Introduction
Antiphospholipid syndrome (APS) is an autoimmune
disease associated with arterial or venous thrombosis, recurrent
fetal abortion or thrombocytopenia (1). The development of APS involves the
production of antiphospholipid antibodies, such as anticardiolipin
antibodies (ACL), lupus anticoagulant (LA) and anti-β2 glycoprotein
I antibodies (2,3). The major pathological changes seen in
APS caused by antibodies include villus microtubule embolism,
placental infarction and fetal arterial embolism, which can result
in embryo abortion and stillbirth (4,5).
Termination of pregnancy at <28 weeks or fetal weight <1 kg
is referred to as abortion and recurrent spontaneous abortion (RSA)
is defined as continuous spontaneous abortion occurring more than
twice (6). RSA is a common
phenomenon with an incidence rate of 1-3% (7). Risk factors for RSA range from
genetic, hormonal or metabolic factors, to uterine anatomy,
autoimmune dysfunction, thrombosis tendency and infection (8). APS accounts for 7-25% of RSA and the
rate of miscarriage can reach 90% in RSA without treatment
(9-13).
In China, the positive rates of ACL and LA were 2.2
and 0.07-0.27%, while those with RSA history were 4.08 and 5.71%
(2013), respectively, and in Western countries (USA, 1995; UK,
1996; Switzerland, 1987; Italy, 1997; Spain, 1994; The Netherlands,
1996), RSA pregnant women have ACL positive rates of 5-51%
(14,15). For the past 30 years, several
treatment options have been available, such as aspirin, heparin,
plasma exchange, glucocorticoid, immunoglobulin and other combined
or single applications for ACL (16,17),
but the research conclusions about optimal treatment still remains
controversial. At present, heparin combined with aspirin is a
widely used treatment (18,19). The aim of the present study was to
perform a meta-analysis to elucidate the effects of aspirin and
heparin in the treatment of RSA in women with APS and to provide a
basis for informed clinical treatment.
Materials and methods
Search strategy
Previous relevant studies published before May 2019
on the use of aspirin and heparin in the treatment of RSA in women
with APS were obtained from the Cochrane (www.cochranelibrary.com), Pubmed (pubmed.ncbi.nlm.nih.gov), Embase (www.embase.com), CNKI (www.cnki.net),
VANFUN (www.wanfangdata.com.cn/index.html) and VIP (www.cqvip.com) databases. The references of all
identified articles were also reviewed to obtain additional
studies. Search terms were as follows: ‘Antiplatelet’,
‘anticoagulants’, ‘lupus’, ‘antiphospholipid syndrome’,
‘antiphospholipid antibody’, ‘recurrent miscarriage’, ‘recurrent
abortion’, ‘spontaneous fetal loss’, ‘abortion’, ‘habitual’,
‘habitual abortion’, ‘treatment’, ‘heparin’, ‘aspirin’,
‘randomized’, ‘randomized controlled trial’ and ‘RCT’. These terms
were used in combination with ‘AND’ or ‘OR’. The present
meta-analysis was performed independently by two investigators and
disagreements were resolved by a third investigator. The main
disagreement was whether one of the selected articles should be
included or excluded in the meta-analysis. When the disagreement
occurred, the three investigators read the article together and
determined whether to incorporate the study.
Following the Participants, Interventions,
Comparisons, Outcomes and Study design principle (20), the key search terms were: (P) female
with RSA associated with APS; (I) patients in treatment group
treated by aspirin and/or heparin; (C) placebo, aspirin and
prednisone, intravenous immunoglobulin, prednisone or aspirin
alone; (O) fertility outcome indexes, including live birth,
pre-term delivery, miscarriage, birth weight, vaginal delivery,
cesarean delivery, intrauterine death, gestational age at birth,
intrauterine growth restriction (IUGR), gestational diabetes,
thrombocytopenia and pre-eclampsia; and (S) randomized controlled
trial.
Study selection criteria
All included studies met the following criteria: i)
The study was a randomized controlled trial; ii) the research
subjects were women with RSA associated with APS; iii) the
treatment in the experimental group included aspirin and/or
heparin, while the treatment in the control group was not limited;
and iv) the study was written in English or Chinese. The exclusion
criteria were as follows: i) Repeated articles or results; ii)
clear data errors (including wrong index units, inconsistent data,
results exceeding maximum values); iii) case reports, case-control
studies, theoretical research, conference reports, systematic
reviews, meta-analyses, or other forms of research or comments,
which were not designed in a randomized controlled manner; and iv)
irrelevant outcomes.
Data extraction
For each included study, two categories of
information were extracted: Basic information and primary study
outcomes. Basic information relevant to the current meta-analysis
included authors’ names, year of publication, sample size,
patients’ age and sex, results of the treatment of experimental and
control groups, and Jadad score (21). Primary clinical outcomes relevant to
the analysis included: Live birth, pre-term delivery, miscarriage,
birth weight, vaginal delivery, cesarean delivery, intrauterine
death, gestational age at birth, IUGR, gestational diabetes,
thrombocytopenia and pre-eclampsia.
Quality assessment
Study quality was determined on the basis of Jadad
scores, which were assigned according to the following criteria: i)
Whether studies included a specific statement regarding
randomization; ii) whether the method used to randomize patients
was appropriate; iii) whether the study was conducted in a
double-blinded manner; iv) whether the approach to double-blinding
was described appropriately; and v) whether patient information was
complete. A Jadad score <3 was indicative of low quality and
therefore associated with a substantial risk of bias. Data
extraction was performed independently by two investigators and
disagreements were resolved by a third investigator. The main
disagreements included: data extraction of primary clinical
outcomes and the Jadad score of the included studies. When the data
or scores were not consistent, the three investigators read the
article together, analyzed and discussed data, and then extracted
the data again until a consensus was reached.
Statistical analysis
STATA software (version 10.0; StataCorp LP) was used
for all analyses. Heterogeneity in the study results was assessed
using χ2 and I2 tests and appropriate
analytical models (fixed effect or random effect) were determined
accordingly. P≤0.05 and an I2>50% indicated high
heterogeneity and a random effect model was used in this case.
P>0.05 and an I2≤50% indicated acceptable
heterogeneity and a fixed effect model was used instead. Results
for continuous variables are presented as the mean ± standard
deviation and were compared on the basis of weighted mean
difference (WMD), while categorical data are presented as
percentages and compared based on relative risk (RR) and odds
ratio. WMD and 95% CI were used to analyze the birth weight and
gestational age at birth, while RR and 95% CI were used to analyze
live birth, pre-term delivery, miscarriage, vaginal delivery,
cesarean delivery, intrauterine death, IUGR, gestational diabetes,
thrombocytopenia and pre-eclampsia. Pooled data (WMD or RR with 95%
CI) were analyzed to determine the effects of aspirin and heparin
treatment on RSA in women with APS. Publication bias was evaluated
using the funnel plot method. A symmetrical funnel plot that was
narrow at the top and wide at the bottom indicated no publication
bias in relation to the analyzed index. Begg's and Mazumdar's rank
test was to analyze the direct correlation between standardized
effect size and its variance. A value of 0 indicates that there is
no direct correlation between effect size and accuracy; while a
value higher than 0 indicates that there is a correlation, with an
increasing value signifying a stronger correlation. Begg's rank
correlation test and Egger's linear regression method were used to
determine the possible publication bias through visually inspecting
funnel plots. P<0.05 was considered statistically
significant.
Results
Overview of included studies
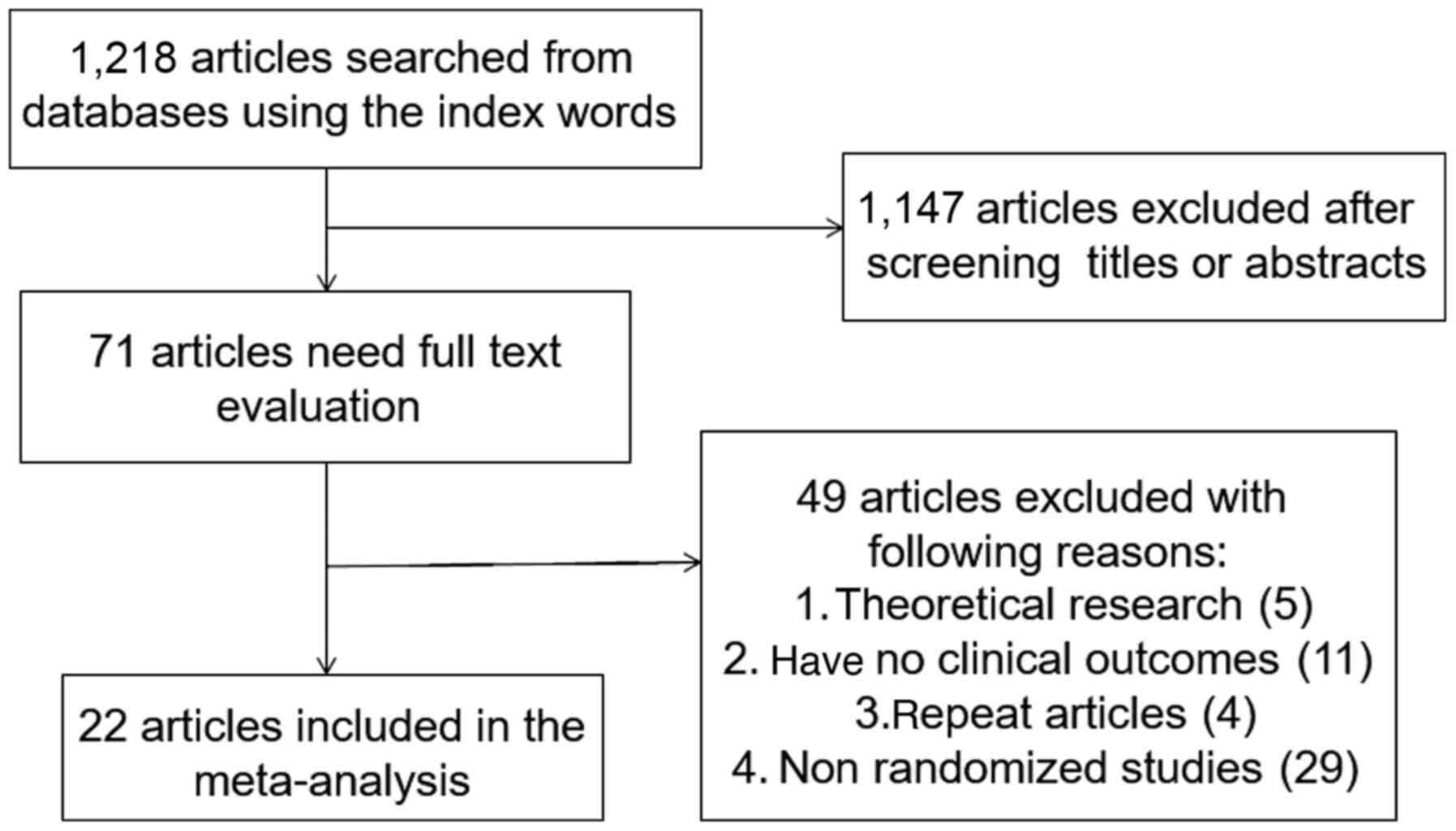
A total of 1,218 articles were identified, of which
1,147 were excluded by primary title and abstract review. The
remaining 71 articles were subject to a complete full-text
assessment, which further excluded 49 articles for failing to meet
study inclusion criteria and the exclusion reasons were as follows:
i) 5 articles with theoretical research; ii) 11 articles with no
clinical outcomes, iii) 4 repeated articles; and iv) 29
non-randomized trials. Thus, a total of 22 studies (22-43)
were ultimately identified that met the inclusion criteria for the
present meta-analysis. These 22 studies involved 1,515 patients in
the treatment group and 1,531 in the control group. The study
selection process is outlined in Fig.
1.
Table I summarizes
basic information for each study, including authors’ names, year of
publication, sample, age, sex, treatment methods and Jadad score.
The mean Jadad score for these selected studies was 3.39 (>3),
indicating that, on average, these were of high quality.
 | Table IBasic characteristics of the included
studies. |
Table I
Basic characteristics of the included
studies.
| | No. of
patients | Interventions | |
|---|
| Author, year | T | C | T | C | Jadad score | Refs. |
|---|
| Rai et al,
1997 | 45 | 45 | Heparin and
aspirin | Aspirin | 3 | (25) |
| Farquharson et
al, 2002 | 51 | 47 | Heparin and
aspirin | Aspirin | 5 | (26) |
| Triolo et
al, 2003 | 19 | 21 | Heparin and
aspirin | Intravenous
immunoglobulin | 3 | (23) |
| Goel et al,
2006 | 33 | 39 | Heparin and
aspirin | Aspirin | 4 | (27) |
| Dendrinos et
al, 2009 | 40 | 38 | Heparin and
aspirin | Intravenous
immunoglobulin | 3 | (28) |
| Ismail et
al, 2016 | 90 | 90 | Heparin and
aspirin | Placebo | 3 | (30) |
| Tulppala et
al, 1997 | 33 | 33 | Aspirin | Placebo | 5 | (32) |
| Pattison et
al, 2000 | 20 | 20 | Aspirin | Placebo | 3 | (22) |
| Cowchock et
al, 1992 | 12 | 8 | Heparin | Prednisone | 3 | (34) |
| Laskin et
al, 2009 | 45 | 43 | Heparin and
aspirin | Aspirin | 5 | (29) |
| Zhou et al,
2012 a | 30 | 31 | Heparin and
aspirin | Aspirin | 3 | (31) |
| Zhou et al,
2012 b | 30 | 30 | Heparin and
aspirin | Placebo | 3 | (31) |
| Zhang et al,
2015 | 27 | 27 | Heparin | Aspirin and
prednisone | 2 | (33) |
| Bu Mingxiu et
al, 2009 | 20 | 20 | Heparin | Prednisone | 2 | (36) |
| Jinhua et
al, 2003 | 24 | 24 | Heparin | Aspirin and
prednisone | 2 | (35) |
| Madani et
al, 2019 | 30 | 30 | Aspirin | Placebo | 4 | (37) |
| Blomqvist et
al, 2018 | 200 | 200 | Aspirin | Placebo | 4 | (38) |
| Bao et al,
2017 | 497 | 518 | Heparin and
aspirin | Aspirin | 4 | (39) |
| Maged et al,
2016 | 90 | 90 | Heparin and
aspirin | Placebo | 5 | (40) |
| Zhang et al,
2018 | 44 | 44 | Heparin and
aspirin | Aspirin | 3 | (41) |
| Zhaojuan et
al, 2018 | 28 | 28 | Heparin and
aspirin | Aspirin | 3 | (42) |
| Tang et al,
2012 | 44 | 42 | Heparin and
aspirin | Aspirin and
prednisone | 3 | (24) |
| Liang et al,
2015 | 63 | 63 | Heparin | Aspirin and
prednisone | 3 | (43) |
All the indexes were divided into the following
sub-groups for further analysis: Heparin and aspirin vs. aspirin
alone, heparin and aspirin vs. intravenous immunoglobulin, heparin
and aspirin vs. aspirin and prednisone, heparin and aspirin vs.
placebo, aspirin vs. placebo, heparin vs. prednisone and heparin
vs. aspirin and prednisone.
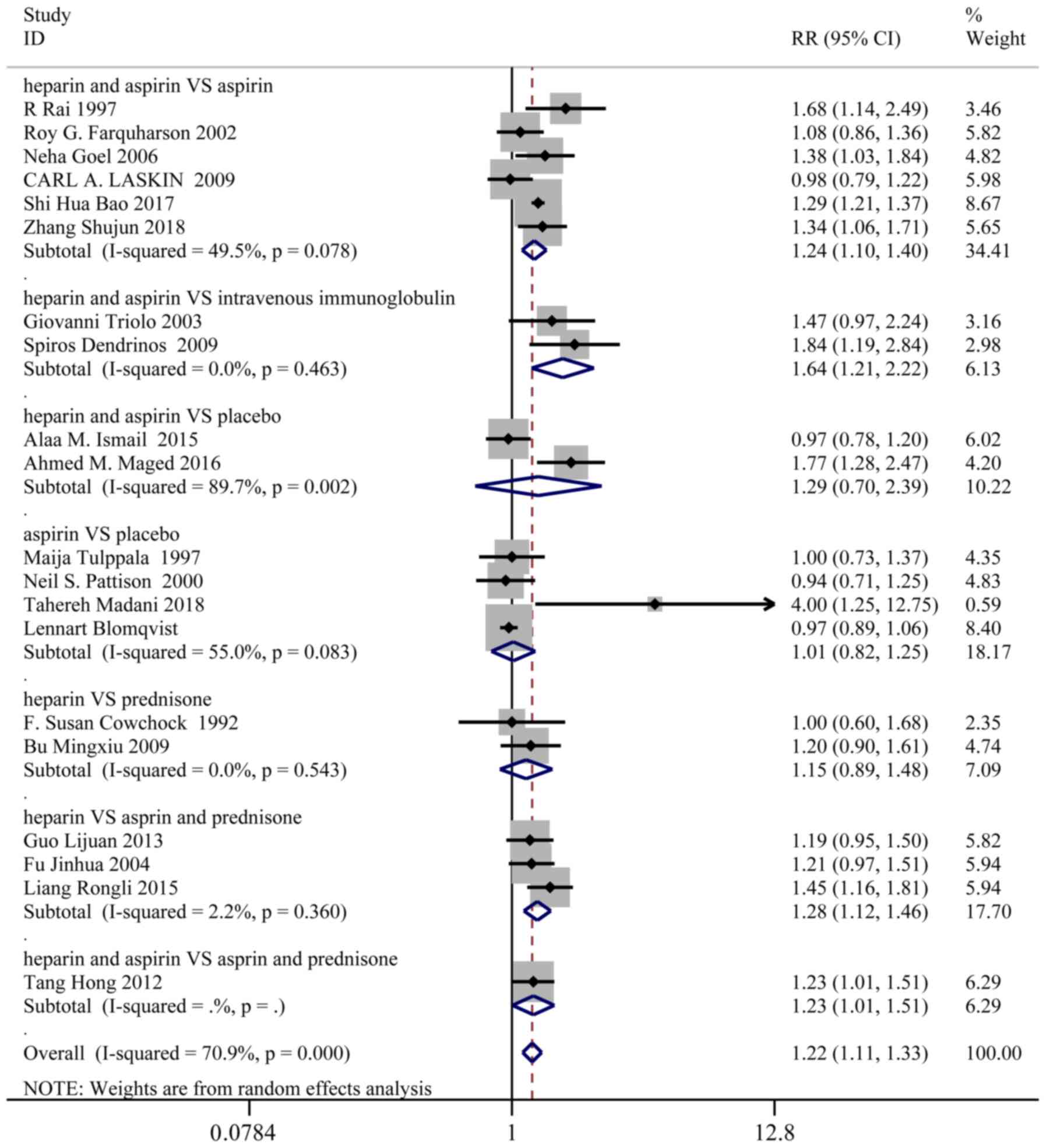
Live birth
A total of 20 studies, representing 1,427 patients
in the treatment group and 1,442 patients in the control group,
reported the live birth rates. Based on a χ2 P<0.0001
and an I2=70.9%, a random effect model was used to
assess live birth. Overall, the live birth rate was significantly
higher in the treatment group than the control group (RR: 1.22; 95%
CI: 1.11-1.33). In the sub-group analysis, live birth was markedly
improved in the following groups (Fig.
2): Heparin and aspirin vs. aspirin (RR: 1.24; 95% CI:
1.10-1.40), heparin and aspirin vs. intravenous immunoglobulin (RR:
1.64; 95% CI: 1.21-2.22), heparin and aspirin vs. aspirin and
prednisone (RR: 1.23; 95% CI: 1.01-1.51), heparin vs. aspirin and
prednisone (RR: 1.28; 95% CI: 1.12-1.46).
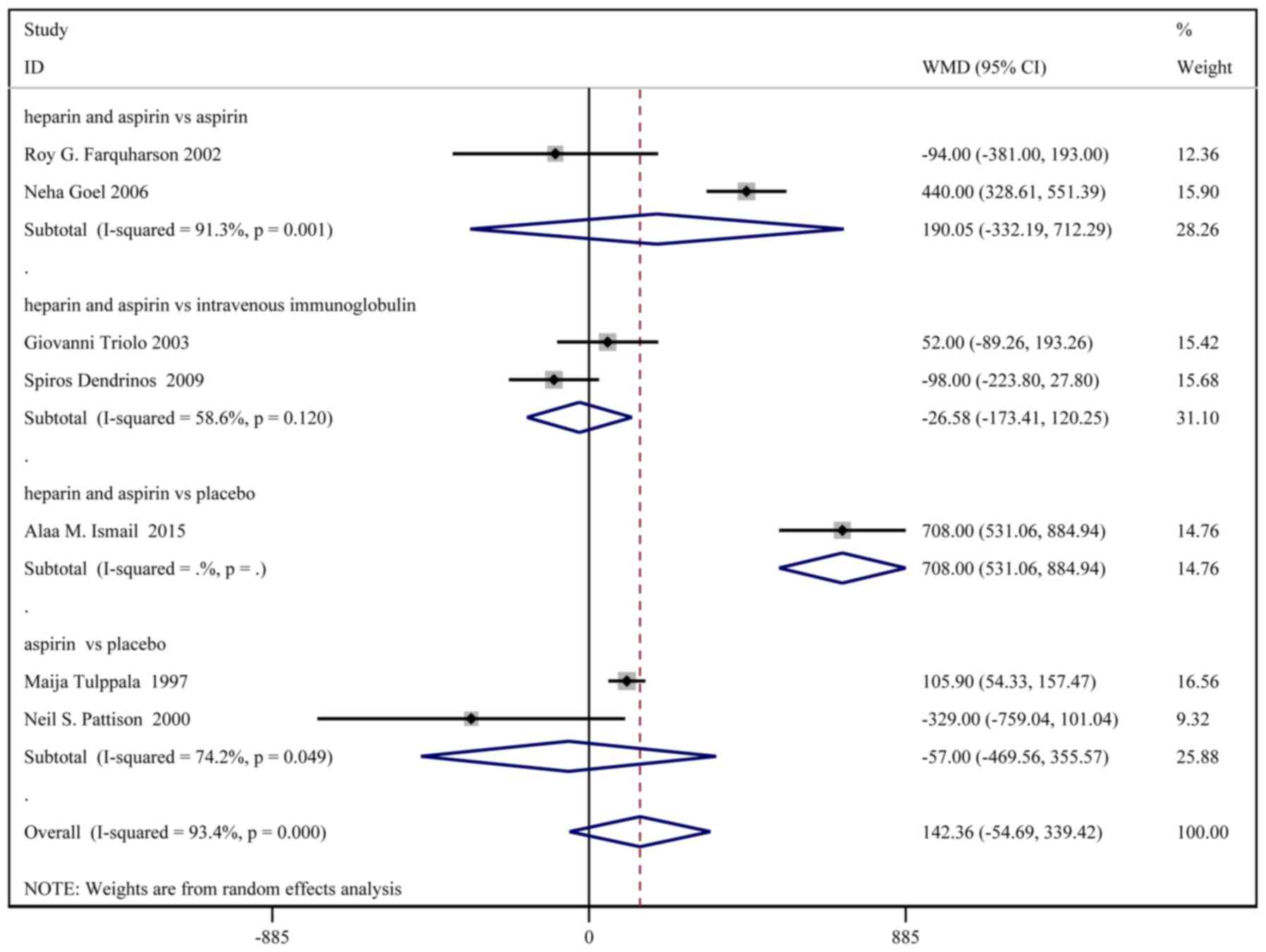
Birth weight
A total of 8 studies, with a total of 331 patients
in the treatment group and 333 patients in the control group,
reported the birth weights. Based on a χ2 P<0.0001
and an I2=93.4%, a random effect model was used to
assess the birth weight (Fig. 3).
No significant difference in birth weight was identified between
the two groups [weighted MD (WMD): 154.65 g; 95% CI:
-27.75-337.06]. In the sub-group analysis, birth weight was
significantly higher in the heparin and aspirin group than the
placebo group (WMD: 708.00; 95% CI: 531.06-884.94).
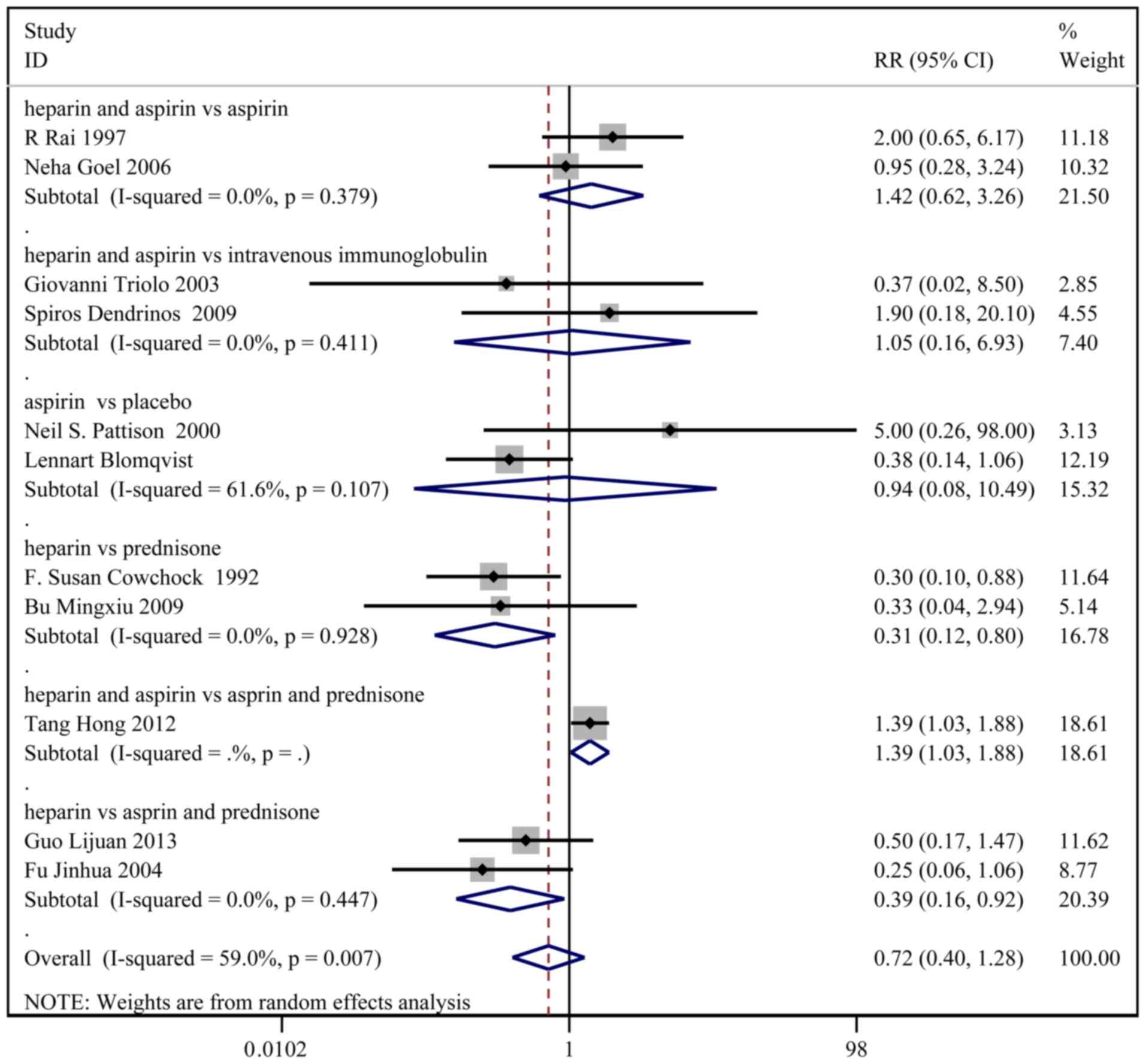
Pre-term delivery
A total of 11 studies, with 480 patients in the
treatment group and 482 patients in the control group, reported the
incidence of pre-term delivery. Based on a χ2 P=0.007
and an I2=59.0%, a random effect model was used to
assess pre-term delivery (Fig. 4).
No significant difference in pre-term delivery was observed between
the two groups (RR: 0.72; 95% CI: 0.40-1.28). In the sub-group
analysis, the incidence of pre-term delivery was higher in the
heparin and aspirin group than the aspirin and prednisone group
(RR: 1.39; 95% CI: 1.03-1.88).
Other results
No significant differences in the rates of cesarean
delivery, intrauterine death, gestational diabetes and
thrombocytopenia were identified between the two groups.
Vaginal delivery was significantly higher in the
heparin and aspirin group than the intravenous immunoglobulin group
(RR: 2.07; 95% CI: 1.19-3.62). The gestational age at birth was
markedly higher in the heparin and aspirin group compared with the
placebo group (WMD: 4.11 week; 95% CI: 3.68-4.53).
The incidence of IUGR was lower in the treatment
group than the control group (RR: 0.42; 95% CI: 0.20-0.88). In the
sub-group analysis, the incidence of IUGR was also lower in the
heparin and aspirin group compared with the placebo group (RR:
0.33; 95% CI: 0.14-0.80).
Moreover, the incidence of miscarriages was lower in
the treatment group compared with the control group (RR: 0.60; 95%
CI: 0.49-0.73), as well as lower in the heparin and aspirin group
than the aspirin (RR: 0.59; 95% CI: 0.40-0.87) and placebo groups
(RR: 0.47; 95% CI: 0.33-0.67) and lower in the heparin group
compared with the aspirin and prednisone groups (RR: 0.32; 95% CI:
0.17-0.62).
Furthermore, the incidence of pre-eclampsia was
lower in the treatment group than the control group (RR: 0.51; 95%
CI: 0.31-0.87) and the placebo group (RR: 0.48; 95% CI: 0.25-0.93).
The aforementioned results are summarized in Table II.
 | Table IIThe others results of
meta-analysis. |
Table II
The others results of
meta-analysis.
| | P-value |
|---|
| Index | Interventions | RR (95% CI) | bP-value | I2,
% | cP-value | Begg's | Egger's |
|---|
| Vaginal
delivery | Overall | 1.37
(0.63-3.00) | 0.022 | 80.9 | 0.432 | 1.000 | - |
| | HA vs. II | 2.07
(1.19-3.62) | - | - | 0.011 | - | - |
| | A vs. Pl | 0.95
(0.64-1.42) | - | - | 0.802 | - | - |
| Cesarean
delivery | Overall | 1.09
(0.68-1.75) | 0.346 | 10.5 | 0.729 | 0.462 | 0.574 |
| | HA vs. A | 0.75
(0.38-1.47) | - | - | 0.402 | - | - |
| | HA vs. II | 2.39
(0.71-8.05) | 0.187 | 42.6 | 0.159 | 0.317 | - |
| | A vs. Pl | 1.13
(0.48-2.65) | 0.750 | 0.0 | 0.788 | 0.317 | - |
| Intrauterine
death | HA vs. II | 0.44
(0.07-2.79) | 0.387 | 0.0 | 0.382 | 1.000 | - |
| Gestational age at
birth | Overall | 1.24
(-0.46-2.93)a | 0.000 | 97.9 | 0.154 | 0.764 | 0.976 |
| | HA vs. A | 0.04
(-1.66-1.73)a | 0.000 | 94.7 | 0.967 | 0.602 | 0.936 |
| | HA vs. II | 0.40
(-1.00-1.80)a | - | - | 0.577 | - | - |
| | HA vs. Pl | 4.11
(3.68-4.53)a | 0.925 | 0.0 | <0.001 | 0.317 | - |
| | A vs. Pl | 0.00
(-0.49-0.49)a | - | - | 1.000 | - | - |
| IUGR | Overall | 0.42
(0.20-0.88) | 0.267 | 18.7 | 0.021 | 1.000 | - |
| | HA vs. Pl | 0.33
(0.14-0.80) | - | - | 0.014 | - | - |
| | A vs. Pl | 0.89
(0.20-3.96) | - | - | 0.873 | - | - |
| Miscarriages | Overall | 0.60
(0.49-0.73) | 0.033 | 49.2 | 0.000 | 0.436 | 0.437 |
| | HA vs. A | 0.59
(0.40-0.87) | 0.605 | 0.0 | 0.007 | 0.602 | 0.703 |
| | A vs. Pl | 1.20
(0.80-1.81) | 0.862 | 0.0 | 0.382 | 0.317 | - |
| | HA vs. Pl | 0.47
(0.33-0.67) | 0.946 | 0.0 | <0.001 | 0.317 | - |
| | HA vs. Apr | 0.35
(0.12-1.01) | - | - | 0.051 | - | - |
| | H vs. Apr | 0.32
(0.17-0.62) | 0.962 | 0.0 | 0.001 | 0.317 | - |
| | H vs. Pl | 0.20
(0.03-1.56) | - | - | 0.125 | - | - |
| Gestational
diabetes | | | | | | | |
| | Overall | 0.26
(0.06-1.14) | 0.966 | 0.0 | 0.073 | 1.000 | - |
| | A vs. Pl | 0.25
(0.03-2.07) | - | - | 0.199 | - | - |
| | H vs. Pl | 0.27
(0.03-2.10) | - | - | 0.209 | - | |
|
Thrombocytopenia | | | | | | | |
| | Overall | 0.69
(0.30-1.57) | 0.798 | 0.0 | 0.373 | 0.806 | 0.373 |
| | HA vs. A | 0.51
(0.16-1.63) | 0.698 | 0.0 | 0.255 | 0.602 | 0.899 |
| | HA vs. Pl | 0.50
(0.05-5.22) | - | - | 0.563 | - | - |
| | HA vs. Apr | 1.27
(0.30-5.35) | - | - | 0.742 | - | - |
| Pre-eclampsia | | | | | | | |
| | Overall | 0.51
(0.31-0.87) | 0.936 | 0.0 | 0.012 | 0.260 | 0.438 |
| | HA vs. A | 0.52
(0.05-5.40) | - | - | 0.581 | - | - |
| | HA vs. Pl | 0.48
(0.25-0.93) | 0.582 | 0.0 | 0.029 | 0.317 | - |
| | A vs. Pl | 0.58
(0.24-1.43) | 0.666 | 0.0 | 0.240 | 0.602 | 0.643 |
Quality and bias assessment
An assessment of study quality and risk of bias was
performed using multiple complementary methods, including funnel
plots, Begg's and Mazumdar's rank test, and Egger's test. There was
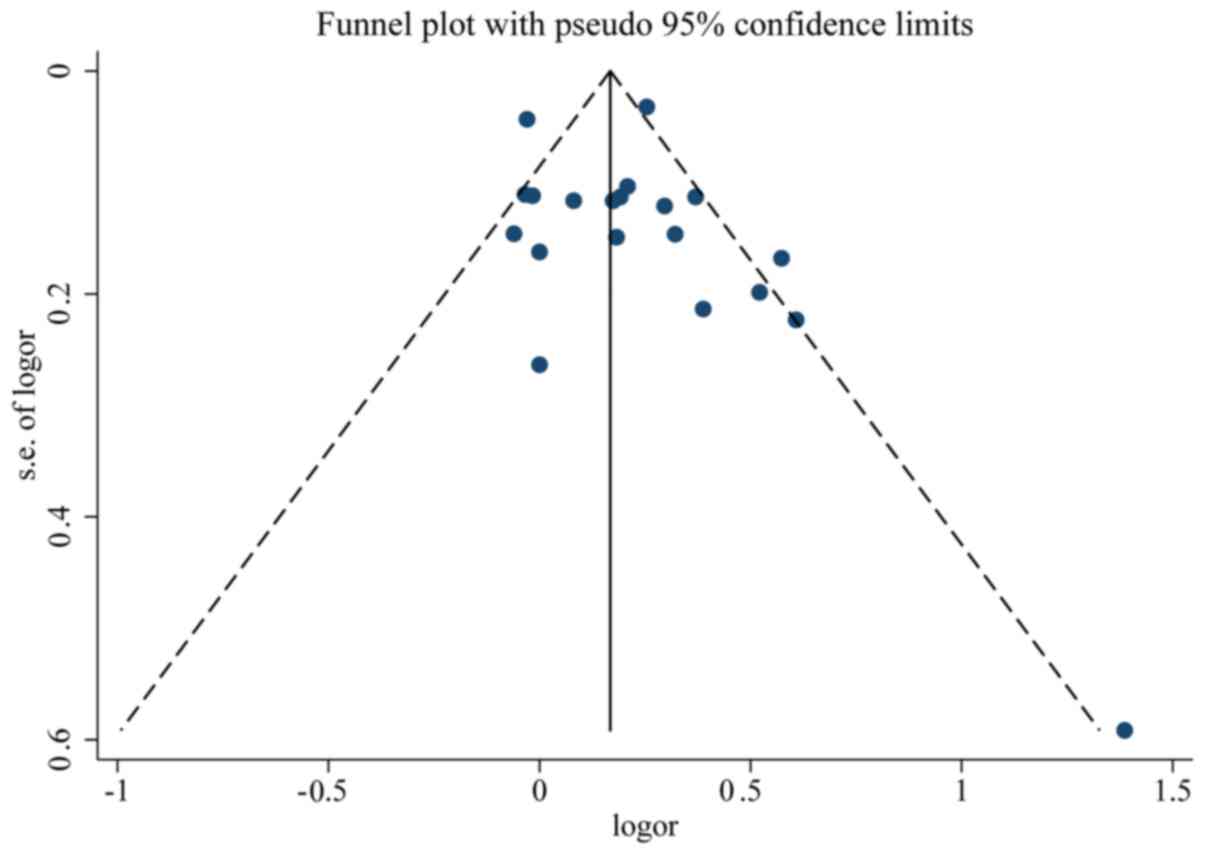
clear symmetry in the log RR funnel plot for included studies of
live birth, suggesting a low publication bias risk (Fig. 5). The results of Begg's and
Mazumdar's rank test (Z=1.52; P=0.127) and Egger's test (P=0.351)
demonstrated that there was no significant risk of bias among the
studied results.
Discussion
The mechanisms underlying the effects of heparin on
RSA are unclear. However, several possibilities have been suggested
in the literature. It has been demonstrated that the combination of
heparin and heparin antithrombin III can exert direct anticoagulant
effects (44). Alternatively,
heparin can combine with antiphospholipid antibody (APA) and reduce
its biological activity to protect the phospholipid components of
blastocyst trophoblast cells from damage (45). APA can activate the complement
system, especially C3 and C5, which in turn can promote
inflammation and immune responses (46-48).
In this respect, heparin can not only reduce inflammation, affect
antigen processing, inhibit the formation of antibodies and
complement-mediated abnormal immune responses, but also improve the
implantation rate of embryos (49).
Additionally, heparin can reduce the activity of aspartic acid
caspase-3, inhibit the apoptosis of trophoblast cells and
participate in the adhesion and invasion of blastocysts to
endometrial epithelium, thereby promoting the proliferation of
trophoblast cells and the formation of the placenta (50). As heparin has a relatively high
molecular weight, it is difficult to pass through the placenta and
has no teratogenic effects, thus is safe for the fetus and can be
used in early pregnancy (51-53).
Anticoagulant therapy is mainly used for pregnant
patients with a history of abortion and positive APA (54). The primary goal of this therapy is
to protect the mother from thrombosis and reduce the risk of
miscarriage (55). Aspirin can
inhibit platelet aggregation, increase prostacyclin levels and has
an anticoagulant effect (56,57).
Hertz-Picciotto et al (58)
observed that only high doses of aspirin (650-2, 600 mg/day) can
lead to obvious fetal malformation, while low doses of aspirin
(<150 mg/day) do not affect the quality of fetal birth or
increase perinatal fetal mortality and therefore is safe to use.
However, it should be noted that long-term use of aspirin may
increase the risk of peptic ulcer disease (59). Therefore, aspirin should be used
with caution when coagulation-related indicators are abnormal and
APA is positive.
In the present study, it was observed that heparin
and aspirin significantly improved live birth, compared with
aspirin and prednisone. Compared with the placebo group, the
heparin and aspirin group displayed markedly improved gestational
age at birth, decreased the incidence of IUGR and miscarriage and a
lower incidence of pre-eclampsia. Compared with the aspirin group,
the heparin and aspirin group significantly improved live birth and
decreased the incidence of miscarriages. Furthermore, heparin and
aspirin significantly increased the birth weight relative to
placebo. Heparin and aspirin markedly improved live birth and
vaginal delivery, compared with intravenous immunoglobulin.
However, there were certain limitations in the
present analysis. Indeed, only randomized studies were included and
individual studies varied in their exclusion and inclusion
criteria, as well as dosage. In addition, only a limited number of
studies were included. Lastly, pooled data were analyzed, as
individual patient data were not available; therefore, excluding
the possibility of a more comprehensive analysis.
Overall, heparin and aspirin may be an optimal
combination for treating RSA in women with APS. Nonetheless, the
limited number of studies included in the present meta-analysis
warrants further validation.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XY made substantial contributions to the conception
and design of the current study. XY drafted the article and
critically revised the draft for important intellectual content.
Data acquisition, analysis and interpretation were performed by LH.
All authors read and approved the final manuscript. All authors
agreed to be accountable for all aspects of the work and ensuring
that questions related to the accuracy or integrity of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Linnemann B: Antiphospholipid syndrome -
an update. Vasa. 47:451–464. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Uthman I, Noureldine MH, Ruiz-Irastorza G
and Khamashta M: Management of antiphospholipid syndrome. Ann Rheum
Dis. 78:155–161. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Carmi O, Berla M, Shoenfeld Y and Levy Y:
Diagnosis and management of catastrophic antiphospholipid syndrome.
Expert Rev Hematol. 10:365–374. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Garcia D and Erkan D: Diagnosis and
management of the antiphospholipid syndrome. N Engl J Med.
379(1290)2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Fujieda Y, Amengual O and Atsumi T:
Pathogenic role of antiphospholipid antibodies: An update. Lupus.
27:2012–2013. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
McIntyre JA, Coulam CB and Faulk WP:
Recurrent spontaneous abortion. Am J Reprod Immunol. 21:100–104.
1989.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Subgroup O: Chinese society of obstetrics
and gynecology, Chinese medical association. Zhonghua Fu Chan Ke Za
Zhi. 51:3–9. 2016.
|
|
8
|
Lin QD and Qiu LH: Pathogenesis,
diagnosis, and treatment of recurrent spontaneous abortion with
immune type. Front Med China. 4:275–279. 2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Miyakis S, Lockshin MD, Atsumi T, Branch
DW, Brey RL, Cervera R, Derksen RH, de Groot PG, Koike T, Meroni
PL, et al: International consensus statement on an update of the
classification criteria for definite antiphospholipid syndrome
(APS). J Thromb Haemost. 4:295–306. 2006.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Carrington B, Sacks G and Regan L:
Recurrent miscarriage: Pathophysiology and outcome. Curr Opin
Obstet Gynecol. 17:591–597. 2005.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kuon RJ, Wallwiener LM, Germeyer A,
Strowitzki T, Daniel V and Toth B: Establishment of a standardized
immunological diagnostic procedure in RM patients. J Reprod
Immunol. 1(55)2012.
|
|
12
|
Christiansen OB: Evidence-based
investigations and treatments of recurrent pregnancy loss. Curr
Opin Obstet Gynecol. 18:304–312. 2006.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Regan L and Rai R: Epidemiology and the
medical causes of miscarriage. Best Pract Res Clin Obstet Gynaecol.
14:839–854. 2000.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Vinatier D, Dufour P, Cosson M and Houpeau
JL: Antiphospholipid syndrome and recurrent miscarriages. Eur J
Obstet Gynecol Reprod Biol. 96:37–50. 2001.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Qiujuan Wang and Wenhua Xu: Pregnancy
complicated with antiphospholipid syndrome. Zhong Hua Fu Chan Ke Za
Zhi Bian Ji Bu. 30:117–119. 1995.(In Chinese).
|
|
16
|
Cervera R: Antiphospholipid syndrome.
Thromb Res. 151 (Suppl 1):S43–S47. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Schreiber K and Hunt BJ: Managing
antiphospholipid syndrome in pregnancy. Thromb Res. 181 (Suppl
1):S41–S46. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Del Ross T, Ruffatti A, Visentin MS,
Tonello M, Calligaro A, Favaro M, Hoxha A and Punzi L: Treatment of
139 pregnancies in antiphospholipid-positive women not fulfilling
criteria for antiphospholipid syndrome: A retrospective study. J
Rheumatol. 40:425–429. 2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Rai RS, Clifford K, Cohen H and Regan L:
High prospective fetal loss rate in untreated pregnancies of women
with recurrent miscarriage and antiphospholipid antibodies. Hum
Reprod. 10:3301–3304. 1995.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zeng X, Sun Z and Tang H: Series ten of
meta-analysis: Formulation of eligibility criteria. Chin J Evid
Based Cardiovasc Med. 5:6–9. 2013.
|
|
21
|
McCormick F, Cvetanovich GL, Kim JM,
Harris JD, Gupta AK, Abrams GD, Romeo AA and Provencher MT: An
assessment of the quality of rotator cuff randomized controlled
trials: Utilizing the Jadad score and CONSORT criteria. J Shoulder
Elbow Surg. 22:1180–1185. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Pattison NS, Chamley LW, Birdsall M,
Zanderigo AM, Liddell HS and McDougall J: Does aspirin have a role
in improving pregnancy outcome for women with the antiphospholipid
syndrome? A randomized controlled trial. Am J Obstet Gynecol.
183:1008–1012. 2000.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Triolo G, Ferrante A, Ciccia F,
Accardo-Palumbo A, Perino A, Castelli A, Giarratano A and Licata G:
Randomized study of subcutaneous low molecular weight heparin plus
aspirin versus intravenous immunoglobulin in the treatment of
recurrent fetal loss associated with antiphospholipid antibodies.
Arthritis Rheum. 48:728–731. 2003.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Tang H, Huang MY and Yi T: Effect of low
molecular weight heparin combined with low-dose aspirin for ACA
positive recurrent spontaneous abortion. J Hainan Med.
19-20:2012.
|
|
25
|
Rai R, Cohen H, Dave M and Regan L:
Randomised controlled trial of aspirin and aspirin plus heparin in
pregnant women with recurrent miscarriage associated with
phospholipid antibodies (or antiphospholipid antibodies). BMJ.
314:253–257. 1997.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Farquharson RG, Quenby S and Greaves M:
Antiphospholipid syndrome in pregnancy: A randomized, controlled
trial of treatment. Obstet Gynecol. 100:408–413. 2002.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Goel N, Tuli A and Choudhry R: The role of
aspirin versus aspirin and heparin in cases of recurrent abortions
with raised anticardiolipin antibodies. Med Sci Monit.
12:CR132–CR136. 2006.PubMed/NCBI
|
|
28
|
Dendrinos S, Sakkas E and Makrakis E:
Low-molecular-weight heparin versus intravenous immunoglobulin for
recurrent abortion associated with antiphospholipid antibody
syndrome. Int J Gynaecol Obstet. 104:223–225. 2009.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Laskin CA, Spitzer KA, Clark CA, Crowther
MR, Ginsberg JS, Hawker GA, Kingdom JC, Barrett J and Gent M: Low
molecular weight heparin and aspirin for recurrent pregnancy loss:
Results from the randomized, controlled HepASA Trial. J Rheumatol.
36:279–287. 2009.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ismail AM, Hamed AH, Saso S, Abu-Elhasan
AM, Abu-Elghar MM and Abdelmeged AN: Randomized controlled study of
pre-conception thromboprophylaxis among patients with recurrent
spontaneous abortion related to antiphospholipid syndrome. Int J
Gynaecol Obstet. 132:219–223. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhou X: The effect of aspirin combined
with low molecular weight heparin in treatment of 30 patients with
habitual abortion and antiphospholipid syndrome. Cn Mod Doct.
50:60–62. 2012.
|
|
32
|
Tulppala M, Marttunen M,
Söderstrom-Anttila V, Foudila T, Ailus K, Palosuo T and Ylikorkala
O: Low-dose aspirin in prevention of miscarriage in women with
unexplained or autoimmune related recurrent miscarriage: Effect on
prostacyclin and thromboxane A2 production. Hum Reprod.
12:1567–1572. 1997.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhang G and Cheng L: Effect of low
molecular weight heparin combined with low dose aspirin on hormone
level and immune function in patients with recurrent spontaneous
abortion. Zhongguo Sheng Hua Yao Wu Za Zhi. 67-69:2015.(In
Chinese).
|
|
34
|
Cowchock FS, Reece EA, Balaban D, Branch
DW and Plouffe L: Repeated fetal losses associated with
antiphospholipid antibodies: A collaborative randomized trial
comparing prednisone with low-dose heparin treatment. Am J Obstet
Gynecol. 166:1318–1323. 1992.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Jinhua F, Wang Z and Lang F: Study of
heparin on pregnant woman with recurrent pregnant loss and positive
anti-phospholipid antibody. Zhonghua Wei Chan Yi Xue Za Zhi
2003.
|
|
36
|
Clinical observation on 40 autoimmune
habitual abortion patients with low molecular weight heparin
calcium. China Practical Medicine 2009.
|
|
37
|
Madani T, Ahmadi F, Jahangiri N,
Bahmanabadi A and Bagheri Lankarani N: Does low-dose aspirin
improve pregnancy rate in women undergoing frozen-thawed embryo
transfer cycle? A pilot double-blind, randomized placebo-controlled
trial. J Obstet Gynaecol Res. 45:156–163. 2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Blomqvist L, Hellgren M and Strandell A:
Acetylsalicylic acid does not prevent first-trimester unexplained
recurrent pregnancy loss: A randomized controlled trial. Acta
Obstet Gynecol Scand. 97:1365–1372. 2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Bao SH, Sheng SL, Liao H, Zhou Q, Frempong
ST and Tu WY: Use of D-dimer measurement to guide anticoagulant
treatment in recurrent pregnancy loss associated with
antiphospholipid syndrome. Am J Reprod Immunol.
78(78)2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Maged AM, Abdelhafiz A, Mostafa WA,
El-Nassery N, Fouad M, Salah E and Kotb A: The role of prophylactic
use of low dose aspirin and calheparin in patients with unexplained
recurrent abortion. Gynecol Endocrinol. 32:970–972. 2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Zhang Shuyun ZH: Clinical analysis of low
molecular weight heparin and aspirin in treating recurrent
pregnancy loss caused by antiphospholipid antibodies. Hebei Med.
24:422–426. 2018.
|
|
42
|
Zhaojuan S: Evaluation of the efficacy of
low molecular weight heparin combined with aspirin in the treatment
of recurrent abortion caused by anti-phospholipid antibody. J Pract
Gynecol Endocrinol. 5:53–54. 2018.
|
|
43
|
Liang RH: Effect of low molecular weight
heparin combined with Zishen Yutai pill for RSA caused by positive
ACA. Chin Youjiang Med J. 43:471–473. 2015.
|
|
44
|
Schreiber K, Sciascia S, de Groot PG,
Devreese K, Jacobsen S, Ruiz-Irastorza G, Salmon JE, Shoenfeld Y,
Shovman O and Hunt BJ: Antiphospholipid syndrome. Nat Rev Dis
Primers. 4(17103)2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Galli M and Barbui T: Antiphospholipid
antibodies and pregnancy. Best Pract Res Clin Haematol. 16:211–225.
2003.PubMed/NCBI View Article : Google Scholar
|
|
46
|
D'Ippolito S, Di Simone N, Di Nicuolo F,
Castellani R and Caruso A: Antiphospholipid antibodies: Effects on
trophoblast and endothelial cells. Am J Reprod Immunol. 58:150–158.
2007.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Katsuragawa H, Kanzaki H, Inoue T, Hirano
T, Mori T and Rote NS: Monoclonal antibody against
phosphatidylserine inhibits in vitro human trophoblastic hormone
production and invasion. Biol Reprod. 56:50–58. 1997.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Di Simone N, Raschi E, Testoni C,
Castellani R, D'Asta M, Shi T, Krilis SA, Caruso A and Meroni PL:
Pathogenic role of anti-beta 2-glycoprotein I antibodies in
antiphospholipid associated fetal loss: Characterisation of beta
2-glycoprotein I binding to trophoblast cells and functional
effects of anti-beta 2-glycoprotein I antibodies in vitro. Ann
Rheum Dis. 64:462–467. 2005.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Chamley LW: Antiphospholipid antibodies:
Biological basis and prospects for treatment. J Reprod Immunol.
57:185–202. 2002.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Lopez-Pedrera CH, Aguirre MA, Ruiz-Limon
P, Pérez-Sánchez C, Jimenez-Gomez Y, Barbarroja N and Cuadrado MJ:
Immunotherapy in antiphospholipid syndrome. Int Immunopharmacol.
27:200–208. 2015.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Sebire NJ, Fox H, Backos M, Rai R,
Paterson C and Regan L: Defective endovascular trophoblast invasion
in primary antiphospholipid antibody syndrome-associated early
pregnancy failure. Hum Reprod. 17:1067–1071. 2002.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Franklin RD and Kutteh WH: Effects of
unfractionated and low molecular weight heparin on antiphospholipid
antibody binding in vitro. Obstet Gynecol. 101:455–462.
2003.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Girardi G: Heparin treatment in pregnancy
loss: Potential therapeutic benefits beyond anticogulation. J
Reprod Lmmunol. 66:45–51. 2005.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Bates SM, Greer IA, Middeldorp S, Veenstra
DL, Prabulos AM and Vandvik PO: VTE, thrombophilia, antithrombotic
therapy, and pregnancy: Antithrombotic therapy and prevention of
thrombosis, 9th Ed: American College of Chest Physicians
Evidence-Based Clinical Practice Guidelines. Chest. 141 (Suppl
2):e691S–e736S. 2012.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Makino A and Sugiura-Ogasawara M:
Anticoagulant therapy and pregnancy. Reprod Med Biol. 7:1–10.
2008.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Girardi G, Redecha P and Salmon JE:
Hepafin prevents antiphospholipid antibody-induced fetal loss by
inhibiting complement activation. Nat Med. 10:1222–1226.
2004.PubMed/NCBI View
Article : Google Scholar
|
|
57
|
Hills FA, Abrahams VM, González-Timón B,
Francis J, Cloke B, Hinkson L, Rai R, Mor G, Regan L, Sullivan M,
et al: Heparin prevents programmed cell death in human trophoblast.
Mol Hum Reprod. 12:237–243. 2006.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Hertz-Picciotto I, Hopenhayn-Rich C, Golub
M and Hooper K: The risks and benefits of taking aspirin during
pregnancy. Epidemiol Rev. 12:108–148. 1990.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Shim YK and Kim N: Nonsteroidal
anti-inflammatory drug and aspirin-induced peptic ulcer disease.
Korean J Gastroenterol. 67:300–312. 2016.PubMed/NCBI View Article : Google Scholar
|