Introduction
Diabetes mellitus (DM) is a group of metabolic
diseases characterized by hyperglycemia, which is caused by
impaired insulin secretion or dysregulated biological function, or
both (1). DM is the leading risk
factor of cardiovascular disease, which has the highest mortality
rate in China (2,3). Moreover the prevalence of diabetes and
prediabetes among the Chinese population increased substantially
from 9.7 and 15.5% in 2010 to 11.6 and 50.1% in 2013, respectively
(3,4). Thus, developing a strategy for disease
control in diabetes at the prediabetic stage is urgently required
(5,6).
Insulin resistance is a pathological condition in
which cells fail to respond physiologically to the hormone insulin,
leading to excess secretion of insulin as compensation to maintain
the stability of blood glucose, eventually accelerating the
development of type 2 DM (T2DM) (7,8).
Targeting insulin resistance has been used as a first line strategy
to treat diabetes (2). The insulin
receptor (IR) is a tetramer, which consists of two α subunits and
two β subunits connected via disulfide bonds. The α subunit is
extracellular and presents binding sites of insulin, while the β
subunit is composed of a transmembrane domain and a intracellular
kinase domain, acting as a signal transducer (9,10). By
binding to IR, the PI3K-dependent signaling pathway is initiated,
which leads to the recruitment and interaction of the IR substrate
and PI3K, eventually resulting in the activation of AKT via
phosphoinositide-dependent kinase 1 (PDK1) (9,10).
In traditional Chinese medicine (TCM) theory, herbs
such as Polygonatum odoratum, Pueraria lobata and
Astragalus membranaceus, are considered as treatments of
diabetes for 1,000s of years (11),
and demonstrate fewer side effects compared with conventional
medicine (12,13). In plants, various bioflavonoids have
been reported to mitigate hyperglycemia or diabetes (14-17).
Hesperidin is a flavanone glycoside that abundantly exists in lemon
and sweet orange (18). Previous
studies have revealed anti-hyperglycemic and anti-hyperlipidemic
effects of hesperidin in diabetic rats, but the molecular mechanism
remains unknown (19-22).
The present study aimed to investigate the potential preventive
effect of hesperidin against T2DM using a rat model of alloxan and
high fat diet (HFD)-induced insulin resistance. Furthermore, the
current study examined the underlying molecular mechanism via which
hesperidin improved glucose metabolism by activating the IR/PDK1
pathway.
Materials and methods
Reagents
Hesperidin was purchased from Sigma-Aldrich (Merck
KGaA), and was suspended in 0.5% sodium carboxy methyl cellulose
(CMC-Na) for animal study or DMSO for cell-based assay.
Glucose uptake assay
Healthy rat subcutaneous adipocytes were isolated as
previously described (23) and
cultured in DMEM (Gibco; Thermo Fisher Scientific, Inc.) with 10%
FBS (Gibco; Thermo Fisher Scientific, Inc.) at 37˚C in a humidified
5% CO2 atmosphere. The cells were treated with 3, 10, 30
or 100 µg/ml hesperidin at 37˚C and subsequently incubated
without-serum at 37˚C overnight. Following the treatment, 1 nM
insulin (Sigma-Aldrich; Merck KGaA) and a cocktail containing
2-deoxyglucose and 3H-2-deoxyglucose were applied to the
cells and incubated at 37˚C for 2 h. Cytochalasin B (10 μM;
Sigma-Aldrich; Merck KGaA) served as non-specific 2-deoxyglucose
uptake to account for non-insulin induced glucose uptake under the
same conditions. Cells were then washed and lysed. Glucose uptake
was measured using Tri-Carb Liquid Scintillation Counter
(PerkinElmer, Inc.) as counts per well and calculated by setting 0%
as the effect of 1 nM insulin (negative control) and 100% as the
effect of 100 nM insulin (positive control).
Animals and insulin resistance
model
A total of 24 male Sprague Dawley rats (weight,
150-200 g; age, 6-7 weeks) were purchased from Vital River
Laboratories, Co., Ltd., and housed in a temperature and
humidity-controlled room (22-23˚C; 45-65%; 12 h light/dark cycle)
with free access to food and water. Rats were administered either a
HFD or a standard rat chow (control diet; Vital River Laboratories
Co., Ltd.). All animals received humane care, and all experimental
protocols were approved by Institutional Animal Care and Use
Committee of Heilongjiang University of Chinese Medicine.
The insulin resistance model was induced following
the protocol approved by China Food and Drug Administration
(24). After 1-week acclimation
with the control diet, the rats were divided into three groups (n=8
per group), including naïve group, model group and hesperidin
group. The naïve and model groups were orally administered with
vehicle (CMC-Na; 5 ml/kg) for 35 days, while the hesperidin group
was treated with 100 mg/kg hesperidin for the same period. After
being fed with control diet for 1 week, both model and hesperidin
groups were given HFD for another 3 weeks. Then, they were fasted
for 24 h with free access to water prior to intraperitoneal
injection of alloxan (103-105 mg/kg; Sigma-Aldrich; Merck KGaA).
HFD was given for another 3-5 days after the injection. At the end
of the model establishment, an oral glucose tolerance test (OGTT)
was performed before the animals were sacrificed via CO2
euthanasia. Samples of blood (1 ml), liver and epididymal adipose
tissue were collected for further analysis.
OGTT
After fasting for 3-4 h, the rats were administered
2.5 g/kg glucose via oral gavage. Then, drop blood (15 µl) was
sampled via the tail vein at 0, 15, 30, 60, 90 and 120 min
post-glucose challenge, after which blood glucose was determined
using a glucometer (ACCU-CHEK; Roche Diabetes Care, Inc.). The area
under curve (AUC) was calculated using GraphPad Prism 6.0 (GraphPad
Software, Inc.).
Biochemistry analysis
The serum levels of glucose (cat. no. F006-1-1),
insulin (cat. no. H203), triglyceride (TG; cat. no. F001-1-1),
total cholesterol (TC; cat. no. F002-1-1) and free fatty acid (FFA;
cat. no. A042-2-1) were determined with commercially available kits
from Nanjing Jiancheng Bioengineering Institute, according to the
manufacturer's instructions.
Hepatic enzyme activity assay
Glucokinase activity was determined as described by
Davidson and Arion (25). Liver
samples were homogenized in buffer containing 50 mmol/l Tris-HCl
(pH 7.4), 100 mmol/l KCl, 10 mmol/l mercaptoethanol and 1 mmol/l
EDTA (Sigma-Aldrich; Merck KGaA). Homogenates were centrifuged at
100,000 x g at 4˚C for 1 h before the post-microsomal supernatant
was used for the spectrophotometric continuous assay (Thermo Fisher
Scientific, Inc.), in which the formation of glucose-6-phosphate
from glucose at 27˚C for 1 h was coupled to its oxidation by
glucose-6-phosphate dehydrogenase and NAD. Glucose-6-phosphatase
activity was determined in the hepatic microsome using a
spectrophotometric assay developed by Alegre et al (26). The reaction mixture contained 100
mmol/l sodium HEPES, 26.5 mmol/l glucose-6-phospate, 1.8 mmol/l
EDTA, 2 mmol/l NADP+, 0.6 kIU/l mutarotase and 6 kIU/l
glucose dehydrogenase. Phosphoenolpyruvate carboxykinase activity
was measured using the spectrophotometric assay developed by Bentle
and Lardy (27). The reaction
mixture contained 50 mmol/l sodium HEPES, 1 mmol/l IDP, 1 mmol/l
MnCl2, 1 mmol/l dithiothreitol, 0.25 mmol/l NADH, 2
mmol/l phosphoenolpyruvate, 50 mmol/l NaHCO3 and 7.2 U
malic dehydrogenase. The enzyme activity was measured at 25˚C for 1
h, based on a decrease in the absorbance at 340 nm using a
microplate reader (Thermo Fisher Scientific, Inc.).
Western blotting
Protein extracted from adipose tissue using RIPA
lysis buffer (Beyotime Institute of Biotechnology) for 30 min, and
then boiled with 5X loading buffer (Beyotime Institute of
Biotechnology). Total protein was quantified using a bicinchoninic
acid assay and 50 µg protein was separated via 12% SDS-PAGE. The
separated proteins were transferred to PVDF membranes before being
blocked with 5% non-fat dry milk in TBT-0.1% Tween-20 for 1 h at
room temperature. The PVDF membrane was then incubated with primary
antibodies of IR (cat. no. 23413; 1:1,000), phosphorylated (p)-IR
(cat. no. 2969; 1:1,000), PDK1 (cat. no. 13037; 1:1,000,) p-PDK1
(cat. no. 3438; 1:1,000) or GAPDH (cat. no. 5174; 1:1,000; each,
Cell Signaling Technology.) overnight at 4˚C. Horseradish
peroxidase (HRP)-conjugated anti-mouse IgG (cat. no. 7076; 1:2,000)
and HRP-conjugated anti-rabbit IgG antibodies (cat. no. 7074;
1:2,000; Cell Signaling Technology, Inc.) were then added and
incubated for 2 h at room temperature, after which the horseradish
peroxidase-conjugated protein was detected using a chemiluminescent
horseradish peroxidase substrate solution (EMD Millipore). The
specificity of the p-IR antibody was Tyr1150/1151, and the
specificity of the p-PDK1 antibody was Ser241. The expression of
protein was quantified using Image-Pro Plus software (version 6.0;
Media Cybernetics, Inc.).
Statistical analysis
Quantitative data are presented as the mean ± SD,
and were analyzed using GraphPad Prism 6.0 (GraphPad Software,
Inc.). Differences between groups were analyzed using a two-way
repeated measures ANOVA for OGTT, or one-way ANOVA with Tukey's or
Dunnett's multiple comparison test for other studies. P<0.05 was
considered to indicate a statistically significant difference.
Experiments were repeated ≥3 times.
Results
Hesperidin prevents hyperglycemia in
diabetic rats without changing insulin level
In order to examine the preventive effect of
hesperidin on diabetes, a rat model of insulin resistance was
induced using alloxan and HFD, which can mimic the natural progress
of diabetes. After model induction, compared with the naïve group,
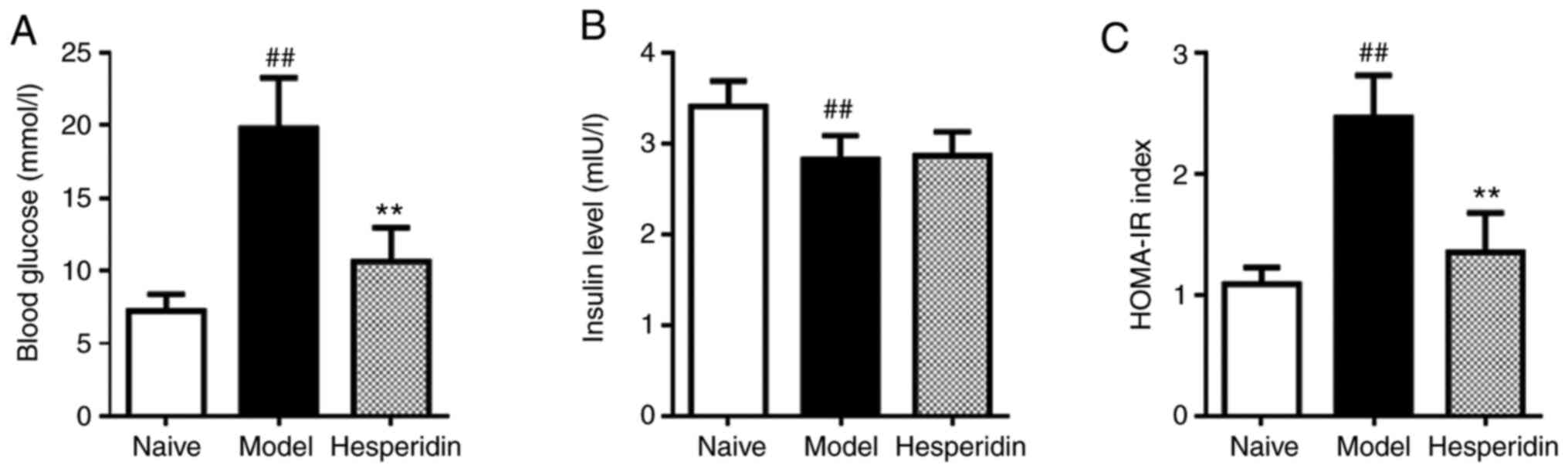
the level of fasting blood glucose was significantly increased from
7.2 to 19.8 mmol/l (Fig. 1A), while
the insulin level was decreased from 3.4 to 2.8 mIU/l (Fig. 1B), causing the insulin resistance
index to almost double (Fig. 1C).
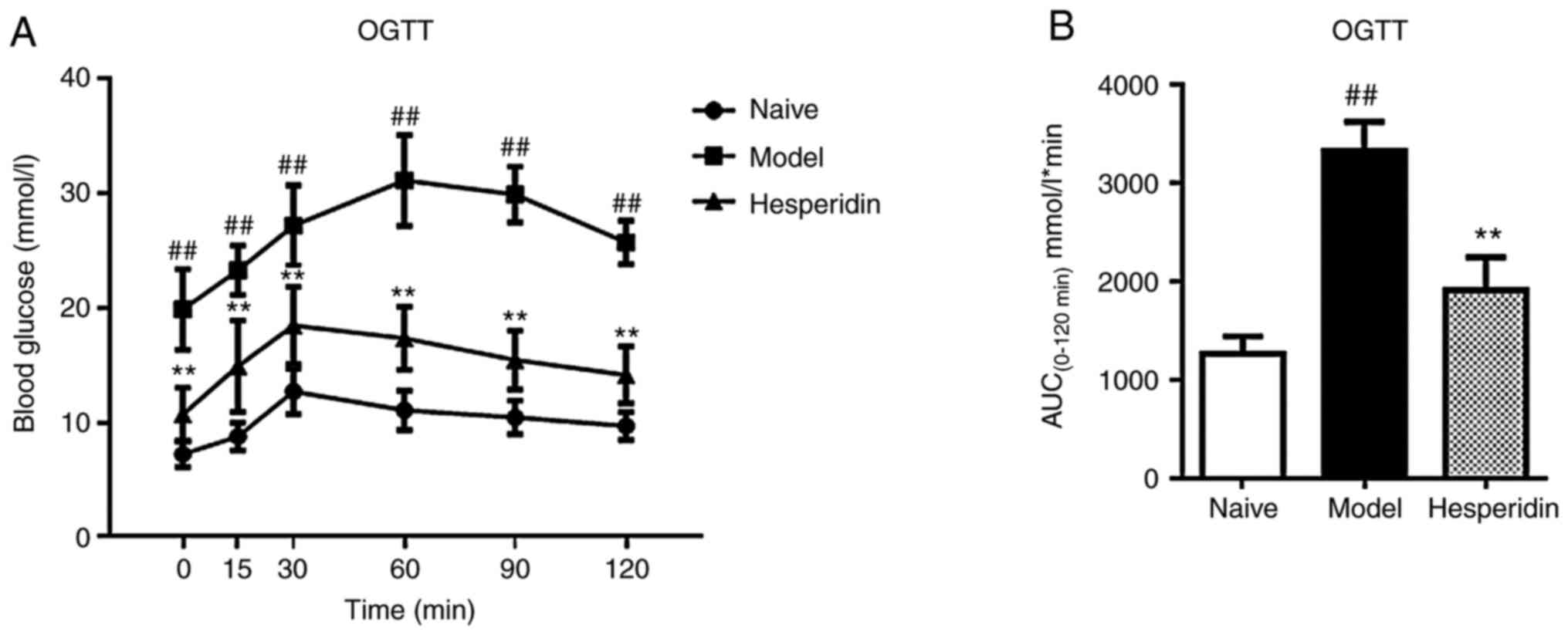
In addition, OGTT demonstrated that the levels of blood glucose at
different time points after glucose challenge were significantly
increased compared with the naïve group (Fig. 2A). The AUC was increased by 160%
compared with naïve group (Fig.
2B).
In diabetic rats, 100 mg/kg hesperidin significantly
reduced blood glucose level from 19.8 to 10.6 mmol/l compared with
the model group (Fig. 1A).
Furthermore, compared with the model group, blood glucose levels at
different time points after glucose challenge were significantly
reduced, with the AUC value decreasing by 42% (Fig. 2), but blood insulin levels remained
similar (Fig. 1B). These effects
led to a decrease in the insulin resistance index in the hesperidin
group compared with the model group (Fig. 1C), suggesting that hesperidin may
alleviate hyperglycemia by improving insulin sensitivity.
Therefore, the results indicated that hesperidin prevented
hyperglycemia after 5-week-treatment in diabetic rats, suggesting
possible diabetes prevention using a natural product.
Hesperidin has no significant effect
on lipid metabolism
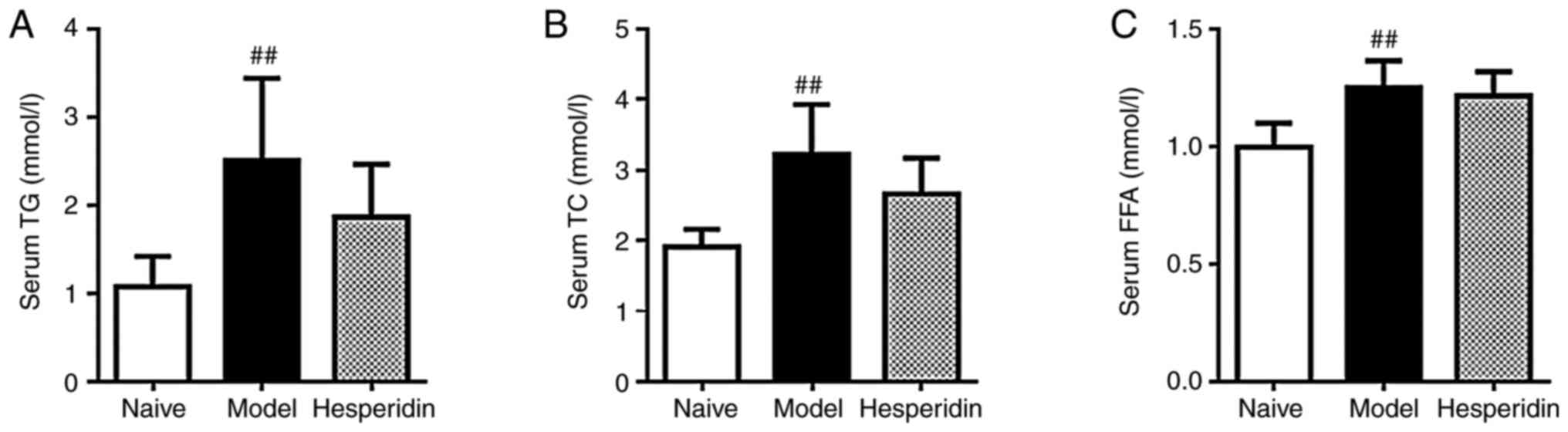
The levels of TG, TC and FFA were significantly
elevated in the model group compared with the naïve group,
indicating dysfunctional lipid metabolism in this model (Fig. 3). Hesperidin treatment did not alter
TG, TC or FFA levels compared with the model group, indicating that
hesperidin had limited efficacy in improving lipid metabolism
(Fig. 3).
Role of hesperidin on glucose
regulating enzymes
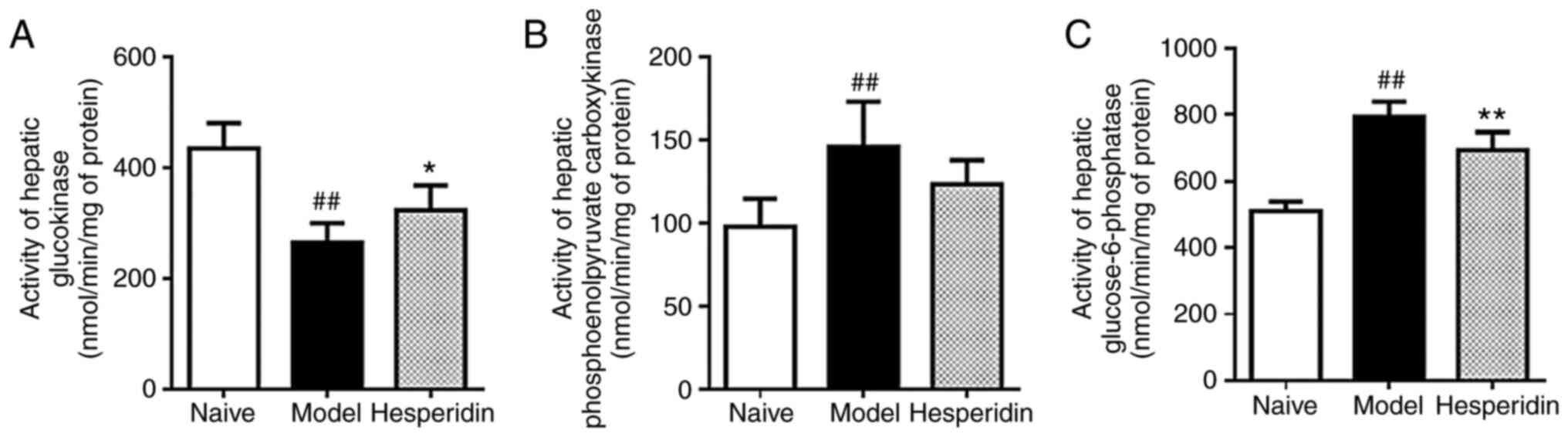
In the liver, significant glucokinase activity,
glucose-6-phosphatase and phosphoenolpyruvate carboxykinase were
identified between the naïve group and the model group (Fig. 4). Hesperidin induced glucokinase
activity, but decreased the activity of glucose-6-phosphatase and
phosphoenolpyruvate carboxykinase compared with the model group
(Fig. 4), which are vital hepatic
glucose regulating enzymes involved in glycolysis and
gluconeogenesis (28).
Hesperidin improves insulin
sensitivity by activating the insulin receptor pathway
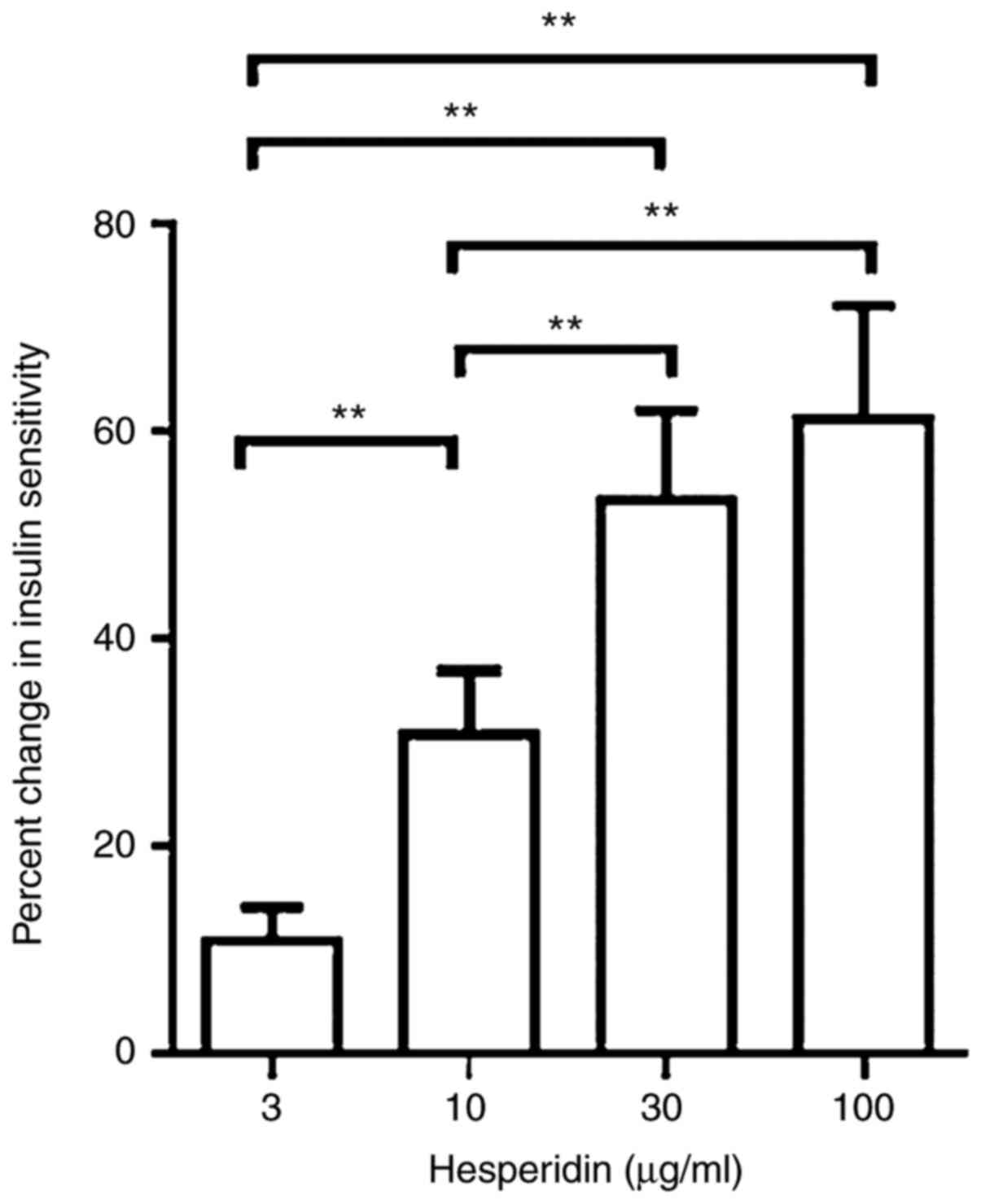
In the glucose uptake assay, a significant
dose-dependent effect was observed, in which 3, 10, 30 and 100
µg/ml hesperidin increased insulin sensitivity by 11, 31, 54 and
61%, respectively, compared with the negative control (Fig. 5).
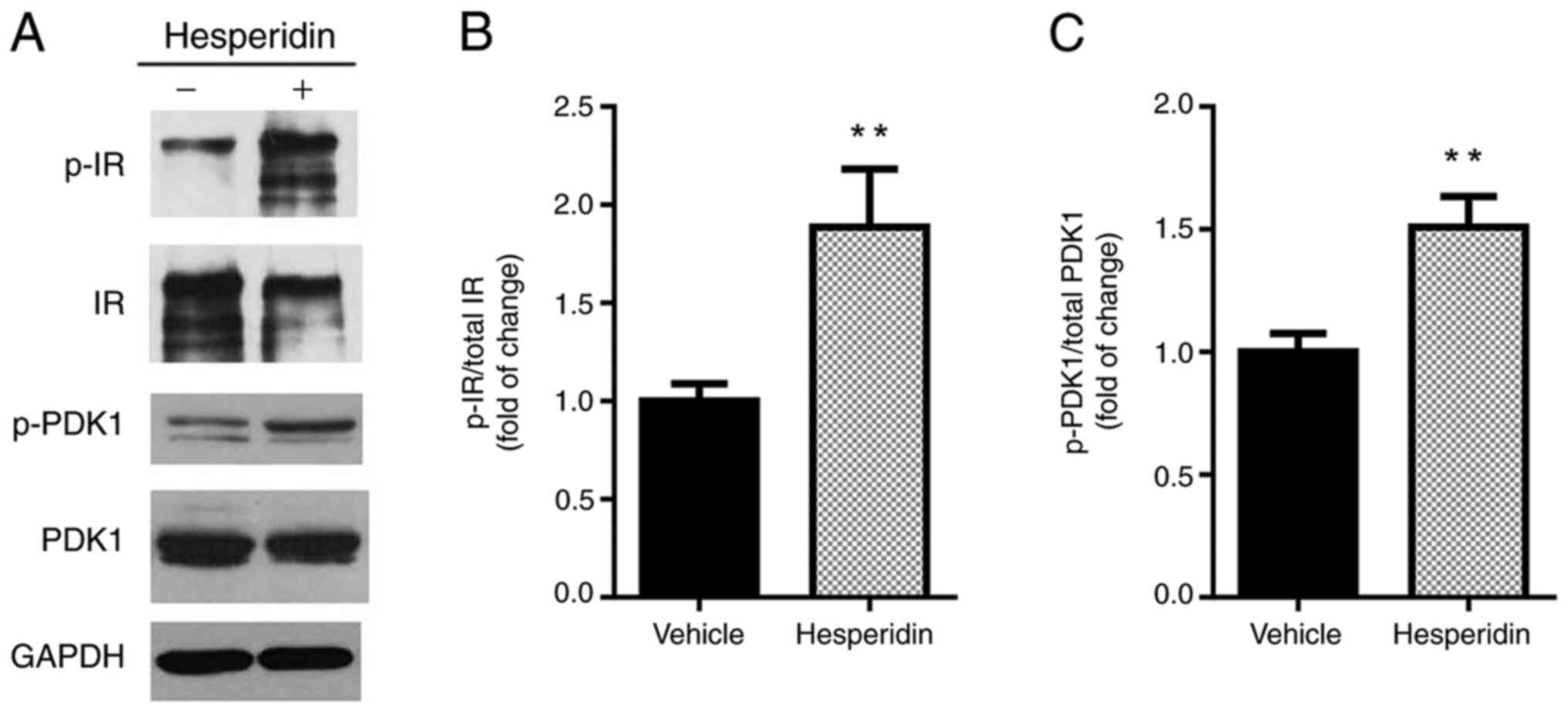
Western blot analysis was conducted to investigate
the underlying molecular mechanism via which hesperidin regulated
insulin sensitivity. The total expression levels of IR and PDK1
were not changed after hesperidin treatment, but the
phosphorylation of these proteins was significantly increased by 89
and 51%, respectively (Fig. 6).
Collectively, these findings suggested that hesperidin may improve
insulin sensitivity by activating the IR/PDK1 pathway.
Discussion
Previous studies have reported antidiabetic effects
of hesperidin in HFD + streptozocin rats, Goto-Kakizaki rats and
C57BL/KsJ-db/db mice (19-22).
The present study established a diabetic rat model using HFD and
alloxan, which represents a closer mimic of the pathogenesis of
insulin resistance (24). Moreover,
this model is well-established and recommended by the China Food
and Drug Administration to evaluate the efficacies of TCM (29,30).
Homeostatic model assessment (HOMA) is a widely reported method to
quantify insulin resistance (31).
The cut-off values in the current study of HOMA-insulin resistance
may be utilized for identifying insulin resistance, indicating the
clinical and epidemiological importance (32). In addition, according to the
Experimental Methodology of Pharmacology (4th edition), rats with
fasting blood glucose level of >16.7 mmol/l are considered as
diabetic rats (33). The present
study successfully induced insulin resistance in the model with an
increased fasting blood glucose level (19.8 mmol/l) and
HOMA-insulin resistance (2.5) in the model group. It was identified
that hesperidin treatment significantly improved fasting blood
glucose and oral glucose tolerance, but had limited effects on TG,
TC and FFA levels, suggesting its antidiabetic functionality was
exerted exclusively by regulating glucose metabolism.
Hyperglycemia may be attributed to decreased hepatic
glycogen synthesis and increased hepatic glucose production, which
may be the result of decreased glucokinase activities and increased
glucose-6-phosphatase and phosphoenolpyruvate carboxykinase
activities in a diabetic state (34,35).
Hepatic glucokinase can be the most sensitive indicator of the
glycolytic pathway in diabetes and its increase can accelerate the
utilization of blood glucose for glycogen storage in the liver
(36). Glucose-6-phosphatase and
phosphoenolpyruvate carboxykinase are two critical enzymes in the
metabolic pathway of gluconeogenesis to release glucose by the
liver (37-39).
In the current study, hesperidin induced glucokinase activity,
while decreased the activities of glucose-6-phosphatase and
phosphoenolpyruvate carboxykinase in liver to maintain glucose
homeostasis.
If not transported across the cell membrane for
further utilization, blood glucose will undergo glycolysis or
aerobic oxidation in the circulating system (40). A commonly used approach to treat
diabetes is to enhance glucose uptake (41). Hesperidin and naringin are
glycosides of hesperitin and naringenin, sharing similar flavanone
structures (18). Dhanya et
al (42) observed a 2-fold
increase in the uptake of fluorescent labeled glucose after
naringin treatment in differentiated L6 myoblast, while Zygmunt
et al (43) reported that
naringenin stimulated glucose uptake in L6 myotubes in a dose- and
time-dependent manner. The present study identified increased
glucose uptake induced by 3-100 µg/ml hesperidin in rat
subcutaneous adipocytes. However, in contrast, Yang et al
(44) revealed that hesperetin
decreased IR-phosphorylation and impaired glucose uptake in human
breast cancer cells. Therefore, these flavanones and their
glycosides may have complicated effects in different tissues or
cells.
The IR signal pathway serves a key role in the
regulation of glucose homeostasis (9,10).
Molecular docking assays have been used to investigate the
interaction between IR tyrosine kinase with individual flavonoids,
and based on Autodock binding energies it was hypothesized that
flavonones, such as hesperitin and naringenin, are potent
activators of IR tyrosine kinase (45). However, there is lacking evidence
from in vivo studies to support this hypothesis. The present
study identified significantly increased phosphorylation of IR in
adipose tissues after hesperidin treatment, as well as the enhanced
phosphorylation of PDK1, which is a critical kinase responsible to
transduce the signal from IR to AKT (46). Therefore, these findings suggest
that hesperidin may prevent hyperglycemia and diabetes by
activating the IR/PDK1 pathway.
In conclusion, the present study demonstrated the
potent preventive effect of hesperidin using a rat model of alloxan
and HFD-induced insulin resistance, as well as identified the
underlying mechanism via which hesperidin alleviated hyperglycemia
by activating the IR/PDK1 signaling pathway. Considering the
increasing diabetic population and the fewer side effects of
natural products, hesperidin administration may be an effective
strategy for preventing diabetes and alleviating hyperglycemia.
Acknowledgements
Not applicable.
Funding
This work was supported by the General Program of
National Natural Science Foundation of China (grant no. 81573935),
Scientific and Technological Projects of Educational Bureau of
Heilongjiang Province (grant no. 12531604) and Research and
Development Program of Application Technology of Harbin (Out
Standing Academic Leader; Category B; grant no. 2015RAXYJ053).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
LL and DZ designed the study. PP, JJ and GZ acquired
the data. PP, YS and YH analyzed the data. PP and DZ drafted the
manuscript. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The present study and all animal experiments were
approved by the Ethics Committee Institutional Animal Care and Use
Committee of Heilongjiang University of Chinese Medicine.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Elizabeth MM, Alarcon-Aguilar J F, Clara
OC, Escobar-Villanueva and Carmen M: Pancreatic β-cells and
type 2 diabetes development. Curr Diabetes Rev. 13:108–121.
2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
American Diabetes Association. 2.
Classification and diagnosis of diabetes. Diabetes Care. 40 (Suppl
1):S11–S24. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Xu Y, Wang L, He J, Bi Y, Li M, Wang T,
Wang L, Jiang Y, Dai M, Lu J, et al: 2010 China Noncommunicable
Disease Surveillance Group: Prevalence and control of diabetes in
Chinese adults. JAMA. 310:948–959. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yang SH, Dou KF and Song WJ: Prevalence of
diabetes among men and women in China. N Engl J Med. 362:2425–2426;
author reply 2426. 2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wang L, Gao P, Zhang M, Huang Z, Zhang D,
Deng Q, Li Y, Zhao Z, Qin X, Jin D, et al: Prevalence and ethnic
pattern of diabetes and prediabetes in China in 2013. JAMA.
317:2515–2523. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yang L, Shao J, Bian Y, Wu H, Shi L, Zeng
L, Li W and Dong J: Prevalence of type 2 diabetes mellitus among
inland residents in China (2000-2014): A meta-analysis. J Diabetes
Investig. 7:845–852. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yamauchi T, Kamon J, Waki H, Terauchi Y,
Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N,
et al: The fat-derived hormone adiponectin reverses insulin
resistance associated with both lipoatrophy and obesity. Nat Med.
7:941–946. 2001.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
DeFronzo RA and Ferrannini E: Insulin
resistance A multifaceted syndrome responsible for NIDDM, obesity,
hypertension, dyslipidemia, and atherosclerotic cardiovascular
disease. Diabetes Care. 14:173–194. 1991.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Leto D and Saltiel AR: Regulation of
glucose transport by insulin: Traffic control of GLUT4. Nat Rev Mol
Cell Biol. 13:383–396. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
10
|
Pollak M: The insulin and insulin-like
growth factor receptor family in neoplasia: An update. Nat Rev
Cancer. 12:159–169. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Zhang H, Tan C, Wang H, Xue S and Wang M:
Study on the history of Traditional Chinese Medicine to treat
diabetes. Eur J Integr Med. 2:41–46. 2010.
|
|
12
|
Calixto JB: Efficacy, safety, quality
control, marketing and regulatory guidelines for herbal medicines
(phytotherapeutic agents). Braz J Med Biol Res. 33:179–189.
2000.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Valli G and Giardina EG: Benefits, adverse
effects and drug interactions of herbal therapies with
cardiovascular effects. J Am Coll Cardiol. 39:1083–1095.
2002.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ong KC and Khoo HE: Insulinomimetic
effects of myricetin on lipogenesis and glucose transport in rat
adipocytes but not glucose transport translocation. Biochem
Pharmacol. 51:423–429. 1996.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hsu FL, Liu IM, Kuo DH, Chen WC, Su HC and
Cheng JT: Antihyperglycemic effect of puerarin in
streptozotocin-induced diabetic rats. J Nat Prod. 66:788–792.
2003.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Choi JS, Yokozawa T and Oura H:
Improvement of hyperglycemia and hyperlipemia in
streptozotocin-diabetic rats by a methanolic extract of Prunus
davidiana stems and its main component, prunin. Planta Med.
57:208–211. 1991.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Shisheva A and Shechter Y: Quercetin
selectively inhibits insulin receptor function in vitro and the
bioresponses of insulin and insulinomimetic agents in rat
adipocytes. Biochemistry. 31:8059–8063. 1992.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Garg A, Garg S, Zaneveld LJ and Singla AK:
Chemistry and pharmacology of the Citrus bioflavonoid hesperidin.
Phytother Res. 15:655–669. 2001.PubMed/NCBI View
Article : Google Scholar
|
|
19
|
Jung UJ, Lee MK, Jeong KS and Choi MS: The
hypoglycemic effects of hesperidin and naringin are partly mediated
by hepatic glucose-regulating enzymes in C57BL/KsJ-db/db mice. J
Nutr. 134:2499–2503. 2004.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Akiyama S, Katsumata S, Suzuki K, Nakaya
Y, Ishimi Y and Uehara M: Hypoglycemic and hypolipidemic effects of
hesperidin and cyclodextrin-clathrated hesperetin in Goto-Kakizaki
rats with type 2 diabetes. Biosci Biotechnol Biochem. 73:2779–2782.
2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ahmed OM, Mahmoud AM, Abdelmoneim A and
Ashour MB: Antidiabetic effects of hesperidin and naringin in type
2 diabetic rats. Diabetol Croat. 41:53–67. 2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Shi X, Liao S, Mi H, Guo C, Qi D, Li F,
Zhang C and Yang Z: Hesperidin prevents retinal and plasma
abnormalities in streptozotocin-induced diabetic rats. Molecules.
17:12868–12881. 2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wu X, Motoshima H, Mahadev K, Stalker TJ,
Scalia R and Goldstein BJ: Involvement of AMP-activated protein
kinase in glucose uptake stimulated by the globular domain of
adiponectin in primary rat adipocytes. Diabetes. 52:1355–1363.
2003.PubMed/NCBI View Article : Google Scholar
|
|
24
|
China Food and Drug Administration (CFDA):
Technical standards for testing and assessment of health food.
CFDA, 2003.
|
|
25
|
Davidson AL and Arion WJ: Factors
underlying significant underestimations of glucokinase activity in
crude liver extracts: Physiological implications of higher cellular
activity. Arch Biochem Biophys. 253:156–167. 1987.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Alegre M, Ciudad CJ, Fillat C and
Guinovart JJ: Determination of glucose-6-phosphatase activity using
the glucose dehydrogenase-coupled reaction. Anal Biochem.
173:185–189. 1988.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Bentle LA and Lardy HA: Interaction of
anions and divalent metal ions with phosphoenolpyruvate
carboxykinase. J Biol Chem. 251:2916–2921. 1976.PubMed/NCBI
|
|
28
|
Nordlie RC and Foster JD: A retrospective
review of the roles of multifunctional glucose-6-phosphatase in
blood glucose homeostasis: Genesis of the tuning/retuning
hypothesis. Life Sci. 87:339–349. 2010.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Liu Y, Li X, Xie C, Luo X, Bao Y, Wu B, Hu
Y, Zhong Z, Liu C and Li M: Prevention effects and possible
molecular mechanism of mulberry leaf extract and its formulation on
rats with insulin-insensitivity. PLoS One.
11(e0152728)2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zhang Y, Dong H, Wang M and Zhang J:
Quercetin isolated from Toona sinensis leaves attenuates
hyperglycemia and protects hepatocytes in
high-carbohydrate/high-fat diet and alloxan induced experimental
diabetic mice. J Diabetes Res. 2016(8492780)2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wallace TM, Levy JC and Matthews DR: Use
and abuse of HOMA modeling. Diabetes Care. 27:1487–1495.
2004.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Geloneze B, Vasques AC, Stabe CF, Pareja
JC, Rosado LE, Queiroz EC and Tambascia MA: BRAMS Investigators.
HOMA1-IR and HOMA2-IR indexes in identifying insulin resistance and
metabolic syndrome: Brazilian Metabolic Syndrome Study (BRAMS). Arq
Bras Endocrinol Metabol. 53:281–287. 2009.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wei W and Wu XM: Li: Experimental
Methodology of Pharmacology. 4th edition. People's Medical
Publishing House, Beijing, 2010 (In Chinese).
|
|
34
|
DeFronzo RA: Lilly lecture 1987. The
triumvirate: Beta-cell, muscle, liver. A collusion responsible for
NIDDM. Diabetes. 37:667–687. 1988.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Reaven GM: Pathophysiology of insulin
resistance in human disease. Physiol Rev. 75:473–486.
1995.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Iynedjian PB, Gjinovci A and Renold AE:
Stimulation by insulin of glucokinase gene transcription in liver
of diabetic rats. J Biol Chem. 263:740–744. 1988.PubMed/NCBI
|
|
37
|
Massillon D, Chen W, Barzilai N,
Prus-Wertheimer D, Hawkins M, Liu R, Taub R and Rossetti L: Carbon
flux via the pentose phosphate pathway regulates the hepatic
expression of the glucose-6-phosphatase and phosphoenolpyruvate
carboxykinase genes in conscious rats. J Biol Chem. 273:228–234.
1998.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Lochhead PA, Salt IP, Walker KS, Hardie DG
and Sutherland C: 5-aminoimidazole-4-carboxamide riboside mimics
the effects of insulin on the expression of the 2 key gluconeogenic
genes PEPCK and glucose-6-phosphatase. Diabetes. 49:896–903.
2000.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Al-Quraishy S, Dkhil MA and Abdel Moneim
AE: Anti-hyperglycemic activity of selenium nanoparticles in
streptozotocin-induced diabetic rats. Int J Nanomedicine.
10:6741–6756. 2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Govers R: Cellular regulation of glucose
uptake by glucose transporter GLUT4. Adv Clin Chem. 66:173–240.
2014.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Natali A and Ferrannini E: Effects of
metformin and thiazolidinediones on suppression of hepatic glucose
production and stimulation of glucose uptake in type 2 diabetes: A
systematic review. Diabetologia. 49:434–441. 2006.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Dhanya R, Arun KB, Nisha VM, Syama HP,
Nisha P, Santhosh Kumar TR and Jayamurthy P: Preconditioning L6
muscle cells with naringin ameliorates oxidative stress and
increases glucose uptake. PLoS One. 10(e0132429)2015.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Zygmunt K, Faubert B, MacNeil J and Tsiani
E: Naringenin, a citrus flavonoid, increases muscle cell glucose
uptake via AMPK. Biochem Biophys Res Commun. 398:178–183.
2010.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Yang Y, Wolfram J, Boom K, Fang X, Shen H
and Ferrari M: Hesperetin impairs glucose uptake and inhibits
proliferation of breast cancer cells. Cell Biochem Funct.
31:374–379. 2013.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Ganugapati J, Baldwa A and Lalani S:
Molecular docking studies of banana flower flavonoids as insulin
receptor tyrosine kinase activators as a cure for diabetes
mellitus. Bioinformation. 8:216–220. 2012.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Cohen P, Alessi DR and Cross DAE: PDK1,
one of the missing links in insulin signal transduction? FEBS Lett.
410:3–10. 1997.PubMed/NCBI View Article : Google Scholar
|