Introduction
Tic disorders (TDs) constitute a spectrum of
heritable neuropsychiatric conditions, including Tourette syndrome
and attention deficit hyperactivity disorder, that are
characterized by the presence of tics that begin in childhood,
typically peaking in severity just before adolescence before
improving in adulthood (1). Tics
can cause subjective discomfort in the patient such as pain or
injury, sustained social problems such as social isolation,
bullying; emotional problems such as reactive depressive symptoms
and functional interference such as academic underachievement
(2). A systematic review and
meta-analysis of tic disorders in children in China indicated that
the prevalence of TDs was up to 6.1%, which differed based on sex,
age and geographical location (3).
Many factors hinder the assessment of TD severity, including
spontaneous variations in tics in an individual over time, large
variability in the impact of a given level of physical tic severity
on an individual or their family and the tendency of patients to
suppress their tics, especially when in the presence of a clinician
(4). Therefore, the evaluation of
tics and their associated comorbidities relies upon self-reported
scales and clinician-derived interviews (5). However, investigation into TDs using
neuroimaging is becoming a popular topic of research, as functional
abnormalities in the striatum of the brain are considered to be
closely associated with TDs (6,7).
The pathophysiological model of tics generally
involves disruptions in γ-aminobutyric acid transmission (8). The model, which we term the tic
generation model (TGM), is based on two kernels that are used for
scaling the factors (cortical input and striatal feedback). The
model is a simplified version of the simplified spike response
model (SRM) that has been used extensively to model neuronal
activity (9). Haloperidol, which
can effectively inhibit the excitability of the cortical motor area
by restraining the activity of dopamine receptors (10), has been approved by the US Food and
Drug Administration for the treatment of TDs. In addition,
Traditional Chinese medicine (TCM) is also believed by some to be
suitable for the treatment of psychiatric disorders (11). A number of studies in China have
previously suggested that 18F-Fluorodeoxyglucose (FDG) can be
combined with the Yale Global Tic Severity Scale (YGTSS) to
evaluate patients with TD (12,13).
Therefore, the aim of the present study was to assess the
relationship between FDG signals in the striatum of the brain and
the stereotypy scores of TD model rats following treatment with
either TCM or haloperidol.
Materials and methods
Rat model
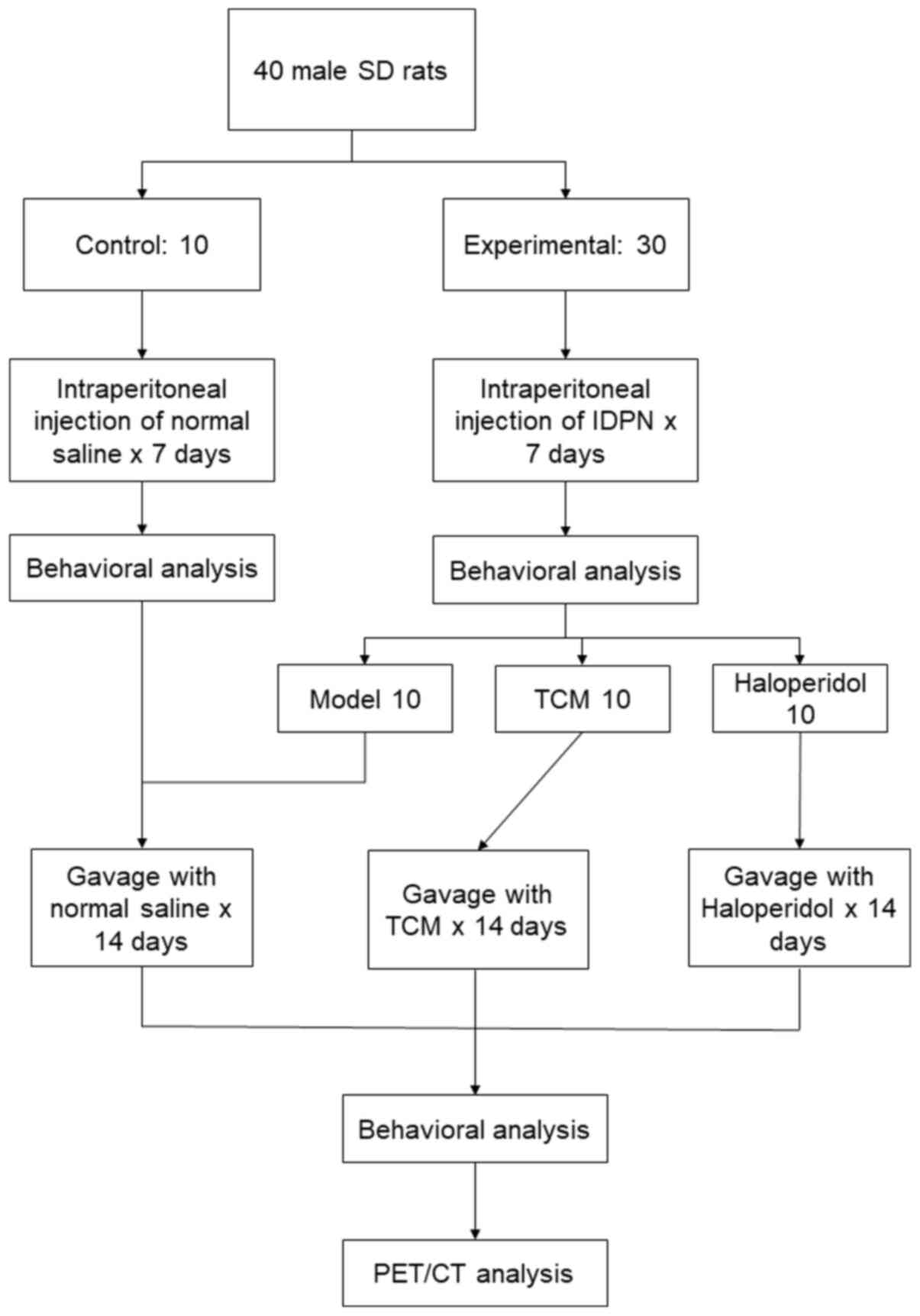
A total of 40 male Sprague-Dawley rats (age, 4
weeks; mean weight, 110±12 g; Xinhua Hospital Experimental Animal
Center) were housed in an air-conditioned animal room on a 12-h
light/dark cycle at 22±3˚C and 50±10% humidity. The rats were
provided with a laboratory diet and water ad libitum. After
1 week of adaptation, rats were randomly divided into the control
(n=10) and experimental (n=30) groups. Rats in the experimental
group received daily intraperitoneal (i.p.) injections of
iminodipropionitrile (IDPN; Sigma-Aldrich; Merck KGaA) at 150 mg/kg
for 7 days. Rats in the control group were injected with normal
saline (0.9%; 5 ml/kg, i.p.). The relationship between stimulation
and behavior was assessed by using peristimulus tic histograms with
0.01 sec bins. To calculate the success percentage in inducing
tics, unsuccessful stimuli that were immediately (500 millisec)
preceded by a tic were removed from the total number of stimuli in
the calculation. After 7 days, the behavior of rats in the
experimental group was analyzed and they were further divided into
the following three groups (n=10 per group): i) TCM; ii)
haloperidol; and iii) model only. Rats in the TCM group received a
TCM concoction including 10 g Tian Ma (Rhizoma Gastrodiae),
10 g Gou Teng (Ramulus Uncariae cum Uncis), 10 g Shen Jin
Cao (Herba Lycopodii), 5 g Quan Xie (Scorpio) and 10
g Xin Yi (Flos Magnoliae), a mixture of compounds as
described to treat tic disorders in a previous study (14). TCM compounds were provided as
granules, which were mixed and boiled in 100 ml water for 60 min.
All TCM compounds were supplied by Jiangyin Tianjiang
Pharmaceutical Co., Ltd. Rats were administered with the TCM
compound daily by oral gavage at 9 g/kg, whilst rats in control
group received normal saline and rats in the haloperidol group
received haloperidol (0.1 mg/ml) daily by oral gavage. The
intervention lasted for 2 weeks before behavioral analysis and
Micro-PEC/CT were conducted for each group. All experimental and
animal care procedures were approved by the Ethics Committee of
Xinhua Hospital Affiliated to Shanghai Jiaotong University School
of Medicine (Shanghai, China). An overview of the experimental
procedures is shown in Fig. 1.
Behavioral analysis
Animals were observed during 30-min sessions by two
trained observers who were blind to the experimental conditions
(7). Observations were made at 5-10
min, 15-20 min and 25-30 min for 1-min periods, for a total of
three periods. The items of behavioral stereotypies considered in
this analysis were sniffing, focused sniffing and licking/shaking.
Several classifications of oral stereotypies, including wood chip
eating, self-gnawing, biting, licking that was not associated with
grooming and ‘taffy pulling’ (i.e. repetitive paw-to-mouth
movements), were also recorded. A rat that engaged in ≥1 episodes
of the same behavior received a score of 1. Animals that exhibited
≥1 behaviors of any other classification received an additional
point for each stereotypy observed. Scores ranged from 0-5 and were
averaged for each experimental group. Higher scores indicated
increasing severity.
Micro-positron emission tomography/CT
(PET/CT)
Micro-PET/CT (Siemens Inveon MM PET/CT scanner;
Siemens AG) was conducted at Ruijin Hospital Affiliated to Shanghai
Jiao Tong University School of Medicine (Shanghai, China). All
animals behavioral analysis before PET/CT was performed. The
animals received a dose of FDG via tail vein injection before
imaging. FDG, with an activity of 500 Ci/mmol, was prepared. 1.0
mCi of pyrogen-free 18FDG was injected through the tail vein and
the rats were returned to their home cage in a room with minimal
ambient noise for the uptake period. Subsequently, the rats were
anesthetized with 10% chloral hydrate (350 mg/kg; i.p.) by
intraperitoneal injection and were placed in the micro PET scanner
with their feet extended. No rats displayed signs of peritonitis
following chloral hydrate administration. A 15-min static PET
acquisition was performed followed by a 5-min anatomical CT scan
acquisition. In these conditions, their metabolic rate in the
region of interest (ROI) was indirectly assessed to determine the
FDG uptake rate, which was quantified as follows: i) Specific
uptake values (SUVs) were collected three times across each ROI in
the bilateral striatum; and ii) mean SUVs were obtained across each
ROI in the striatum (SUV mean). The ROI size was 15-16 pixels and
the data were expressed as Becquerel (Bq)/cubic centimeter
(CC).
where x- striatum is the mean tracer
activity within the ROI of the striatum, Idose is the
injected dose and BW is the body weight. This was accomplished
using the PMOD software package (version 2.1; PMOD Technologies
LLC) in conjunction with the W. Schiffer rat brain template and
atlas (15).
Statistical analysis
The data were analyzed using the SPSS version 24.0
software (IBM Corp.). The normality of data distribution of the
continuous variables was assessed using the Shapiro-Wilk test.
Continuous variables with normal distribution are presented as the
mean ± standard deviation (SD). The means of normally distributed
continuous variables were compared using Tukey's test and one way
ANOVA (Respectively, if the results of ANOVA was significant,
Tukey's test was used to compare the differences between the two
means.). Tukey's test was used to compare between groups and assess
variation between timepoints. Correlation between the stereotypy
scores and SUVs was assessed using Spearman's rank correlation
analysis. P<0.05 was considered to indicate a statistically
significant difference. Repeated measures ANOVA was used to analyze
stereotypy scores at different time points among the groups.
Results
Stereotypy scores in a rat model of
TD
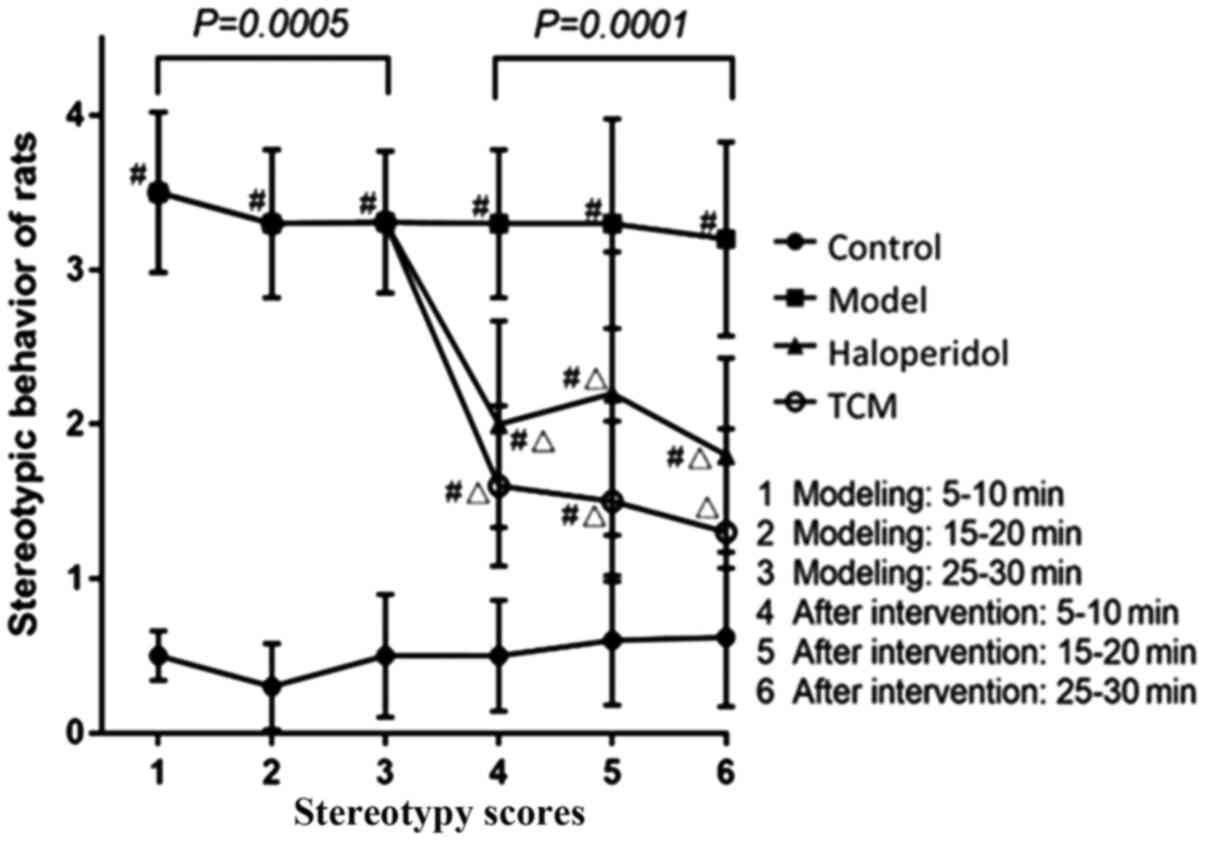
Stereotypy scores of rats in the experimental group
were found to be significantly higher compared with those in the
control group (repeated measures ANOVA; P=0.0005; Table I). In the first period of
observation (5-10 min), the scores of the model group were higher
(3.5±0.52) compared with those in the control group (0.5±0.16;
P<0.05). In the remaining two periods of observation, scores in
the model group were also higher (15-20, 3.30±0.48; 25-30 min,
3.31±0.46) compared with those in the control group (15-20,
0.30±0.28; 25-30 min, 0.50±0.40; P<0.05; Table I).
 | Table IComparison of stereotypy behavior in
control and tic disorder model rats. |
Table I
Comparison of stereotypy behavior in
control and tic disorder model rats.
| | | Stereotypy score at
each timepoint |
|---|
| Group | N | 5-10 min | 15-20 min | 25-30 min |
|---|
| Control | 10 | 0.50±0.16 | 0.30±0.28 | 0.50±0.40 |
| Experimental | 30 |
3.50±0.52a |
3.30±0.48a |
3.31±0.46a |
Stereotypy scores after TCM
intervention
After intervention, the scores of rats that received
TCM and haloperidol were found to be partially but significantly
reduced compared with scores in the model only group (Repeated
measures ANOVA; P<0.05). The magnitude of reduction in
stereotypy score in the TCM group (5-10, 1.60±0.5; 10-15 min,
1.50±0.52; 25-30 min, 1.30±0.67) was found to be higher compared
with that in the haloperidol group (5-10, 2.00±0.67; 10-15 min,
2.20±0.92; 25-30 min, 1.80±0.63; Table
II). The stereotypy scores of TCM group and haloperidol group
were significantly lower than that of model only group (both
P<0.05), while they were still significantly higher than control
group (P<0.05; Table II;
Fig. 2).
 | Table IIComparison of stereotypy behavior in
control and tic disorder rats after intervention. |
Table II
Comparison of stereotypy behavior in
control and tic disorder rats after intervention.
| | Stereotypy scores at
each timepoint |
|---|
| Group | N | 5-10 min | 15-20 min | 25-30 min |
|---|
| Control | 10 | 0.50±0.36 | 0.60±0.42 | 0.62±0.45 |
| Model | 10 |
3.30±0.48a |
3.30±0.68a |
3.20±0.63a |
| Haloperidol | 10 |
2.00±0.67a,b |
2.20±0.92a,b |
1.80±0.63a,b |
| TCM | 10 |
1.60±0.52a,b |
1.50±0.52a,b |
1.30±0.67b |
SUV of the striatum ROI
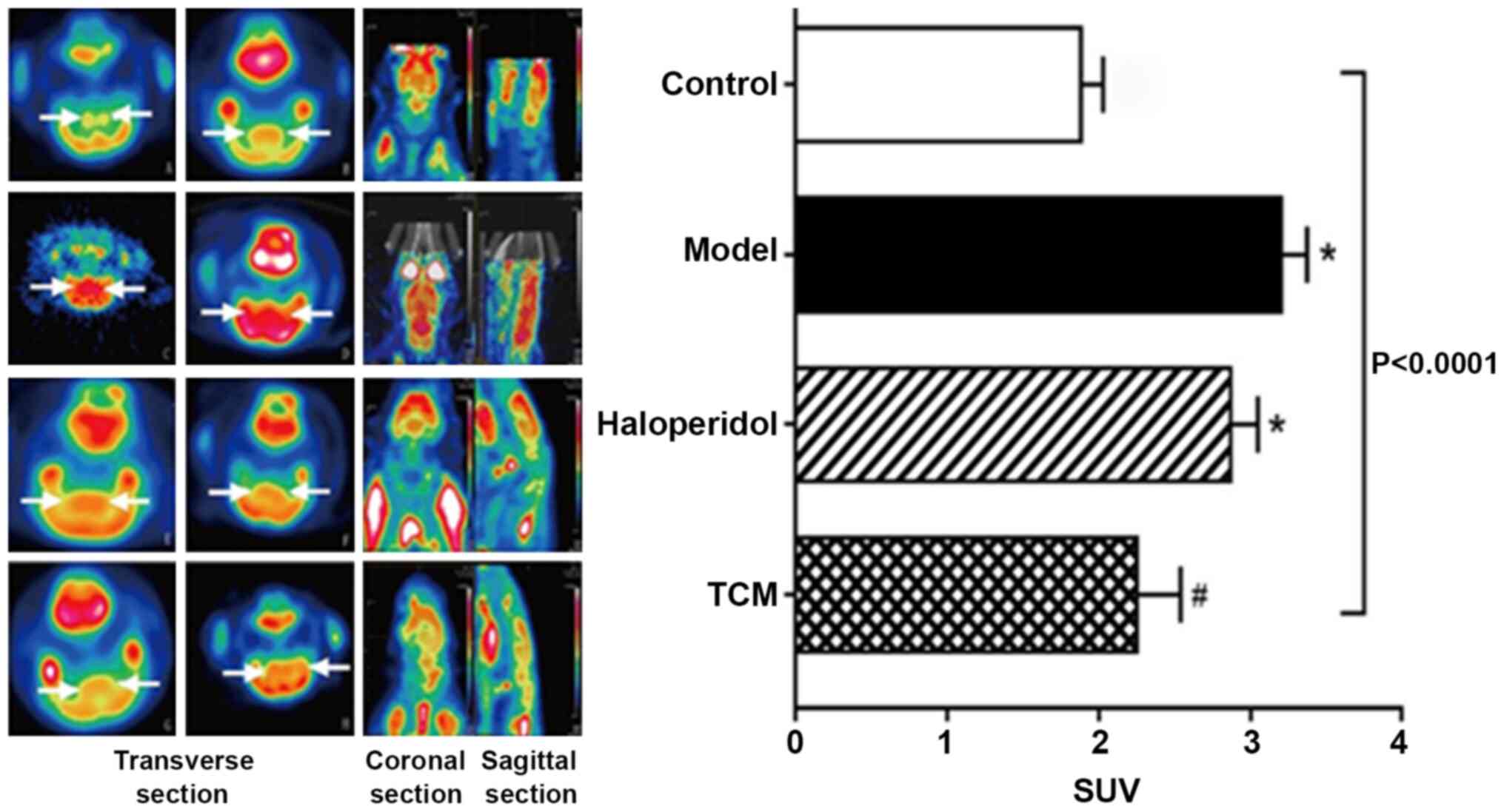
FDG PET/CT scans were performed on the rats in the
four treatment groups (Fig. 3). The
PET signal showed severe hypo-metabolism in the striatum with a
marked reduction in FDG uptake in rats from the haloperidol and TCM
groups when compared with those in the model only group (control,
1.88±0.15; model only, 3.20±0.35; haloperidol, 2.87±0.18; TCM,
2.25±0.29). Tukey's test indicated that FDG uptake in the model
group was significantly higher than that of control group
(P<0.05), while FDG uptake in the TCM group was significantly
lower than in the model group (P<0.05).
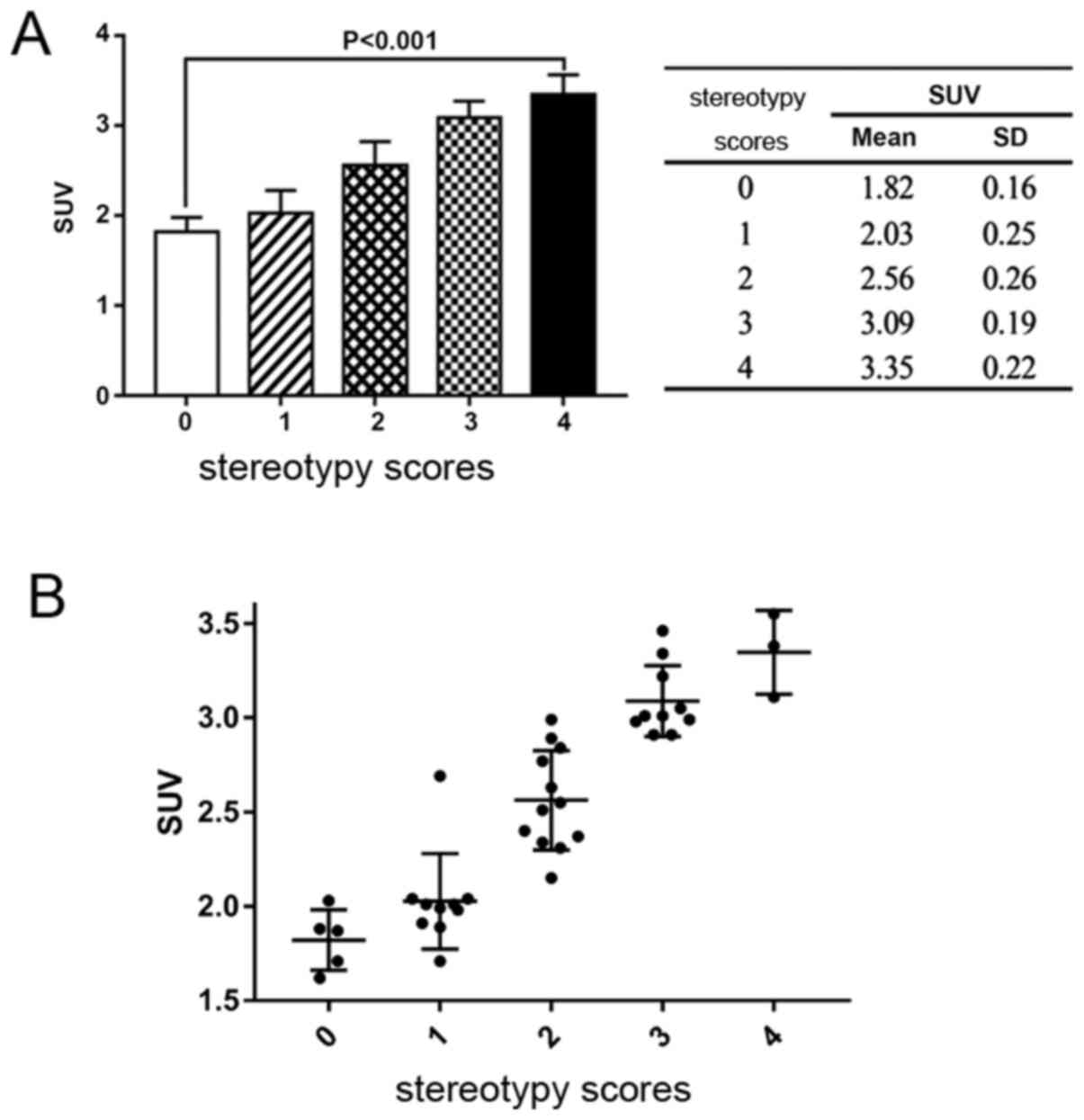
SUV and stereotypy scores
Correlation between the stereotypy scores after
intervention and the SUVs were then analyzed. A moderately positive
moderate but significant correlation was identified between the
SUVs and the stereotypy scores (Spearman's rank correlation
analysis; R=0.926; P=0.001; Fig.
4). One-way ANOVA showed that the differences in the SUVs
between each score category was statistically significant
(P<0.05).
Discussion
Tourette's syndrome (TS) is the most severe form of
TD that is a childhood-onset condition and features multiple motor
and ≥1 phonic tic lasting >1 year (16). In total, ~85% patients with TS
suffer from psychiatric comorbidities including, in order of
prevalence, attention deficit hyperactivity and
obsessive-compulsive disorder, followed by anxiety, mood, autistic
spectrum, oppositional defiant and conduct disorders and
personality disorders (17). Those
disorders may impact patients' academic progress and family
relationships of the patients. There are several potential
interventions for TDs, including pharmacological treatment
(2) and behavioral and psychosocial
conditioning (18). However, no
universal objective indices currently exist for evaluating the
severity of these diseases. In general, the evaluation of tics and
comorbidities in children and adults is reliant on self-reporting
scales and clinician-derived interviews. The most widely used
checklists of tic characteristics and severity that combine an
observation component with historical information obtained from the
patients' parents and/or spouses include the YGTSS (19), Shapiro Tourette syndrome severity
scale and the Hopkins motor and vocal tic scale (20).
IDPN has been widely used as a tool for
neuropathological studies, since it can induce a series of
neurobehavioral disturbances, including dyskinesia and repetitive
motor-defects, characteristics comparable to that of TS (10). In the present study, IDPN was used
to develop a rat model of TD. However, there are limitations to
this animal model. The heritability patterns of TS and other tic
disorders indicate that the pathogenesis of these conditions is
also strongly influenced by genetic factors (21). TD animal models based on genetic
manipulation have emerged, which may be more accurate compared with
drug intervention-induced TD animal models (16).
Abnormalities in the striatum is an emerging topic
in studies into TD pathogenesis. Abnormal dopamine uptake in the
striatum of TD patients has been reported, where enhanced dopamine
uptake within the striatum is believed to be associated with TDs
(22). Makki et al (23) previously found that microstructural
abnormalities of the striatum in children contribute to the
pathophysiology of TS. A transcriptome analysis of the human
striatum in TS also suggested that metabolic alterations may be
linked to the condition (24). FDG
PET/CT is a well-established method for characterizing functional
activity in the living human brain. Regional glucose metabolism,
coupled with energy-requiring processes driven by the synaptic
activity of neurons, appears to be a reliable index of functional
activity within the central nervous system (25). The present study suggests that FDG
PET/CT can be a useful tool for screening metabolic alterations in
the striatum.
An appropriate evaluation indicator would be
beneficial in the evaluation of TD treatment. The aim of the
present study was to determine the diagnostic performance of FDG
PET/CT in the evaluation of TD in rat models. The results of the
present study suggested that FDG PET/CT of the striatum can be used
to evaluate the effectiveness of TCM and haloperidol treatment for
TD in rats, where the SUVs positively correlated with the
stereotypy scores. However, the present study has a number of
limitations. Since the optimal cutoff values of the mean max SUV
were calculated from the sum of the maximum sensitivity and
specificity, the performance ability may have been overestimated.
Additionally, the number of rats used in the present study was low.
This method of evaluation was tested only on a rat model of TD.
Further study involving human patients is required.
In summary, the present study suggests that FDG
PET/CT of the striatum may be useful for the evaluation of the
effectiveness of treatment of TDs. However, further clinical
studies are needed to clarify the sensitivity of FDG PET/CT in the
evaluation of patients with TD and to investigate the association
between metabolic abnormalities and the severity of clinical
symptoms.
Acknowledgements
The authors would like to thank Dr Ming Ruan (Ruijin
Hospital affiliated to Shanghai Jiao Tong University School of
Medicine) for his help and guidance in the use of Micro PET/CT.
Funding
The present research was supported by grants from
The Shanghai Municipal Commission of Health and Family Planning
(grant no. ZYKC201701011) and the National Natural Science
Foundation of China (grant no. 8187150436).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
PZ and MW designed the study; PZ, BM and SW
performed the experiments; PZ and MW collected the data; PZ and MY
analyzed the data; and BM and SW prepared the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
All procedures were approved by Xinhua Hospital
Affiliated to Shanghai Jiao Tong University School of Medicine
(approval no. XHEC-F-2020-017).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Steeves T, McKinlay BD, Gorman D,
Billinghurst L, Day L, Carroll A, Dion Y, Doja A, Luscombe S,
Sandor P and Pringsheim T: Canadian guidelines for the
evidence-based treatment of tic disorders: Behavioural therapy,
deep brain stimulation, and transcranial magnetic stimulation. Can
J Psychiatry. 57:144–151. 2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Roessner V, Plessen KJ, Rothenberger A,
Ludolph AG, Rizzo R, Skov L, Strand G, Stern JS, Termine C and
Hoekstra PJ: ESSTS Guidelines Group. European clinical guidelines
for Tourette syndrome and other tic disorders. Part II:
Pharmacological treatment. Eur Child Adolesc Psychiatry.
20:173–196. 2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Yang C, Zhang L, Zhu P, Zhu C and Guo Q:
The prevalence of tic disorders for children in China: A systematic
review and meta-analysis. Medicine. 95(e4354)2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Abramovitch A, Reese H, Woods DW, Peterson
A, Deckersbach T, Piacentini J, Scahill L and Wilhelm S:
Psychometric properties of a self-report instrument for the
assessment of tic severity in adults with tic disorders. Behav
Ther. 46:786–796. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Martino D, Pringsheim TM, Cavanna AE,
Colosimo C, Hartmann A, Leckman JF, Luo S, Munchau A, Goetz CG,
Stebbins GT, et al: Systematic review of severity scales and
screening instruments for tics: Critique and recommendations. Mov
Disord. 32:467–473. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Rapanelli M, Frick L, Pogorelov V, Ohtsu
H, Bito H and Pittenger C: Histamine H3R receptor activation in the
dorsal striatum triggers stereotypies in a mouse model of tic
disorders. Transl Psychiatry. 7(e1013)2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Vinner E, Israelashvili M and Bar-Gad I:
Prolonged striatal disinhibition as a chronic animal model of tic
disorders. J Neurosci Methods. 292:20–29. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ding L, Yang Z, Liu G, Ran N, Yi M, Li H,
Zhao H, Tang L, Cheng H, Zhao J, et al: Safety and efficacy of
taurine as an add-on treatment for tics in youngsters. Eur J
Neurol. 27:490–497. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Gerstner W: Time structure of the activity
in neural network models. Phys Rev E Stat Phys Plasmas Fluids Relat
Interdiscip Topics. 51:738–758. 1995.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhao L, Qi F, Zhang F, Wang Z, Mu L, Wang
Y, En Q, Li J, Du Y and Li A: Dual regulating effect of Ningdong
granule on extracellular dopamine content of two kinds of
Tourette's syndrome rat models. Biosci Trends. 9:245–251.
2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wang Y, Li M, Liang Y, Yang Y, Liu Z, Yao
K, Chen Z and Zhai S: Chinese herbal medicine for the treatment of
depression: Applications, efficacies and mechanisms. Curr Pharm
Des. 23:5180–5190. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Braun AR, Stoetter B, Randolph C, Hsiao
JK, Vladar K, Gernert J, Carson RE, Herscovitch P and Chase TN: The
functional neuroanatomy of Tourette's syndrome: An FDG-PET study.
I. Regional changes in cerebral glucose metabolism differentiating
patients and controls. Neuropsychopharmacology. 9:277–291.
1993.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Jeffries KJ, Schooler C, Schoenbach C,
Herscovitch P, Chase TN and Braun AR: The functional neuroanatomy
of Tourette's syndrome: An FDG PET study III: Functional coupling
of regional cerebral metabolic rates. Neuropsychopharmacology.
27:92–104. 2002.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Sun LY, Li QP, Zhao LL and Ding YQ:
Traditional Chinese medicine inheritance system analysis of
professor Ding Yuanqing in treating tic disorder medication based
on experience. Zhongguo Zhong Yao Za Zhi. 40:3314–3318.
2015.PubMed/NCBI(In Chinese).
|
|
15
|
Schiffer WK, Mirrione MM, Biegon A,
Alexoff DL, Patel V and Dewey SL: Serial microPET measures of the
metabolic reaction to a microdialysis probe implant. J Neurosci
Methods. 155:272–284. 2006.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Godar SC, Mosher LJ, Di Giovanni G and
Bortolato M: Animal models of tic disorders: A translational
perspective. J Neurosci Methods. 238:54–69. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Martino D, Ganos C and Pringsheim TM:
Tourette syndrome and chronic Tic disorders: The clinical spectrum
beyond Tics. Int Rev Neurobiol. 134:1461–1490. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Verdellen C, van de Griendt J, Hartmann A
and Murphy T: ESSTS Guidelines Group. European clinical guidelines
for Tourette syndrome and other tic disorders. Part III:
Behavioural and psychosocial interventions. Eur Child Adolesc
Psychiatry. 20:197–207. 2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Leckman JF, Riddle MA, Hardin MT, Ort SI,
Swartz KL, Stevenson J and Cohen DJ: The Yale Global Tic severity
scale: Initial testing of a clinician-rated scale of tic severity.
J Am Acad Child Adolesc Psychiatry. 28:566–573. 1989.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Walkup JT, Rosenberg LA, Brown J and
Singer HS: The validity of instruments measuring tic severity in
Tourette's syndrome. J Am Acad Child Adolesc Psychiatry.
31:472–477. 1992.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Qi Y, Zheng Y, Li Z, Liu Z and Xiong L:
Genetic studies of Tic disorders and Tourette syndrome. Methods Mol
Biol. 2011:547–571. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Singer HS, Hahn IH and Moran TH: Abnormal
dopamine uptake sites in postmortem striatum from patients with
Tourette's syndrome. Ann Neurol. 30:558–562. 1991.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Makki MI, Behen M, Bhatt A, Wilson B and
Chugani HT: Microstructural abnormalities of striatum and thalamus
in children with Tourette syndrome. Mov Disord. 23:2349–2356.
2008.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Lennington JB, Coppola G, Kataoka-Sasaki
Y, Fernandez TV, Palejev D, Li Y, Huttner A, Pletikos M, Sestan N,
Leckman JF and Vaccarino FM: Transcriptome analysis of the human
striatum in Tourette syndrome. Biol Psychiatry. 79:372–382.
2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Chugani HT: Imaging brain metabolism in
the newborn. J Child Neurol. 33:851–860. 2018.PubMed/NCBI View Article : Google Scholar
|