Introduction
Patients with liver cirrhosis frequently experience
non-specific symptoms and report a severe reduction in their
quality of life (QOL) (1,2). Patient-reported outcome (PRO) is an
outcome-reporting system directly reported by the patient to
describe their own experience of the condition, which differs from
that reported by physicians and nurses (3). PRO method, similar to questionnaires,
is used in clinical trials to gain a better understanding of the
efficacy or effectiveness of a treatment modality (4). There are several questionnaires
concerning liver disease, including the Chronic Liver Disease (CLD)
Questionnaire (CLD-Q) (5), Liver
Disease Quality of Life Questionnaire (6) and the Liver Disease Symptom Index 2.0
(LDSI 2.0) (7), which are
designated to assess the QOL of patients with CLD. Although the
CLD-Q and LDSI 2.0 contain questions regarding abdominal symptoms,
their primary aim is not to assess the symptoms of ascites. By
contrast, Ascites Symptom Inventory-7 (ASI-7) is specific to
ascites symptoms, which directly reflects the abdominal symptoms of
the patients (8). The effectiveness
of ASI-7 has been previously reported (9), though the existence of the
questionnaire in not widely known.
In a previous study of ascending levels of tolvaptan
(SALT) in 2006, an orally active vasopressin V2-receptor
antagonist, tolvaptan, was first reported as safe and effective for
increasing serum sodium concentrations among patients with
hyponatremia (9,10). Since December 2013, tolvaptan has
been used in Japan owing to a mechanism of action that is different
from those of conventional diuretics, including furosemide or
spironolactone (11). Tolvaptan
acts by suppressing the expression of Aquaporin (AQP)-2, inhibiting
water reabsorption in the renal collecting ducts (12). Unlike other diuretics, this drug
does not stimulate sodium channels; instead, it increases free
water excretion without affecting urinary sodium levels (13).
A number of reports have previously assessed the QOL
of patients with refractory ascites (14,15).
In addition, there is an insufficient number of assessments on the
effectiveness of ASI-7 following the administration of tolvaptan in
treating cirrhotic ascites, where decreases in ascites may reduce
the ASI-7 score (8). Therefore, the
aim of the present cohort study was to apply the ASI-7
questionnaire to evaluate the symptoms subjectively after tolvaptan
administration in patients with cirrhotic ascites.
Materials and methods
Therapeutic protocol
The protocol was registered to the clinical trials
registry managed by the University Hospital Medical Information
Network in Japan (registration no. UMIN000013095). This trial was
conducted between December 2013 and April 2018 and consisted of
patients with liver cirrhosis accompanied by ascites who fulfilled
the following criteria in accordance with a Japanese phase III
study (11): (i) >20 years of
age; and (ii) experienced persistent ascites despite conventional
diuretic treatments with ≥20 mg/day furosemide and/or ≥25 mg/day
spironolactone. The following exclusion criteria were adopted from
a phase III Japanese study (11):
i) Having other complications or malignancies such as hepatic
encephalopathy (West haven criteria ≥grade II) (16); ii) vascular invasive hepatocellular
carcinoma, esophageal or gastric varices requiring new treatment,
repeated hemorrhoid bleeding due to rectal varices, congestive
heart failure and anuria or impaired urination (10); (iii) a history of cerebrovascular
disorders; (iv) hemoglobin levels <8.0 g/dl, total serum sodium
<120 or >147 mEq/l or serum potassium >5.5 mEq/l; (v)
inability to take oral medication; and (vi) patients otherwise
adjudged by the investigator to be inappropriate for inclusion into
the study.
The present study consisted of a 2-day pretreatment
observation period and a 7-day treatment period. All patients
received 3.75 or 7.5 mg/day tolvaptan for 7 days as an adjunct to
conventional diuretics (10-60 mg/day furosemide and/or 25-100
mg/day spironolactone) during sodium restriction (<7 g/day). Day
1 was defined as the first day of tolvaptan administration. Data
obtained immediately before the administration of tolvaptan were
used as baseline data. Day 8 was defined as the end of the
tolvaptan treatment. The primary outcome of this study is to
investigate if body weight loss correlates with improvements in the
ASI-7 scores following tolvaptan administration, whilst the
secondary outcome is to assess the relationship between the ASI-7
score and parameters such as urine volume or hepatic reserve.
Patient enrollment
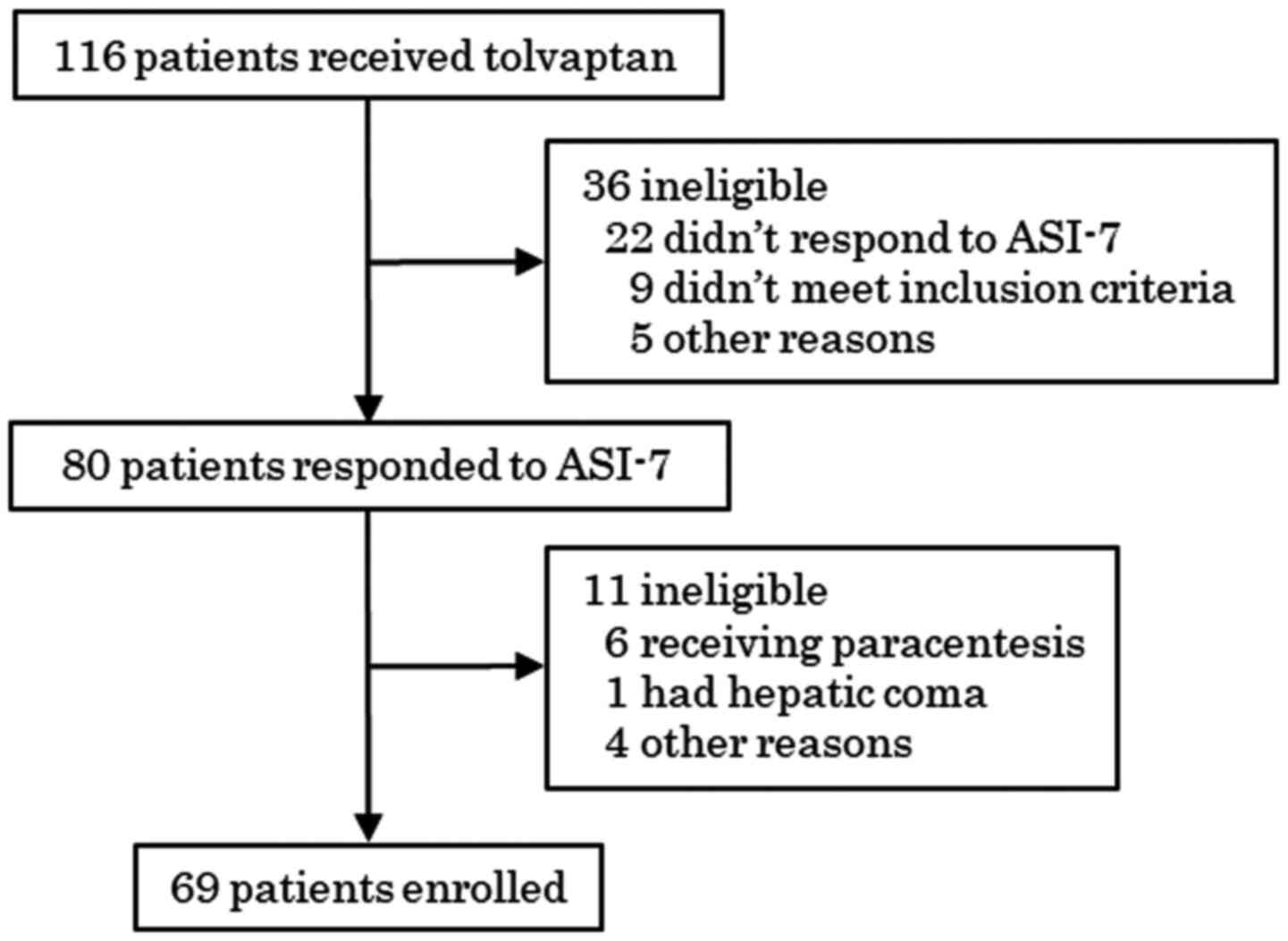
In this prospective, single-arm, multi-center study,
a total of 116 consecutive patients with cirrhotic ascites treated
with tolvaptan were enrolled. However, only 80 patients completed
the ASI-7 questionnaire. Patients that underwent paracentesis,
experienced hepatic coma during the treatment period or submitted
questionnaires with missing data were excluded. In total, 11
patients dropped out of the study due to various reasons (Fig. 1). Finally, a total of 69 patients
were included for the analysis. The clinical characteristics of the
patients with cirrhotic ascites are shown in Table I.
 | Table IClinical characteristics of
patients. |
Table I
Clinical characteristics of
patients.
| Parameter | Dataa |
|---|
| Age (years) | 68.8±11.5 |
| Sex
(male/female) | 43/26 |
| Cause of cirrhosis
(HBV/HCV/Alc/others) | 7/24/17/21 |
| Ascites volume
(mild/moderate/severe) | 10/25/34 |
| Child-Pugh
classification (B/C) | 33/36 |
| Child-Pugh score | 10.1±1.7 |
| Dose of tolvaptan
received (3.75/7.5 mg) | 7/62 |
| Dose of furosemide
(mg) | 25.6±11.7 |
| Dose of
spironolactone (mg) | 50.9±16.2 |
| With HCC | 29 (42.0%) |
| With portal vein
thrombus | 18 (26.1%) |
Ethics
The present study was approved by the Institutional
Review Board (IRB) of Nara Medical University (IRB approval no.
2-9). This study was conducted in accordance with the ethical
standards in the Declaration of Helsinki. Written informed consent
was obtained from all patients prior to their participation in the
study.
Physical examination findings
The body weight (BW) and urine output of each
participant were measured every day of the study. Consistent with
previous reports (17), responders
to the treatment were defined as patients exhibiting >1.5-kg
loss in BW after 1-week tolvaptan administration whereas
non-responders were defined as those having exhibiting weight loss
<1.5 kg or weight gain. Child-Pugh scores were calculated from
the five clinical measures of liver disease. Each measure was
allocated a score of 1-3, with 3 indicating severe derangement. The
five clinical measures were as follows: Total bilirubin, serum
albumin, prothrombin time, ascites and hepatic encephalopathy
(18).
Questionnaire
The ASI-7 was used on days 1 and 8 of the study. It
consists of seven items on a 5-point Likert scale: 0, does not
apply; 1, slightly applies; 2, somewhat applies; 3, strongly
applies; and 4, very strongly applies. The following was written on
each questionnaire: ‘Please answer the following questions about
your ascites (abdominal fluid or fullness) and related symptoms, as
they are today’. The seven items comprising the scale shown in
Table II cover various domains and
were arranged in a sequence from mild to severe symptoms,
facilitating clinical interpretation. The cumulative score was
calculated by summing up the scores of each of the 7 items (0-4
points), which can range from 0 to 28 points. The scale of the
ASI-7 scoring system showed asymmetric distribution within a range
of 0-28 points. The score system of ASI-7 was defined as follows:
i) Slight ascites, 0-11; ii) mild ascites, 12-18; iii) moderate
ascites, 19-22; and iv) severe ascites, 23-28. ΔASI-7 scores were
defined as ‘ASI-7 score on day 8’, ‘ASI-7 score on day 1’.
 | Table IIItems in the ASI-7
questionnairea. |
Table II
Items in the ASI-7
questionnairea.
| Number | Item |
|---|
| 1 | My stomach feels
heavy. |
| 2 | My stomach is bloated
and I feel discomfort. |
| 3 | My stomach is
bloated, so it is difficult to move around. |
| 4 | My stomach feels
heavy when lying down. |
| 5 | I have difficulty
breathing after walking for 2-3 min. |
| 6 | I cannot eat because
my stomach is bloated. |
| 7 | I cannot take deep
breaths. |
Statistical analysis
Patient backgrounds and clinical parameters are
presented as the number of patients (%) or means ± SD. Mann-Whitney
U test was used for comparisons between two groups. Kruskal-Wallis
test followed by Dunn's test was performed for comparisons among
three groups. Spearman's rank correlation analysis was performed to
determine the correlation coefficient (R) and P-value. All
statistical analyses were performed using SPSS (SPSS, Inc.) for
Windows version 16.0. P<0.05 were considered to indicate a
statistically significant difference.
Results
Baseline characteristics of
patients
In total, 69 patients with difficult-to-treat
ascites were analyzed. Table I
shows the baseline (day 1) clinical characteristics of the patients
enrolled. All enrolled patients had liver cirrhosis, with the mean
age of 68.8±11.5 years (range: 34-85 years); 43 patients (62.3%)
were male and 26 patients (37.7%) were female. In total, 60
patients (87.0%) received furosemide (mean dose, 25.6±11.7 mg/day;
range, 10-60 mg/day) and 62 patients (89.9%) received
spironolactone (mean dose, 50.9±16.2 mg/day; range: 25-100 mg/day).
Of the 69 patients, 62 received 7.5 mg tolvaptan, whilst the
remaining 7 received 3.75 mg tolvaptan. The cause of liver
cirrhosis in the participants were as follows: i) 7 patients had
hepatitis B; ii) 24 patients had hepatitis C; iii) 17 patients had
a history of alcoholism; and iv) 21 patients had other disorders,
including primary biliary cholangitis and autoimmune hepatitis. In
total, 33 patients had Child-Pugh grade B and 36 patients had
Child-Pugh grade C. Of the 69 participants, 18 patients (26.1%) had
portal vein thrombus and 29 (42.0%) had complications with
hepatocellular carcinoma; these patients but did not experience
invasion of the portal vein. The amount of ascites was classified
into the mild, moderate and severe groups, proposed by the
International Club of Ascites (19), as estimated using CT or
ultrasonography (US). In total, 10 patients had mild ascites, 25
patients had moderate ascites, and the remaining 34 patients had
severe ascites.
Correlation among each baseline
parameter
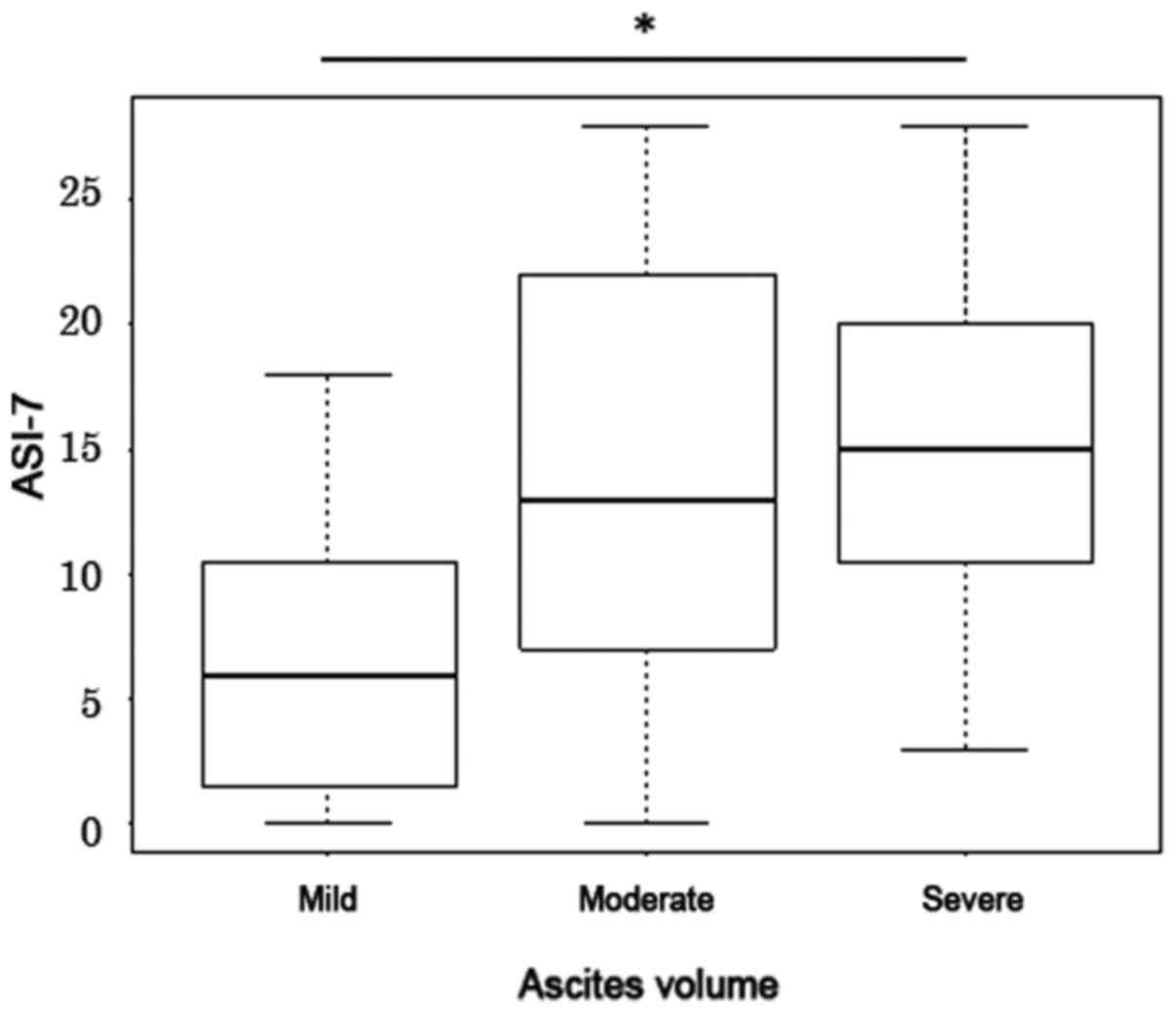
Baseline ASI-7 score was first obtained on day 1,
where mean score of 13.7±8.1 was documented and the ASI-7 score
increased with ascites volume. For patients with mild, moderate and
severe ascites, the median ASI-7 scores were found to be 6, 13 and
15 respectively (Fig. 2).
Comparison of the three groups revealed a significant difference by
Kruskal-Wallis test (P=0.02). The ASI-7 scores of patients with
severe ascites were significantly higher compared with those of
patients with mild ascites (P=0.01; Fig. 2). There were no difference between
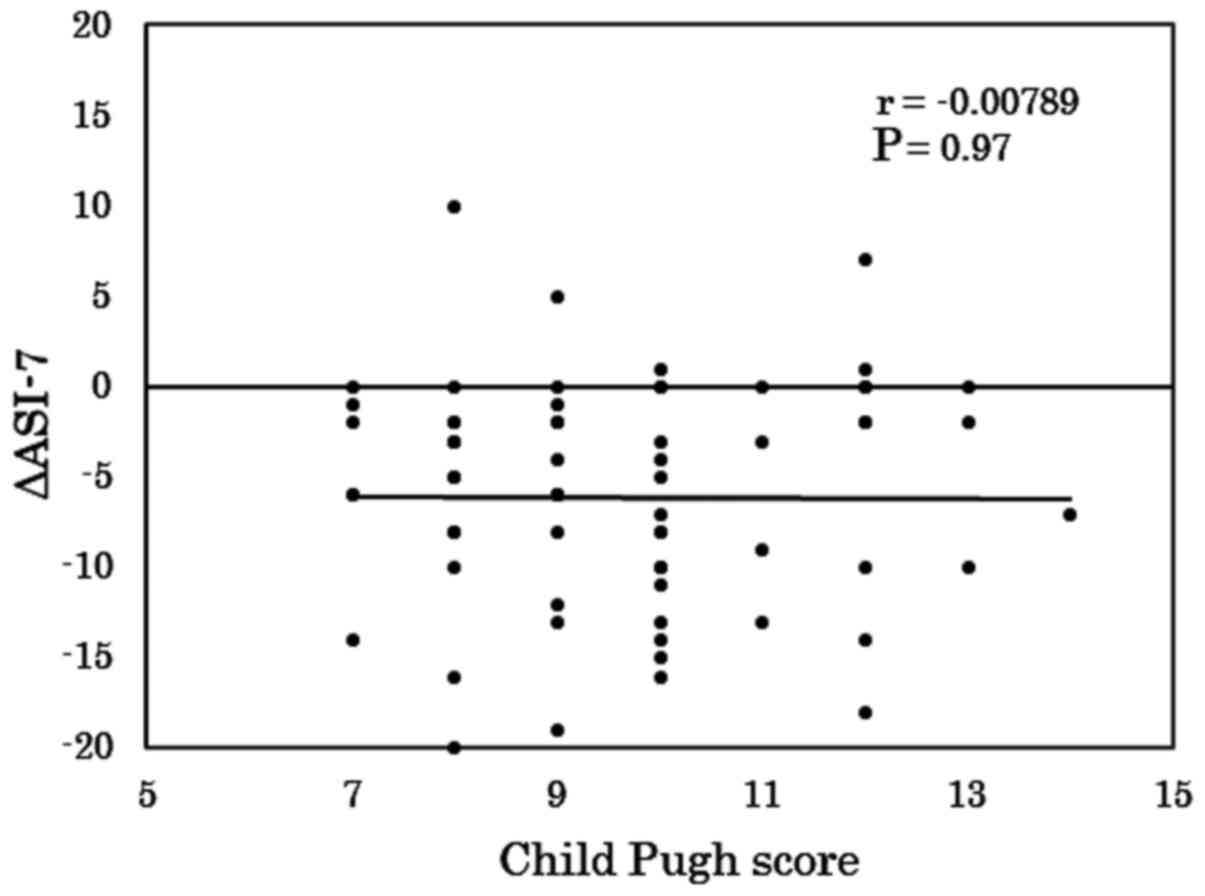
moderate vs. mild and moderate vs. severe. Additionally, there was
no correlation between the Child-Pugh and ASI-7 scores between day
8 and day 1 (r<0.001; Fig.
3).
Effectiveness of 7-day treatment with
tolvaptan
In total, 49 patients (71.0%) experienced a
reduction in BW (>1.5 kg) after 7 days, with 32 patients (46.4%)
experiencing >500-ml increase in urine volume the day 1 after
tolvaptan treatment. The remaining participants experienced a
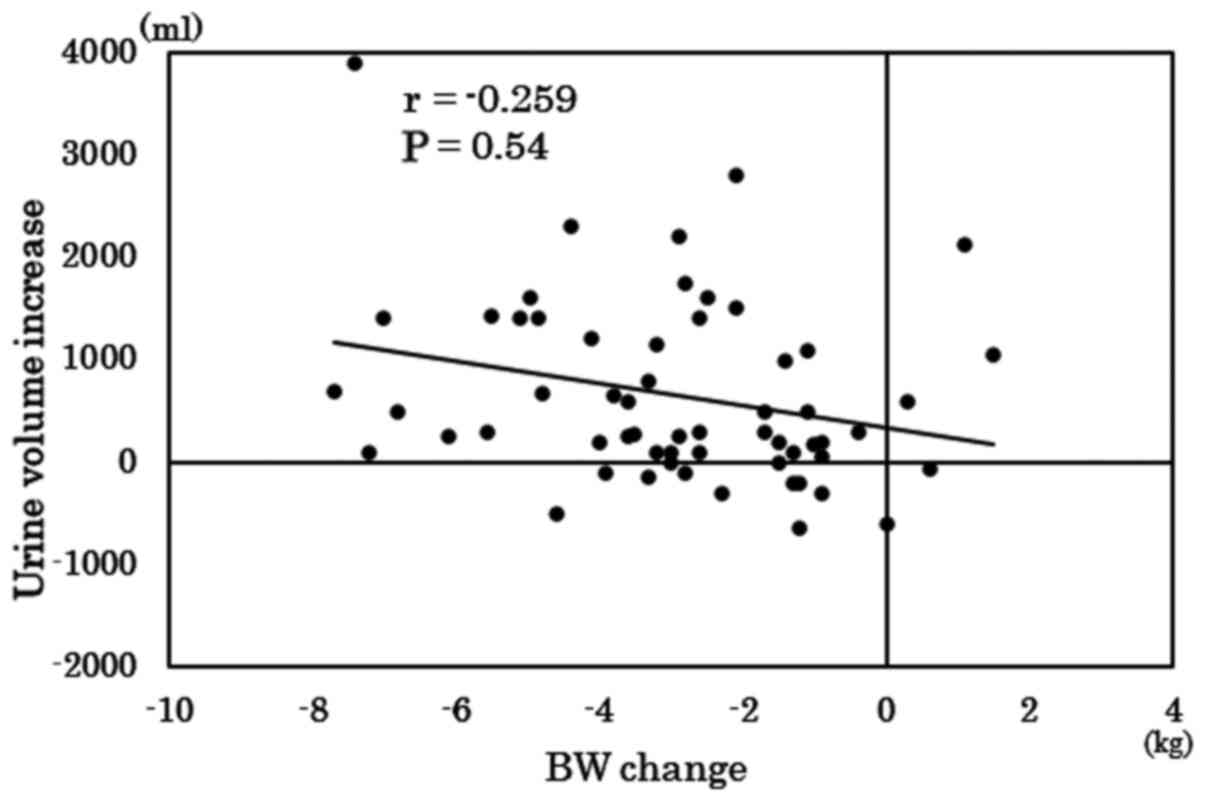
decrease in urine volume. Correlation between BW change in 7 days
and changes in urine volume the day 1 after tolvaptan treatment was
assessed, A weak but insignificant correlation between changes in
BW and urine volume was observed (r=-0.079; Fig. 4). The mean ASI-7 scores also
decreased from 13 (Q3-Q1:20-6.5) to 5 (Q3-Q1:11-0) after 7 days of
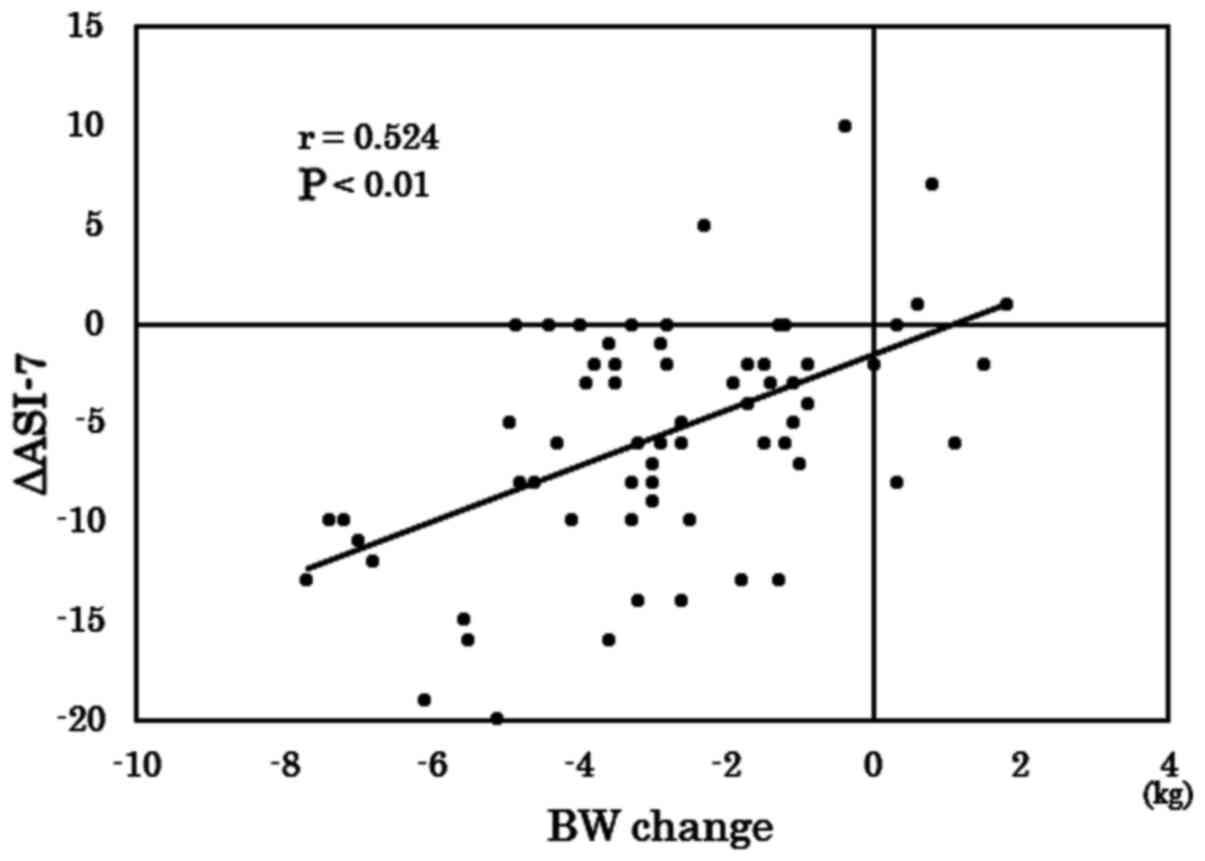
treatment. Correlation between the changes in the BW and ASI-7
scores in 7 days was next assessed, where a moderate and
significant correlation between the BW change in 7 days and ASI-7
score change (r=0.524; P<0.01; Fig.
5) was noted. By contrast, no correlation was observed between
the BW change after 7 days and change of urine volume at day 1
(data not shown; r=0.137; P=0.15). The treatment response was
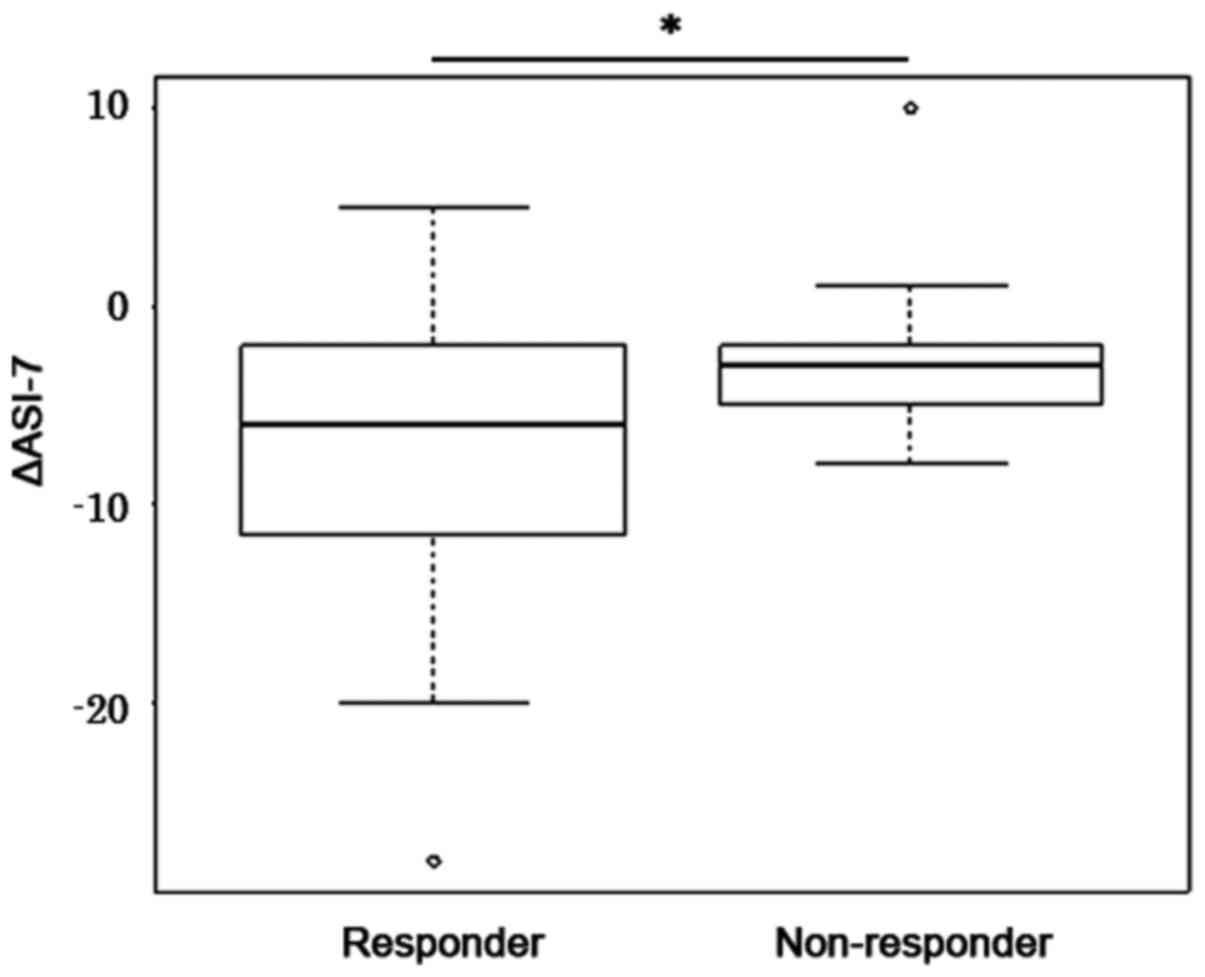
evaluated on the basis of changes in the BW after tolvaptan
treatment. Among the responders, 43/49 patients (87.8%) experienced
reduced ASI-7, whilst 13/20 non-responder patients (65.0%) had
decreased ASI-7. Responders and non-responders had an median ASI-7
score change of -6 (Q3-Q1:-11.5--2) and -3 (Q3-Q1:-5--2),
respectively, with a significant difference reported (P=0.012;
Fig. 6).
Consideration of each parameter of
ASI-7
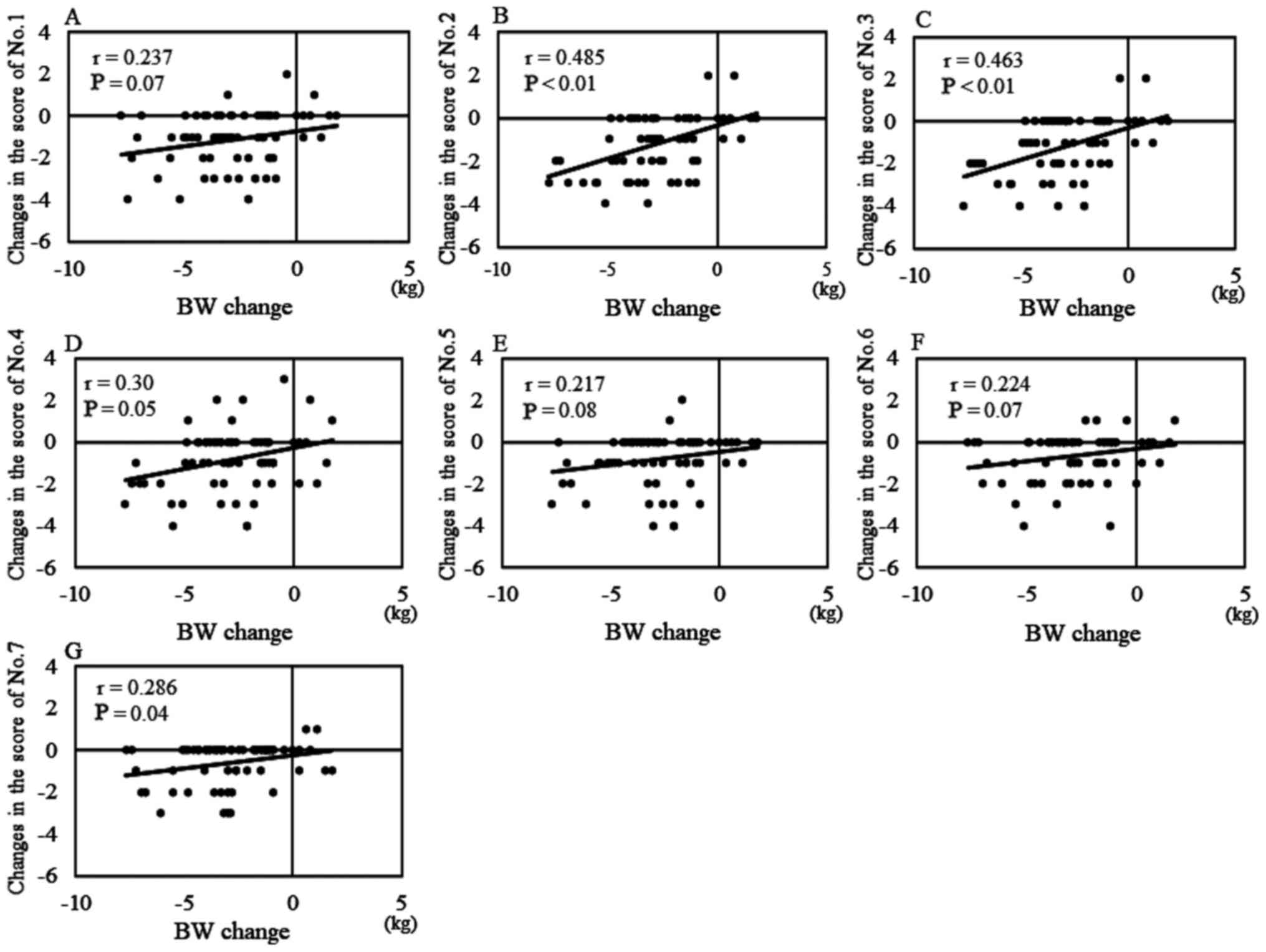
Correlation between the ΔASI-7 in each item and
changes in BW is shown in Fig. 7.
The strongest correlation was observed between item no. 2, which
stated ‘my stomach is bloated and uncomfortable’ and changes in BW
(r=0.485; P<0.01; Fig. 7B). Item
no. 3, which stated ‘my stomach is bloated, so it is difficult to
move around’, also exhibited a second most notable correlation with
changes in BW (r=0.463; P<0.01; Fig.
7C). Item 7, which stated ‘I cannot take deep breaths’
exhibited a third most notable correlation with changes in BW
(r=0.286; P<0.05; Fig. 7G). The
ΔASI-7 of the remaining items (nos. 1, 4, 5, 6 and 7) had either
weak or no correlation with changes in BW.
Discussion
The results from the present cohort study
demonstrated that following 7 days tolvaptan treatment, responders
lost weight, such that changes in the their ASI-7 scores correlated
with changes in BW. However, changes in urine volume did not
correlate with the changes in the ASI-7 scores. Responders showed a
further decrease in the ASI-7 scores after 7 days compared with
those in non-responders.
ASI-7 is primarily focused on the symptoms related
to the ascites of the patients. Only two reports have previously
discussed the use of ASI-7 in assessing the symptoms and wellbeing
of patients with ascites (8,15).
Objective parameters, including BW, urine volume, abdominal
circumference and ascites volume measured using US or CT, were
measured before and after tolvaptan treatment. The primary goal of
ascites treatment is to relieve the symptoms suffered by the
patients and consequently improve the QOL (20). The present study was the first to
use ASI-7 in evaluating cirrhotic patients with ascites who have
been treated with tolvaptan, which confirmed that the ASI-7 is a
valuable tool for assessing the response to and effectiveness of
tolvaptan administration after a 1-week study period.
Findings from the present study demonstrated that
hepatic reserves (Child-Pugh score) did not correlate with the
ASI-7 score. Generally, patients with severe ascites have high
ASI-7 scores (8). In the present
study, an elevation in the ASI-7 score was found to associate with
an increase in the ascitic volume. In total, 4 of the 10 patients
with mild ascites had severe hepatic hydrothorax with most having
high ASI-7 scores, which may have influenced the severity of
hepatic hydrothorax. However, some patients with severe ascites had
low ASI-7 scores. This may be attributed to the presence of ascites
for an extended period of time with only few abdominal symptoms. A
dispersion is therefore observed between the volume of ascites and
ASI-7 scores, which may be affected by the length of time the
patient has had severe ascites by extension of the abdominal wall.
To the best of our knowledge, however, there is no previous studies
reporting the extension of the abdominal wall caused by the
distension of severe ascites for a few weeks.
In patients with liver cirrhosis, the intrahepatic
resistance is increased by disruption in the intrahepatic blood
flow (21). Consequently, the
renin-angiotensin-aldosterone system, sympathetic nervous system
and antidiuretic hormone are activated, thereby reducing renal
blood flow, sodium and water reabsorption, leading to the
development of ascites and edema (22). The most effective treatment for
ascites, other than diuretics, is the transjugular intrahepatic
portosystemic shunt procedure (23)
or large-volume paracentesis (24),
which also improve the QOL of the patients. A reduction in BW
instead of an increase in urine output has been used as the primary
endpoint in clinical trials with tolvaptan (25). This is due to BW changes being
considered to be the clinically more important parameter, since
they can accurately reflect the volume overload in patients with
ascites (26). In the present
study, a weak correlation was noted between BW change and urine
output increase, suggesting that the increase in urine output does
not always reflect BW change. The phase III trial showed an average
decrease of ~2.0 kg/week in BW (11). However, a recent report concluded
that a 1.5-kg decrease in 7 days was the best cut-off value in
patients with hepatic edema receiving tolvaptan treatment, as
symptom reduction was achieved (17). A responder was therefore defined in
the study as those who experienced a 1.5-kg decrease in BW in 7
days. Patients who were tolvaptan responders had an 87.8% decrease
in the ASI-7 score whereas patients termed non-responders
experienced a 65.0% decrease in the ASI-7 score. A significant
difference was observed between responders and non-responders,
suggesting that tolvaptan served an important role in the reduction
in the ASI-7 scores. There was a significant correlation between
the BW change and the ASI-7 score change. Responders to tolvaptan
had an average ASI-7 score change of -7.8, whilst non-responders
exhibited an average change in the ASI-7 score of -2.8, meaning
that some patients showed a decrease in the ASI-7 score without a
concomitant BW decrease. Although the reasons for this are highly
complex, some cases could have been affected by the mental impact.
Patients who experienced an increase in urine output without a
reduction in BW occasionally feel an improvement in their symptoms
associated with ascites. Additionally, results from the present
study suggest that assessments using the ASI-7 items 2 and 3 may
accurately reflect the impact on abdominal symptoms. These
observations suggest that changes in the ASI-7 score is reflected
by a reduction in patient symptoms after 7 days of tolvaptan
treatment and item 2 of the ASI-7 scoring system was the most
influential item for indicating ascites symptoms.
There is a number of limitations associated with the
present study. They include the small number of patients and
utilization of only one type of questionnaire, ASI-7. Since there
are other questionnaires, including the SF-36, CLD-Q, or LDSI 2.0,
difficulty remains in concluding ASI-7 to be the most useful tool
for assessing tolvaptan treatment. However, as other questionnaires
do not target ascites, using the ASI-7 appeared to be more useful
in assessing the symptoms of ascites. The present study was also
limited in that all patients enrolled were Japanese. It has been
previously reported that the health-related quality of life
patterns differs between Asian and Western populations owing to
several different patient-reported outcomes (27).
In conclusion, the present study confirmed the
potential use of the ASI-7 questionnaire as an effective tool for
assessing patients with hepatic ascites. The majority of patients
who responded to treatment with tolvaptan also exhibited a
reduction in the ASI-7 score after 7 days of tolvaptan
administration, which may serve as a reflection of BW change. ASI-7
has proven to be a valuable tool for assessing information, such as
the abdominal symptoms associated with cirrhotic ascites.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HK, NS, KK, HT, YF, SSat, SSai and TK collected the
data. KM, TN and YS analyzed the data. HK and TA interpreted the
data. HF and HY contributed to the design of the work. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board of Nara Medical University. Written informed consents
were obtained for the present study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bhanji RA, Carey EJ and Watt KD: Review
article: Maximising quality of life while aspiring for quantity of
life in end-stage liver disease. Aliment Pharmacol Ther. 46:16–25.
2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Marchesini G, Bianchi G, Amodio P, Salerno
F, Merli M, Panella C, Loguercio C, Apolone G, Niero M, et al:
Factors associated with poor health-related quality of life of
patients with cirrhosis. Gastroenterology. 120:170–178.
2001.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Studenic P, Radner H, Smolen JS and
Aletaha D: Discrepancies between patients and physicians in their
perceptions of rheumatoid arthritis disease activity. Arthritis
Rheum. 64:2814–2823. 2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Gutteling JJ, de Man RA, van der Plas SM,
Schalm SW, Busschbach JJ and Darlington AS: Determinants of quality
of life in chronic liver patients. Aliment Pharmacol Ther.
23:1629–1635. 2006.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Prinsen CA, Mokkink LB, Bouter LM, Alonso
J, Patrick DL, de Vet HC and Terwee CB: COSMIN guideline for
systematic reviews of patient-reported outcome measures. Qual Life
Res. 27:1147–1157. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Cheung AC, Patel H, Meza-Cardona J, Cino
M, Sockalingam S and Hirschfield GM: Factors that influence
health-related quality of life in patients with primary Sclerosing
cholangitis. Dig Dis Sci. 61:1692–1699. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Klompenhouwer AJ, Sprengers D, Willemssen
FE, Gaspersz MP, Ijzermans JN and De Man RA: Evidence of good
prognosis of hepatocellular adenoma in post-menopausal women. J
Hepatol. 65:1163–1170. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Onishi Y, Wakita T, Fukuhara S, Noguchi Y,
Okada M, Sakaida I, Sasaki Y and Kobayashi K: Development and
validation of a symptom scale specific for ascites accompanied with
cirrhosis: The ASI-7. Clin Transl Gastroenterol.
5(e48)2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Schrier RW, Gross P, Gheorghiade M, Berl
T, Verbalis JG, Czerwiec FS and Orlandi C: SALT Investigators.
Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for
hyponatremia. N Engl J Med. 355:2099–2112. 2006.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Miyazaki T, Yamamura Y, Onogawa T,
Nakamura S, Kinoshita S, Nakayama S, Fujiki H and Mori T:
Therapeutic effects of tolvaptan, a potent, selective nonpeptide
vasopressin V2 receptor antagonist, in rats with acute and chronic
severe hyponatremia. Endocrinology. 146:3037–3043. 2005.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sakaida I, Yanase M, Kobayashi Y, Yasutake
T, Okada M, Okita K, Kageyama F, Miyaoka H, Nakamura H, Sakaeda H,
et al: ASCITES Clinical Pharmacology Group: The pharmacokinetics
and pharmacodynamics of tolvaptan in patients with liver cirrhosis
with insufficient response to conventional diuretics: A
multicentre, double-blind, parallel-group, phase III study. J Int
Med Res. 40:2381–2393. 2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yamamura Y, Nakamura S, Itoh S, Hirano T,
Onogawa T, Yamashita T, Yamada Y, Tsujimae K, Aoyama M, Kotosai K,
et al: OPC-41061, a highly potent human vasopressin V2-receptor
antagonist: Pharmacological profile and aquaretic effect by single
and multiple oral dosing in rats. J Pharmacol Exp Ther.
287:860–867. 1998.PubMed/NCBI
|
|
13
|
Clark WF, Devuyst O and Roussel R: The
vasopressin system: new insights for patients with kidney diseases:
Epidemiological evidence and therapeutic perspectives. J Intern
Med. 282:310–321. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Gülberg V, Liss I, Bilzer M, Waggershauser
T, Reiser M and Gerbes AL: Improved quality of life in patients
with refractory or recidivant ascites after insertion of
transjugular intrahepatic portosystemic shunts. Digestion.
66:127–130. 2002.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Neijenhuis M, Gevers TJG, Atwell TD,
Gunneson TJ, Schimek AC, Kievit W, Drenth JPH and Kamath PS:
Development and validation of a patient-reported outcome
measurement for symptom assessment in cirrhotic ascites. Am J
Gastroenterol. 113:567–575. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Vilstrup H, Amodio P, Bajaj J, Cordoba J,
Ferenci P, Mullen KD, Weissenborn K and Wong P: Hepatic
encephalopathy in chronic liver disease: 2014 Practice Guideline by
the American Association for the Study of Liver Diseases and the
European Association for the Study of the Liver. Hepatology.
60:715–735. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hiramine Y, Uojima H, Nakanishi H,
Hiramatsu A, Iwamoto T, Kimura M, Kawaratani H, Terai S, Yoshiji H,
Uto H, et al: Response criteria of tolvaptan for the treatment of
hepatic edema. J Gastroenterol. 53:258–268. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Pugh RN, Murray-Lyon IM, Dawson JL,
Pietroni MC and Williams R: Transection of the oesophagus for
bleeding oesophageal varices. Br J Surg. 60:646–649.
1973.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Moore KP, Wong F, Gines P, Bernardi M,
Ochs A, Salerno F, Angeli P, Porayko M, Moreau R, Garcia-Tsao G, et
al: The management of ascites in cirrhosis: Report on the consensus
conference of the International Ascites Club. Hepatology.
38:258–266. 2003.PubMed/NCBI View Article : Google Scholar
|
|
20
|
European Association for the Study of the
Liver. EASL clinical practice guidelines on the management of
ascites, spontaneous bacterial peritonitis, and hepatorenal
syndrome in cirrhosis. J Hepatol. 53:397–417. 2010.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Iwakiri Y, Shah V and Rockey DC: Vascular
pathobiology in chronic liver disease and cirrhosis - current
status and future directions. J Hepatol. 61:912–924.
2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Schrier RW: Water and sodium retention in
edematous disorders: Role of vasopressin and aldosterone. Am J Med.
119 (Suppl 1):S47–S53. 2006.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wong F: The use of TIPS in chronic liver
disease. Ann Hepatol. 5:5–15. 2006.PubMed/NCBI
|
|
24
|
Bureau C, Adebayo D, Chalret de Rieu M,
Elkrief L, Valla D, Peck-Radosavljevic M, McCune A, Vargas V,
Simon-Talero M, Cordoba J, et al: Alfapump® system vs.
large volume paracentesis for refractory ascites: A multicenter
randomized controlled study. J Hepatol. 67:940–949. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kawaratani H, Fukui H, Moriya K, Noguchi
R, Namisaki T, Uejima M, Kitade M, Takeda K, Okura Y, Kaji K, et
al: Predictive parameter of tolvaptan effectiveness in cirrhotic
ascites. Hepatol Res. 47:854–861. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kawaratani H, Fukui H and Yoshiji H:
Treatment for cirrhotic ascites. Hepatol Res. 47:166–177.
2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Fukuhara S, Bito S, Green J, Hsiao A and
Kurokawa K: Translation, adaptation, and validation of the SF-36
Health Survey for use in Japan. J Clin Epidemiol. 51:1037–1044.
1998.PubMed/NCBI View Article : Google Scholar
|