Introduction
Dry eye syndrome is characterized by multifactorial
tear film instability, which consequently triggers inflammation and
damage to conjunctival epithelial cells (1). The expression levels of inflammatory
factors, including interleukin (IL)-1 and tumor necrosis factor
(TNF)-α, are upregulated in dry eye syndrome. In turn, this
activates the mitogen-activated protein kinase (MAPK) signaling
pathway and induces a series of downstream changes, ultimately
resulting in dry eyes (2,3). The MAPK signaling pathway is crucial
for signaling transduction in mammalian cells. Via the MAPK
pathway, signals from extracellular stimuli maybe transduced into
nuclei and are able to mediate cellular responses (4-8).
There are several branches of the MAPK pathway, among which the
MAPK-ERK pathway has a role in amplifying extracellular signals
(9,10).
Fluoxetine is a selective serotonin reuptake
inhibitor (SSRI) that is widely administered as an anti-depressant
drug. A previous study by the present group investigated the
prevalence of dry eye diseases among patients suffering from
depression from 2009 to 2015, and the results demonstrated that dry
eye syndrome was significantly more common when patients received
SSRI treatment. SSRIs exert their anti-depressant effect via
regulating the MAPK-ERK signaling pathway, and may also trigger
ocular surface inflammation and cellular apoptosis by elevating the
expression of IL-β and TNF-α via MAPK-ERK (11). This is a potential mechanism by
which anti-depressant drugs promote dry eye syndrome. In this
present study, it was speculated that the pathogenesis of dry eye
syndrome caused by anti-depressant drugs may be due to SSRIs
inhibiting 5-hydroxytryptamine (5-HT) reuptake by presynaptic
membrane 5-HT pumps in neurons, increasing the number of 5-HT
receptors. This would activate the MAPK signaling pathway,
increasing the levels of phosphorylated (p-)ERK, which may in turn
induce an increase in matrix metalloproteinases (MMPs) and
inflammatory factors and promote apoptosis. This would ultimately
result in the symptom of dry eyes. However, the mechanisms by which
an increase in 5-HT receptors activates the MAPK-ERK signaling
pathway have remained elusive (6,7,12). In
the present study, cellular apoptosis was observed via the gene
expression of Bcl-2, Bax and MMPs in order to provide novel
evidence for the mechanism by which anti-depressant drugs induce
dry eye diseases.
Materials and methods
Materials
Human conjunctival epithelial cells (HConEpiCs) were
derived from the primary culture of conjunctival tissue from
healthy adult human donors, which was provided by the Eye Center of
Renmin Hospital at Wuhan University (Wuhan, China), collected
between January 2016 and October 2016. A total of 20 patients (10
males and 10 females) with ocular fundus diseases, aged from 20-45
years, requiring surgical treatment were recruited. They were
required to have healthy ocular surface with no conjunctival
lesions and no cardiovascular disease. The selected healthy adults
were confirmed not to have dry eye syndrome. The standard of
exclusion contained the following aspects: Tear break-up time
(BUT>10 sec), tear river width (>0.3 mm) determined with an
Ocular surface analyzer and Schirmer test 2 (>10 mm at 5 min).
Low-glucose Dulbecco's modified Eagle's medium (DMEM)was purchased
from Boster Biological Technology Co., Ltd. Patient consent was
obtained and ethical approval of the present study was granted by
the Clinical Research Ethics Review Board of Wuhan University
(Wuhan, China). The present study collected 20 cases of abandoned
conjunctival tissues during the operations. Each tissue size was
about 5x5 mm and were stored at 4˚C in sterile glass bottles.
Trypsin-EDTA was manufactured by Shanghai Chemical Reagent Co.,
Ltd. Antibodies against Cytokeratin-13 (CK-13; cat. no. YM6162R)
were purchased from Immunoway Biotechnology Company. Antibodies
against Bax (cat. no. 9942), Bcl2 (cat. no. 9941), MMP2 (cat. no.
4022), MMP9 (cat. no. 3852) and GAPDH (cat. no. 5174) were
purchased from Cell Signaling Technology (all 1:1,000).
TRIzol® was produced by Invitrogen (Thermo Fisher
Scientific, Inc.); fluoxetine was purchased from Sigma-Aldrich
(Merck KGaA); Cell Counting Kit-8 (CCK-8) cell proliferation kits
were purchased from Nanjing Jiancheng Bioengineering Institute;
mouse-anti-human ERK1/2 monoclonal antibody (cat. no. 9926) and
mouse-anti-human p-ERK1/2 monoclonal antibody (cat. no. 9910; both
used at 1:1,000) were purchased from Upstate Biotechnology, Inc.;
horseradish peroxidase (HRP)-conjugated goat anti-mouse secondary
antibody was purchased from Boster Biological Technology Co., Ltd.
(cat. no. BA1054); the enhanced chemiluminescence reagent kit was
purchased from Pierce (Thermo Fisher Scientific, Inc.).
Culture and characterization of
HConEpiCs
HConEpiCs were isolated for further culture using
trypsin-EDTA mixture digestion solution. Conjunctival tissue was
obtained from healthy adult human donors. The tissue was chopped
into small pieces using scissors, followed by treatment with
trypsin at 37˚C for 10 min. The trypsin digestion was neutralized
by addition of medium. The tissue chunks were then further broken
down by pipetting and the flakes of tissue were aspirated away.
Subsequently, the cell suspension was centrifuged at 72 x g for 6
min at room temperature. The supernatant was removed and the cell
density was adjusted to 4-6x104 cells/ml. The cells were
seeded at 4-6x104 cells/ml (a total of 50,000 cells)
into culture flasks and cultured in an incubator at 5%
CO2 and 37˚C. The cells were cultured in low-glucose
DMEM with 10% FBS, (Gibco; Thermo Fisher Scientific, Inc.; cat. no.
10099) The medium was replaced every 3-4 days and the growth of the
cells was closely monitored under an inverted light microscope.
Cells were passaged after confluence was reached. Experiments were
performed using cells at passages 2-4. The growth of the HConEpiCs
was observed under an inverted microscope and images were captured.
The expression profile of human conjunctival epithelium indicated
that the most abundant gene transcript was that of CK-13(13). Hence, CK-13 immunofluorescence
detection was used to examine the cultured cells.
CCK-8 assay
HConEpiCs in the logarithmic phase were seeded in
96-well plates with 100 µl medium per well and incubated with 5%
CO2 at 37˚C. After 24 h of incubation, fluoxetine at
different dosages (0, 1, 2.5, 5, 10, 20 and 40 µM) was added to the
culture, followed by incubation for another 24 h at 37˚C. For each
dose, three replicates were performed. The culture medium was
removed and 100 µl serum-free DMEM basal medium with 10 µl CCK-8
was added to each well, and cells were further cultured for 2 h.
The integrated optical density (IOD) values were measured at 450 nm
using an ELISA reader and converted into proliferation rates. Cell
proliferation rates were calculated as
(IODuntreated-IODtreated)/IODuntreated
x100%. The appropriate fluoxetine dose for subsequent experiments
was selected according to the cell proliferation rates.
Transwell invasion and migration
assays
For the invasion assay, 100 µl serum-free DMEM
containing 10 µl Matrigel was added to the wells of Transwell
chambers. The chambers were incubated for 3 h in an incubator to
allow for the Matrigel to solidify. The following experimental
groups were set up: Control group and 5 µM fluoxetine group. The
cells were re-suspended at 1x105/ml with serum-free
DMEM. Subsequently, 200 µl cell suspension was added to the
Matrigel-coated wells and 500 µl DMEM containing 20% FBS was added
to the lower chambers. After 24 h of incubation, cells were fixed
with 4% paraformaldehyde (PFA) for 1 h at room temperature and
stained with 0.25% crystal violet at the room temperature for 20
min. Cells on the upper chamber of the transwell membrane were
wiped away using a cotton swab and five fields were randomly
selected for examination under an inverted microscope
(magnification, x100). Images were obtained and the number of cells
was counted. Six replicates were performed for each condition.
For the migration assay, the cells were divided into
a control group and a 5 µM fluoxetine group. Cells
(2x104) were re-suspended in serum-free medium 24 h
post-treatment and seeded into the top wells of the Transwell
chambers, and 500 µl of RPMI-1640 medium containing 20% fetal
bovine serum was added to the lower chambers. Samples were
collected after 20 h of incubation, fixed with 4% PFA for 20 min at
room temperature and stained with 0.25% crystal violet for 20 min
at room temperature. The cells in the upper chamber that did not
pass through the matrix glue were wiped off, leaving the cells that
passed through the membrane using a cotton swab and five fields
were randomly selected for examination under an inverted microscope
(magnification, x100). Images were obtained and the number of cells
was counted. For each condition, six replicates were performed.
Immunofluorescent staining for ERK1/2
and p-ERK1/2 in HConEpiCs
Cells in the logarithmic growth phase were seeded on
gelatin-coated coverslips. The inoculation density was
1x104 cells/ml, and the cells were allowed to adhere for
24 h and fixed with 4% PFA for 15 min at room temperature. The
gelatin coating solution was purchased from Beijing Reagan
Biotechnology Co., Ltd. (cat. no. IH0205). The fixed samples were
washed three times with PBS for 5 min and permeabilized with 1%
Triton-X-100 for 10 min. The cells were blocked with 1% bovine
serum albumin (Beyotime Institute of Biotechnology; cat. no. P0007)
for 1 h at room temperature and incubated with primary antibody
against ERK1/2 or p-ERK1/2 (1:100 dilution) overnight at 4˚C. The
samples were then washed with PBS three times and incubated with
HRP conjugated goat anti-rabbit secondary antibodies (1:100
dilution; Thermo Fisher Scientific, Inc.; cat. no. 31460) for 1 h
at room temperature. The nuclei were counterstained with DAPI for
15 min and the cells were washed with PBS three times for 15 min.
Finally, the slides were covered with cover slips, sealed with
glycerol and subjected to imaging using a fluorescence microscope
(BX51; Olympus Corporation). The nuclei were visualized in blue and
ERK1/2 or p-ERK1/2 were labeled with red fluorescence.
Western blot analysis
The protein levels of intracellular ERK, p-ERK, Bax,
Bcl-2 and MMP-2/9 were evaluated. HConEpiCs were treated with a
gradient of doses of fluoxetine (0, 1, 2.5, 5, 10, 20 and 40 µM)
for 24 h and total protein was then extracted. The Pierce™ BCA
Protein Assay Kit (Thermo Fisher Scientific, Inc.; cat. no. 23227)
was used to quantify protein levels. Protein samples (20 µg) were
mixed with loading buffer and loaded onto 10% separating gels and
5% stacking gels for SDS-PAGE. The proteins were then transferred
to a nitrocellulose membrane (Beyotime Institute of Biotechnology;
cat. no. FFN08). The membrane was blocked with 5% fat-free milk in
PBS for 1 h at room temperature and incubated with primary
antibodies overnight at 4˚C. The membrane was then washed in PBS
three times and incubated with a HRP-conjugated secondary antibody
(1:10,000; Thermo Fisher Scientific, Inc.; cat. no. 31430) for 1 h
at room temperature. After three rounds of membrane washing,
enhanced chemiluminescence was performed to visualize the protein.
The dilution the antibodies against ERK, p-ERK, Bax, Bcl-2 and
MMP-2/9 was 1:1,000.
Statistical analysis
Statistical analysis was performed with SPSS12.0
(SPSS, Inc.). Values are expressed as the mean ± standard error.
Student's t-tests were used for comparisons between two groups.
One-way ANOVAs were used for comparisons between multiple groups
and post-hoc analysis was performed using Tukey's highly
significant differences test.
Results
Isolation and characterization of
primary HConEpiCs
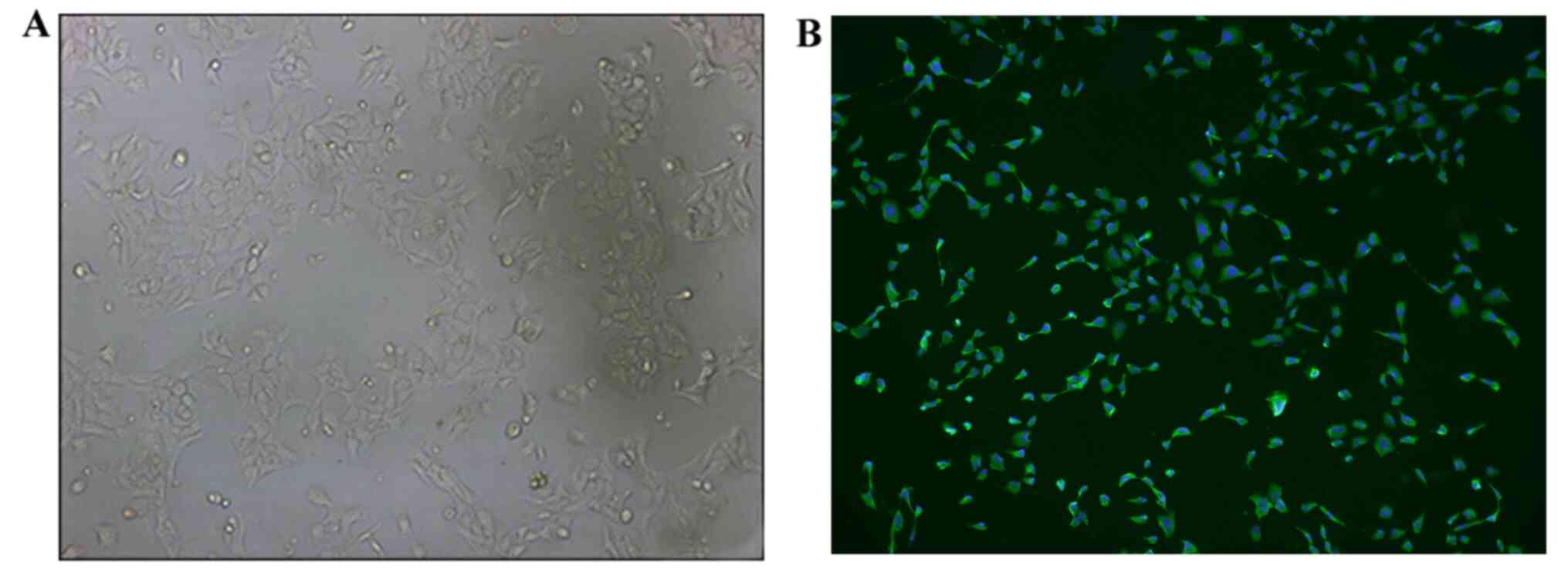
It was observed via inverted microscopy that the
recovered HConEpiCs started adhering to the culture dish and
proliferating at 0.5 h post-cell seeding. HConEpiCs appeared
morphologically round with clear cell membranes and nuclei in the
middle of the cells. These cells started to migrate toward the
periphery areas at 1 h after cell seeding. At 24 h, cells were
fully attached and exhibited a polygonal shape, growing
individually or clumping together (Fig.
1A). Immunostaining assays demonstrated that most of the cells
were positive for CK-13 staining, which indicated that the isolated
cells were HConEpiCs (Fig. 1B).
Effect of fluoxetine on the viability
of HConEpiCs
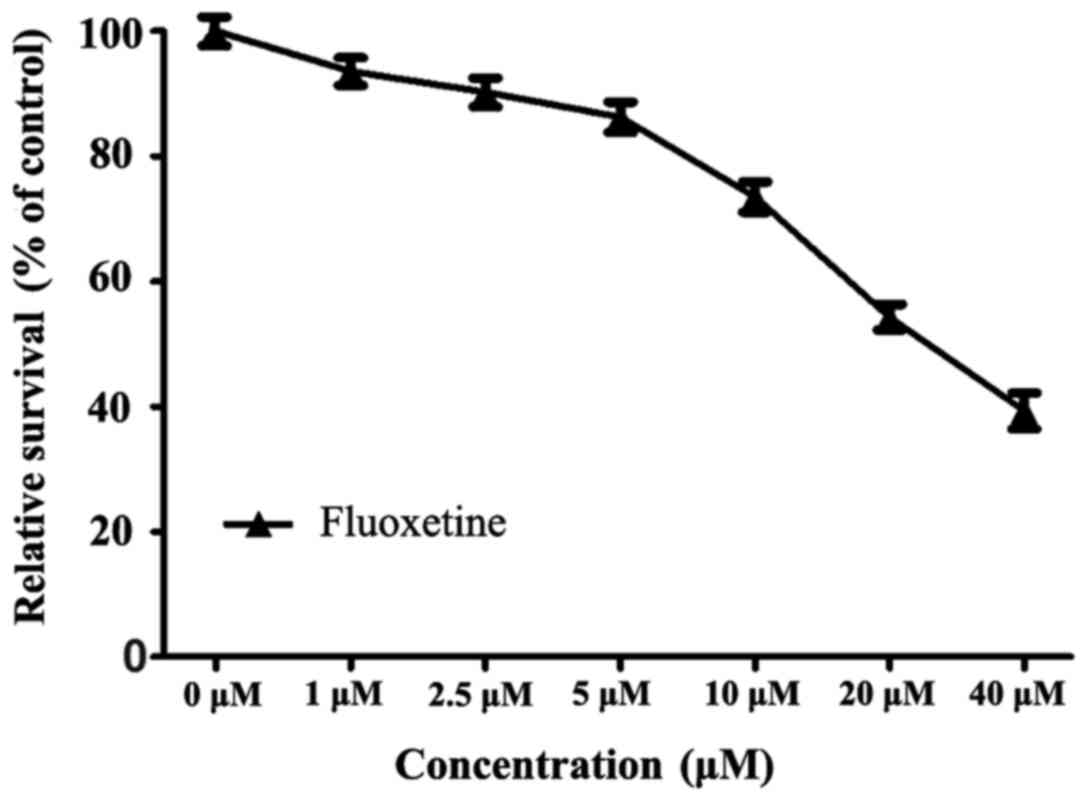
The results of the CCK-8 assay revealed that after
24 h of treatment with a series of fluoxetine dosages (0, 1, 2.5,
5, 10, 20 and 40 µM), the viability of HConEpiCs gradually
decreased. A slight decline in cell viability was observed when
cells were exposed to fluoxetine at 1, 2.5 or 5 µM, with
viabilities of 93.62±3.12, 90.25±3.26 and 86.28±3.42% of the
control group, respectively. The cell viability markedly declined
when cells were exposed to fluoxetine at 10, 20 or 40 µM, with
viabilities of 73.53±3.37, 54.32±2.87 and 39.33±4.06%, of the
control group, respectively. These results indicated that
fluoxetine at ≥10 µM exerted an evident cytotoxic effect on
HConEpiCs. Therefore, 5 µM fluoxetine was applied in the subsequent
experiments (Fig. 2).
Fluoxetine inhibits cell migration and
invasion
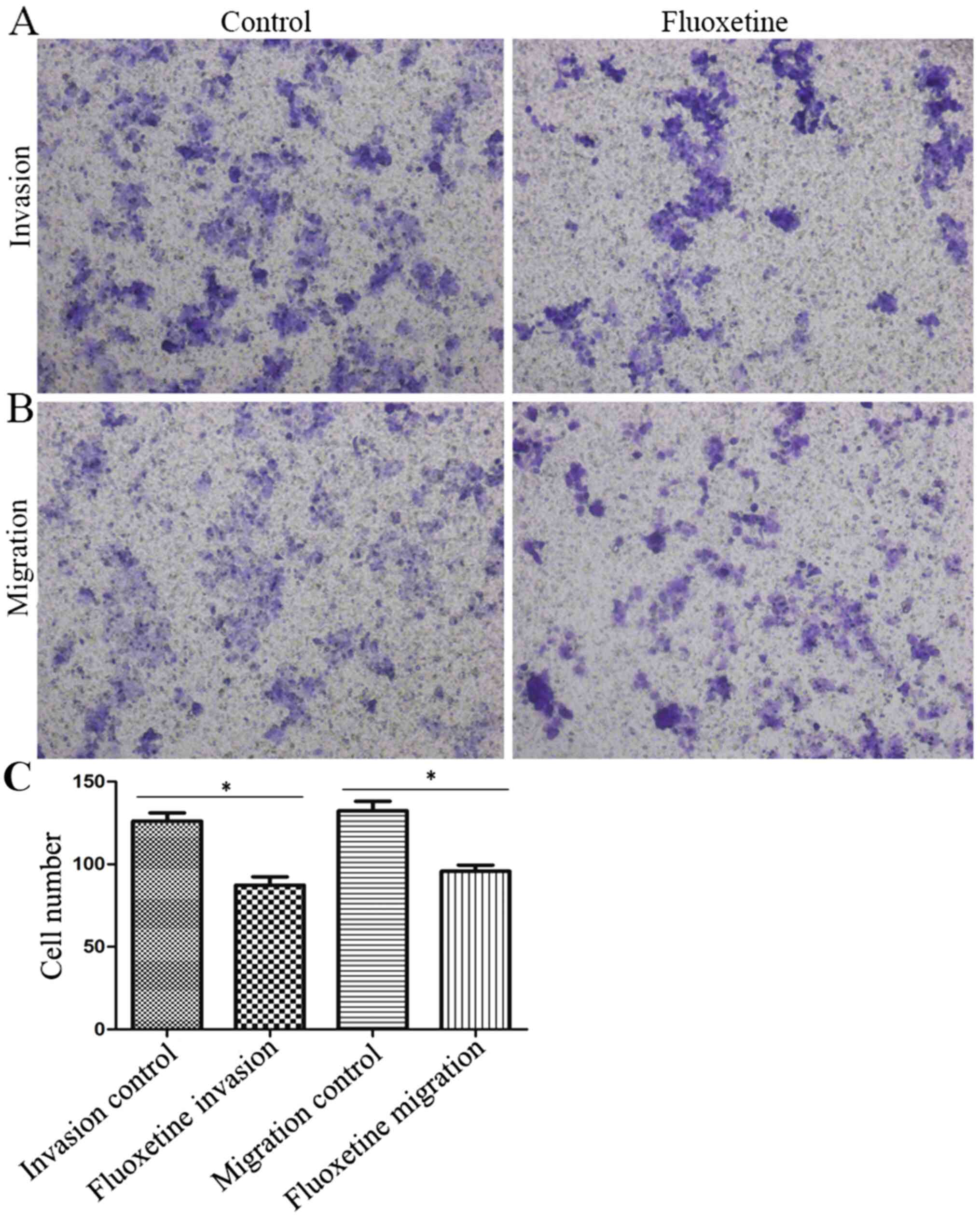
Transwell assays were performed to assess the effect
of fluoxetine on the invasive and migratory capacity of HConEpiCs
(Fig. 3). The invasion assay
indicated that the number of cells that penetrated the
Matrigel-coated membrane significantly decreased in the 5 µM
fluoxetine group, with the invasion capacity decreased to 69.31% of
that in the control group (Fig. 3A
and C). Furthermore, the Transwell
migration assay demonstrated that the rate of cells that penetrated
the membrane in the fluoxetine-treated group was significantly
reduced, with the migration capacity decreased to 72.29% relative
to that in the control group (Fig.
3B and C).
Expression of ERK1/2 and p-ERK1/2 in
HConEpiCs via immunostaining and the effect of fluoxetine on their
expression
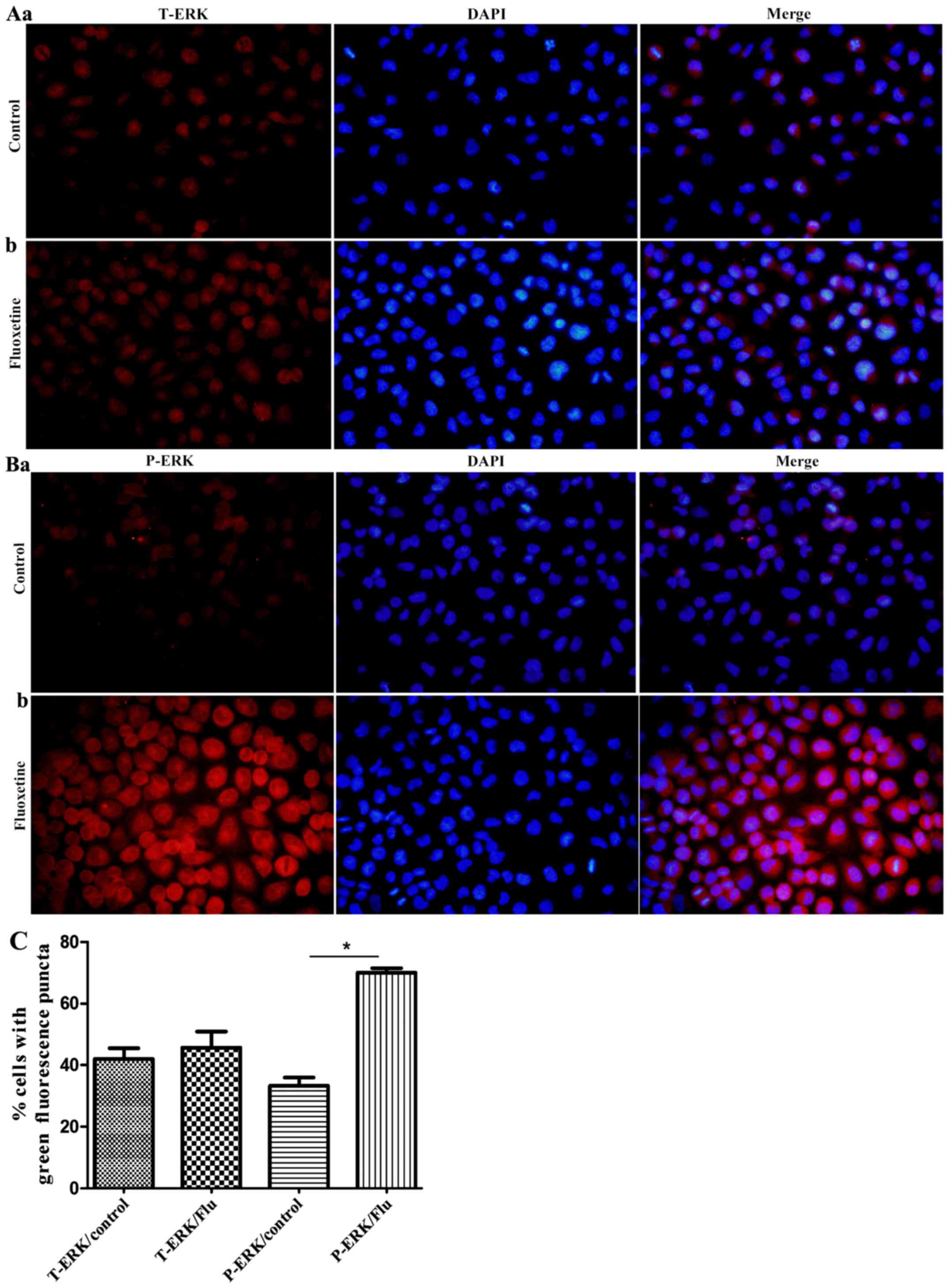
As indicated in Fig.
4, total ERK and p-ERK were widely abundant in the cytoplasm of
HConEpiCs. However, HConEpiCs treated with 5 µM fluoxetine for 24 h
exhibited a notably increased protein level of p-ERK, while the
level of total ERK did not significantly change.
Effect of fluoxetine on the protein
levels of ERK1/2, p-ERK1/2, Bax, Bcl-2 and MMPs in HConEpiCs
HConEpiCs were treated with a gradient of fluoxetine
doses (0, 1, 2.5, 5, 10, 20 and 40 µM) for 24 h and their protein
lysates were subjected to western blot analysis to assess the
protein levels of ERK, p-ERK, Bax, Bcl-2 and MMPs (Fig. 5). A representative western blot
image is provided in Fig. 5A. The
quantification results revealed that the protein levels of total
ERK and MMP-2/9 were not significantly different between the
fluoxetine-treated groups and the control (Fig. 5B and D). However, significantly elevated protein
levels of p-ERK and Bax were observed as the fluoxetine
concentration increased and the maximum p-ERK level was achieved
when fluoxetine was increased to 20 µM. Furthermore, the
fluoxetine-treated groups exhibited significantly lower levels of
Bcl-2 in comparison with the control group (Fig. 5B and C).
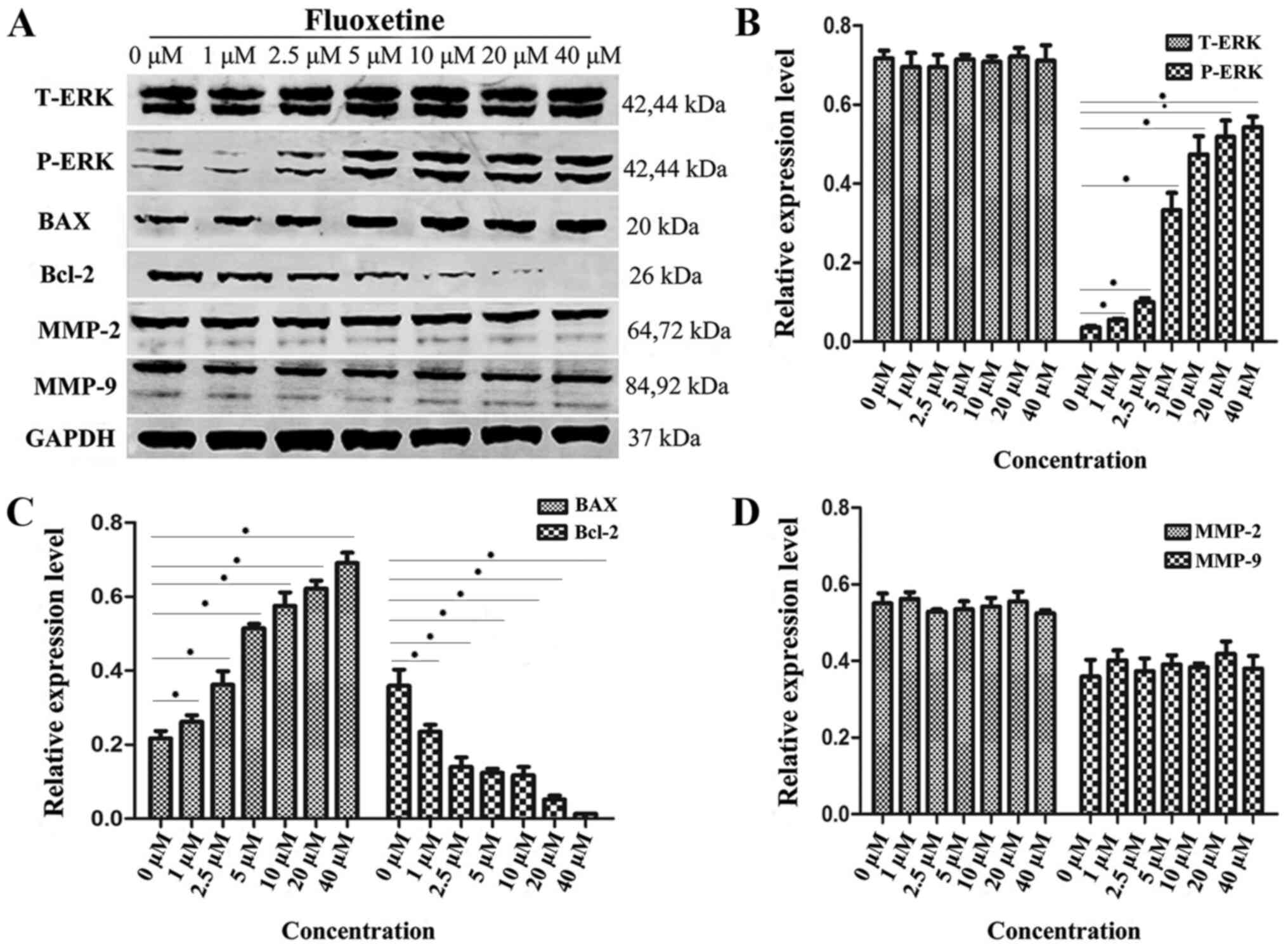 | Figure 5Expression levels of proteins
associated with the MAPK-ERK1/2 pathway, apoptosis and MMPs in
HConEpiCs treated by fluoxetine. (A) The protein levels of T-ERK,
P-ERK, BAX, Bcl-2, MMP-2, MMP-9 and GAPDH in HConEpiCs treated by
fluoxetine at different concentrations (0, 1, 2.5, 5, 10, 20 and 40
µM). (B) Quantification of protein levels of T-ERK and P-ERK in
HConEpiCs prior to and after treatment with fluoxetine at a
gradient of concentrations for 24 h. (C) Quantification of protein
levels of BAX and Bcl-2 in HConEpiCs prior to and after the
24-htreatment with fluoxetine at a gradient of concentrations. (D)
Quantification of the protein levels of MMP-2 and MMP-9 in
HConEpiCs prior to and after the 24-htreatment with fluoxetine at
different concentrations. *P<0.05. HConEpiCs, human
conjunctival epithelial cells; MMP, matrix metalloproteinase;
T/P-ERK, total/phosphorylated ERK. |
Discussion
MAPKs are highly conserved among eukaryotic cells
and have significant roles in signal transduction. They participate
in a variety of physiological and pathological processes, including
cell proliferation, differentiation, apoptosis, stress responses
and inflammatory responses (14).
MAPK pathways maybe triggered by diverse exogenous cytokines or
environmental factors, including hormones (insulin), growth factors
(platelet-derived factors), inflammatory factors and TNFs, and
behave as critical pathways that transduce the signal from the cell
surface to the nucleus (14). There
are three major MAPK family members: ERK, c-Jun N-terminal kinase
and p38MAPK (14). The MAPK-ERK
signaling pathway is able to amplify extracellular signals and has
been the most widely studied pathway in recent years.
Fluoxetine is an SSRI that is frequently
administered as an anti-depressant drug. Fluoxetine selectively
inhibits 5-HT re-uptake by presynaptic membrane 5-HT pumps in
neurons, thus increasing the concentration of 5-HT in the synaptic
space and enhancing the biological functions of 5-HT pumps. Dwivedi
et al (15) reported that
the activity of ERK1/2 was significantly lower in brain tissues
from patients who died from depression-associated suicide. 5-HT
receptors are able to activate the MAPK pathway via
phosphorylation, while anti-depressants may antagonize depression
by manipulating activation of the MAPK pathway (11,16).
In addition, long-term treatment for depression may cause an
intracellular increase of acute and chronic inflammatory factors,
including IL-β and TNF-α, which may cause inflammation on the eye
surface and promote dry eye syndrome (16).
In the present study, it was observed that the
proliferation rates of HConEpiC gradually decreased in the presence
of increasing dosages of fluoxetine. A dosage of 5 µM was used in
subsequent experiments. The results indicated that the
phosphorylation of ERK was enhanced in the fluoxetine-treated group
compared with that in the control group, while no significant
difference was observed in the protein levels of total ERK.
Furthermore, the protein levels of Bax and Bcl-2 were respectively
upregulated and downregulated in the fluoxetine-treated group.
These results suggest that fluoxetine at certain concentrations is
able to activate the MAPK-ERK signaling pathway via triggering the
phosphorylation of ERK1/2, subsequently causing apoptosis. In turn,
this increase in cell death may further stimulate the MAPK-ERK
pathway, establish a positive feedback loop of signaling
transduction and resulting in dry eye syndrome. Bax and Bcl-2 are
well-studied apoptosis-associated genes. Bax is a pro-apoptotic
factor among Bcl-2 family proteins and its overexpression promotes
cell death (17). By contrast, the
Bcl-2 gene has an anti-apoptotic role during the process of
programmed cell death and is widely accepted as an apoptosis
inhibitor (17) Bcl-2 and Bax
proteins also antagonistically form a heterodimer to regulate the
progression of apoptosis.
According to previous studies, the ocular surface
cells in patients with dry eye disease are abnormal with
inflammatory cell infiltration, inducing the increase of
inflammatory factors and the expression of MMPs. These studies
indicate that desiccating stress stimulates the expression of
MMP-9, IL-1α, IL-1β and TNF-α mRNA, as well as activates MAPK
signaling pathways in the corneal epithelium. MAPKs are known to
stimulate the production of inflammatory cytokines and MMPs, and
they may have an important role in the induction of these factors
that have been implicated in the pathogenesis of dry eye disease
(12,18). In the present study, after treatment
of HConEpiCs with a gradient of fluoxetine concentrations for 24 h,
no significant difference in the protein expression levels of MMPs
was observed, which was contrary to the expected outcome and
requires further research.
In conclusion, fluoxetine at certain concentrations
markedly induced apoptosis of HConEpiCs. The present study
preliminarily examined the role of MAPK but due to the complexity
of MAPK signaling pathway regulation, it was not possible to
clarify the mechanism of 5-HT receptor increase in activating the
MAPK-ERK pathway. The existence of 5-HT receptors in HConEpiCs is a
limitation of the present study. 5-HT receptors exist in the
neurons of brain tissues. Anti-depressant drugs may exert their
effects by activating the MAPK-ERK pathway to then increase the
concentration of 5-HT receptors. In the epithelial cells of the eye
conjunctiva, anti-depressant drugs increase inflammatory cytokines
and induce cell apoptosis by activating the MAPK-ERK pathway,
resulting in dry eye conjunctiva. It may be speculated that
HConEpiCs also express 5-HT receptor, and therefore, the present
study does not reveal whether HConEpiCs express the 5-HT
transporter, which may be replenished afterwards. Therefore,
further study of the regulatory mechanism of the MAPK signal
transduction pathway is required to identify proteins that directly
regulate the MAPK-ERK pathway, and the association between
apoptotic proteins and dry eye syndrome should be clarified. This
will assist in developing drugs with better targeting abilities and
fewer adverse reactions. The present study provided a foundation to
investigate the mechanisms by which anti-depressant drug induces
dry eye syndrome and may assist in developing novel therapies for
dry eye syndrome.
Acknowledgements
Thanks is given to Professor Liao Hua from the
Department of Otolaryngology Head and Neck Surgery, Renmin Hospital
of Wuhan University, for his strong experimental technical support
for this study. I would like to thank Mr Yan Jiangbo, a graduate
student of ophthalmology at the school of medicine of Wuhan
University, for his valuable experience in the preliminary
experiments.
Funding
The present study received support from the Natural
Science Foundation of Hubei province (grant no. 2016CKB708).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TC, who was a major contributor to the manuscript,
participated in the design and operation of the experiment, and
obtained, analyzed and interpreted the relevant data. YY
participated in the conception and design of the experiment, and
revised the contents of the manuscript. BC participated in the
operation of the experiment and recorded the experimental process
and data, while XW participated in the specific operations of the
experiments and the drafting of the manuscript. All the authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Clinical Research
Ethics Committee of Renmin Hospital of Wuhan University (reference
no. AF SOP/3.6-01/5.1). All patients in the study who provided
disused conjunctival tissue blocks signed informed consent to the
use of their tissue.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Schiffman RM, Walt JG, Jacobsen G, Doyle
JJ, Lebovics G and Sumner W: Utility assessment among patients with
dry eye disease. Ophthalmology. 110:1412–1419. 2003.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Han SB, Hyon JY, Woo SJ, Lee JJ, Kim TH
and Kim KW: Prevalence of dry eye disease in an elderly Korean
population. Arch Ophthalmol. 129:633–638. 2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lin PY, Cheng CY, Hsu WM, Tsai SY, Lin MW,
Liu JH and Chou P: Association between symptoms and signs of dry
eye among an elderly Chinese population in Taiwan: The shihpai eye
study. Invest Ophthalmol Vis Sci. 46:1593–1598. 2005.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Management and therapy of dry eye disease.
Report of the management and therapy subcommittee of the
international dry eye WorkShop. Ocul Surf. 5:163–178.
2007.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Luo C, Wang F, Qin S, Chen Q and Wang QK:
Coronary artery disease susceptibility gene ADTRP regulates cell
cycle progression, proliferation, and apoptosis by global gene
expression regulation. Physiol Genomics. 48:554–564.
2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Paul A, Wilson S, Belham CM, Robinson CJ,
Scott PH, Gould GW and Plevin R: Stress-activated protein kinases:
Activation, regulation and function. Cell Signal. 9:403–410.
1997.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Pflugfelder SC, de Paiva CS, Tong L, Luo
L, Stern ME and Li DQ: Stress-activated protein kinase signaling
pathways in dry eye and ocular surface disease. Ocul Surf. 3 (4
Suppl):S154–S157. 2005.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Li DQ, Luo L, Chen Z, Kim HS, Song XJ and
Pflugfelder SC: JNK and ERK MAP kinases mediate induction of
IL-1beta, TNF-alpha and IL-8 following hyperosmolar stress in human
limbal epithelial cells. Exp Eye Res. 82:588–596. 2006.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Tang CH and Tsai CC: CCL2 increases MMP-9
expression and cell motility in human chondrosarcoma cells via the
Ras/Raf/MEK/ERK/NF-κB signaling pathway. Biochem Pharmacol.
83:335–344. 2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chang F, Steelman LS, Shelton JG, Lee JT,
Navolanic PM, Blalock WL, Franklin R and McCubrey JA: Regulation of
cell cycle progression and apoptosis by the Ras/Raf/MEK/ERK pathway
(Review). Int J Oncol. 22:469–480. 2003.PubMed/NCBI
|
|
11
|
Raison CL, Capuron L and Miller AH:
Cytokines sing the blues: Inflammation and the pathogenesis of
depression. Trends Immunol. 27:24–31. 2006.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Luo L, Li DQ, Doshi A, Farley W, Corrales
RM and Pflugfelder SC: Experimental dry eye stimulates production
of inflammatory cytokines and MMP-9 and activates MAPK signaling
pathways on the ocular surface. Invest Ophthalmol Vis Sci.
45:4293–4301. 2004.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Dota A, Nishida K, Adachi W, Nakamura T,
Koizumi N, Kawamoto S, Okubo K and Kinoshita S: An expression
profile of activegenes in human conjunctival epithelium. Exp Eye
Res. 72:235–241. 2001.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Krishna M and Narang H: The complexity of
mitogen-activated protein kinases (MAPKs) made simple. Cell Mol
Life Sci. 65:3525–3544. 2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Dwivedi Y, Rizavi HS, Zhang H, Roberts RC,
Conley RR and Pandey GN: Aberrant extracellular signal-regulated
kinase (ERK)1/2 signalling in suicide brain: Role of ERK kinase 1
(MEK1). Int J Neuropsychopharmacol. 12:1337–1354. 2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kim KW, Han SB, Han ER, Woo SJ, Lee JJ,
Yoon JC and Hyon JY: Association between depression and dry eye
disease in an elderly population. Invest Ophthalmol Vis Sci.
52:7954–7958. 2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kroemer G: The proto-oncogene Bcl-2 and
its role in regulating apoptosis. Nat Med. 3:614–620.
1997.PubMed/NCBI View Article : Google Scholar
|
|
18
|
De Paiva CS, Corrales RM, Villarreal AL,
Farley WJ, Li DQ, Stern ME and Pflugfelder SC: Corticosteroid and
doxycycline suppress MMP-9 and inflammatory cytokine expression,
MAPK activation in the corneal epithelium in experimental dry eye.
Exp Eye Res. 83:526–535. 2006.PubMed/NCBI View Article : Google Scholar
|