Introduction
Cyanotic congenital heart disease (CHD) is a type of
congenital defect that develops during gestation and affects
1/1,000 newborns representing ~10% of all CHD worldwide (1,2).
Progress in surgery and other interventions has improved the
treatment of purpuric CHD, however, treatment failure often occurs
due to the persistence of cyanosis (3). Chronic hypoxia is the basic
pathophysiological process associated with cyanotic CHD (4). Thus, investigation of the protective
mechanisms of cardiomyocytes under chronic hypoxia may provide
novel treatment strategies for patients with cyanotic CHD.
MicroRNAs (miRNAs/miRs) are a class of highly
conserved, non-coding RNA molecules that are ~22 nucleotides in
length. miRNAs bind to the 3'-untranslated region (UTR) of target
mRNAs to regulate gene expression in a post-transcriptional manner
(5). Recent studies have proposed
that miRNAs play a key role in the progression of CHD (6,7).
Existing research has confirmed that miRNA-145 can target frataxin
to regulate the development of CHD (6). miR-182 was confirmed to play a
protective role in cyanotic CHD by suppressing transcription factor
HES1(7). Studies have reported that
miR-219-5p is a tumor suppressor in various cancers, such as
glioblastoma and colon cancer (8,9).
However, the potential role of miR-219-5p in cyanotic CHD remains
unclear.
Liver receptor homolog-1 (LRH-1) is a nuclear
receptor which has been implicated in a variety of biological
processes, including cell development, lipid homeostasis,
embryogenesis and steroidogenesis (10). LRH-1 expression was identified in
mouse and human tissues derived from the endoderm, including the
liver, intestine and exocrine pancreas, as well as in the ovary and
brain (11,12). LRH-1 was reported to play a role in
cancer development and progression (13-15).
Functional studies have shown that LRH-1 regulated cancer cell
growth, apoptosis and invasiveness (14,15).
However, the physiological function of LRH-1 in cyanotic CHD
remains to be elucidated.
The present study aimed to investigate the role of
miR-219-5p in the proliferation and apoptosis of hypoxic
cardiomyocytes and its potential mechanisms in the development of
cyanotic CHD.
Materials and methods
Patients
A total of 30 children diagnosed with CHD in Huai'an
First People's Hospital (Huai'an, China) between January 2017 and
March 2018, including 15 children with cyanotic CHD (8 females, 7
males; age range, 3.6-16 years) and 15 children with acyanotic CHD
(8 females, 7 males; age range, 2.9-15 years), were enrolled in the
current study. All participants and their legal guardians agreed to
the use of their samples in the present study, and written informed
consents were obtained from all the legal guardians of all
participants. The study protocol was approved by the Ethics
Committee of Huai'an First People's Hospital. Standardized
anesthesia and operation were performed according to a previous
study (16). A biopsy sample was
obtained from the right ventricular outflow tract. Myocardium
samples were rapidly frozen in liquid nitrogen and stored at -70˚C
until further use.
Cell culture and treatment
Embryonic rat ventricular myocardial H9C2 cells were
purchased from the American Type Culture Collection and cultured in
DMEM (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10%
FBS (Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin/streptomycin. H9C2 cells were first serum-starved
overnight before being incubated in an incubator containing a
gaseous mixture of 5% CO2, 94% N2 and 1%
O2 (Thermo Fisher Scientific, Inc.) at 37˚C for 12, 24,
48 and 72 h.
Cell transfection
miR-219-5p inhibitor (5'-AGAAUUGCG UUUGGACAAUCA-3')
and inhibitor control (5'-CAGUAC UUUUGUGUAGUACAA-3') were
synthesized by Shanghai GenePharma Co., Ltd. LRH-1-small
interfering RNA (siRNA; cat no. CRR1673) and control-siRNA (cat no.
9500C-20) were obtained from Guangzhou Weijia Technology Co., Ltd
(https://whiga.biomart.cn/). A total of
100 nM miR-219-5p inhibitor and 100 nM inhibitor control, 0.2 µM
LRH-1-siRNA and 0.2 µM control-siRNA or 100 nM miR-219-5p inhibitor
+ 0.2 µM LRH-1siRNA were transfected into H9C2 cells using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. Cells
were left incubated for 72 h before transfection efficiency was
analyzed with reverse transcription-quantitative PCR (RT-qPCR).
Cells without any treatment were used as the control.
Western blot analysis
Total protein from H9C2 cells was extracted using
RIPA buffer (Beyotime Institute of Biotechnology) containing
protease and phosphatase inhibitors. A bicinchoninic acid protein
assay kit (Thermo Fisher Scientific, Inc.) was used to detect
protein concentration. Protein lysates (40 µg per lane) were
subjected to 12% SDS-PAGE and transferred onto PVDF membranes (EMD
Millipore). Membranes were blocked for 1 h at room temperature
using 5% skim milk and subsequently incubated with the following
primary antibodies at 4˚C overnight: LRH-1 (cat no. 12800; 1:1,000;
Cell Signaling Technology, Inc.), cyclin D1 (cat no. 2978; 1:1,000;
Cell Signaling Technology, Inc.), β-catenin (cat no. 8480; 1:1,000;
Cell Signaling Technology, Inc.) and β-actin (cat no. 4970;
1:1,000; Cell Signaling Technology, Inc.). The membranes were then
incubated with horseradish peroxidase-coupled secondary antibody
(anti-rabbit immunoglobulin G, cat no. 7074; 1:1,000; Cell
Signaling Technology, Inc.) at room temperature for 2 h. Protein
bands were visualized with an ECL western blotting kit (EMD
Millipore).
RT-qPCR
TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) was used to isolate total RNA according to
the manufacturer's instructions. cDNA was reverse transcribed from
total RNA using a PrimeScript RT Reagent kit (Takara Biotechnology
Co., Ltd.) according to the manufacturer's instructions. qPCR was
subsequently performed using the SYBR Premix Ex Taq GC kit (Takara
Bio, Inc.) on an ABI 7500 thermocycler (Applied Biosystems; Thermo
Fisher Scientific, Inc.). The thermocycling conditions were as
follows: Initial denaturation 95˚C for 5 min, followed by 38 cycles
of denaturation at 95˚C for 15 sec and annealing/elongation at 60˚C
for 30 sec. The following primer pairs were used for the qPCR:
miR-219-5p forward, 5'-ACACTCCAGCTGGGTGAT TGTCCAAACGCAAT-3' and
reverse, 5'-CTCAACTGGTGT CGTGGA3'; LRH-1 forward,
5'-GCACGGACTTACACCTAT TGTG-3' and reverse,
5'-TGTCAATTTGGCAGTTCTGG-3'; cyclin D1 forward, 5'-AACTACCTG
GACCGCTTCCT-3 and reverse, 5'-CCACTTGAGCTTGTTCACCA-3'; U6 forward,
5'-CTCGCTTCGGCAGCACATAT-3' and reverse, 5'-TTG CGTGTCATCCTTGCG-3'
and GAPDH forward, 5'-CTG GGCTACACTGAGCACC-3' and reverse,
5'-AAGTGGTCG TTGAGGGCAATG-3'. GAPDH and U6 were employed as
internal controls. Relative expression of genes was calculated
using the 2-ΔΔCq method (17).
MTT assay
MTT assay was used to measure cell viability. H9C2
cells (4x103 cells/well) were seeded into 96-well
culture plates. Subsequently, H9C2 cells were pre-transfected with
miR-219-5p inhibitor, inhibitor control or miR-219-5p inhibitor +
LRH-1-siRNA for 6 h. The cells were incubated for a further 72 h in
hypoxic and non-hypoxic conditions. Finally, cells were incubated
with MTT (5 mg/ml) for 4 h. A total of 150 µl DMSO was added to
each well and incubated at 37˚C for 30 min. A microplate
spectrophotometer (Thermo Fisher Scientific, Inc.) was used to
measure the absorbance at a wavelength of 450 nm.
Luciferase reporter assay
The potential target genes of miR-219-5p were
identified using bioinformatics prediction software MicroRNA.org (http://www.microrna.org/mircrorna/home.do). The
results indicated the binding sites between LRH-1 and miR-219-5p,
which was further confirmed with a dual luciferase reporter assay.
The 3'-UTR of LRH-1 containing the seed sequence of the wild-type
(WT) or a mutated (MUT) binding site of miR-219-5p was cloned into
psiCHECK-2 vectors (Promega Corporation) to generate
psiCHECK-LRH-1-3'UTR-WT and MUT luciferase reporter plasmids.
Subsequently, Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) was used to co-transfect plasmid DNA and
miR-219-5p mimic/mimic control into H9C2 cells for 48 h. A dual
luciferase reporter assay system (Promega Corporation) was then
used to measure the luciferase activity and Renilla luciferase
activity was used for normalization.
Flow cytometry assay
Flow cytometry using the Annexin V-FITC apoptosis
kit (Beyotime Institute of Biotechnology) was performed to
determine cell apoptosis. H9C2 cells were pre-transfected with
miR-219-5p inhibitor, inhibitor control or miR-219-5p inhibitor +
LRH-1-siRNA for 6 h. Subsequently, the cells were incubated for a
further 72 h in hypoxic and non-hypoxic conditions. Following
treatment, cells were harvested and suspended in 500 µl binding
buffer. Cells were then double-stained with 5 µl Annexin V-FITC and
5 µl propidium iodide for 10 min in the dark at room temperature.
FACS flow cytometer (BD Biosciences) was used to analyze each
sample. Data were analyzed using FlowJo software (version 7.6.1;
FlowJo LLC).
Statistical analysis
SPSS 18.0 (SPSS, Inc.) was used to analyze the data.
All experiments were performed three times. Results were expressed
as the mean ± SD. Comparisons between groups were determined using
Student's t-test or one-way ANOVA with Tukey's post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
miR-219-5p is upregulated in patients
with cyanotic CHD and hypoxic cardiomyocytes
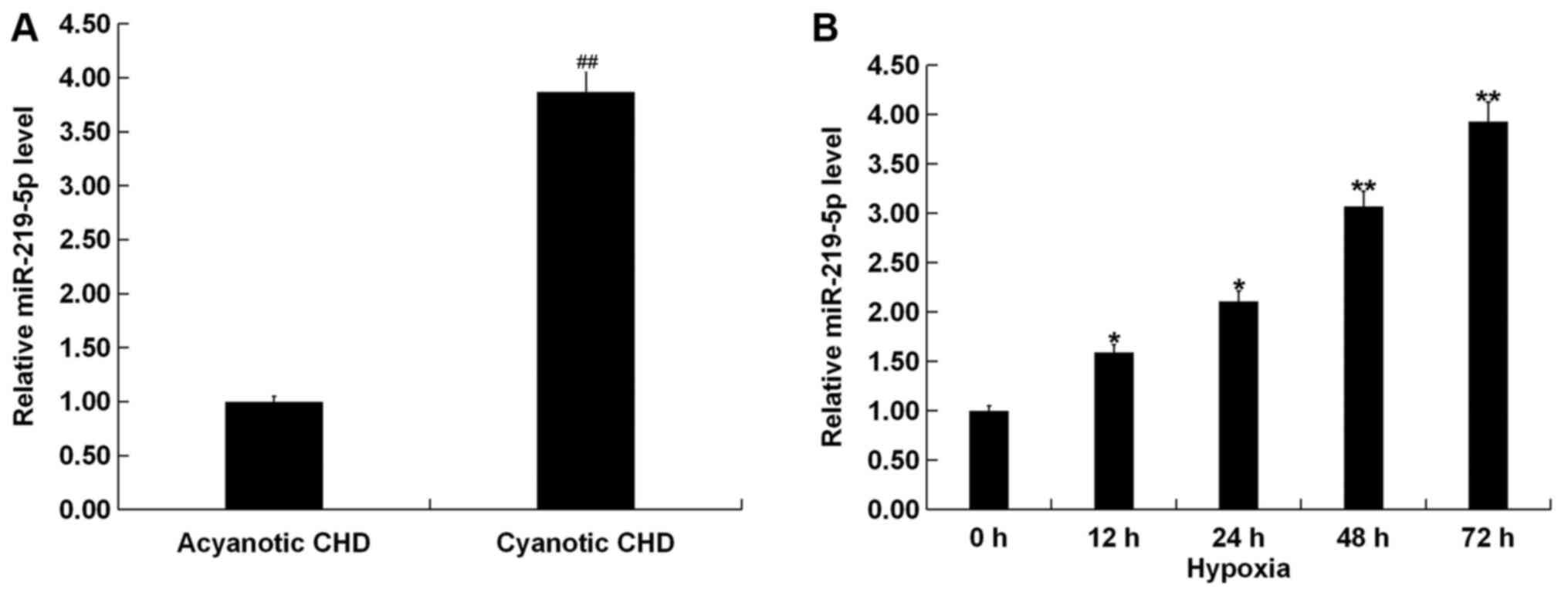
RT-qPCR results revealed that the expression of
miR-219-5p in patients with cyanotic CHD was significantly higher
compared with patients with acyanotic CHD (Fig. 1A). Additionally, H9C2 cells were
exposed to hypoxic conditions for 0 (cells under normal oxygen
conditions), 12, 24, 48 and 72 h. miR-219-5p was found to gradually
increase in a time-dependent manner (Fig. 1B).
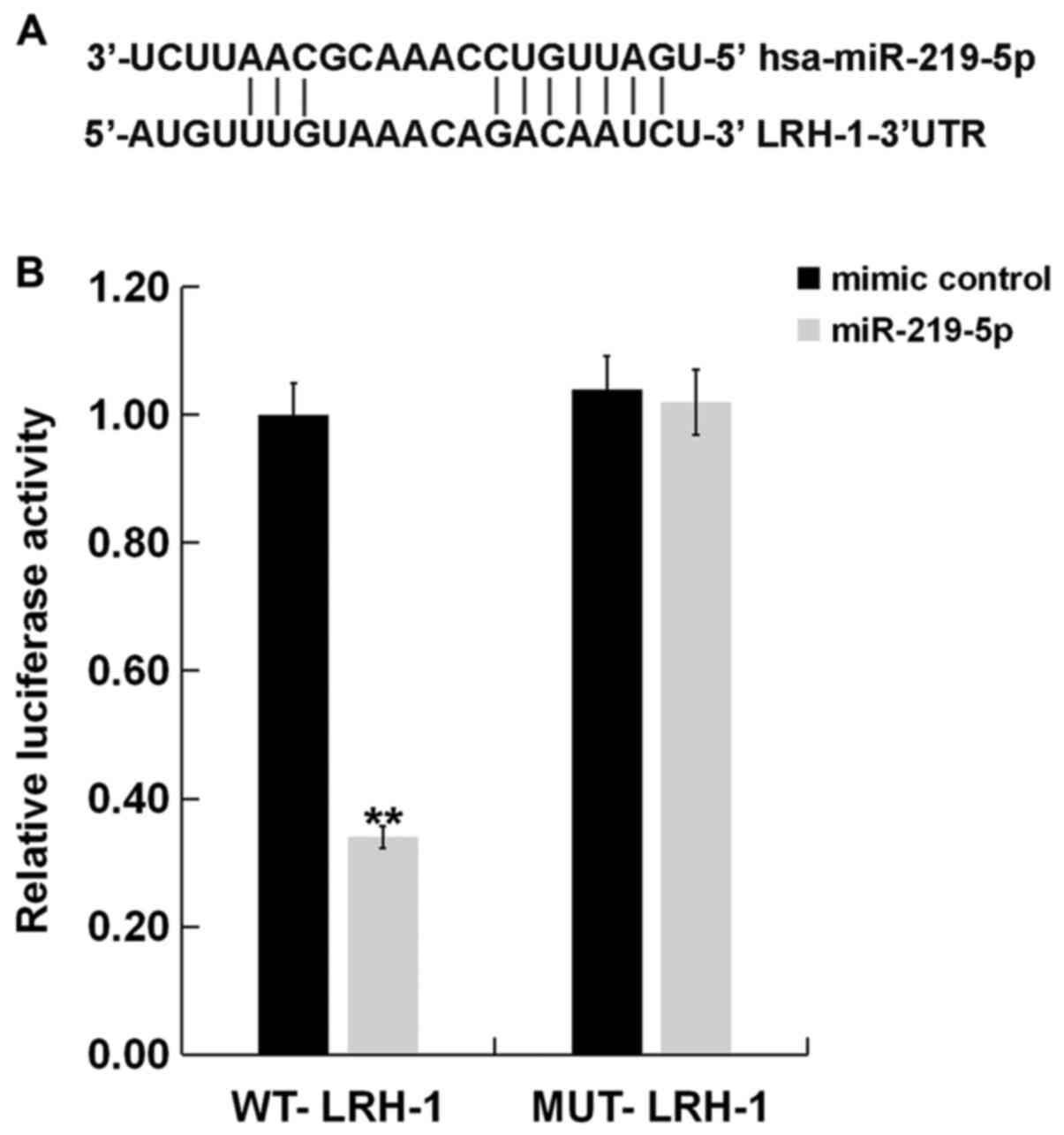
LRH-1 is a target of miR-219-5p
Bioinformatic results from MicroRNA.org predicted that LRH-1 contained
theoretical miR-219-5p binding sites in its 3'-UTR (Fig. 2A). To confirm whether miR-219-5p
could directly target the LRH-1 3'-UTR, a luciferase reporter
plasmid containing WT and MUT LRH-1 3'-UTR were co-transfected with
the miR-219-5p mimic or mimic control into H9C2 cells. As shown in
Fig. 2B, co-expression of
miR-219-5p mimic significantly inhibited the luciferase reporter
activity of the wild-type LRH-1 3'-UTR but not of the mutant LRH-1
3'-UTR. These results suggested that LRH-1 is a direct target of
miR-219-5p.
Effects of miR-219-5p inhibitor on
cell survival under hypoxic conditions
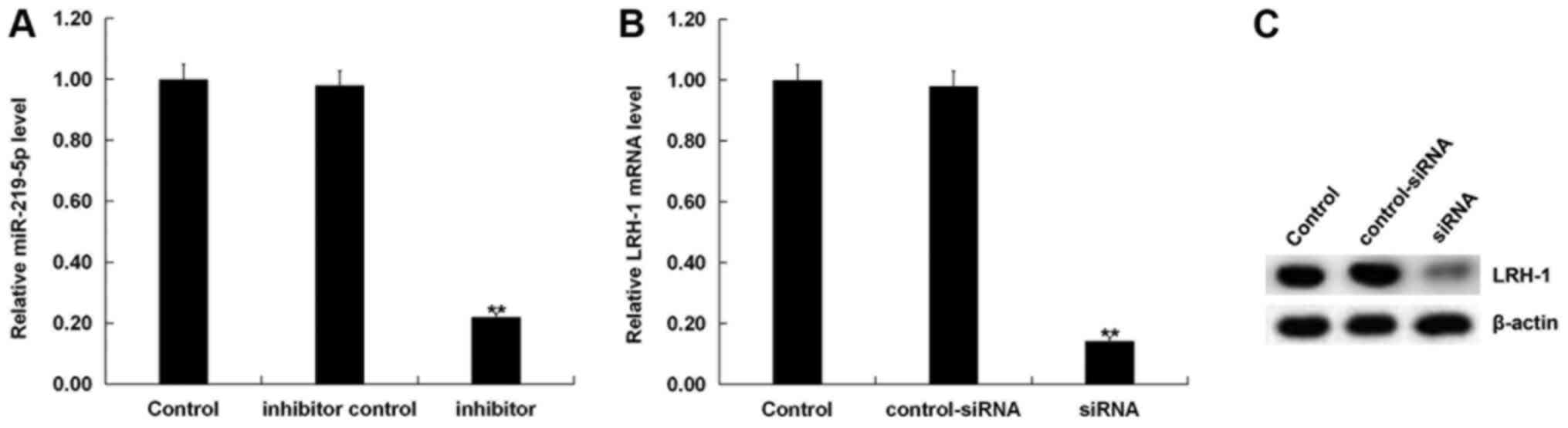
The potential role of miR-219-5p in hypoxic
cardiomyocytes was then investigated. H9C2 cells were transfected
with miR-219-5p inhibitor, inhibitor control, LRH-1-siRNA or
control-siRNA for 72 h. Transfection efficiencies were detected by
RT-qPCR. As shown in Fig. 3A,
miR-219-5p expression significantly decreased in miR-219-5p
inhibitor-transfected cells compared with the control group. Levels
of both mRNA (Fig. 3B) and protein
(Fig. 3C) expression of LRH-1
markedly decreased in the LRH-1-siRNA transfection group compared
with the control group.
An MTT assay was then performed to detect cell
viability. As shown in Fig. 4A,
inhibition of miR-219-5p significantly enhanced H9C2 cell viability
in hypoxic conditions at 72 h compared with cells under hypoxic
conditions. Co-transfection of cells with LRH-1-siRNA resulted in
significantly reduced cell viability compared with the miR-219-5p
inhibitor group.
Downregulation of miR-219-5p inhibits
hypoxia-induced cardiomyocyte apoptosis
To investigate whether miR-219-5p played a role in
hypoxia-induced apoptosis, flow cytometry was used. Flow cytometry
results showed that hypoxia induction significantly enhanced H9C2
cell apoptosis compared with the control group. Hypoxia-induced
apoptosis was significantly reduced by miR-219-5p inhibitor, and
this reduction was partially reversed by LRH-1-siRNA transfection
(Figs. 4B and C).
Effects of miR-219-5p inhibition on
the LRH-1/Wnt/β-catenin pathway in hypoxic cardiomyocytes
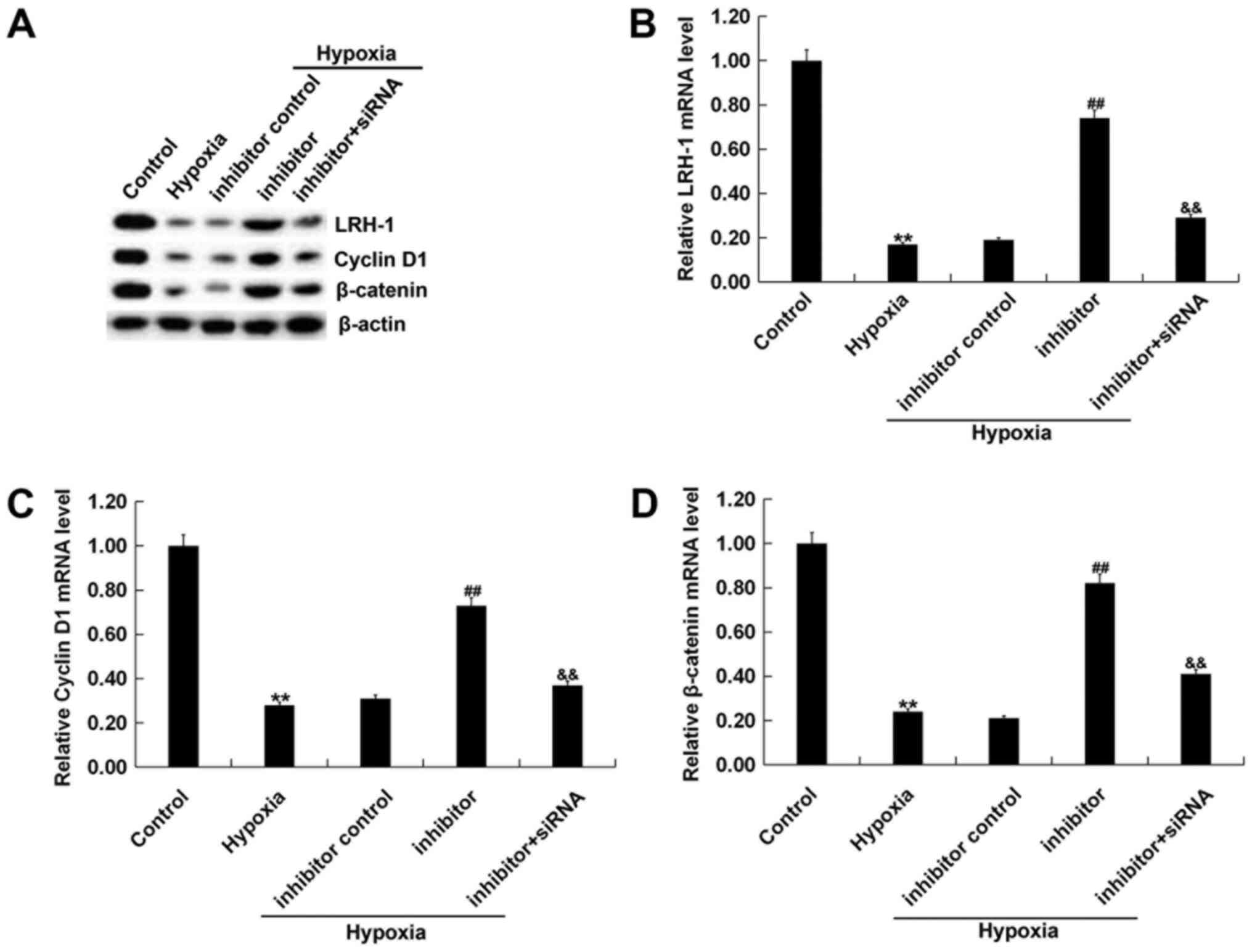
In order to investigate the possible mechanisms
behind the role of miR-219-5p in hypoxic cardiomyocytes, the
LRH-1/Wnt/β-catenin pathway was studied. H9C2 cells were first
transfected with miR-219-5p inhibitor, inhibitor control or
miR-219-5p inhibitor + LRH-1-siRNA for 6 h. Subsequently, the cells
were incubated for a further 72 h in hypoxic and non-hypoxic
conditions. The mRNA and protein expression levels of LRH-1, cyclin
D1 and β-catenin in H9C2 cells were then determined. The results
indicated that hypoxia induction reduced protein (Fig. 5A) and mRNA (Fig. 5B-D) levels of LRH-1, cyclin D1 and
β-catenin in H9C2 cells. Transfection with miR-219-5p inhibitor
increased protein and mRNA levels of LRH-1, cyclin D1 and β-catenin
compared with inhibitor controls. These effects were partially
reversed by transfecting cells with LRH-1-siRNA.
Discussion
In the present study, miR-219-5p was discovered to
possibly be involved in the development of cyanotic CHD. The
present research showed that the expression of miR-219-5p was
significantly upregulated in patients with cyanotic CHD and hypoxic
cardiomyocytes compared with patients with acyanotic CHD and normal
untreated cardiomyocytes, respectively. Downregulation of
miR-219-5p increased hypoxia-induced cardiomyocyte viability and
inhibited hypoxia-induced cardiomyocyte apoptosis by targeting
LRH-1. Therefore, inhibition of miR-219-5p expression may be a
potential mechanism for cardioprotection against chronic
hypoxia.
Chronic hypoxia is the basic pathophysiological
process associated with cyanotic CHD (18). A previous study reported that
reduction of cell viability, invasion and migration, and increased
cell apoptosis could be induced by hypoxia in H9C2 cells (19). In addition, hypoxia could enhance
cardiomyocyte apoptosis to induce cytotoxicity (20). Hypoxia treatment triggered a variety
in miRNA expression abnormalities, such as miR-181 and miR-532-5p,
in cardiomyocytes (21,22). In the present study, H9C2 cell
viability was inhibited and apoptosis was increased after 72 h of
hypoxia induction. miR-219-5p expression in patients with cyanotic
CHD and in hypoxia-induced cardiomyocytes was significantly
upregulated compared with patients with acyanotic CHD and normal
cardiomyocytes, respectively.
miR-219-5p has been extensively studied in several
disease processes. A previous study reported that miR-219-5p
inhibitor protected against spinal cord injury by regulating the
LRH-1/Wnt/β-catenin signaling pathway (23). miR-219-5p promoted the metastasis
and growth of hepatocellular carcinoma via downregulating cadherin
1(24). Additionally, a recent
study found that miR-219-5p was markedly increased in hypoxic
keratinocytes and targeted transmembrane protein 98 to inhibit
wound healing in keratinocytes under hypoxic conditions (25). However, to the best of our
knowledge, few studies have reported the potential role of
miR-219-5p in heart diseases. In the present research, the data
showed that inhibition of miR-219-5p reduced cell apoptosis and
enhanced cell viability in hypoxia-induced cardiomyocytes,
indicating that inhibition of miR-219-5p might play a protective
role in the progression of cyanotic CHD.
In the present research, LRH-1 was shown to be a
direct target of miR-219-5p. LRH-1 is known to be a co-activator of
the Wnt/β-catenin signaling pathway (26). Dysregulation of Wnt/β-catenin
signaling is involved in various cellular and biological processes,
including cell differentiation, proliferation and apoptosis
(27). Dysregulated Wnt/β-catenin
pathway is implicated in the onset and progression of hypertensive
heart disease (28). A previous
study reported that hypoxia-inducible factor 2α induced
cardiomyogenesis via enhancing the activation of the Wnt/β-catenin
signaling in mouse embryonic stem cells (29). These studies indicated a positive
role of Wnt/β-catenin in cardiac protection. In the present study,
the results showed that inhibition of the LRH-1/Wnt/β-catenin
pathway in cardiomyocytes by hypoxia could be reversed by
miR-219-5p inhibitor transfection. Moreover, LRH-1 siRNA
transfection could reverse the effects of miR-219-5p inhibitor on
hypoxia-induced cardiomyocytes. However, a limitation of the
current research is the absence of another experimental condition
such as a hypoxia group co-transfected with both inhibitor and
control siRNA.
In conclusion, the present research demonstrated
that the expression of miR-219-5p was increased in patients with
cyanotic CHD and hypoxic cardiomyocytes. Downregulation of
miR-219-5p increased cell viability and reduced apoptosis of
hypoxia-induced cardiomyocytes via affecting the
LRH-1/Wnt/β-catenin signaling pathway. These results suggested that
miR-219-5p may be a potential target in the clinical therapeutic
treatment of cyanotic CHD. However, the study is only a preliminary
analysis of the role of miR-219-5p in congenital heart disease. To
further confirm the role of miR-219-5p in CHD, more in-depth
experimental research is still required. For example, the role of
miR-219-5p/LRH-1 in other types of cardiomyocytes (such as human
primary cardiomyocytes and mouse cardiomyocytes) could be
investigated. The role of miR-219-5p/LRH-1 in CHD could be
investigated in vivo. The relationship between the
expression of miR-219-5p/LRH-1 in patients with CHD and the
clinical pathological features of patients with CHD could also be
studied.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All datasets used and/or generated during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
CH contributed to the conception and design of the
study. SH and FW contributed to the data acquisition, analysis and
interpretation. HD analyzed the data and prepared the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Ethics
Committee of Huai'an First People's Hospital (Huai'an, China).
Written informed consents were obtained from the legal guardians of
all participants.
Patient consent for publication
All patients' guardians provided consent for
publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cassidy AR, White MT, DeMaso DR, Newburger
JW and Bellinger DC: Executive function in children and adolescents
with critical cyanotic congenital heart disease. J Int Neuropsychol
Soc. 21:34–49. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hoffman JI and Kaplan S: The incidence of
congenital heart disease. J Am Coll Cardiol. 39:1890–1900.
2002.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Haseba S, Sakakima H, Nakao S, Ohira M,
Yanagi S, Imoto Y, Yoshida A and Shimodozono M: Early postoperative
physical therapy for improving short-term gross motor outcome in
infants with cyanotic and acyanotic congenital heart disease.
Disabil Rehabil. 40:1694–1701. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Cordina RL and Celermajer DS: Chronic
cyanosis and vascular function: Implications for patients with
cyanotic congenital heart disease. Cardiol Young. 20:242–253.
2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297.
2004.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wang L, Tian D, Hu J, Xing H, Sun M, Wang
J, Jian Q and Yang H: miRNA-145 regulates the development of
congenital heart disease through targeting FXN. Pediatr Cardiol.
37:629–636. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhang Y, Peng B and Han Y: miR-182
alleviates the development of cyanotic congenital heart disease by
suppressing HES1. Eur J Pharmacol. 836:18–24. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Cheng J, Deng R, Zhang P, Wu C, Wu K, Shi
L, Liu X, Bai J, Deng M, Shuai X, et al: miR-219-5p plays a tumor
suppressive role in colon cancer by targeting oncogene Sall4. Oncol
Rep. 34:1923–1932. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Jiang Y, Yin L, Jing H and Zhang H:
MicroRNA-219-5p exerts tumor suppressor function by targeting ROBO1
in glioblastoma. Tumour Biol. 36:8943–8951. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Fayard E, Auwerx J and Schoonjans K:
LRH-1: An orphan nuclear receptor involved in development,
metabolism and steroidogenesis. Trends Cell Biol. 14:250–260.
2004.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wang ZN, Bassett M and Rainey WE: Liver
receptor homologue-1 is expressed in the adrenal and can regulate
transcription of 11 beta-hydroxylase. J Mol Endocrinol. 27:255–258.
2001.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Grgurevic N, Tobet S and Majdic G:
Widespread expression of liver receptor homolog 1 in mouse brain.
Neuro Endocrinol Lett. 26:541–547. 2005.PubMed/NCBI
|
|
13
|
Wu C, Feng J, Li L, Wu Y, Xie H, Yin Y, Ye
J and Li Z: Liver receptor homologue 1, a novel prognostic marker
in colon cancer patients. Oncol Lett. 16:2833–2838. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Bianco S, Jangal M, Garneau D and Gévry N:
LRH-1 controls proliferation in breast tumor cells by regulating
CDKN1A gene expression. Oncogene. 34:4509–4518. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Xiao L, Wang Y, Xu K, Hu H, Xu Z, Wu D,
Wang Z, You W, Ng CF, Yu S, et al: Nuclear receptor LRH-1 functions
to promote castration-resistant growth of prostate cancer via its
promotion of intratumoral androgen biosynthesis. Cancer Res.
78:2205–2218. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Jian Z, Li JB, Ma RY, Chen L, Zhong QJ,
Wang XF, Wang W, Hong Y and Xiao YB: Increase of macrophage
migration inhibitory factor (MIF) expression in cardiomyocytes
during chronic hypoxia. Clin Chim Acta. 405:132–138.
2009.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Piccoli M, Conforti E, Varrica A, Ghiroldi
A, Cirillo F, Resmini G, Pluchinotta F, Tettamanti G, Giamberti A,
Frigiola A, et al: NEU3 sialidase role in activating HIF-1α in
response to chronic hypoxia in cyanotic congenital heart patients.
Int J Cardiol. 230:6–13. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Gong L, Xu H, Chang H, Tong Y, Zhang T and
Guo G: Knockdown of long non-coding RNA MEG3 protects H9c2 cells
from hypoxia-induced injury by targeting microRNA-183. J Cell
Biochem. 119:1429–1440. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Park M, Youn B, Zheng XL, Wu D, Xu A and
Sweeney G: Globular adiponectin, acting via AdipoR1/APPL1, protects
H9c2 cells from hypoxia/reoxygenation-induced apoptosis. PLoS One.
6(e19143)2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hao P, Cao X, Zhu Z, Gao C, Chen Y and Qi
D: Effects of miR-181a targeting XIAP gene on apoptosis of
cardiomyocytes induced by hypoxia/reoxygenation and its mechanism.
J Cell Biochem: Nov 28, 2018 (Epub ahead of print).
|
|
22
|
Ma J, Zhang J, Wang Y, Long K, Wang X, Jin
L, Tang Q, Zhu L, Tang G, Li X, et al: miR-532-5p alleviates
hypoxia-induced cardiomyocyte apoptosis by targeting PDCD4. Gene.
675:36–43. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Li J, Li L and Shen Y: Protective role of
microRNA-219-5p inhibitor against spinal cord injury via liver
receptor homolog-1/Wnt/β-catenin signaling pathway regulation. Exp
Ther Med. 15:3563–3569. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yang J, Sheng YY, Wei JW, Gao XM, Zhu Y,
Jia HL, Dong QZ and Qin LX: MicroRNA-219-5p promotes tumor growth
and metastasis of hepatocellular carcinoma by regulating cadherin
1. BioMed Res Int. 2018(4793971)2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Tang Q and Ran H: MicroRNA-219-5p inhibits
wound healing by targeting TMEM98 in keratinocytes under normoxia
and hypoxia condition. Eur Rev Med Pharmacol Sci. 22:6205–6211.
2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Botrugno OA, Fayard E, Annicotte JS, Haby
C, Brennan T, Wendling O, Tanaka T, Kodama T, Thomas W, Auwerx J,
et al: Synergy between LRH-1 and beta-catenin induces G1
cyclin-mediated cell proliferation. Mol Cell. 15:499–509.
2004.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Fodde R and Brabletz T: Wnt/beta-catenin
signaling in cancer stemness and malignant behavior. Curr Opin Cell
Biol. 19:150–158. 2007.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhao Y, Wang C, Wang C, Hong X, Miao J,
Liao Y, Zhou L and Liu Y: An essential role for Wnt/β-catenin
signaling in mediating hypertensive heart disease. Sci Rep.
8(8996)2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Sun X, Pang L, Shi M, Huang J and Wang Y:
HIF2α induces cardiomyogenesis via Wnt/β-catenin signaling in mouse
embryonic stem cells. J Transl Med. 13(88)2015.PubMed/NCBI View Article : Google Scholar
|