matrigel-based tube formation assay to assess the vasculogenic
activity of tumor cells. J Vis Exp. 55(e3040)2011.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
Hernández de la Cruz ON, López-González
JS, García-Vázquez R, Salinas-Vera YM, Muñiz-Lino MA,
Aguilar-Cazares D, López-Camarillo C and Carlos-Reyes Á: Regulation
networks driving vasculogenic mimicry in solid tumors. Front Oncol.
9(1419)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Haibe Y, Kreidieh M, El Hajj H, Khalifeh
I, Mukherji D, Temraz S and Shamseddine A: Resistance Mechanisms to
Anti-angiogenic Therapies in Cancer. Front Oncol.
10(221)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wu HB, Yang S, Weng HY, Chen Q, Zhao XL,
Fu WJ, Niu Q, Ping YF, Wang JM, Zhang X, et al: Autophagy-induced
KDR/VEGFR-2 activation promotes the formation of vasculogenic
mimicry by glioma stem cells. Autophagy. 13:1528–1542.
2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ge H and Luo H: Overview of advances in
vasculogenic mimicry - a potential target for tumor therapy. Cancer
Manag Res. 10:2429–2437. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Xu X, Jia R, Zhou Y, Song X and Fan X:
Investigation of vasculogenic mimicry in sebaceous carcinoma of the
eyelid. Acta Ophthalmol. 88:e160–e164. 2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhang Z, Han Y, Zhang K and Teng L:
Investigation of vasculogenic mimicry in intracranial
hemangiopericytoma. Mol Med Rep. 4:1295–1298. 2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Robertson FM, Simeone AM, Lucci A,
McMurray JS, Ghosh S and Cristofanilli M: Differential regulation
of the aggressive phenotype of inflammatory breast cancer cells by
prostanoid receptors EP3 and EP4. Cancer. 116 (Suppl):2806–2814.
2010.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hendrix MJ, Seftor EA, Hess AR and Seftor
RE: Vasculogenic mimicry and tumour-cell plasticity: Lessons from
melanoma. Nat Rev Cancer. 3:411–421. 2003.PubMed/NCBI View
Article : Google Scholar
|
|
22
|
Seftor RE, Seftor EA, Koshikawa N, Meltzer
PS, Gardner LM, Bilban M, Stetler-Stevenson WG, Quaranta V and
Hendrix MJ: Cooperative interactions of laminin 5 gamma2 chain,
matrix metalloproteinase-2, and membrane
type-1-matrix/metalloproteinase are required for mimicry of
embryonic vasculogenesis by aggressive melanoma. Cancer Res.
61:6322–6327. 2001.PubMed/NCBI
|
|
23
|
Qiao L, Liang N, Zhang J, Xie J, Liu F, Xu
D, Yu X and Tian Y: Advanced research on vasculogenic mimicry in
cancer. J Cell Mol Med. 19:315–326. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Hess AR, Seftor EA, Seftor RE and Hendrix
MJ: Phosphoinositide 3-kinase regulates membrane type 1-matrix
metalloproteinase (MMP) and MMP-2 activity during melanoma cell
vasculogenic mimicry. Cancer Res. 63:4757–4762. 2003.PubMed/NCBI
|
|
25
|
Liu Y, Li F, Yang YT, Xu XD, Chen JS, Chen
TL, Chen HJ, Zhu YB, Lin JY, Li Y, et al: IGFBP2 promotes
vasculogenic mimicry formation via regulating CD144 and MMP2
expression in glioma. Oncogene. 38:1815–1831. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Bai R, Ding T, Zhao J, Liu S, Zhang L, Lan
X, Yu Y and Yin L: The effect of PI3K inhibitor LY294002 and
gemcitabine hydrochloride combined with ionizing radiation on the
formation of vasculogenic mimicry of Panc-1 cells in vitro and in
vivo. Neoplasma. 63:80–92. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhang S, Li M, Gu Y, Liu Z, Xu S, Cui Y
and Sun B: Thalidomide influences growth and vasculogenic mimicry
channel formation in melanoma. J Exp Clin Cancer Res.
27(60)2008.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wang JY, Sun T, Zhao XL, Zhang SW, Zhang
DF, Gu Q, Wang XH, Zhao N, Qie S and Sun BC: Functional
significance of VEGF-a in human ovarian carcinoma: Role in
vasculogenic mimicry. Cancer Biol Ther. 7:758–766. 2008.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Qin L, Ren Y, Chen AM, Guo FJ, Xu F, Gong
C, Cheng P, Du Y and Liao H: Peroxisome proliferator-activated
receptor γ ligands inhibit VEGF-mediated vasculogenic mimicry of
prostate cancer through the AKT signaling pathway. Mol Med Rep.
10:276–282. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Mei J, Gao Y, Zhang L, Cai X, Qian Z,
Huang H and Huang W: VEGF-siRNA silencing induces apoptosis,
inhibits proliferation and suppresses vasculogenic mimicry in
osteosarcoma in vitro. Exp Oncol. 30:29–34. 2008.PubMed/NCBI
|
|
31
|
Yu JQ, Zhou Q, Zhu H, Zheng FY and Chen
ZW: Overexpression of astrocyte elevated gene-1 (AEG-1) in cervical
cancer and its correlation with angiogenesis. Asian Pac J Cancer
Prev. 16:2277–2281. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Huang LL, Wang Z, Cao CJ, Ke ZF, Wang F,
Wang R, Luo CQ, Lu X and Wang LT: AEG-1 associates with metastasis
in papillary thyroid cancer through upregulation of MMP2/9. Int
JOncol. 51:812–822. 2017.PubMed/NCBI View Article : Google Scholar
|