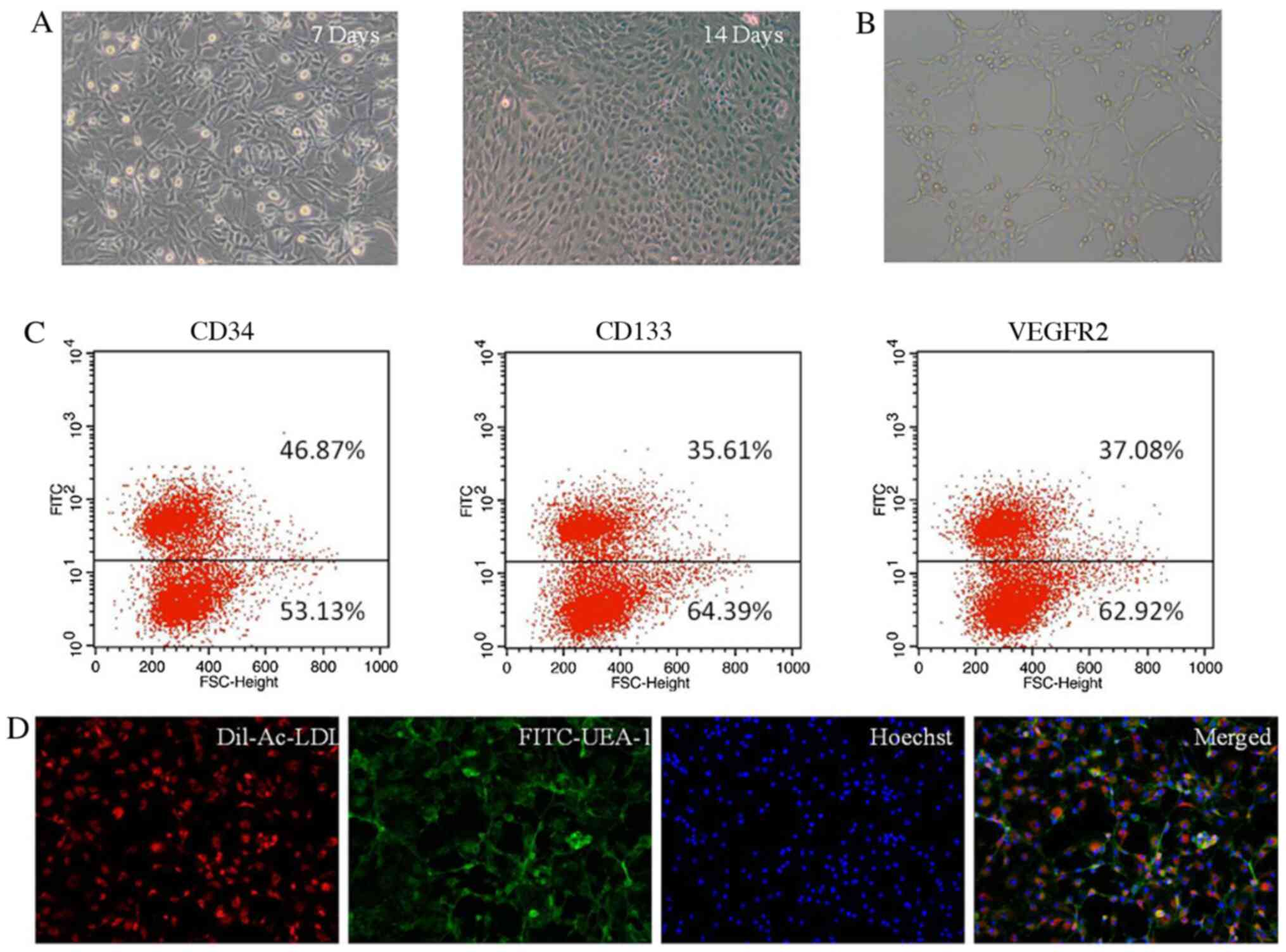
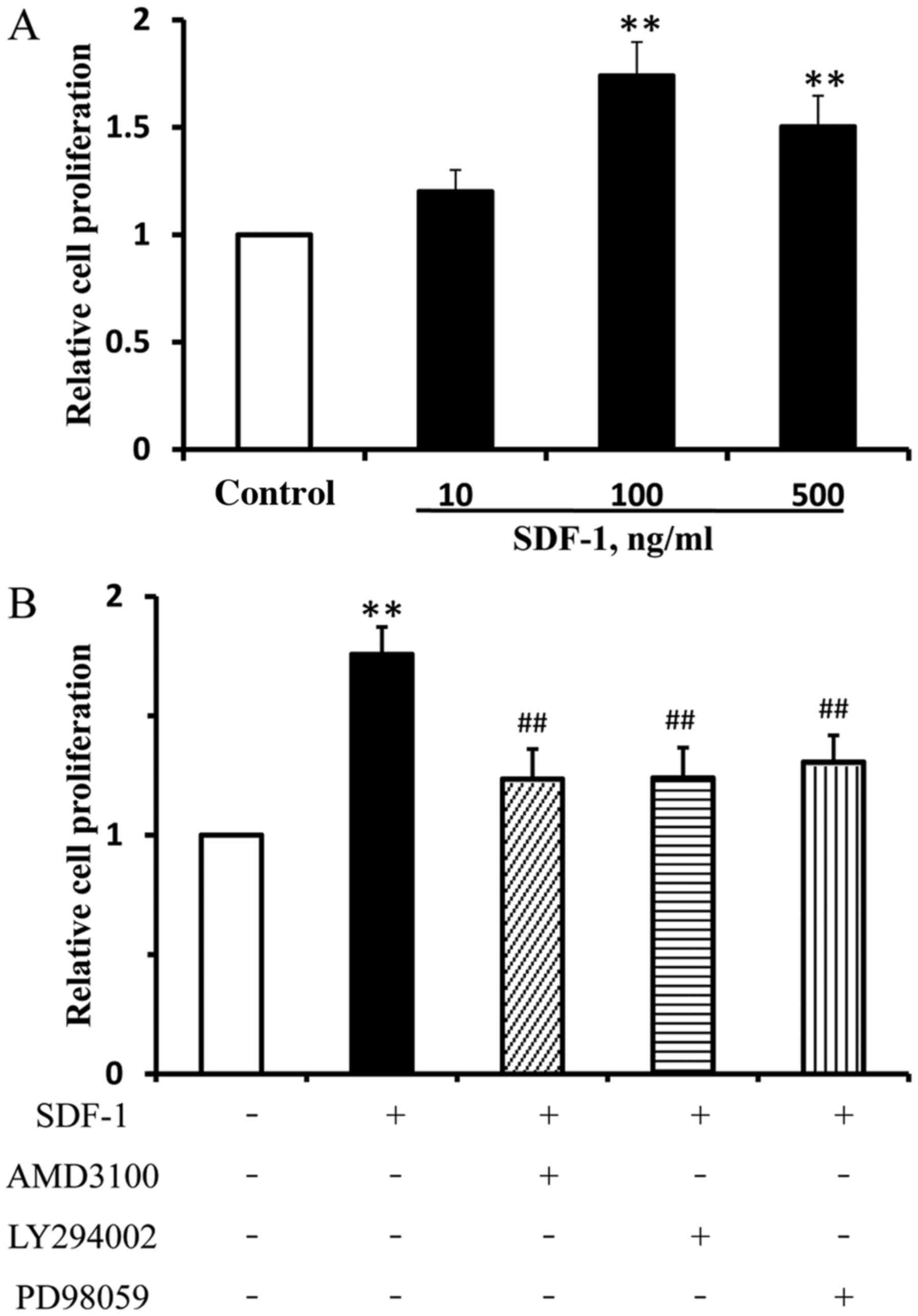
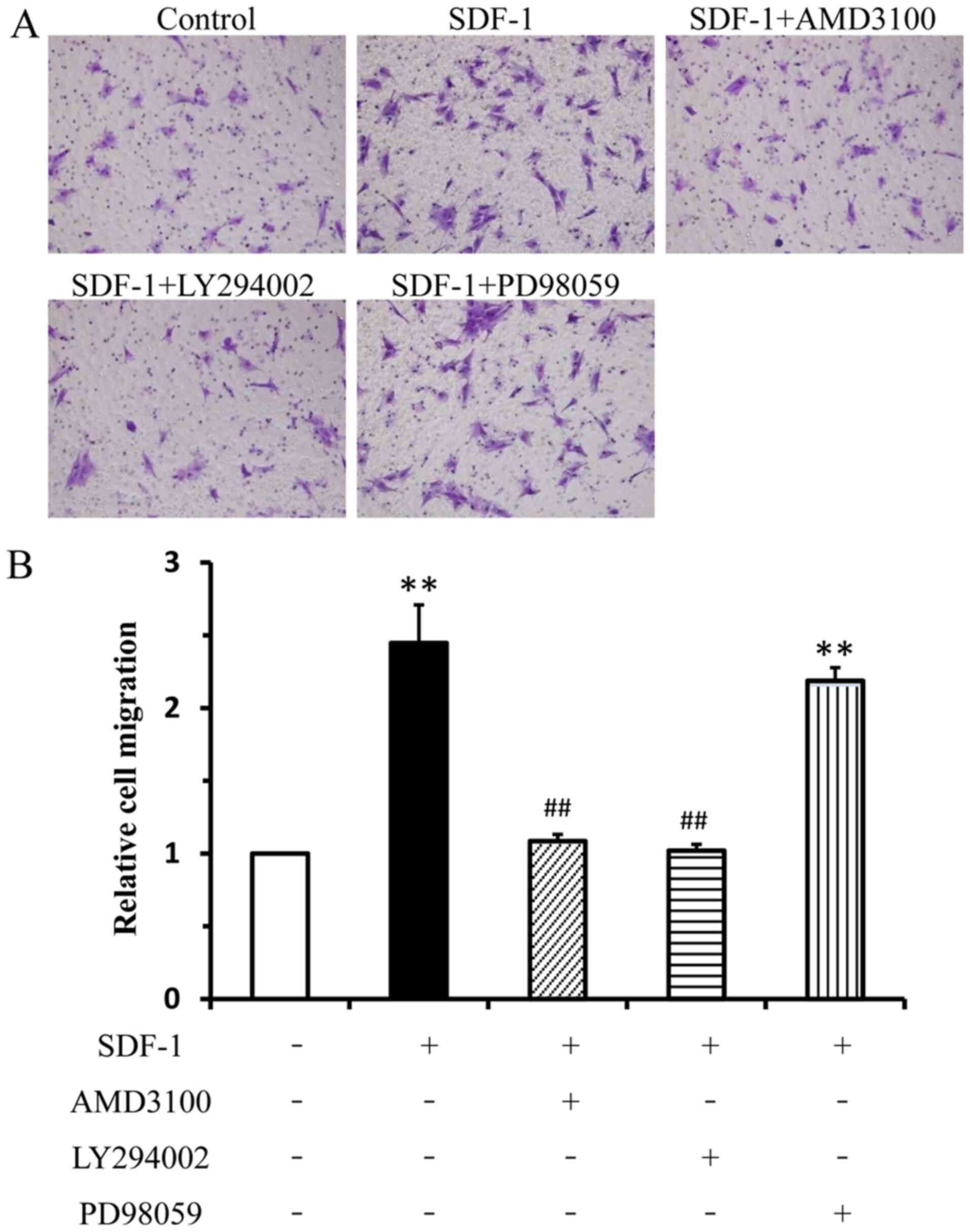
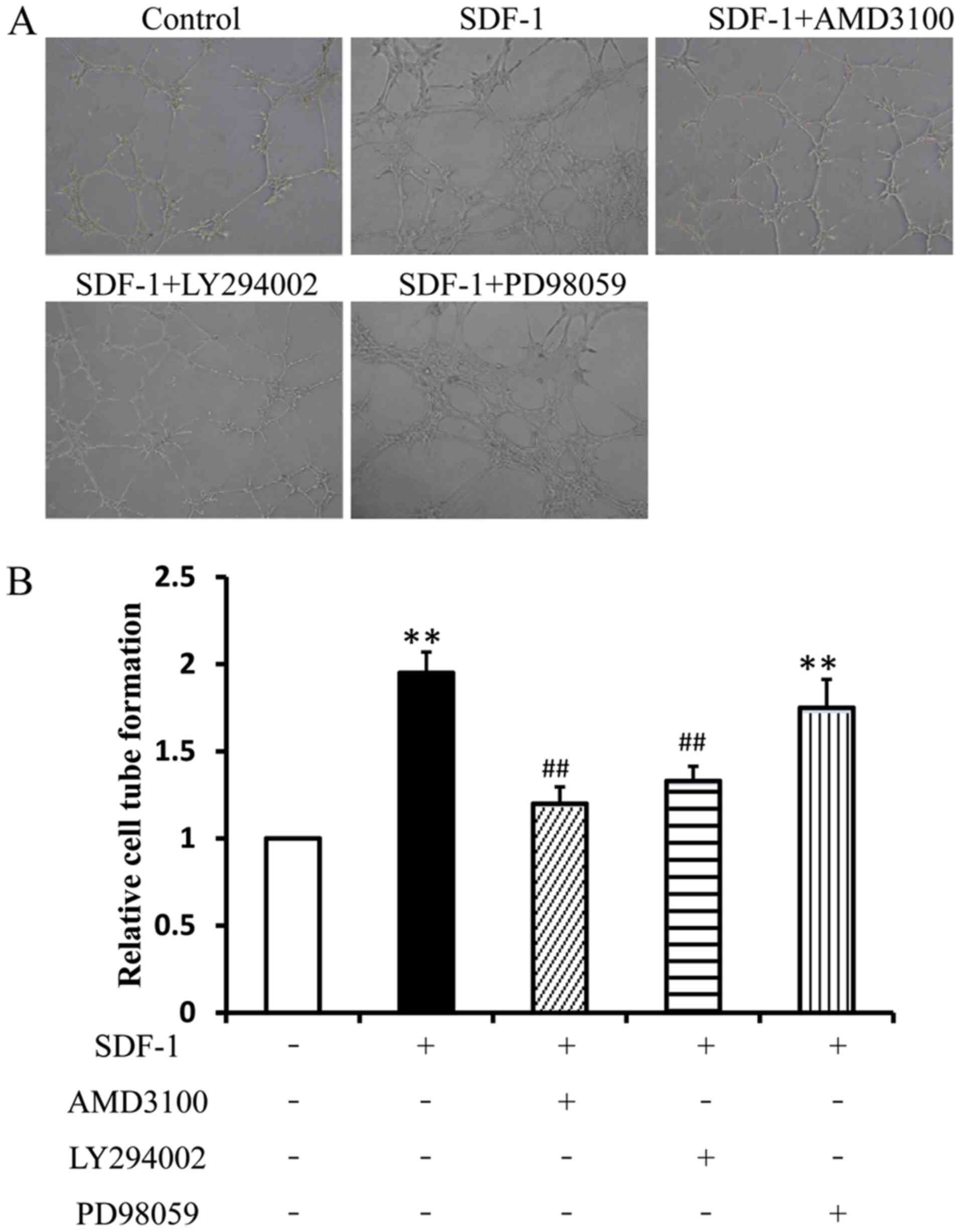
|
1
|
Asahara T, Murohara T, Sullivan A, Silver
M, van der Zee R, Li T, Witzenbichler B, Schatteman G and Isner JM:
Isolation of putative progenitor endothelial cells for
angiogenesis. Science. 275:964–966. 1997.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zhang M, Rehman J and Malik AB:
Endothelial progenitor cells and vascular repair. Curr Opin
Hematol. 21(224)2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Li Y, Wang Z, Mao M, Zhao M, Xiao X, Sun
W, Guo J, Liu C, Yang D, Qiao J, et al: Velvet antler mobilizes
endothelial progenitor cells to promote angiogenesis and repair
vascular endothelial injury in rats following myocardial
infarction. Front Physiol. 9(1940)2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hu Q, Ke X, Zhang T, Chen Y, Huang Q, Deng
B, Xie S, Wang J and Nie R: Hydrogen sulfide improves vascular
repair by promoting endothelial nitric oxide synthase-dependent
mobilization of endothelial progenitor cells. J Hypertens.
37:972–984. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hu Z, Wang H, Fan G, Zhang H, Wang X, Mao
J, Zhao Y, An Y, Huang Y, Li C, et al: Danhong injection mobilizes
endothelial progenitor cells to repair vascular endothelium injury
via upregulating the expression of Akt, eNOS and MMP-9.
Phytomedicine. 61(152850)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wei H, Mao Q, Liu L, Xu Y, Chen J, Jiang
R, Yin L, Fan Y, Chopp M, Dong J and Zhang J: Changes and function
of circulating endothelial progenitor cells in patients with
cerebral aneurysm. J Neurosci Res. 89:1822–1828. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Xu Y, Tian Y, Wei HJ, Chen J, Dong JF,
Zacharek A and Zhang JN: Erythropoietin increases circulating
endothelial progenitor cells and reduces the formation and
progression of cerebral aneurysm in rats. Neuroscience.
181:292–299. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Urbich C and Dimmeler S: Endothelial
progenitor cells: Functional characterization. Trends Cardiovasc
Med. 14:318–322. 2004.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Moccia F, Zuccolo E, Poletto V, Cinelli M,
Bonetti E, Guerra G and Rosti V: Endothelial progenitor cells
support tumour growth and metastatisation: Implications for the
resistance to anti-angiogenic therapy. Tumor Biol. 36:6603–6614.
2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Flamini V, Jiang WG, Lane J and Cui YX:
Significance and therapeutic implications of endothelial progenitor
cells in angiogenic-mediated tumour metastasis. Crit Rev Oncol
Hematol. 100:177–189. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhao X, Liu HQ, Li J and Liu XL:
Endothelial progenitor cells promote tumor growth and progression
by enhancing new vessel formation. Oncol Lett. 12:793–799.
2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Jung KH and Roh JK: Circulating
endothelial progenitor cells in cerebrovascular disease. J Clin
Neurol. 4:139–147. 2008.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sheng ZQ, Li YF, Zheng KL, Lu HH, Xie J,
Wu H and Xu B: The relationship between number and function of EPCs
and concentration of VEGF165 and SDF-1 in coronary artery spasm.
Eur Rev Med Pharmacol Sci. 22:2767–2777. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Coppolino G, Cernaro V, Placida G,
Leonardi G, Basile G and Bolignano D: Endothelial progenitor cells
at the interface of chronic kidney disease: From biology to
therapeutic advancement. Curr Med Chem. 25:4545–4551.
2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ammendola M, Leporini C, Luposella M,
Sacco R, Sammarco G, Russo E, Patruno R, De Sarro G and Ranieri G:
Targeting endothelial progenitor cells in cancer as a novel
biomarker and anti-angiogenic therapy. Curr Stem Cell Res Ther.
10:181–187. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Nagasawa T, Kikutani H and Kishimoto T:
Molecular cloning and structure of a pre-B-cell growth-stimulating
factor. Proc Natl Acad Sci USA. 91:2305–2309. 1994.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ratajczak M, Zuba-Surma E, Kucia M, Reca
R, Wojakowski W and Ratajczak J: The pleiotropic effects of the
SDF-1-CXCR4 axis in organogenesis, regeneration and tumorigenesis.
Leukemia. 20:1915–1924. 2006.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Teicher BA and Fricker SP: CXCL12
(SDF-1)/CXCR4 pathway in cancer. Clin Cancer Res. 16:2927–2931.
2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Möhle R, Bautz F, Rafii S, Moore MA,
Brugger W and Kanz L: The chemokine receptor CXCR-4 is expressed on
CD34+ hematopoietic progenitors and leukemic cells and mediates
transendothelial migration induced by stromal cell-derived
factor-1. Blood. 91:4523–4530. 1998.PubMed/NCBI
|
|
20
|
Walter DH, Haendeler J, Reinhold J,
Rochwalsky U, Seeger F, Honold J, Hoffmann J, Urbich C, Lehmann R,
Arenzana-Seisdesdos F, et al: Impaired CXCR4 signaling contributes
to the reduced neovascularization capacity of endothelial
progenitor cells from patients with coronary artery disease. Circ
Res. 97:1142–1151. 2005.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hattori K, Heissig B, Tashiro K, Honjo T,
Tateno M, Shieh JH, Hackett NR, Quitoriano MS, Crystal RG, Rafii S
and Moore MA: Plasma elevation of stromal cell-derived factor-1
induces mobilization of mature and immature hematopoietic
progenitor and stem cells. Blood. 97:3354–3360. 2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hoenig MR, Bianchi C and Sellke FW:
Hypoxia inducible factor-1α, endothelial progenitor cells,
monocytes, cardiovascular risk, wound healing, cobalt and
hydralazine: A unifying hypothesis. Curr Drug Targets. 9:422–435.
2008.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yamaguchi JI, Kusano KF, Masuo O, Kawamoto
A, Silver M, Murasawa S, Bosch-Marce M, Masuda H, Losordo DW, Isner
JM and Asahara T: Stromal cell-derived factor-1 effects on ex vivo
expanded endothelial progenitor cell recruitment for ischemic
neovascularization. Circulation. 107:1322–1328. 2003.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kawakami Y, Ii M, Matsumoto T, Kuroda R,
Kuroda T, Kwon SM, Kawamoto A, Akimaru H, Mifune Y, Shoji T, et al:
SDF-1/CXCR4 axis in Tie2-lineage cells including endothelial
progenitor cells contributes to bone fracture healing. J Bone Miner
Res. 30:95–105. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Carmeliet P: Mechanisms of angiogenesis
and arteriogenesis. Nat Med. 6:389–395. 2000.PubMed/NCBI View
Article : Google Scholar
|
|
26
|
Ganju RK, Brubaker SA, Meyer J, Dutt P,
Yang Y, Qin S, Newman W and Groopman JE: The α-chemokine, stromal
cell-derived factor-1alpha, binds to the transmembrane
G-protein-coupled CXCR-4 receptor and activates multiple signal
transduction pathways. J Biol Chem. 273:23169–23175.
1998.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Libura J, Drukala J, Majka M, Tomescu O,
Navenot JM, Kucia M, Marquez L, Peiper SC, Barr FG,
Janowska-Wieczorek A and Ratajczak MZ: CXCR4-SDF-1 signaling is
active in rhabdomyosarcoma cells and regulates locomotion,
chemotaxis, and adhesion. Blood. 100:2597–2606. 2002.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zeng Y, Wang X, Yin B, Xia G, Shen Z, Gu W
and Wu M: Role of the stromal cell derived factor-1/CXC chemokine
receptor 4 axis in the invasion and metastasis of lung cancer and
mechanism. J Thorac Dis. 9:4947–4959. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yang P, Wang G, Huo H, Li Q, Zhao Y and
Liu Y: SDF-1/CXCR4 signaling up-regulates survivin to regulate
human sacral chondrosarcoma cell cycle and epithelial-mesenchymal
transition via ERK and PI3K/AKT pathway. Med Oncol.
32(377)2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Liao A, Shi R, Jiang Y, Tian S, Li P, Song
F, Qu Y, Li J, Yun H and Yang X: SDF-1/CXCR4 axis regulates cell
cycle progression and epithelial-mesenchymal Transition via
up-regulation of survivin in glioblastoma. Mol Neurobiol.
53:210–215. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Porcile C, Bajetto A, Barbieri F, Barbero
S, Bonavia R, Biglieri M, Pirani P, Florio T and Schettini G:
Stromal cell-derived factor-1alpha (SDF-1alpha/CXCL12) stimulates
ovarian cancer cell growth through the EGF receptor
transactivation. Exp Cell Res. 308:241–253. 2005.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Lu Y, Hu B, Guan GF, Chen J, Wang CQ, Ma
Q, Wen YH, Qiu XC, Zhang XP and Zhou Y: SDF-1/CXCR4 promotes F5M2
osteosarcoma cell migration by activating the Wnt/β-catenin
signaling pathway. Med Oncol. 32(194)2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Peng SB, Peek V, Zhai Y, Paul DC, Lou Q,
Xia X, Eessalu T, Kohn W and Tang S: Akt activation, but not
extracellular signal-regulated kinase activation, is required for
SDF-1α/CXCR4-mediated migration of epitheloid carcinoma cells. Mol
Cancer Res. 3:227–236. 2005.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Segal MS, Shah R, Afzal A, Perrault CM,
Chang K, Schuler A, Beem E, Shaw LC, Li Calzi S, Harrison JK, et
al: Nitric oxide cytoskeletal-induced alterations reverse the
endothelial progenitor cell migratory defect associated with
diabetes. Diabetes. 55:102–109. 2006.PubMed/NCBI
|
|
35
|
Zheng H, Fu G, Dai T and Huang H:
Migration of endothelial progenitor cells mediated by stromal
cell-derived factor-1α/CXCR4 via PI3K/Akt/eNOS signal transduction
pathway. J Cardiovasc Pharmacol. 50:274–280. 2007.PubMed/NCBI View Article : Google Scholar
|
|
36
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals: In: Guide for the Care and Use of Laboratory Animals
National Academies Press (US) Copyright©. 2011, National
Academy of Sciences, Washington (DC), 2011.
|
|
37
|
Kruger NJ: The Bradford method for protein
quantitation. Methods Mol Biol. 32:9–15. 1994.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Balaji S, King A, Crombleholme TM and
Keswani SG: The role of endothelial progenitor cells in postnatal
vasculogenesis: Implications for therapeutic neovascularization and
wound healing. Adv Wound Care (New Rochelle). 2:283–295.
2013.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Lu C, Zhang J, Zhang D, Uzan G and Li M:
EPCs in vascular repair: How can we clear the hurdles between bench
and bedside? Front Biosci (Landmark Ed). 19(34)2014.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Li L, Liu H, Xu C, Deng M, Song M, Yu X,
Xu S and Zhao X: VEGF promotes endothelial progenitor cell
differentiation and vascular repair through connexin 43. Stem Cell
Res Ther. 8(237)2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Li DW, Liu ZQ, Wei J, Liu Y and Hu LS:
Contribution of endothelial progenitor cells to neovascularization.
Int J Mol Med. 30:1000–1006. 2012.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Dimmeler S and Zeiher AM: Vascular repair
by circulating endothelial progenitor cells: The missing link in
atherosclerosis? J Mol Med (Berl). 82:671–677. 2004.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Liu SQ, Tefft BJ, Zhang D, Roberts D,
Schuster DJ and Wu A: Cardioprotective mechanisms activated in
response to myocardial ischemia. Mol Cell Biomech. 8:319–338.
2011.PubMed/NCBI
|
|
44
|
Ahluwalia A, Jones MK, Matysiakbudnik T
and Tarnawski AS: VEGF and colon cancer growth beyond angiogenesis:
Does VEGF directly mediate colon cancer growth via a non-angiogenic
mechanism? Curr Pharm Des. 20:1041–1044. 2014.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Odent Grigorescu G, Rosca AM, Preda MB,
Tutuianu R, Simionescu M and Burlacu A: Synergic effects of VEGF-A
and SDF-1 on the angiogenic properties of endothelial progenitor
cells. J Tissue Eng Regen Med. 11:3241–3252. 2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Lipfert J, Odemis V, Wagner D, Boltze J
and Engele J: CXCR4 and CXCR7 form a functional receptor unit for
SDF-1/CXCL12 in primary rodent microglia. Neuropathol Appl
Neurobiol. 39:667–680. 2013.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Balabanian K, Lagane B, Infantino S, Chow
KY, Harriague J, Moepps B, Arenzana-Seisdedos F, Thelen M and
Bachelerie F: The chemokine SDF-1/CXCL12 binds to and signals
through the orphan receptor RDC1 in T lymphocytes. J Biol Chem.
280:35760–35766. 2005.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Burns JM, Summers BC, Wang Y, Melikian A,
Berahovich R, Miao Z, Penfold ME, Sunshine MJ, Littman DR, Kuo CJ,
et al: A novel chemokine receptor for SDF-1 and I-TAC involved in
cell survival, cell adhesion, and tumor development. J Exp Med.
203:2201–2213. 2006.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Rajagopal S, Kim J, Ahn S, Craig S, Lam
CM, Gerard NP, Gerard C and Lefkowitz RJ: β-arrestin-but not G
protein-mediated signaling by the ‘decoy’ receptor CXCR7. Proc Natl
Acad Sci USA. 107:628–632. 2010.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Puchert M and Engele J: The peculiarities
of the SDF-1/CXCL12 system: In some cells, CXCR4 and CXCR7 sing
solos, in others, they sing duets. Cell Tissue Res. 355:239–253.
2014.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Badillo AT, Chung S, Zhang L, Zoltick P
and Liechty KW: Lentiviral gene transfer of SDF-1α to wounds
improves diabetic wound healing. J Surg Res. 143:35–42.
2007.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Yoshida D, Nomura R and Teramoto A:
Signalling pathway mediated by CXCR7, an alternative chemokine
receptor for stromal-cell derived factor-1α, in AtT20 mouse
adrenocorticotrophic hormone-secreting pituitary adenoma cells. J
Neuroendocrinol. 21:481–488. 2009.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Hartmann TN, Grabovsky V, Pasvolsky R,
Shulman Z, Buss EC, Spiegel A, Nagler A, Lapidot T, Thelen M and
Alon R: A crosstalk between intracellular CXCR7 and CXCR4 involved
in rapid CXCL12-triggered integrin activation but not in
chemokine-triggered motility of human T lymphocytes and CD34+
cells. J Leukoc Biol. 84:1130–1140. 2008.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Boldajipour B, Mahabaleshwar H, Kardash E,
Reichman-Fried M, Blaser H, Minina S, Wilson D, Xu Q and Raz E:
Control of chemokine-guided cell migration by ligand sequestration.
Cell. 132:463–473. 2008.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Pozzobon T, Goldoni G, Viola A and Molon
B: CXCR4 signaling in health and disease. Immunol Lett. 177:6–15.
2016.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Chen L, Wu F, Xia WH, Zhang YY, Xu SY,
Cheng F, Liu X, Zhang XY, Wang SM and Tao J: CXCR4 gene transfer
contributes to in vivo reendothelialization capacity of endothelial
progenitor cells. Cardiovasc Res. 88:462–470. 2010.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Wu Q, Shao H, Darwin Eton D, Li J, Li J,
Yang B, Webster KA and Yu H: Extracellular calcium increases CXCR4
expression on bone marrow-derived cells and enhances
pro-angiogenesis therapy. J Cell Mol Med. 13:3764–3773.
2009.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Rai SN, Dilnashin H, Birla H, Singh SS,
Zahra W, Rathore AS, Singh BK and Singh SP: The role of PI3K/Akt
and ERK in neurodegenerative disorders. Neurotox Res. 35:775–795.
2019.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Everaert BR, Van Craenenbroeck EM, Hoymans
VY, Haine SE, Van Nassauw L, Conraads VM, Timmermans JP and Vrints
CJ: Current perspective of pathophysiological and interventional
effects on endothelial progenitor cell biology: Focus on
PI3K/AKT/eNOS pathway. Int J Cardiol. 144:350–366. 2010.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Keshavarz S, Nassiri SM, Siavashi V and
Alimi NS: Regulation of plasticity and biological features of
endothelial progenitor cells by MSC-derived SDF-1. Biochim Biophys
Acta Mol Cell Res. 1866:296–304. 2019.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Guo S, Yu L, Cheng Y, Li C, Zhang J, An J,
Wang H, Yan B, Zhan T, Cao Y, et al: PDGFRβ triggered by bFGF
promotes the proliferation and migration of endothelial progenitor
cells via p-ERK signalling. Cell Biol Int. 36:945–950.
2012.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Wang P, Zhang H, Li Z, Liu X, Jin Y, Lei
M, Jiao Z, Bi Y and Guo W: Low-dose radiation promotes the
proliferation and migration of AGE-treated endothelial progenitor
cells derived from bone marrow via activating SDF-1/CXCR4/ERK
signaling pathway. Radiat Res. 191:518–526. 2019.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Rosell A, Arai K, Lok J, He T, Guo S,
Navarro M, Montaner J, Katusic ZS and Lo EH: Interleukin-1β
augments angiogenic responses of murine endothelial progenitor
cells in vitro. J Cereb Blood Flow Metab. 29:933–943.
2009.PubMed/NCBI View Article : Google Scholar
|