Introduction
Psoriasis is a chronic inflammatory skin disease
with a long course and a tendency to relapse. Various factors,
including bacterial infection, and immune-mediated, genetic and
environmental factors serve an important role in the development of
psoriasis (1). In the general
population, the incidence of psoriasis ranges between 0.6 and 4.8%
(2). The clinical manifestations of
psoriasis are red scaly plaques that can occur on any part of the
body (3). Disfiguration, disability
and associated comorbidity affect the physical health and mental
status of the patients considerably (4). Therefore, the development of novel
therapeutic strategies is imperative for the successful treatment
of psoriasis.
Interleukin-17 (IL-17) is an inflammatory cytokine
with diverse roles in immune protection and immunopathology
(5,6). IL-17 promotes the activation of T
cells and stimulates epithelial cells, endothelial cells and
fibroblasts to produce a variety of cytokines, which leads to
inflammation (7). However, during
abnormal expression or overexpression of IL-17, chronic
inflammation is caused that is associated with several inflammatory
disorders and autoimmune diseases (8). IL-17 participates in the occurrence
and development of various immune diseases and inflammatory
conditions by binding to the IL-17 receptor. This causes signal
transduction and the induction of pro-inflammatory factors in order
to mediate defense responses (9).
Therefore, IL-17 is considered an attractive therapeutic
target.
Tumor necrosis factor (TNF) receptor-associated
factors (TRAFs) are key regulatory proteins in the TNF
receptor-associated signaling pathway. They are characterized by a
helical coiled structure and a conserved TRAF domain at the
C-terminus (10). TRAF3 is one of
the most versatile members of the TRAF family. It is an
intracellular protein that primarily acts as an adaptor protein
with a large variety of binding partners (11). TRAF3 interacting protein 2
(TRAF3IP2), also called CIKS or Act1, is a key intermediate in the
normal inflammatory response and is involved in the pathogenesis of
various autoimmune and inflammatory diseases (12). Furthermore, TRAF3IP2 is a protein
acting downstream of the IL-17 receptor that binds to and
stabilizes mRNAs encoding key inflammatory proteins (9).
Human keratinocytes account for >95% of human
epidermal cells. Human immortalized epidermal cells (HaCaT) cells
are immortalized cells with human keratinocyte differentiation and
proliferation characteristics that have been widely used for
studies involving skin biology and differentiation (13). Therefore, the present study aimed to
investigate the effects of IL-17 on the proliferation, apoptosis
and inflammation of HaCaT cells and to further investigate its
mechanism of action. The experiments aimed to identify novel
molecular targeted drugs or therapeutic targets that may
effectively treat psoriasis.
Materials and methods
Cell culture
HaCaT cells were purchased from the Shanghai
Institute of Cell Biology, Chinese Academy of Sciences. The cells
were cultured in Dulbecco's modified Eagle's medium (Gibco; Thermo
Fisher Scientific, Inc.) containing 10% fetal bovine serum (Beijing
Solarbio Science & Technology Co., Ltd.) and 1%
penicillin-streptomycin (Beijing Solarbio Science & Technology
Co., Ltd.) at 37˚C, in the presence of 5% CO2. Growth
phase cells were used for subsequent experiments.
Cell transfection and grouping
HaCaT cells in the logarithmic growth phase were
adjusted to a density of 5x105 cells/ml with trypsin and
incubated in a 6-well plate. When the cells reached 80% confluence,
transfection was performed according to manufacturer's protocols of
the lip2000 lentiviral transfection kit (Shanghai Jikai
Biotechnology Co., Ltd.). The cells were divided into the 5
following groups: i) sham control group (Sham), which included
cells transfected with an empty vector (Shanghai Jikai
Biotechnology Co., Ltd.); ii) lentiviral-mediated
TRAF3IP2-knockdown group (si-TRAF3IP2; 10/250 µl), which included
cells transfected with si-TRAF3IP2 (Shanghai Jikai Biotechnology
Co., Ltd.); iii) sham control + IL-17 group (Sham + IL-17), which
included cells transfected with empty carrier and cultured with 10
ng/ml IL-17A; iv) lentiviral-mediated TRAF3IP2-knockdown+IL-17
group (si-TRAF3IP2+IL-17), which included cells transfected with
si-TRAF3IP2 and cultured with 10 ng/ml IL-17A; and v) negative
control siRNA group (si-NC), which included cells transfected with
scrambled siRNA (Shanghai Jikai Biotechnology Co., Ltd). All cell
groups were cultured for 48 h. The si-NC group was used as the
transfection control. The target sequence of si-TRAF3IP2 was
5'-CCCCAAATACAAACAGGACGT-3'. The carrier frame structure of
si-TRAF3IP2 was GV248 hU6-MCS-Ubiquitin-EGFP-IRES-puromycin. The
si-NC sense strand was 5'-UUCUCCGAACGUGUCACGUTT-3' and antisense
strand was 5'-ACGUGACACGUUCGGAGAATT-3'.
MTT assay
A total of 100 µl of cells were incubated in 96-well
plates. The cells were incubated for 24, 36, 48 and 72 h following
transfection and IL-17 administration, and 100 µg MTT was added.
The cells were incubated at 37˚C for 4 h and the supernatant was
dissolved with 200 µl dimethyl sulfoxide (Sigma-Aldrich; Merck
KGaA). A plate reader (Bio-Rad Laboratories, Inc.) was used to
assess the optical density of each well at 495 nm. The relative
absorbance of each group was calculated by the following
equation:
Relative
absorbance=(ODExperiment-ODBlank)/(ODControl-ODBlank)
Flow cytometry
The cells in the different groups were collected and
resuspended with pre-chilled PBS (1X) and centrifuged at 11,180 x g
for 5 min at 4˚C. Following washing twice with precooled (4˚C)
sterile PBS solution, the cells were fixed overnight with 70%
ethanol and incubated at 4˚C. Following another wash with PBS, 100
µl Rnase A was added to resuspend the cells. The cells were heated
in a waterbath at 37˚C for 30 min. Subsequently, 400 µl propidium
iodide (PI) was added and the samples were incubated in the dark
for 30 min. The cell cycle was detected using a flow cytometer
(Gallios; Beckman Coulter, Inc.). The AnnexinV-FITC apoptosis assay
kit (BD Biosciences) was used for apoptosis detection. Following
washing, 100 µl binding buffer (1X) was added to the cells, and the
resulting mixture was incubated with 5 µl Annexin V-FITC in the
dark for 15 min. Subsequently, 10 µl PI was added and mixed with
the samples. The CellQuest 5.1 software (BD Biosciences) was used
to analyze the data.
ELISA
The cell supernatants were collected and the levels
of IL-6 (cat. no. HEA079Hu03; v.), IL-8 (cat. no. SEA080Si03;
Saihongrui Technology Co., Ltd.), IL-23 (cat. no. SEA384Mu02;
Saihongrui Technology Co., Ltd.), TNF-α (cat. no. E-EL-M0049c;
Elabscience Biotechnology Inc.) and VEGF (cat. no. GRJ13-009;
Guangrui Biotechnology Co., Ltd.) in the cells were detected in
strict accordance with the manufacturer's protocols of the ELISA
kit. The optical densities at 450 nm were measured using a
microplate reader (RT-6100; Lei Du).
Reverse transcription-quantitate
polymerase chain reaction (RT-qPCR)
The cells in the different groups were collected and
centrifuged at 4˚C, at 12,000 rpm for 15 min. Total RNA was
extracted using the TRIzol reagent (Takara Bio, Inc.). The cDNA was
synthesized using the reverse transcription kit (Applied
Biosystems; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. The SYBR-Green PCR kit (Qiagen, Inc.) was
used as a fluorophore. The RT-qPCR assay was performed in a
Mastercycler® nexus X2 (Eppendorf) using 2 µl cDNA as a
template under the following conditions: 95˚C for 10 min, 95˚C for
15 sec, and 60˚C for 1 min for 40 cycles. The data were processed
using the 2-ΔΔCq method (14) and the relative expression levels
were calculated using GAPDH mRNA as an internal reference. The
sequences of the primers (Shanghai Sheng gong Bioengineering
Technology Service Co., Ltd.) used were as follows: TRAF3IP2
forward, 5'-CTGCGTCTGAGTCTGTGGTT-3' and reverse,
5'-TATCCCGTGTCTATGGTTGG-3'; caspase-3 forward,
5'-ATGGAGAACAATAAAACCT-3' and reverse, 5'-CTAGTGATAAAAGTAGAGTTC-3';
GAPDH forward, 5'-TGACTTCAACAGCGACACCCA-3' and reverse,
5'-CACCCTGTTGCTGTAGCCAAA-3'.
Western blot analysis
The cells were lysed in RIPA buffer (Beyotime
Institute of Biotechnology) and the protein concentration was
measured using the bicinchoninic acid kit (Beijing Solarbio Science
& Technology Co., Ltd.). Each protein sample (~40 µg/lane) was
separated on 10% sodium dodecyl sulfate polyacrylamide gels by
electrophoresis (Mini-protean-3; Bio-Rad Laboratories, Inc.) and
transferred onto polyvinylidene difluoride membranes (Merck &
Co., Inc.). Subsequently, the membranes were blocked with 5%
skimmed milk and incubated overnight at 4˚C with primary rabbit
anti-human antibodies against caspase-3 (dilution, 1:1,000; cat.
no. ab13847; Abcam) and GAPDH (dilution, 1:1,000; cat. no. ab70699;
Abcam). The following day, horseradish peroxidase-conjugated goat
anti-rabbit immunoglobulin G secondary antibody (dilution, 1:1,000;
cat. no. ABIN101988; Antibodies Online) was used at room
temperature for 1 h. The membranes were observed and recorded using
the enhanced chemiluminescence system (ImageQuant LAS 4000; General
Electric Company) for 3-5 min. Protein expression levels were
normalized to GAPDH, scanned and quantified using the ImageJ 1.46r
software (National Institutes of Health).
Statistical analysis
All experiments were repeated at least three times.
Statistical analysis was performed using SPSS 19.0 (IBM Corp.). The
mean ± standard deviation was used to measure the data. Significant
differences between two groups were assessed using a Student's
t-test. Pairwise comparisons were performed using the one-way
analysis of variance test, followed by Tukey's post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression of TRAF3IP2 mRNA in
different groups
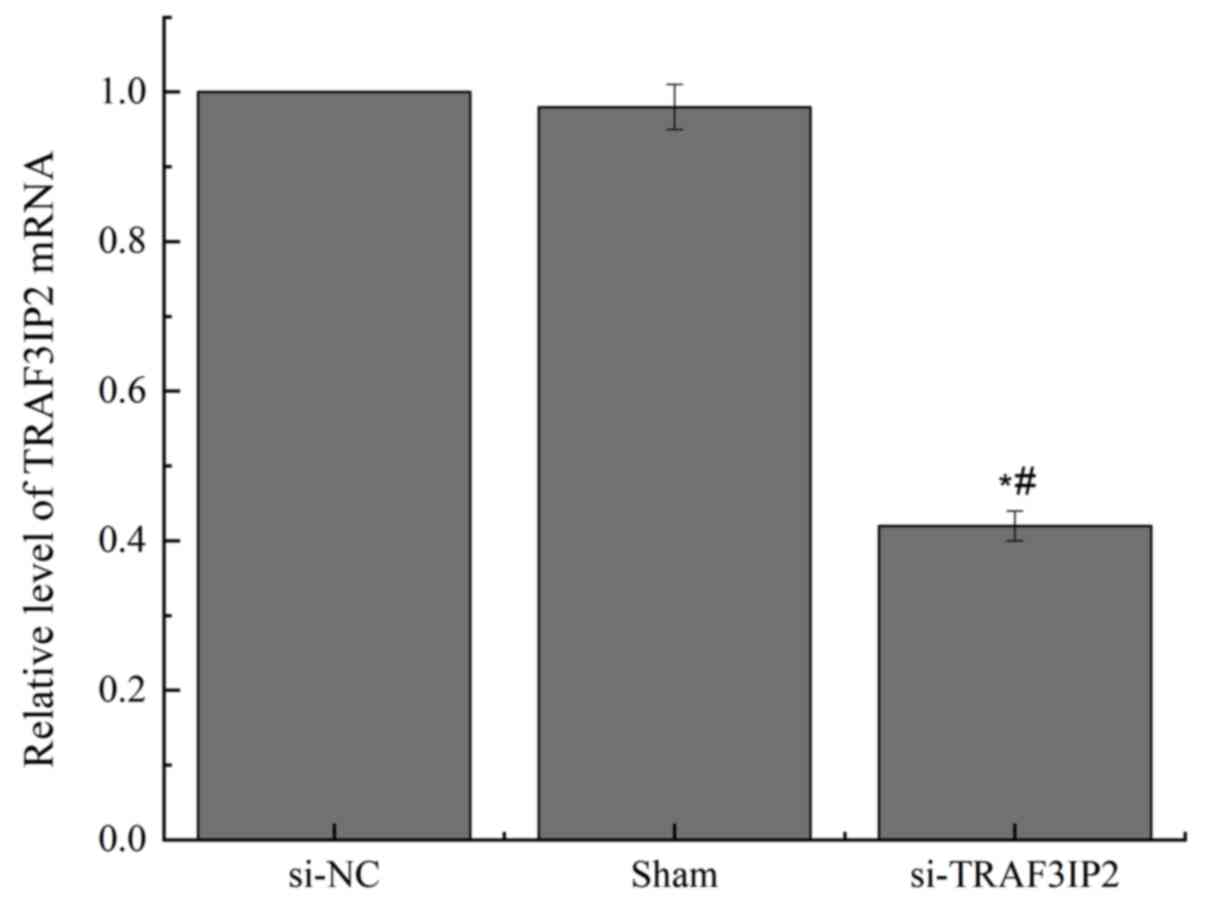
The transfection efficiency was detected by RT-qPCR
(Fig. 1). The TRAF3IP2 mRNA
expression in the si-NC and Sham groups were not significantly
different (P>0.05). By contrast, TRAF3IP2-knockdown using a
lentivirus significantly decreased the expression of TRAF3IP2 mRNA
(P<0.05). This result indicated that the transfections were
successful.
Effects of IL-17 on the proliferation
of HaCaT cells
Cell proliferation ability was detected by MTT
assays (Fig. 2). The si-TRAF3IP2
HaCaT cell group exhibited decreased proliferation compared with
the Sham control cell group. On the other hand, in si-TRAF3IP2
HaCaT cells treated with IL-17 (si-TRAF3IP2 + IL-17), the cell
proliferation was significantly decreased compared with the Sham
control cell group (P<0.05). Furthermore, HaCaT cells treated
with IL-17 exhibited increased proliferation compared with the Sham
group and si-TRAF3IP2 + IL-17 group. In addition, compared with the
Sham + IL-17 group, the proliferation ability was significantly
decreased in the si-TRAF3IP2 + IL-17 group (P<0.05). These
findings suggested that IL-17 promoted the proliferation of HaCaT
cells by interacting with the TRAF3IP2 adaptor protein.
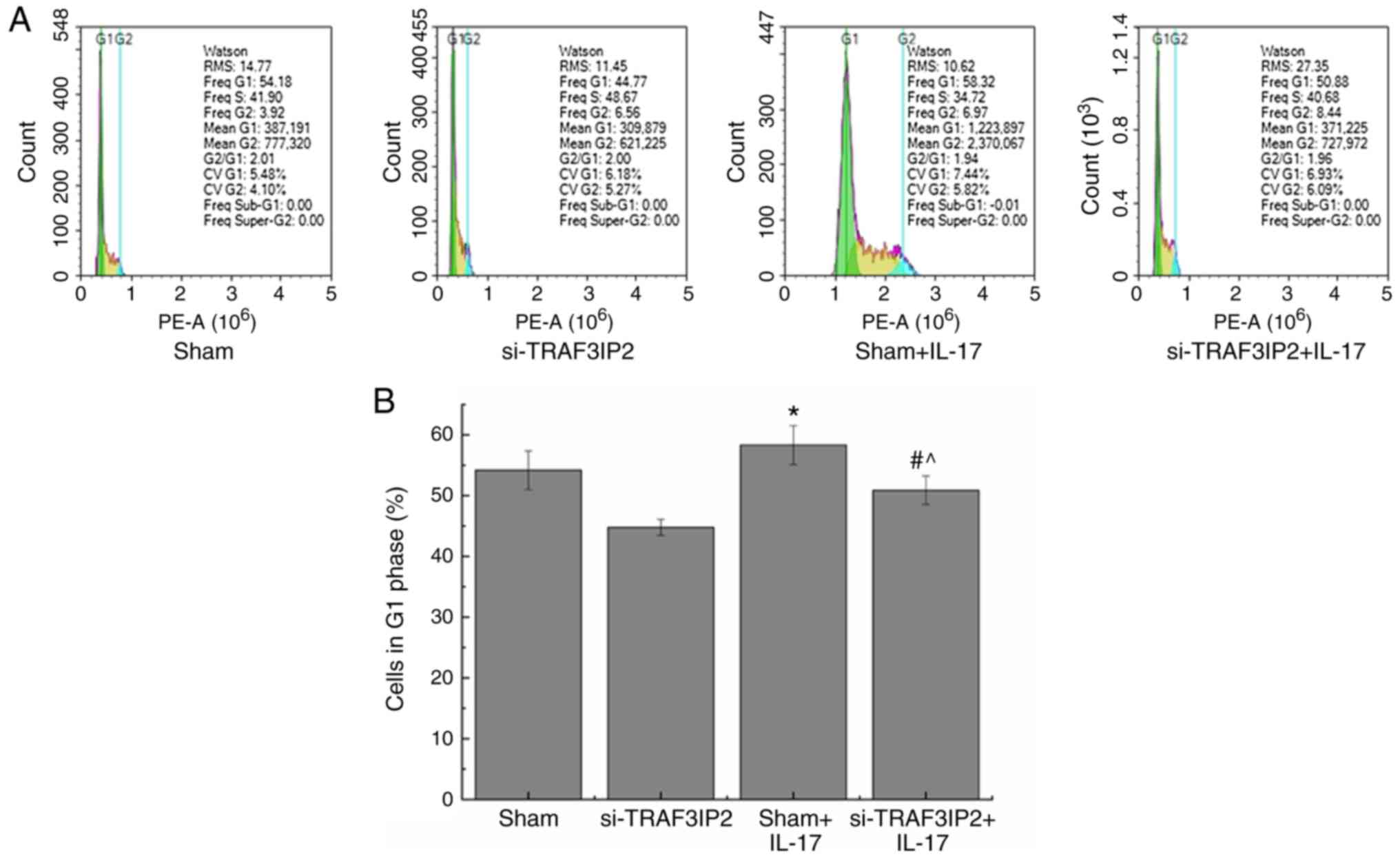
Effects of IL-17 on the cell cycle of
HaCaT cells
The percentage of cells in the G1 phase of the cell
cycle in the Sham+IL-17 group (58.32±3.21%) was significantly
higher than that of the Sham group (Fig. 3; 54.18±3.19%; P<0.05). However,
the percentage of cells in the G1 phase of the si-TRAF3IP2+IL-17
group was decreased to 50.88±2.36%, which was significantly lower
than that in the Sham+IL-17 group (P<0.05). The results further
indicated that IL-17 promoted the transition of the cell cycle from
the G1 to the S phase and the proliferation of HaCaT cells via
interacting with the TRAF3IP2 adaptor protein. Knockdown of
TRAF3IP2 expression decreased the efficacy of IL-17 on the
proliferation of HaCaT cells.
Effects of IL-17 on the induction of
apoptosis of HaCaT cells
Flow cytometric analysis was used to detect the
induction of cell apoptosis (Fig.
4). The number of apoptotic cells in the Sham+IL-17 group
(4.40±0.19%) was significantly lower than that in the Sham group
(5.02±0.23%; P<0.05). However, the percentage of apoptotic cells
in the si-TRAF3IP2+IL-17 group (6.83±0.32%) was significantly
higher than that in the Sham group (P<0.05), indicating that
IL-17 inhibited the apoptosis of HaCaT cells by interacting with
the TRAF3IP2 adaptor protein. Knockdown of the expression of
TRAF3IP2 decreased the efficacy of IL-17 on the induction of
apoptosis of HaCaT cells.
Effects of IL-17 on the levels of
inflammatory factors in HaCaT cells
The cell supernatant cytokine levels were analyzed
in order to reflect the inflammatory response. The expression
levels of IL-6, IL-8, IL-23, TNF-α and VEGF were
significantly increased in the Sham+IL-17 groups compared with
those in the Sham group (Fig. 5;
P<0.05). However, significant decreases in the levels of IL-6,
IL-8, IL-23, TNF-α and VEGF were observed in the
si-TRAF3IP2+IL-17 group compared with those in the Sham+IL-17
groups (P<0.05). These findings indicated that IL-17 promoted
the inflammation of HaCaT cells by interacting with the TRAF3IP2
adaptor protein, while knockdown of TRAF3IP2 expression decreased
the inflammatory efficacy of IL-17 on HaCaT cells.
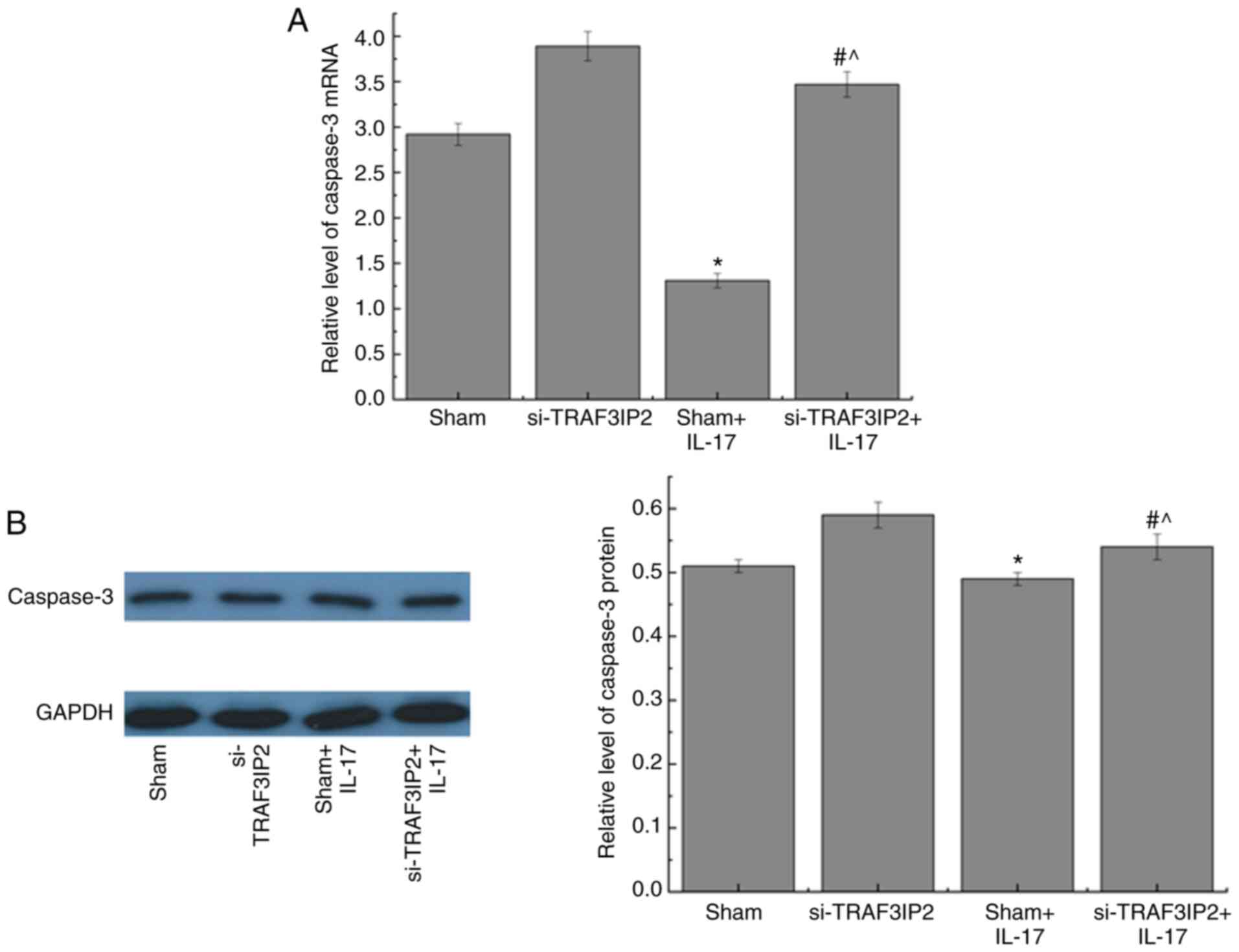
Effects of IL-17 on the expression
levels of caspase-3 in HaCaT cells
RT-qPCR and western blot analyses were used to
investigate the mRNA and protein expression levels of caspase-3 in
HaCaT cells, respectively (Fig. 6).
The mRNA and protein expression levels of caspase-3 were decreased
in the Sham+IL-17 group compared with those in the Sham group.
Furthermore, a significant increase in the mRNA and protein
expression levels of caspase-3 was noted in the si-TRAF3IP2+IL-17
group compared with those of the Sham+IL-17 group. These results
demonstrated that IL-17 decreased the expression levels of
caspase-3 by interacting with the TRAF3IP2 adaptor protein in HaCaT
cells. These effects were reversed by lentiviral-mediated knockdown
of TRAF3IP2.
Discussion
Psoriasis is a programmed pathological interaction
among skin cells, and immune-mediated genetic and environmental
factors (1). The hallmarks of
psoriasis include deep-level epidermal modifications, including
hyperproliferation and changes in keratinocyte differentiation,
along with significant inflammatory cell infiltration and
neovascularization (15). The
concept of using anti-IL-17 as a potential treatment agent is not
novel. The FDA approved secukinumab in 2015 and ixekizumab in 2016
to treat adults with moderate-to-severe plaque psoriasis (16). Furthermore, TRAF3IP2 is one of the
genetic factors implicated in psoriasis that is involved in the
IL23/IL-17 axis (17). Therefore,
the present study investigated the role of IL-17 as a key effector
molecule on psoriasis and investigated its association with
TRAF3IP2.
Cell proliferation is one of the important
physiological functions of viable cells. However, several diseases,
including psoriasis, are associated with abnormal cell
proliferation, migration and invasion (18,19).
The results of the MTT assay indicated that IL-17 promoted the
proliferation of HaCaT cells via interacting with the TRAF3IP2
adaptor protein. The complete cell cycle includes the G1, S and G2
phases and the mitotic phase (20).
Lentiviral-mediated TRAF3IP2-knockdown can affect the cell cycle
and cause abnormal arrest of the cells in the G1 phase, thereby
reducing the number of cells entering the S and G2 phases and
causing a reduction in the proliferation index. Apoptosis is a
basic biological phenomenon of cells that is required to maintain
the stability of the intracellular environment. The caspase family
of enzymes is an important mediator of programmed cell death
(apoptosis) and caspase-3 is directly involved in the transduction
of apoptotic signals (21,22). In the present study, the apoptotic
rate, and mRNA and protein expression levels of caspase-3 were
significantly increased in the IL-17+si-TRAF3IP2 group compared
with those in the Sham+IL-17 group. These findings demonstrated
that IL-17 promoted proliferation and inhibited apoptosis of HaCaT
cells, while knockdown of TRAF3IP2 expression decreased the
efficacy of IL-17.
Psoriasis is a chronic recurrent inflammatory skin
disease that interacts with multiple factors, including
immune-mediated genetic and environmental factors (1). The number of Th17 cells is increased
in the peripheral blood of patients with psoriatic disease and its
effector IL-17 serves an important role in the severity of
psoriasis (15). IL-17 stimulates
the production of VEGF, IL-6, IL-8, granulocyte-macrophage colony
stimulating factor, IL-23, TNF-α and other cytokines from
macrophages (23,24). VEGF is a highly specific vascular
endothelial cell growth factor that promotes increased vascular
permeability, extracellular matrix degeneration, and vascular
endothelial cell migration, proliferation and angiogenesis
(25). IL-6, IL-8, IL-23 and TNF-α
are cytokines with multiple biological effects and are considered
important inflammatory factors. Various cytokines can cause a
chronic inflammatory state in skin cells and alter epidermal
hyperproliferation, differentiation, apoptosis and neoangiogenesis
that result in the production of skin diseases. In the present
study, ELISA indicated that IL-17 promoted the inflammation of
HaCaT cells by interacting with the TRAF3IP2 adaptor protein. Taken
together, the results indicated that IL-17 served a crucial role in
the pathogenesis and development of psoriasis and that knockdown of
TRAF3IP2 may be considered a novel therapeutic target for
psoriasis.
The present study demonstrated that IL-17 promoted
proliferation and inflammation, while inhibiting the apoptosis of
HaCaT cells. The possible mechanism of action may be via its
interaction with the TRAF3IP2 adaptor protein. In addition, the
data provided novel evidence on the role of TRAF3IP2 as a new
therapeutic target for psoriasis. IL-17 may regulate proliferation,
apoptosis and inflammation of human immortalized epidermal cells by
interacting with the TRAF3IP2 adaptor protein. However, the lack of
co-immunoprecipitation experiments to prove the hypothesis that
IL-17 interacts with TRAF3IP2 is a limitation of the present
study.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China Youth Fund (grant no.
81502729).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JiZ conducted the experiments, data collection and
interpretation. YS participated in study design, coordination of
the experiments and data collection. JuZ participated in the study
design, data collection and data analysis and prepared the
manuscript. QL and LC participated in study design, data analysis,
data interpretation and wrote the manuscript. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Shandong Provincial Hospital Affiliated to Shandong
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hugh JM and Weinberg JM: Update on the
pathophysiology of psoriasis. Cutis. 102:6–12. 2018.PubMed/NCBI
|
|
2
|
Naldi L: Epidemiology of psoriasis. Curr
Drug Targets Inflamm Allergy. 3:121–128. 2004.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Boehncke WH: Systemic inflammation and
cardiovascular comorbidity in psoriasis patients: Causes and
consequences. Front Immunol. 9(579)2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Boehncke WH and Schön MP: Psoriasis.
Lancet. 386:983–994. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Brembilla NC, Senra L and Boehncke WH: The
IL-17 family of cytokines in psoriasis: IL-17A and beyond. Front
Immunol. 9(1682)2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Amatya N, Garg AV and Gaffen SL: IL-17
signaling: The Yin and the Yang. Trends Immunol. 38:310–322.
2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kuwabara T, Ishikawa F, Kondo M and
Kakiuchi T: The role of IL-17 and related cytokines in inflammatory
autoimmune diseases. Mediators Inflamm.
2017(3908061)2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Beringer A, Noack M and Miossec P: IL-17
in chronic inflammation: From discovery to targeting. Trends Mol
Med. 22:230–241. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Herjan T, Hong L, Bubenik J, Bulek K, Qian
W, Liu C, Li X, Chen X, Yang H, Ouyang S, et al:
IL-17-receptor-associated adaptor Act1 directly stabilizes mRNAs to
mediate IL-17 inflammatory signaling. Nat Immunol. 19:354–365.
2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Tang X, Zhang L and Wei W: Roles of TRAFs
in NF-κB signaling pathways mediated by BAFF. Immunol Lett.
196:113–118. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yi Z, Wallis AM and Bishop GA: Roles of
TRAF3 in T cells: Many surprises. Cell Cycle. 14:1156–1163.
2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Erikson JM, Valente AJ, Mummidi S,
Kandikattu HK, DeMarco VG, Bender SB, Fay WP, Siebenlist U and
Chandrasekar B: Targeting TRAF3IP2 by genetic and interventional
approaches inhibits ischemia/reperfusion-induced myocardial injury
and adverse remodeling. J Biol Chem. 292:2345–2358. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Deyrieux AF and Wilson VG: In vitro
culture conditions to study keratinocyte differentiation using the
HaCaT cell line. Cytotechnology. 54:77–83. 2007.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Sakkas LI and Bogdanos DP: Are psoriasis
and psoriatic arthritis the same disease? The IL-23/IL-17 axis
data. Autoimmun Rev. 16:10–15. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Campa M, Mansouri B, Warren R and Menter
A: A review of biologic therapies targeting IL-23 and IL-17 for use
in moderate-to-severe plaque psoriasis. Dermatol Ther (Heidelb).
6:1–12. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Sukhov A, Adamopoulos IE and Maverakis E:
Interactions of the immune system with skin and bone tissue in
psoriatic arthritis: A comprehensive review. Clin Rev Allergy
Immunol. 51:87–99. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Greb JE, Goldminz AM, Elder JT, Lebwohl
MG, Gladman DD, Wu JJ, Mehta NN, Finlay AY and Gottlieb AB:
Psoriasis. Nat Rev Dis Primers. 2(16082)2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Iciek MB, Kowalczyk-Pachel D, Kwiecień I
and Dudek MB: Effects of different garlic-derived allyl sulfides on
peroxidative processes and anaerobic sulfur metabolism in mouse
liver. Phytother Res. 26:425–431. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Wee P and Wang Z: Cell cycle
synchronization of HeLa cells to assay EGFR pathway activation.
Methods Mol Biol. 1652:167–181. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Fan TJ, Han LH, Cong RS and Liang J:
Caspase family proteases and apoptosis. Acta Biochim Biophys Sin
(Shanghai). 37:719–727. 2005.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Choudhary GS, Al-Harbi S and Almasan A:
Caspase-3 activation is a critical determinant of genotoxic
stress-induced apoptosis. Methods Mol Biol. 1219:1–9.
2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Coimbra S, Oliveira H, Reis F, Belo L,
Rocha S, Quintanilha A, Figueiredo A, Teixeira F, Castro E,
Rocha-Pereira P and Santos-Silva A: Interleukin (IL)-22, IL-17,
IL-23, IL-8, vascular endothelial growth factor and tumour necrosis
factor-α levels in patients with psoriasis before, during and after
psoralen-ultraviolet A and narrowband ultraviolet B therapy. Br J
Dermatol. 163:1282–1290. 2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Furue K, Ito T and Furue M: Differential
efficacy of biologic treatments targeting the TNF-α/IL-23/IL-17
axis in psoriasis and psoriatic arthritis. Cytokine. 111:182–188.
2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Melincovici CS, Boşca AB, Şuşman S,
Mărginean M, Mihu C, Istrate M, Moldovan IM, Roman AL and Mihu CM:
Vascular endothelial growth factor (VEGF)-key factor in normal and
pathological angiogenesis. Rom J Morphol Embryol. 59:455–467.
2018.PubMed/NCBI
|