Introduction
Atherosclerosis (AS) is a multifactorial disease
that is closely associated with aging and involves a number of
lipid metabolism disorders (1).
Proprotein convertase subtilisin/kexin type 9 (PCSK9) is a key
regulator of cholesterol metabolism, can promote the degradation of
plasma low-density lipoprotein receptor (LDLR) and increase the
level of low-density lipoprotein cholesterol (LDL-C), leading to an
increased risk of AS (2).
Lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1) is
the main receptor of oxidized low-density lipoprotein (ox-LDL),
which interacts with ox-LDL induced endothelial dysfunction,
macrophage-derived foam cell formation and LOX-1 activation to
promote the formation of atherosclerotic plaques and acute
cardiovascular events (3). It has
been previously reported that LOX-1 expression is enhanced by the
overexpression of PCSK9 in human aortic endothelial cells (ECs),
smooth muscle cells (SMCs) and mouse aorta, whereas the LOX-1
expression is reduced by silencing PCSK9(4). Within patients with AS, free radicals
and lipid peroxidation that is produced by lipid oxidation can
cause cell necrosis and apoptosis (5). Tumor suppressor protein p53 (p53),
cyclin-dependent kinase inhibitor 1A (p21), and multiple tumor
suppressor-1 (p16) are associated with the cell cycle regulation,
apoptosis and senescence, which are also associated with AS
(6). A previous study demonstrated
that low shear stress that is mediated by reactive oxygen species
(ROS) can enhance the expression of PCSK9 in human ECs and SMCs,
whereas silencing ROS can inhibit PCSK9 expression and reduce the
effect of LOX-1(7). In addition,
PCSK9 serves an important role in the process of vascular aging,
which can aggravate endothelial dysfunction caused by oxidative
stress and inflammation by stimulating LOX-1 in order to further
accelerate the pathological changes of AS (8).
Fisetin (3,3',4',4,7-tetrahydroxyflavone) is a
natural flavonoid molecule that can regulate abnormal lipid
metabolism, anti-oxidation and anti-aging activities (9). Fisetin has been revealed to regulate
obesity and cardiovascular disease by promoting the expression of
adiponectin in 3T3-L1 preadipocytes (10). Fisetin can also effectively improve
lipid metabolism, oxidative stress and liver injury in mice with
metabolic syndrome that is induced by a high-fructose diet
(11). In a previous study, fisetin
was revealed to reduce the expression of p16 and p21 in progeroid
Ercc1-/∆ mice and aged wild-type mice, as well as
in human adipose tissue senescent cells (12). Our previous study demonstrated that
fisetin can limit ox-LDL-induced lipid accumulation and senescence
in RAW264.7 macrophage-derived foam cells (13). Based on the previous study, the
current study explored how fisetin may regulate lipid metabolism
via PCSK9 and LOX-1 as well as improve the aging status of AS in
apoE-/- mice. An AS model was established in
apoE-/- mice, which was induced by a high-fat diet. The
pathological morphology, lipid accumulation and collagen deposition
of the aortic sinus was subsequently detected in mice.
Additionally, serum levels of total cholesterol (TC), triglyceride
(TG), high-density lipoprotein cholesterol (HDL-C), LDL-C, very
low-density lipoprotein cholesterol (VLDL-C), ox-LDL, superoxide
dismutase (SOD) and malondialdehyde (MDA), alanine aminotransferase
(ALT) and aspartate aminotransferase (AST) activities were
determined and PCSK9, LOX-1, p53, p21 and p16 expressions in the
aorta were assessed.
Materials and methods
Experimental animals
In the current study, a total of 54 12-week-old
specific pathogen-free (SPF) male apoE-/- mice [weight,
20±5 g; animal license no. SCXK (Jing) 2016-0010] and 18 wild-type
male C57BL/6 mice of the same age and genetic background [animal
license no. SCXK (Zhe) 2019-0001] were purchased from Beijing Vital
River Lab Animal Technology Co., Ltd. The mice were kept in an SPF
grade animal facility at the Shanghai Model Organisms Center, Inc.
(Shanghai, China) at a room temperature of 19-22°C,
relative humidity of 50-70% and a 12 h light/dark cycle. All
animals had free access to food and water, and experimental
operations strictly followed the rules and regulations of the
Shanghai Model Organisms Center, Inc. The protocol of the present
study was approved by the Animal Care and Use Committee of the
Shanghai Model Organisms Center, Inc. (IACUC no. 2019-0008).
Modeling and grouping
54 apoE-/- 12-week-old mice were fed a
normal diet for 1 week before being randomly divided into three
groups: apoE-/- mice + high-fat diet (model group;
n=18), apoE-/- mice + high-fat diet + fisetin (fisetin
group; n=18) and the apoE-/- mice + high-fat diet +
atorvastatin (atorvastatin group; n=18). The high-fat diet formula
was a normal mouse diet (0.03% corn, 0.15% secondary meal, 0.03%
alfalfa, 0.08% soybean meal, 0.07% Peru fish meal, 0.05% American
chicken meal, 0.04% animal premix 1, 0.02% gluten, 0.005% calcium
dihydrogen phosphate, 0.5% rice, 0.005% stone powder, 0.02% salad
oil, 0.0045% feed grade sodium chloride and 0.0015% feed grade
magnesium chloride) with the addition of 21% fat and 0.5%
cholesterol. Wild-type C57BL/6 mice of the same genetic background
and age were used as the controls (control group, n=18) and were
fed a normal mouse diet. Mice in the fisetin and atorvastatin
groups were gavaged with aqueous solutions of fisetin and
atorvastatin, respectively. Via conversion with reference to an
adult body weight of 60 kg and the equivalent dose of mice, the
final doses of fisetin and atorvastatin aqueous solutions provided
to mice were 12.5 and 2 mg/kg, respectively. The fisetin was
dissolved in 0.1% DMSO aqueous solution, and the atorvastatin
tablets as a positive control drug were directly dissolved in
distilled water. The control and model groups were treated with the
same volume (0.2 ml/mouse/day) of distilled water via daily oral
gavage, and the intervention period of each group was 12 weeks.
Drugs and reagents
Fisetin (20 mg; cat. no. B20953) was purchased from
Shanghai Yuanye Biotechnology Co., Ltd. Atorvastatin tablets (20
mg; cat. no. H20051408) were purchased from Pfizer, Inc. DMSO (cat.
no. 472301) was obtained from Sigma Aldrich; Merck KGaA. The
protein ladder (cat. no. 26616) was obtained from Thermo Fisher
Scientific, Inc. The BCA Protein Assay kit (cat. no. P0010) and
SDS-PAGE Gel Preparation kit (cat. no. P0012A) were purchased from
Beyotime Institute of Biotechnology. Rabbit anti-PCSK9 (cat. no.
ab95478), mouse anti-p53 (cat. no. ab26), rabbit anti-p21 (cat. no.
ab188224) and rabbit anti-p16 (cat. no. ab51243) were purchased
from Abcam. Mouse anti-LOX-1 (cat. no. AF1564) was supplied by
R&D Systems, Inc. Rabbit anti-GAPDH (cat. no. 5174S), goat
anti-rabbit IgG horseradish peroxidase (HRP)-conjugated secondary
antibody (cat. no. 7074P2) and horse anti-mouse IgG HRP-linked
secondary antibody (cat. no. 7076P2) were purchased from Cell
Signaling Technology, Inc. Mouse TC (cat. no. Ek-M20591), mouse TG
(cat. no. Ek-M20590), mouse HDL-C (cat. no. Ek-M20589), mouse LDL-C
(cat. no. Ek-M20588), mouse VLDL-C (cat. no. Ek-M21066), mouse
ox-LDL (cat. no. Ek-M21214), SOD (cat. no. Ek-M21269) and MDA (cat.
no. Ek-M21268) ELISA kits were purchased from Ek-Bioscience
Biotechnology Co., Ltd. An ALT Assay kit (cat. no. C009-2-1) and an
AST Assay kit (cat. no. C010-2-1) were purchased from Nanjing
Jiancheng Bioengineering Institute.
Hematoxylin and eosin (H&E), Oil
Red O and Masson staining of the aortic sinus
Mice (36 apoE-/- mice and 12 C57BL/6
mice) were anesthetized by intraperitoneal injection of 2%
pentobarbital sodium (50 mg/kg) and euthanized by infusion with 10%
formalin. The heart and blood vessels between the thoracic aorta
and abdominal aorta were removed and preserved in 10% formalin
(fixed in 10% formalin at 4°C for one day) for
paraffinization or frozen sectioning, in which paraffin sections
(thickness of sections: 5 µm) were used for H&E staining
(hematoxylin staining solution, 5 min; eosin staining solution, 5
min; both room temperature) and Masson staining (Masson Stain kit,
cat. no. D026-1-3; Nanjing Jiancheng Bioengineering Institute;
nuclear staining solution 60 sec, slurry staining solution 60 sec,
color separation solution 8 min, counterstain solution 5 min; all
room temperature). Frozen sections (thickness of sections: 10-15
µm) were used for Oil Red O staining (Oil Red O solution; 10 min;
room temperature), and the pathological morphology, lipid
accumulation and collagen deposition of mice aorta were observed.
The H&E and Masson staining images were observed using an
optical microscope (BX51; Olympus Corporation; magnification, x40,
x100, x200), and the Oil Red O staining images were acquired using
a scanner (Panoramic MIDI; 3DHISTECH). The images were
semi-quantitatively analyzed using Image-Pro Plus Version 6.0
(Media Cybernetics, Inc.) as follows: Plaque area ratio = plaque
area/total aortic sinus lumen area; red-stained lipid area
ratio=red-stained area/total aortic sinus lumen area; and collagen
fiber content ratio = blue-stained area/total aortic sinus lumen
area.
Detection of lipids, SOD, MDA levels
and ALT and AST activities in the peripheral blood serum of
mice
Mice (18 apoE-/- mice and 6 C57BL/6 mice)
were anesthetized by intraperitoneal injection of 2% pentobarbital
sodium (50 mg/kg) after being fasted for 12 h. Blood was collected
from the retro-orbital plexus and mice were euthanized via cervical
dislocation. Blood was then incubated at 4°C for 12 h,
and centrifuged at 13,523 x g at 4˚C for 30 min to absorb the
serum. The serum levels of TC, TG, HDL-C, LDL-C, VLDL-C, ox-LDL,
SOD and MDA were detected according to the instructions of the
mouse TC, TG, HDL-C, LDL-C, VLDL-C, ox-LDL, SOD and MDA ELISA kits.
The optical density (OD) value of each sample was measured at a
wavelength of 450 nm. The activities of ALT and AST in the serum
were detected according to the instructions of the ALT and AST
Assay kits. The OD value of the sample was measured at a wavelength
of 510 nm. The standard curve was drawn according to the
manufacturer's instructions of mouse TC, TG, HDL-C, LDL-C, VLDL-C,
ox-LDL, SOD, MDA ELISA kits and ALT, AST Assay kits, with the
corresponding concentration range or activity obtained based on the
standard curve.
Detection of PCSK9, LOX-1, p53, p21
and p16 protein expression in the aorta
After sampling blood from the retro-orbital plexus
of mice, the blood vessels between the thoracic and abdominal aorta
were immediately collected. The total protein of mouse aorta was
extracted from aortic tissues with RIPA lysis buffer (cat. no.
P0013B; Beyotime Institute of Biotechnology) containing PMSF (cat.
no. ST506; Beyotime Institute of Biotechnology), samples were mixed
with 5X loading buffer (cat. no. P0015; Beyotime Institute of
Biotechnology) and quantified using the BCA Protein Assay kit. The
proteins were separated by SDS-PAGE Gel Preparation kit (percentage
of the gel, 12%; 80 V for 30 min followed by 120 V for 90 min),
transferred to a PVDF membrane using the wet transfer method (270
mA for 90 min), and blocked with 5% skim milk at room temperature
for 2 h. Membranes were then incubated overnight at 4°C
with rabbit anti-PCSK9 (74 kDa; 1:1,000), mouse anti-LOX-1 (52 kDa;
1:1,000), mouse anti-p53 (53 kDa; 1:1,000), rabbit anti-p21 (21
kDa; 1:1,000), rabbit anti-p16 (16 kDa; 1:1,000) and rabbit
anti-GAPDH (37 kDa; 1:3,000). The membrane was washed three times
(10 min each), incubated with the corresponding HRP-conjugated
secondary antibody (1:3,000) for 1 h at room temperature and then
washed three times additionally (10 min each). Luminescent solution
A and liquid B were mixed 1:1 according to the instructions of the
Tanon™ High-sig ECL Western Blotting Substrate kit
(Tanon Science & Technology Co., Ltd.). The membrane was
scanned using the iBright FL1000 Imaging System (Thermo Fisher
Scientific, Inc). ImageJ 1.48v (National Institutes of Health) was
used to analyze the integrated absorbance (IA) of the protein band
(IA = average light density value*area). The relative expression
level of the target protein was measured by dividing the IA of the
target protein by the IA of GAPDH.
Statistical analysis
Statistical analysis was performed using SPSS 24.0
(IBM Corp.), and figures were created using GraphPad Prism 8.0.2
(GraphPad Software, Inc.), Adobe Illustrator CC 23.0.2 (Adobe
Systems Inc.) and Adobe Photoshop CC 20.0.6 (Adobe Systems Inc.).
Results are presented as the means ± standard deviation (mean ±
SD). The differences among groups were analyzed using one-way
ANOVA, followed by least-significant difference (LSD) test.
Statistical analyses were two-tailed tests with α=0.05. P<0.05
was considered to indicate a statistically significant
difference.
Results
Lipid levels
Compared with the control group, the levels of TC,
LDL-C, VLDL-C, and ox-LDL increased in the model group, whereas the
level of HDL-C decreased (P<0.01; Table I), and no statistically significant
differences were observed in TG content. Compared with the model
group, the mice in the fisetin and atorvastatin groups exhibited
lower levels of TC, LDL-C, VLDL-C, and ox-LDL (P<0.01; Table I), but there were no significant
differences in the levels of TG and HDL-C (P>0.05; Table I). Additionally, there were no
significant differences between the fisetin and atorvastatin groups
(P>0.05; Table I).
 | Table ISerum lipid levels in peripheral
blood of mice in each group. |
Table I
Serum lipid levels in peripheral
blood of mice in each group.
| Group | n | TC (mmol/l) | TG (mmol/l) | LDL-C (mmol/l) | HDL-C (mmol/l) | VLDL-C
(mmol/l) | ox-LDL (µg/ml) |
|---|
| Control | 6 | 7.36±0.52 | 5.23±0.31 | 6.04±0.11 | 1.62±0.08 | 0.95±0.06 | 8.59±1.25 |
| Model | 6 |
8.24±0.33a | 5.73±0.70 |
6.54±0.16a |
1.51±0.04a |
1.20±0.21a |
10.16±0.29a |
| Fisetin | 6 |
7.50±0.41b | 5.36±0.21 |
6.19±0.35b | 1.56±0.04 |
0.99±0.06b |
8.95±0.50b |
| Atorvastatin | 6 |
7.38±0.37b | 5.31±0.22 |
6.14±0.11b | 1.57±0.04 |
0.97±0.05b |
8.88±0.33b |
SOD and MDA levels
In the model group, SOD levels significantly
decreased and MDA levels significantly increased compared with the
control group (P<0.001; Table
II). However, compared with the model group, SOD levels
increased and MDA levels decreased in the fisetin and atorvastatin
groups (P<0.01; Table II).
There were no significant differences between the fisetin and
atorvastatin groups (P>0.05; Table
II).
 | Table IISOD and MDA levels in peripheral
blood serum of mice in each group. |
Table II
SOD and MDA levels in peripheral
blood serum of mice in each group.
| Group | n | SOD (U/ml) | MDA (nmol/ml) |
|---|
| Control | 6 | 268.44±4.19 | 13.86±0.94 |
| Model | 6 |
245.66±7.03a |
17.18±0.58a |
| Fisetin | 6 |
260.53±6.28b |
15.01±1.42b |
| Atorvastatin | 6 |
261.49±8.69b |
14.94±0.79b |
ALT and AST activity
The activities of ALT and AST in the model group
were significantly increased compared with the control group
(P<0.001). However, in the fisetin and atorvastatin groups, the
activities of ALT and AST were significantly lower compared to the
model group (P<0.001; Table
III). There were no significant differences between the fisetin
and atorvastatin groups (P>0.05; Table III).
 | Table IIIALT and AST activities in peripheral
blood serum of mice in each group. |
Table III
ALT and AST activities in peripheral
blood serum of mice in each group.
| Group | n | ALT (U/l) | AST (U/l) |
|---|
| Control | 6 | 19.97±2.93 | 23.85±4.02 |
| Model | 6 |
72.06±5.38a |
58.42±5.76a |
| Fisetin | 6 |
25.24±5.00b |
29.09±4.08b |
| Atorvastatin | 6 |
24.02±4.37b |
25.76±3.43b |
Detection of the aortic sinus plaque
area
After the mice were fed with a high-fat diet for 12
weeks, a greater amount of atherosclerotic plaque formed in the
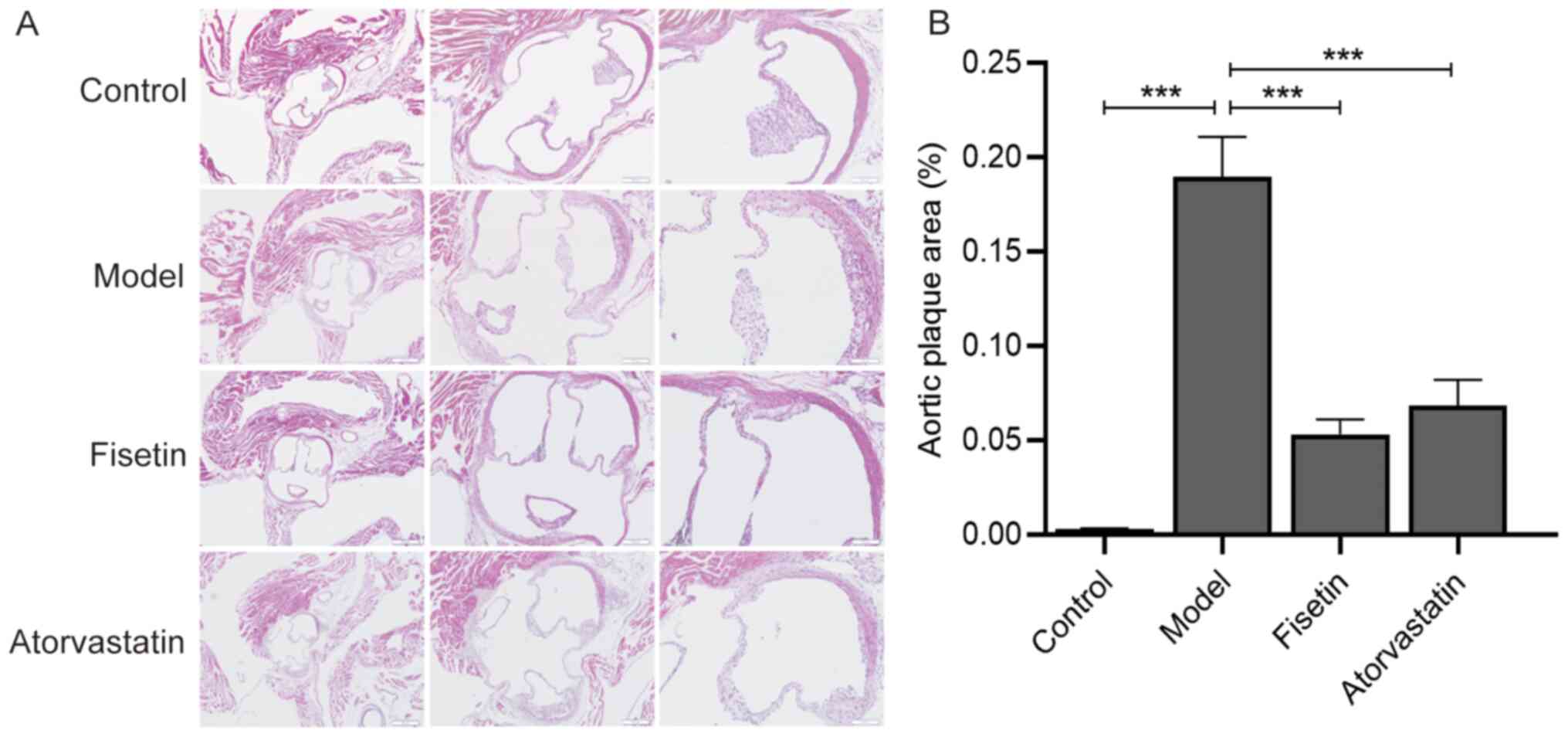
aortic sinus compared with the control group (P<0.001; Fig. 1A and B). Compared with the model group, AS
plaque significantly decreased in the fisetin and atorvastatin
groups (P<0.001; Fig. 1A and
B). There were no significant
differences between the fisetin group and the atorvastatin group
(P>0.05; Fig. 1A and B).
Detection of aortic sinus lipid
accumulation
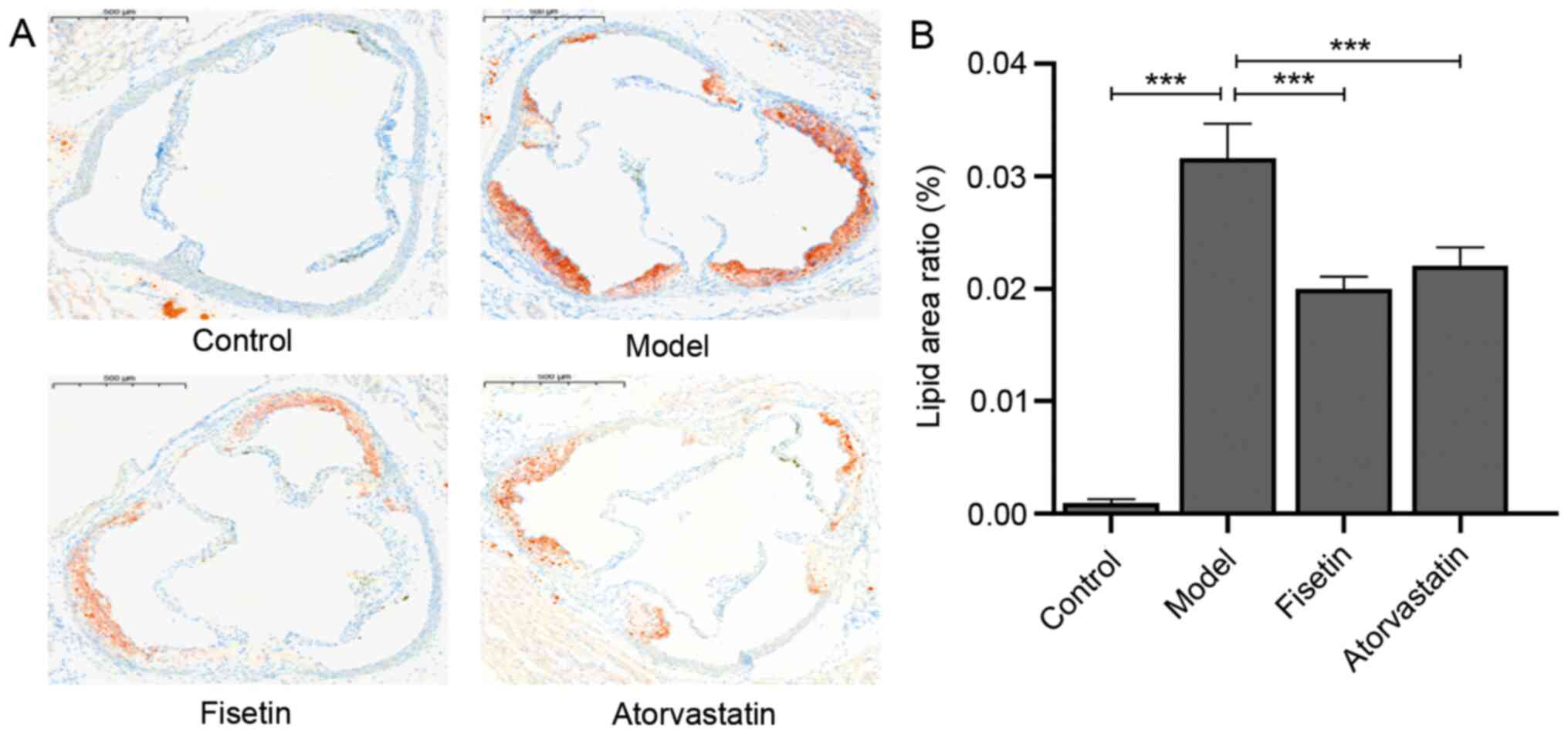
Compared with the control group, an increased amount
of red-stained lipids were indicated in the aortic sinus of the
model group (P<0.001; Fig. 2A
and B). However, the mice in the
fisetin group and atorvastatin group exhibited significantly
decreased lipid accumulation compared with the model group
(P<0.001; Fig. 2A and B). There were no significant differences
in lipid accumulation between the fisetin group and atorvastatin
group (P>0.05; Fig. 2A and
B).
Detection of aortic sinus collagen
content
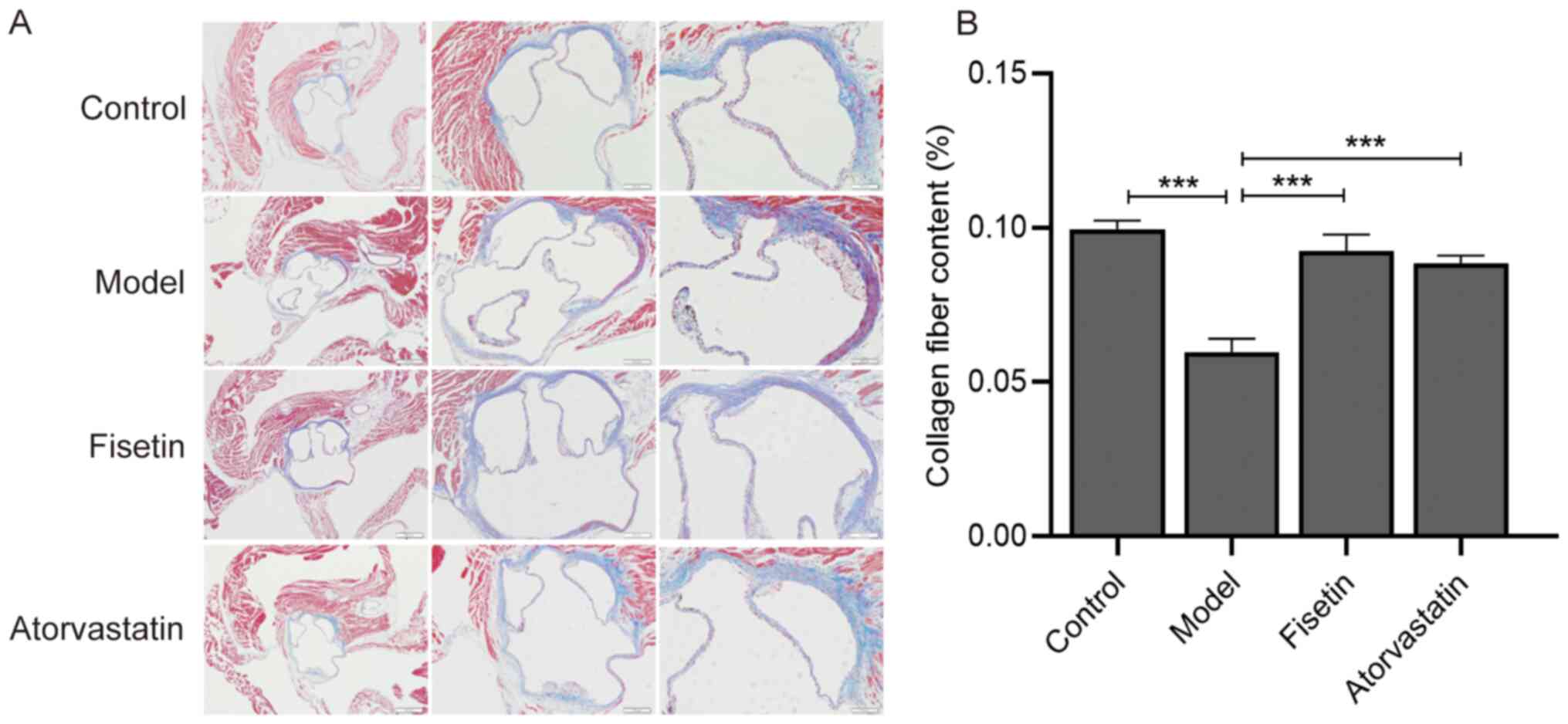
The atherosclerotic plaques in the model group were
observed to exhibit a thinner fibrous cap and decreased collagen
fiber components compared with the control group (P<0.001;
Fig. 3A and B). Following fisetin and atorvastatin
treatment, collagen fiber content in the atherosclerotic plaques of
the aortic sinus increased compared with the model group and were
distributed evenly (P<0.001; Fig.
3A and B). There were no
significant differences between collagen fiber content in the
fisetin group and the atorvastatin group (P>0.05; Fig. 3A and B).
Aortic expression of PCSK9, LOX-1,
p53, p21 and p16
Compared with the control group, the expressions of
PCSK9 (P<0.01; Fig. 4A and
F), LOX-1 (P<0.001; Fig. 4B and F), p53 (P<0.05; Fig. 4C and F), p21 (P<0.01; Fig. 4D and F), and p16 (P<0.001; Fig. 4E and F) significantly increased in the model
group. Following fisetin and atorvastatin treatment, the
expressions of PCSK9 (P<0.001; Fig.
4A and F), LOX-1 (P<0.01,
P<0.001; Fig. 4B and F), p53 (P<0.05; Fig. 4C and F), p21 (P<0.001; Fig. 4D and F) and p16 (P<0.001; Fig. 4E and F) significantly decreased compared with
the model group. There were no significant differences between
PCSK9, LOX-1, p53, p21 and p16 expressions in the fisetin group and
the atorvastatin group (P>0.05; Fig.
4A-F).
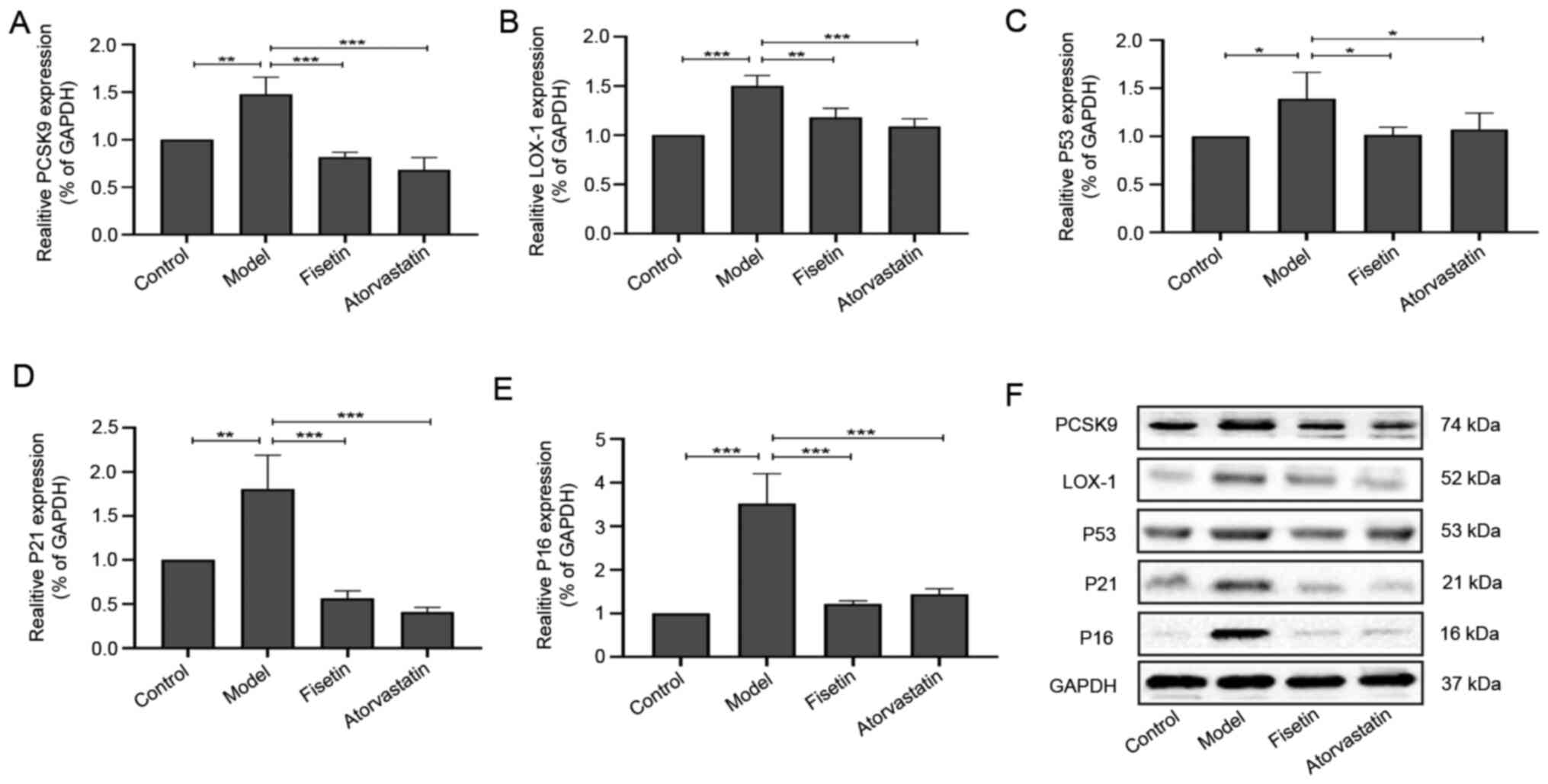 | Figure 4Effect of fisetin on the expression
levels of PCSK9, LOX-1, p53, p21 and p16 in apoE-/- mice
aorta. Expression levels of (A) PCSK9, (B) LOX-1, (C) p53, (D) p21
and (E) p16. (F) Western blot analysis results for PCSK9, LOX-1,
p53, p21 and p16 expressions in the mouse aorta. Data are presented
as mean ± SD (n=3). One-way ANOVA of variance tests followed by
least significant difference test was performed for comparison of
two or more groups, *P<0.05, **P<0.01,
***P<0.001. PCSK9, proprotein convertase
subtilisin/kexin type 9; LOX-1, lectin-like oxidized low-density
lipoprotein receptor-1; p53, tumor suppressor protein p53; p21,
cyclin-dependent kinase inhibitor 1A; p16, multiple tumor
suppressor-1. |
Discussion
AS is mainly characterized by the accumulation of a
large amount of lipids in the arterial wall, and disorders of lipid
metabolism are a risk factor for AS (14). PCSK9 is a serine protease, and its
functional mutation has been positively correlated with plasma
LDL-C concentration in patients with familial hypercholesterolemia
(15). A previous report
demonstrated that the overexpression of PCSK9 in C57BL/6 mice that
were fed a high-fat diet led to the rapid formation of
hyperlipidemia and aggravated the pathological changes of AS
(16). In contrast, knockout of
PCSK9 in Ldlr-/- Apobec1-/- mice has been
revealed to increase the levels of serum lipids, including TC, TG
and cholesterol ester, free cholesterol and the production and
secretion of apoB (17).
Administration of a PCSK9 inhibitor (human monoclonal antibody
RG7652) has also been indicated to reduce the levels of plasma LDL
and VLDL and increase the level of HDL in patients with coronary
heart disease (18). LOX-1 is
essential for the pathogenesis of AS, including renal dysfunction,
endothelial cell apoptosis and senescence, mediated foam cell
formation and regulation of collagen accumulation in AS plaques
(19). Overexpression of
endothelium-specific LOX-1 in apoE-/- mice has been
indicated to increase aortic fat stripe formation and macrophage
recruitment as well as accelerate the formation and development of
AS plaque (20). After the targeted
inhibition of LOX-1 by miRNA-98, the foam and lipid deposition of
peritoneal macrophages in apoE-/- mice has been
demonstrated to decrease (21).
Overexpressed PCSK9 in mouse peritoneal macrophages can promote the
expression of LOX-1 and increase the uptake of ox-LDL, thus
promoting the progression of AS (22). In human aortic ECs and SMCs exposed
to lipopolysaccharide, the expression of LOX-1 has been revealed to
decrease in ECs and SMCs with silenced PCSK9 as well as in
PCSK9-/- mice (7). In
the current study, the AS model induced by high-fat diet in
apoE-/- mice was successfully established, which
included increased AS plaque and lipid accumulation in the aortic
sinus as well as decreased collagen content. Compared with the
control group, serum lipid analysis in the peripheral blood of mice
revealed that the levels of TC, LDL-C, VLDL-C and ox-LDL increased,
whereas the level of HDL-C decreased, and the expressions of PCSK9
and LOX-1 increased in the aorta of mice.
AS is closely associated with aging and often occurs
in middle-aged and elderly individuals (23). In the pathological process of AS,
peroxides produced by oxidative stress can cause damage to DNA,
protein and other cell components, and can lead to vascular
endothelial dysfunction (24). SOD
and MDA are associated with oxidative damage, which can reflect the
severity of cells attacked by free radicals and the ability of
scavenging oxygen free radicals (25,26).
As an antioxidant enzyme for scavenging free radicals in the body,
SOD can scavenge free radicals that can destroy the cells of the
body (25). MDA is the end product
of lipid peroxides, and its accumulation can cause nucleic acid
destruction and protein denaturation, thus promoting cell
senescence (26). P53, p21 and p16
are aging markers that regulate the cell cycle and apoptosis and
participate in the pathway of cell senescence (6). The oxidative stress-mediated p53/p21
signaling pathway can promote the senescence of human aortic ECs
and activate p16 transcription, thus aggravating AS lesions
(27). In addition to participating
in lipid metabolism, PCSK9 serves an important role in the process
of vascular aging and is directly associated with the occurrence
and development of AS (8). It has
been reported that PCSK9 is closely associated with brain
cholesterol level and nerve cell apoptosis (28). LOX-1 can promote the senescence of
mouse cardiac fibroblasts and increase sensitivity to apoptosis
(29). Furthermore, overexpression
of PCSK9 and LOX-1 in human ECs and SMCs is associated with
increased production of mitochondrial ROS (mtROS), whereas knockout
of PCSK9 or LOX-1 is associated with a decrease of mtROS (4). In the present study, the model group
exhibited a decreased level of serum SOD and an increased level of
MDA in the peripheral blood serum of mice. Additionally, the
expressions of p53, p21, and p16 were increased in the model group
compared with the control group.
As aforementioned, fisetin is a flavonol compound
that regulates lipid metabolism disorders, and anti-aging and
anti-oxidation activities (30-32).
A previous research has demonstrated that fisetin can inhibit the
binding of ox-LDL to class B scavenger receptor cluster of
differentiation 36 on macrophages and reduce foam cell formation to
provide anti-AS effects (33).
Fisetin can also inhibit adipose differentiation by reducing mTORC1
activity, thereby reducing adipocyte formation and fat accumulation
(34). Moreover, fisetin can reduce
the effect of ox-LDL in ECs to improve endothelial dysfunction by
downregulating the Erk-5/Mef2c/KLF2 signaling pathway (35). According to a previous report,
hypercholesterolemic rats treated with fisetin exhibited
significantly decreased plasma TC and LDL-C levels (36). In mice with alcoholic liver injury,
fisetin reduced the levels of TG and free fatty acid as well as the
number of lipid droplets (37).
Fisetin also significantly decreased the plasma levels of TC, TG,
LDL-C and VLDL-C, and increased the level of HDL-C in diabetic rats
(38). In addition, fisetin serves
an anti-aging role by inhibiting the proliferation of senescent
cells (39). Fisetin has also been
revealed to significantly improve cognitive and motor dysfunction
and brain inflammation in SAMP8 aging mice (40), and effectively maintain the redox
homeostasis of erythrocytes in aging rats (41). Fisetin can prolong the life span of
yeast, nematode, drosophila melanogaster and mice (42). Furthermore, fisetin can be used as a
cardioprotective agent; a dose of 10 mg/kg was previously revealed
to significantly reduce the cardiotoxicity induced by doxorubicin
in rats (43). Fisetin also
exhibits protective effects on cerebral nerves, and at doses of 25
and 74 mg/kg, can be rapidly distributed in cerebral vessels and
brain parenchyma of mice via intragastric or intraperitoneal
injection (44). Fisetin is the
main chemical component of Lacqueraceae, and its water extract (200
mg/kg) exhibits no obvious anatomical changes or tissue damage and
hepatorenal function insufficiency in BALB/C mice; however, a high
concentration may inhibit bone marrow hematopoiesis long-term
(45). The current study
demonstrated that fisetin could significantly decrease the serum
levels of TC, LDL-C, VLDL-C and ox-LDL, and the detection of ALT
and AST activities in the peripheral blood serum of mice suggested
that the fisetin dosage was within a safe range (Table III).
In summary, the current study concluded that fisetin
exerted an anti-AS effect in apoE-/- mice fed with a
high-fat diet, which reduced the area of atherosclerotic plaques
and lipid accumulation and increased the collagen fiber content in
the aortic sinus. In addition, fisetin treatment resulted in
decreased levels of TC, LDL-C, VLDL-C, ox-LDL and MDA, and
increased the level of SOD compared with the control group.
Finally, fisetin treatment resulted in the downregulation of PCSK9,
LOX-1, p53, p21 and p16 protein expression in aortic tissue,
compared with the model group. Combined with the results of
previous in vitro experiments, these results suggested that
fisetin may serve an atheroprotective role, ameliorating abnormal
lipid metabolism by regulating the expression of PCSK9 and LOX-1,
and improving senescence by regulating the expressions of p53, p21
and p16 (Fig. 5).
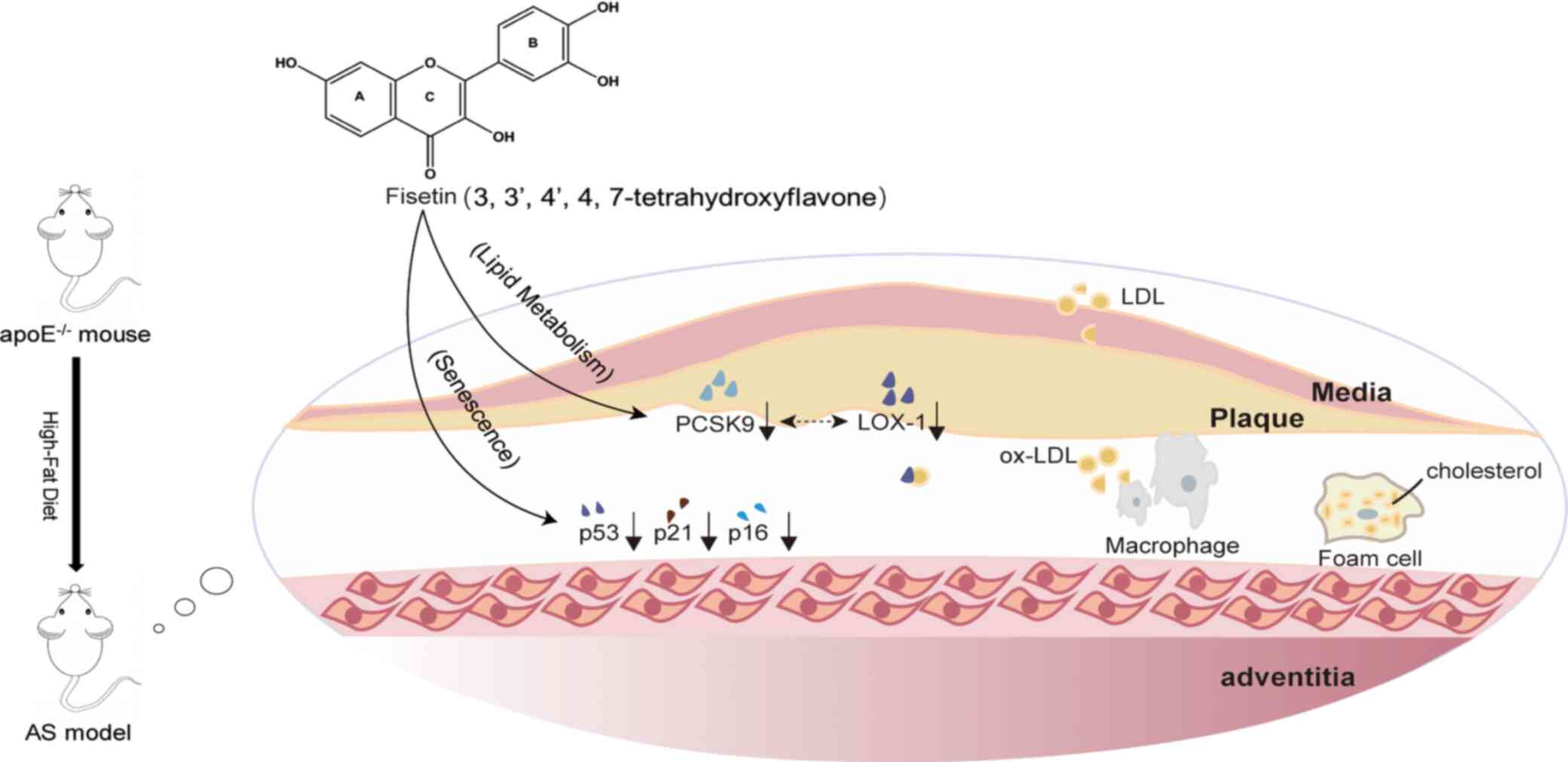 | Figure 5Schematic summary of how fisetin
exerts an anti-AS effect in apoE-/- mice fed with a
high-fat diet. Fisetin treatment ameliorated lipid accumulation in
the atherosclerotic plaque by downregulating PCSK9 and LOX-1, which
reduces the uptake of ox-LDL by macrophages, thereby reducing foam
cell formation. Fisetin also improves senescence by downregulating
the aging-related proteins p53, p21, and p16 in aortic tissue. AS,
Atherosclerosis; PCSK9, proprotein convertase subtilisin/kexin type
9; LOX-1, lectin-like oxidized low-density lipoprotein receptor-1;
ox-LDL, oxidized low-density lipoprotein; p53, tumor suppressor
protein p53; p21, cyclin-dependent kinase inhibitor 1A; p16,
multiple tumor suppressor-1. |
Acknowledgements
Not applicable.
Funding
The current study was supported by the National
Natural Science Foundation of China (grant no. 81873348), the
National Traditional Chinese Medicine Innovation Talent Training
Project [Letter (2019) no. 128], and Shanghai University of
Traditional Chinese Medicine graduate ‘Innovation Ability Training’
special research project (grant no. Y201934).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
DS, LY and QJ conceived the experiments and
experimental plan. LY, QJ and HC performed the experiments and
collected the data. LY, QJ, HC, SX, YH and CC analyzed the data. LY
and QJ wrote the manuscript. DS, HC, CC, SX and YH reviewed the
manuscript critically for important intellectual content. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments were approved and carried out
in strict compliance with the Animal Care and Use Committee of
Shanghai Model Organisms Center, Inc. (IACUC no. 2019-0008).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wang H, Fu H, Zhu R, Wu X, Ji X, Li X,
Jiang H, Lin Z, Tang X, Sun S, et al: BRD4 contributes to
LPS-induced macrophage senescence and promotes progression of
atherosclerosis-associated lipid uptake. Aging (Albany NY).
12:9240–9259. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Del Pinto R, Grassi D, Properzi G,
Desideri G and Ferri C: Low density lipoprotein (LDL) cholesterol
as a causal role for atherosclerotic disease: Potential role of
PCSK9 inhibitors. High Blood Press Cardiovasc Prev. 26:199–207.
2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Tian K, Ogura S, Little PJ, Xu SW and
Sawamura T: Targeting LOX-1 in atherosclerosis and vasculopathy:
Current knowledge and future perspectives. Ann N Y Acad Sci.
1443:34–53. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ding Z, Liu S, Wang X, Deng X, Fan Y,
Shahanawaz J, Shmookler Reis RJ, Varughese KI, Sawamura T and Mehta
JL: Cross-talk between LOX-1 and PCSK9 in vascular tissues.
Cardiovasc Res. 107:556–567. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kattoor AJ, Pothineni NVK, Palagiri D and
Mehta JL: Oxidative stress in atherosclerosis. Curr Atheroscler
Rep. 19(42)2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Shimizu I and Minamino T: Cellular
senescence in cardiac diseases. J Cardiol. 74:313–319.
2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ding Z, Liu S, Wang X, Deng X, Fan Y, Sun
C, Wang Y and Mehta JL: Hemodynamic shear stress via ROS modulates
PCSK9 expression in human vascular endothelial and smooth muscle
cells and along the mouse aorta. Antioxid Redox Signal. 22:760–771.
2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Grobelna MK, Strauss E and Krasiński Z:
The role of proprotein convertase subtilisin-kexin type 9 (PCSK9)
in the vascular aging process-is there a link? Kardiochir
Torakochirurgia Pol. 16:128–132. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Pal HC, Pearlman RL and Afaq F: Fisetin
and Its role in chronic diseases. Adv Exp Med Biol. 928:213–244.
2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Jin T, Kim OY, Shin MJ, Choi EY, Lee SS,
Han YS and Chung JH: Fisetin up-regulates the expression of
adiponectin in 3T3-L1 adipocytes via the activation of silent
mating type information regulation 2 homologue 1
(SIRT1)-deacetylase and peroxisome proliferator-activated receptors
(PPARs). J Agric Food Chem. 62:10468–10474. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Shi YS, Li CB, Li XY, Wu J, Li Y, Fu X,
Zhang Y and Hu WZ: Fisetin attenuates metabolic dysfunction in mice
challenged with a high-fructose diet. J Agric Food Chem.
66:8291–8298. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yousefzadeh MJ, Zhu Y, McGowan SJ,
Angelini L, Fuhrmann-Stroissnigg H, Xu M, Ling YY, Melos KI,
Pirtskhalava T, Inman CL, et al: Fisetin is a senotherapeutic that
extends health and lifespan. EBioMedicine. 36:18–28.
2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Jia Q, Cao H, Shen D, Yan L, Chen C and
Xing S: Fisetin, via CKIP-1/REGγ, limits oxidized LDL-induced lipid
accumulation and senescence in RAW264.7 macrophage-derived foam
cells. Eur J Pharmacol. 865(172748)2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Summerhill VI, Grechko AV, Yet SF, Sobenin
IA and Orekhov AN: The atherogenic role of circulating modified
lipids in atherosclerosis. Int J Mol Sci. 20(3561)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wu NQ and Li JJ: PCSK9 gene mutations and
low-density lipoprotein cholesterol. Clin Chim Acta. 431:148–153.
2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kumar S, Kang DW, Rezvan A and Jo H:
Accelerated atherosclerosis development in C57Bl6 mice by
overexpressing AAV-mediated PCSK9 and partial carotid ligation. Lab
Invest. 97:935–945. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Sun H, Krauss RM, Chang JT and Teng BB:
PCSK9 deficiency reduces atherosclerosis, apolipoprotein B
secretion, and endothelial dysfunction. J Lipid Res. 59:207–223.
2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hilvo M, Simolin H, Metso J, Ruuth M,
Öörni K, Jauhiainen M, Laaksonen R and Baruch A: PCSK9 inhibition
alters the lipidome of plasma and lipoprotein fractions.
Atherosclerosis. 269:159–165. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Xu S, Ogura S, Chen J, Little PJ, Moss J
and Liu P: LOX-1 in atherosclerosis: Biological functions and
pharmacological modifiers. Cell Mol Life Sci. 70:2859–2872.
2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Akhmedov A, Rozenberg I, Paneni F, Camici
GG, Shi Y, Doerries C, Sledzinska A, Mocharla P, Breitenstein A,
Lohmann C, et al: Endothelial overexpression of LOX-1 increases
plaque formation and promotes atherosclerosis in vivo. Eur Heart J.
35:2839–2848. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Dai Y, Wu X, Dai D, Li J and Mehta JL:
MicroRNA-98 regulates foam cell formation and lipid accumulation
through repression of LOX-1. Redox Biol. 16:255–262.
2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ding Z, Liu S, Wang X, Theus S, Deng X,
Fan Y, Zhou S and Mehta JL: PCSK9 regulates expression of scavenger
receptors and ox-LDL uptake in macrophages. Cardiovasc Res.
114:1145–1153. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Fajemiroye JO, da Cunha LC,
Saavedra-Rodríguez R, Rodrigues KL, Naves LM, Mourão AA, da Silva
EF, Williams NEE, Martins JLR, Sousa RB, et al: Aging-induced
biological changes and cardiovascular diseases. Biomed Res Int.
2018(7156435)2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Dzięgielewska-Gęsiak S, Płóciniczak A,
Wilemska-Kucharzewska K, Kokot T, Muc-Wierzgoń M and Wysocka E: The
relationship between plasma lipids, oxidant-antioxidant status, and
glycated proteins in individuals at risk for atherosclerosis. Clin
Interv Aging. 14:789–796. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wang Y, Branicky R, Noë A and Hekimi S:
Superoxide dismutases: Dual roles in controlling ROS damage and
regulating ROS signaling. J Cell Biol. 217:1915–1928.
2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Papac-Milicevic N, Busch CJ and Binder CJ:
Malondialdehyde epitopes as targets of immunity and the
implications for atherosclerosis. Adv Immunol. 131:1–59.
2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhou Z, Yin Y, Chang Q, Sun G and Dai Y:
Downregulation of B-myb promotes senescence via the ROS-mediated
p53/p21 pathway, in vascular endothelial cells. Cell Prolif.
50(e12319)2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Adorni MP, Ruscica M, Ferri N, Bernini F
and Zimetti F: Proprotein convertase subtilisin/kexin type 9, brain
cholesterol homeostasis and potential implication for Alzheimer's
disease. Front Aging Neurosci. 11(120)2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wang X, Khaidakov M, Ding Z, Dai Y,
Mercanti F and Mehta JL: LOX-1 in the maintenance of cytoskeleton
and proliferation in senescent cardiac fibroblasts. J Mol Cell
Cardiol. 60:184–190. 2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Gaballah HH, El-Horany HE and Helal DS:
Mitigative effects of the bioactive flavonol fisetin on
high-fat/high-sucrose induced nonalcoholic fatty liver disease in
rats. J Cell Biochem. 120:12762–12774. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Singh S, Singh AK, Garg G and Rizvi SI:
Fisetin as a caloric restriction mimetic protects rat brain against
aging induced oxidative stress, apoptosis and neurodegeneration.
Life Sci. 193:171–179. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhang L, Wang H, Zhou Y, Zhu Y and Fei M:
Fisetin alleviates oxidative stress after traumatic brain injury
via the Nrf2-ARE pathway. Neurochem Int. 118:304–313.
2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lian TW, Wang L, Lo YH, Huang IJ and Wu
MJ: Fisetin, morin and myricetin attenuate CD36 expression and
oxLDL uptake in U937-derived macrophages. Biochim Biophys Acta.
1781:601–609. 2008.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Jung CH, Kim H, Ahn J, Jeon TI, Lee DH and
Ha TY: Fisetin regulates obesity by targeting mTORC1 signaling. J
Nutr Biochem. 24:1547–1554. 2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Patel R, Varghese JF, Singh RP and Yadav
UCS: Induction of endothelial dysfunction by oxidized low-density
lipoproteins via downregulation of Erk-5/Mef2c/KLF2 signaling:
Amelioration by fisetin. Biochimie. 163:152–162. 2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Shin MJ, Cho Y, Moon J, Jeon HJ, Lee SM
and Chung JH: Hypocholesterolemic effect of daily fisetin
supplementation in high fat fed sprague-dawley rats. Food Chem
Toxicol. 57:84–90. 2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Sun Q, Zhang W, Zhong W, Sun X and Zhou Z:
Dietary fisetin supplementation protects against alcohol-induced
liver injury in mice. Alcohol Clin Exp Res. 40:2076–2084.
2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Prasath GS and Subramanian SP:
Antihyperlipidemic effect of fisetin, a bioflavonoid of
strawberries, studied in streptozotocin-induced diabetic rats. J
Biochem Mol Toxicol. 28:442–449. 2014.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Zhu Y, Doornebal EJ, Pirtskhalava T,
Giorgadze N, Wentworth M, Fuhrmann-Stroissnigg H, Niedernhofer LJ,
Robbins PD, Tchkonia T and Kirkland JL: New agents that target
senescent cells: The flavone, fisetin, and the BCL-XL inhibitors,
A1331852 and A1155463. Aging (Albany NY). 9:955–963.
2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Currais A, Farrokhi C, Dargusch R, Armando
A, Quehenberger O, Schubert D and Maher P: Fisetin reduces the
impact of aging on behavior and physiology in the rapidly aging
SAMP8 mouse. J Gerontol A Biol Sci Med Sci. 73:299–307.
2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Singh S, Garg G, Singh AK, Bissoyi A and
Rizvi SI: Fisetin, a potential caloric restriction mimetic,
attenuates senescence biomarkers in rat erythrocytes. Biochem Cell
Biol. 97:480–487. 2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Wood JG, Rogina B, Lavu S, Howitz K,
Helfand SL, Tatar M and Sinclair D: Sirtuin activators mimic
caloric restriction and delay ageing in metazoans. Nature.
430:686–689. 2004.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Ma T, Kandhare AD, Mukherjee-Kandhare AA
and Bodhankar SL: Fisetin, a plant flavonoid ameliorates
doxorubicin-induced cardiotoxicity in experimental rats: The
decisive role of caspase-3, COX-II, cTn-I, iNOs and TNF-α. Mol Biol
Rep. 46:105–118. 2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Krasieva TB, Ehren J, O'Sullivan T,
Tromberg BJ and Maher P: Cell and brain tissue imaging of the
flavonoid fisetin using label-free two-photon microscopy. Neurochem
Int. 89:243–248. 2015.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Varela-Rodríguez L, Sánchez-Ramírez B,
Rodríguez-Reyna IS, Ordaz-Ortiz JJ, Chávez-Flores D, Salas-Muñoz E,
Osorio-Trujillo JC, Ramos-Martínez E and Talamás-Rohana P:
Biological and toxicological evaluation of Rhus trilobata Nutt.
(Anacardiaceae) used traditionally in Mexico against cancer. BMC
Complement Altern Med. 19(153)2019.PubMed/NCBI View Article : Google Scholar
|