Introduction
Age-related macular degeneration (AMD) is the
primary cause of irreversible visual impairment in elderly
individuals (1). Patients with AMD
are generally >50 years of age and present with progressive
degeneration in both eyes. AMD is roughly divided into two
categories: Dry AMD and wet AMD (2). Wet AMD, also known as exudative AMD or
neovascular AMD (nAMD), is the most severe form of visual
impairment (3,4). The pathological features of nAMD
mainly include progressive atrophy of the retinal pigmented
epithelium (RPE), Bruch's membrane and choroidal capillaries,
resulting in the formation of choroidal neovascularization (CNV)
(5,6). The formation and development of CNV
have a crucial role in the whole process of nAMD. Therefore, in
recent years, anti-VEGF drugs to inhibit CNV have been used for the
clinical treatment of nAMD.
Fundus fluorescein angiography (FFA) and indocyanine
green angiography (ICGA) are thought to be the gold standard in
terms of unambiguous diagnosis, classification, positioning and
active monitoring of nAMD, but the invasiveness of the
examination-due to requiring intravenous dye-may rarely cause
nausea, vomiting and severe allergic reactions. In the past 20
years, optical coherence tomography (OCT), particularly spectral
domain OCT, has fundamentally changed the methods of diagnosis of
macular disease, providing a non-invasive imaging method that is
sensitive to the recognition of retinal and subretinal lesions.
However, as both drusen and subretinal hemorrhage are characterized
by high reflection, OCT has numerous limitations in differentiating
between angiogenesis and dry AMD. OCT angiography (OCT-A) is one of
the most significant advances in fundus imaging. It is able to
display 3D retinal and choroidal vessels without any dye injection,
so it is entirely non-invasive (7,8). In
the past three years, a large number of studies, both in clinical
and experimental environments, have been conducted on the surface
of the eye to evaluate the depth resolution of retinal and
choroidal blood flow through the qualitative and quantitative
analysis of OCT-A measurements (9,10). In
addition, it may be used to observe the activity or the resting
state of CNV (11-13).
In the present study, nAMD cases treated with
ranibizumab were prospectively enrolled and the treatment outcomes
were evaluated following OCT-A-based analyses.
Materials and methods
Patient selection and study
design
The present study was a prospective analysis of
patients undergoing individualized treatment for nAMD recruited at
the Department of Ophthalmology at Shanghai 10th People's Hospital
(Shanghai, China) and was performed in compliance with the tenets
of the Declaration of Helsinki. Approval was obtained from the
Ethics Committee of Shanghai 10th People's Hospital (Shanghai,
China; no. SHSY-IEC-4.0/19-41/01). All patients signed an informed
consent form prior to inclusion. In this prospective study, 23 eyes
of 18 patients with nAMD were examined. The patients first received
three consecutive monthly injections of ranibizumab between April
2018 and November 2018.
Inclusion and exclusion criteria
All patients with nAMD underwent comprehensive
ophthalmic examination, including best-corrected visual acuity
(BCVA), slit lamp biomicroscopy, color fundus photography,
fluorescein angiography, FFA/ICGA using a fundus camera
(Spectralis; Heidelberg Engineering, Inc.) and OCT-A with an RTVue
XR AngioVue Version 2017 (Optovue, Inc.). Furthermore, the
diagnosis of nAMD in all patients was obtained from FFA/ICGA. To
establish the presence of active CNV, patients must exhibit
fluorescence leakage, as seen on FFA/ICGA, and hemorrhage, as seen
on OCT-A, located either within or below the retina or below the
RPE. The inclusion criteria for patients with nAMD in the present
study were as follows: i) aged between 55 and 75 years; ii) nAMD
with no previous treatment by methods including intravitreal
injection (IVI) of drugs, photodynamic therapy and systemic or
topical use of steroids. The exclusion criteria were a history of
retinal diseases other than nAMD, glaucoma, uveitis, diabetes
mellitus, rubeosis iridis, ocular infection, laser photocoagulation
and intraocular surgery. Eyes that could not be imaged clearly by
OCT-A were also excluded.
Study procedures
Patients with nAMD were scheduled to receive three
doses of intravitreal anti-VEGF injection (ranibizumab; IVI of 0.5
mg in 0.05 ml; Lucentis; Genentech, Inc.) monthly.
The patients with nAMD were examined prior to the
first IVI and on days 1, 3, 7, 14, 30, 60 and 90 after the first
IVI. The second injection was administered after the follow-up
examination on day 30 and the third injection was given after the
follow-up on day 60. Follow-up examination included BCVA, slit-lamp
biomicroscopy, color fundus photography and OCT-A.
OCT-A imaging
OCT-A scans of a 6x6 mm2 area centered on
the fovea were acquired using an RT Vue-XR Avanti system (Optovue,
Inc.) with AngioVue software (RTVue-XR version 2017.1.0.155;
Optovue Inc.). This device uses a light source centered on a
wavelength of 840 nm with a full-width at half-maximum bandwidth of
45 nm and an A-scan rate of 70,000 scans per second. At each
location on the retina, the RTVue system captured two consecutive
B-scans, each containing 304 A-scans. OCT-A evaluates the height of
RPE detachment (RPED), the greatest linear dimension (GLD), CNV
flow area, whole retinal thickness and four-quadrant retinal
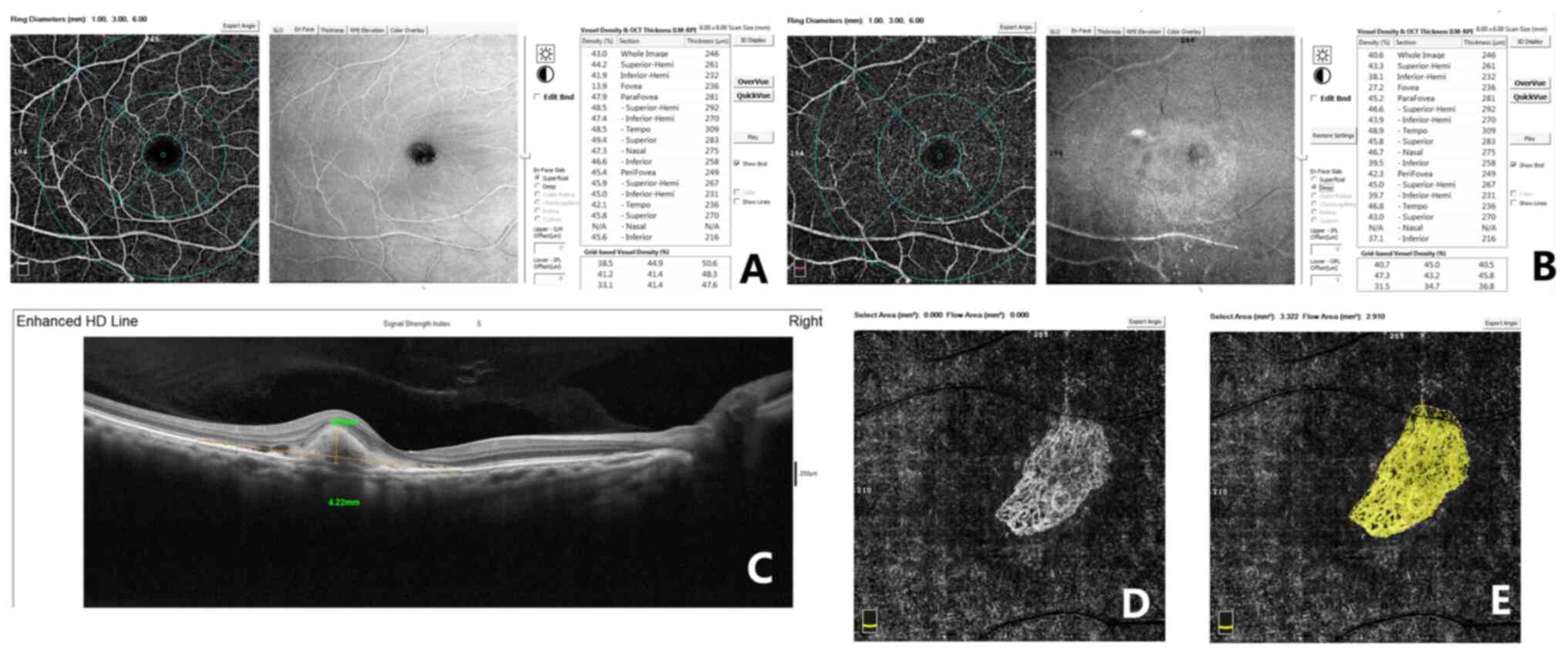
thickness (Fig. 1).
Fig. 1 displays
representative measurements of the macula in an eye with nAMD by
using OCT-A on a 6x6 mm scan. The inner ring and outer ring were
subdivided into four sectors (superior, nasal, inferior and
temporal). In Fig. 1A and B, the superficial and deep retinal
thicknesses were measured in the fovea (1 mm), inner ring (3 mm)
and outer ring (6 mm) following the Early Treatment of Diabetic
Retinopathy Study grid. The OCT scan (horizontal B-scan) provides
segmentation of individual retinal layers, and on this diagram, the
GLD and RPED may be measured. Furthermore, the CNV flow area was
measured by AngioVue software (Fig.
1D and E). The OCT-A images
were graded by two observers.
Statistical analysis
The parameters were presented and analyzed using
typical methods for descriptive statistics. The mean and standard
deviation were calculated for quantitative parameters.
Repeated-measures ANOVA with Bonferroni adjustment was performed to
make comparisons between each follow-up time-point with the
baseline. Pearson's correlation test was used to study the
correlation between BCVA and quantified OCT-A measurements in
patients with nAMD at the baseline prior to the first IVI.
P<0.05 was considered to indicate statistical significance. All
statistical analyses were performed using the IBM SPSS Statistics
(version 21.0; IBM Corp.).
Results
Patient characteristics
A total of 23 eyes from 18 patients (12 males and 6
females; age, 65.61±4.88 years) with CNV were included in the
present study and all eyes had type II CNV. The baseline and
follow-up results of the 23 eyes with nAMD are presented in
Table I.
 | Table IBCVA and optical coherence tomography
angiography quantitative analysis of patients with neovascular
macular degeneration at the eight follow-up points. |
Table I
BCVA and optical coherence tomography
angiography quantitative analysis of patients with neovascular
macular degeneration at the eight follow-up points.
| Index | Baseline | Day 1 | Day 3 | Day 7 | Day 14 | Day 30 | Day 60 | Day 90 |
|---|
| BCVA (LogMAR) | 0.67±0.45 | 0.6±0.44 | 0.6±0.48 | 0.56±0.45 | 0.56±0.42 | 0.51±0.43 |
0.46±0.43a |
0.36±0.30a |
| RPED (µm) | 211.70±127.61 | 208.17±128.13 | 197.78±116.75 | 170.83±103.08 | 177.22±92.49 | 156.52±88.18
a |
142.64±80.47a |
148.52±83.53a |
| GLD (mm) | 2.82±1.20 | 2.72±1.24 | 2.59±1.22 | 2.33±1.22 | 2.36±1.12 |
2,22±1.14a |
2.26±1.13a |
2.25±1.11a |
| CNV
(mm2) | 2.08±2.18 | 2.18±2.17 | 1.92±1.76 | 1.84±1.97 |
1.69±1.90a | 1.44±1.22 | 1.33±1.18 | 1.38±1.17 |
| Whole retinal
thickness (µm) | 310.09±57.99 | 296.91±43.63 | 284.74±24.20 | 278.83±22.06 | 274.91±18.90 | 275.65±21.11 | 271.59±16.93 | 271.26±16.61 |
| Temporal retinal
thickness (µm) | 346.13±83.16 | 334.78±75.78 | 315.78±47.19 | 308.48±39.56 | 298.57±30.73 |
297.43±37.09a | 295.09±31.46 | 295.74±30.89 |
| Superior retinal
thickness (µm) | 342.61±72.36 | 328.78±57.60 | 312.74±30.23 | 304.87±26.11 | 297.78±18.17 | 297.43±37.09 | 298±21.41 | 298±20.92 |
| Nasal retinal
thickness (µm) | 338.09±48.35 | 324.96±43.59 | 314.17±32.37 | 306.70±28.20 |
300.70±24.97a |
297±21.90a | 301.64±26.39 | 302.17±25.92 |
| Inferior retinal
thickness (µm) | 354.78±94.83 | 339.39±58.49 | 330.13±63.12 | 320.78±56.81 |
305.48±34.78a |
302.83±23.97a | 307.45±42.15 | 310.04±43.01 |
OCT-A results in nAMD patients Changes
in BCVA
The BCVA gradually improved after IVI and improved
significantly on days 60 and 90 in comparison to the baseline
(each, P<0.05; Table I).
Changes in CNV
In Table I, the RPED
also improved after IVI with a significant improvement on days 30
and 60 relative to the baseline (each, P<0.05), but it was
slightly increased on day 90 compared with the baseline
(P<0.05). The follow-up observations also indicated that the
length of the GLD was gradually shortened after IVI and the
difference was statistically significant from day 30 to day 90
(all, P<0.05). In addition, the flow area of the CNV exhibited a
trend of improvement after IVI, which also became slightly elevated
again on day 90; however, the difference was statistically
significant only on day 14 after IVI (P<0.05).
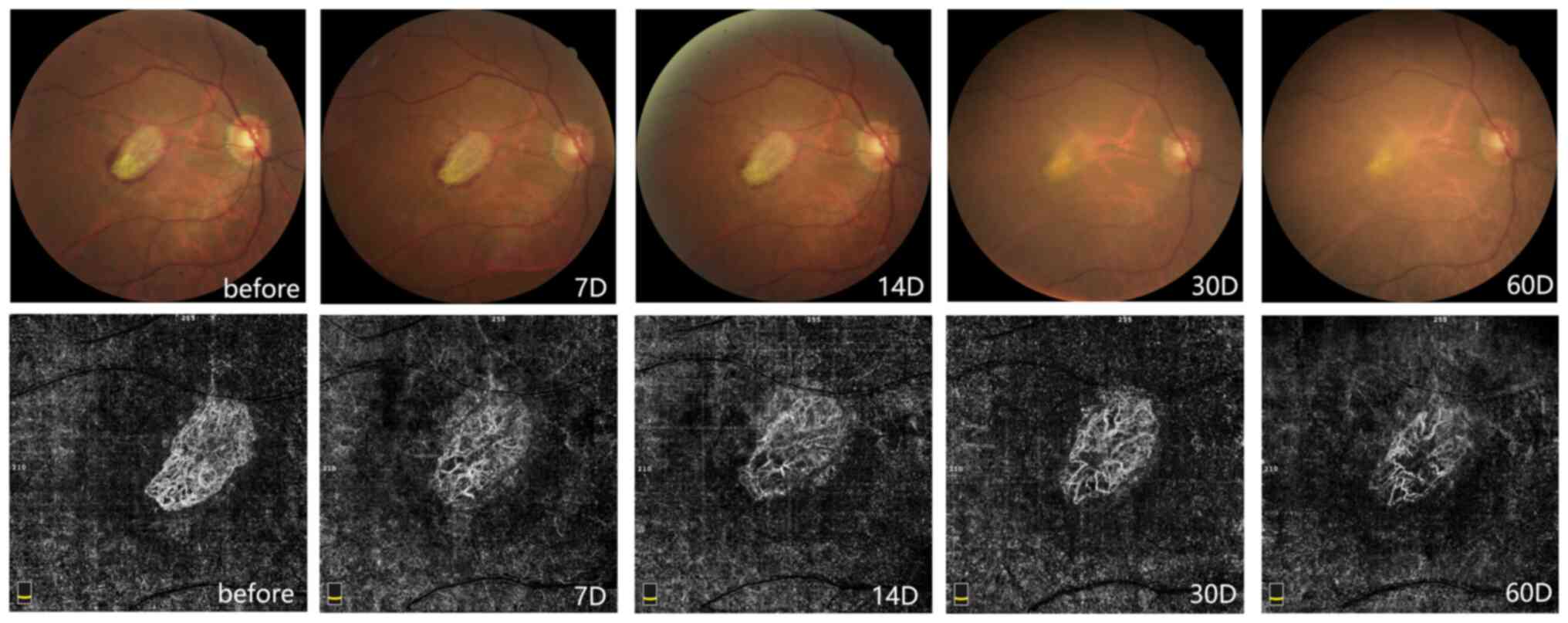
Fig. 2 presents
examination images of a 66-year-old male with nAMD. Fundus color
photographs revealed a yellow-white disc-shaped lesion in the
macula, surrounded by small pieces indicative of bleeding. The
bleeding was gradually absorbed after IVI and there was no obvious
bleeding in the macula at day 60. OCT-A suggested that the CNV had
dense vascular branches prior to IVI, with secondary branches and
an abnormal vascular arch. With the gradual improvement of the
disease after treatment, the CNV presented with a gradually reduced
blood flow density, the vascular branches became more sparse and
the surrounding vascular arch was interrupted. These abnormal new
blood vessels gradually became thick, straight and stiff in
appearance and lacked capillaries.
Changes in retinal thickness
The whole retinal thickness and the retinal
thickness in the four quadrants exhibited a slow thinning on day
30, followed by a slight thickening, with the thickness stabilizing
on days 60-90. At the 7 follow-up time-points, there was no
statistically significant difference in the whole retinal thickness
and superior retinal thickness from that at the baseline. However,
the temporal retinal thickness was significantly different on day
30 (P<0.05), and there were statistically significant
differences in nasal retinal thickness and inferior retinal
thickness on day 14 and day 30 compared with the baseline (all,
P<0.05; Table I).
Correlations between BCVA and the
quantified OCT-A measurements
Finally, the correlations between the quantified
OCT-A measurements and BCVA were analyzed prior to the first time
of IVI by Pearson correlation tests (Table II). The results suggested that the
BCVA was positively correlated with RPED (r=0.26, P=0.001), GLD
(r=0.26, P=0.001), CNV flow area (r=0.24, P=0.002) and nasal
retinal thickness (r=-0.18, P=0.021).
 | Table IICorrelations of best-corrected visual
acuity with quantified optical coherence tomography angiography
measurements in patients with neovascular macular degeneration
prior to the first intravitreal injection. |
Table II
Correlations of best-corrected visual
acuity with quantified optical coherence tomography angiography
measurements in patients with neovascular macular degeneration
prior to the first intravitreal injection.
| Variable | Correlation
parameters |
|---|
| RPED | r=0.26, P=0.001 |
| GLD | r=0.26, P=0.001 |
| CNV | r=0.24, P=0.002 |
| Whole retinal
thickness | r=-0.07, P=0.382 |
| Temporal retinal
thickness | r=0.16, P=0.054 |
| Superior retinal
thickness | r=0.02, P=0.831 |
| Nasal retinal
thickness | r=-0.18, P=0.021 |
| Inferior retinal
thickness | r=0.03, P=0.683 |
Discussion
In the clinic, injection of anti-VEGF is mainly used
to treat nAMD, which is able to effectively reduce the size of the
CNV and the severity of vascular leakage. Fauser et al
(14) indicated that both BCVA
values of patients with nAMD were significantly improved and the
expression level of VEGF was significantly decreased after patients
were injected with anti-VEGF. However, certain studies indicated
that anti-VEGF did not significantly improve the BCVA (15,16).
In the present study, the BCVA was gradually improved after
treatment and the improvement on days 60 and 90 was statistically
significant. Furthermore, the three indicators of RPED, GLD and CNV
flow area, were determined in the present study, which exhibited a
tendency of improvement in the early stage. The reason for this may
be that the patients were treated with ranibizumab for the first
time and were sensitive to the drug, and thus, the visual acuity
was significantly improved. The present results also suggested that
the three indicators were positively correlated with the BCVA,
suggesting that these indicators are able to directly reflect the
disease. The pathophysiological mechanism of nAMD is complex and
known to be associated with oxidative damage (17,18),
immune response, aging, metabolic disorders and chronic
inflammatory reactions (1,19,20).
Therefore, anti-VEGF alone cannot effectively control the
development of nAMD for long periods of time and may require to be
combined with other therapies at later stages, such as
anti-inflammatory therapy.
In 2011, 1-year results of the Comparison of AMD
Treatments Trials (CATT) (21)
supported that both ranibizumab and bevacizumab reduced retinal and
subretinal fluid, but ranibizumab eliminated fluid more. In
addition, there were more serious adverse events in patients
treated with bevacizumab. Therefore, ranibizumab is widely used for
anti-VEGF therapy in patients with nAMD. However, in 2020, one CATT
(22) suggested that ranibizumab
and bevacizumab had similar effects on visual acuity over a 2-year
period, and there were no differences between drugs in rates of
death or arteriothrombotic events. Recently, another study
indicated a similar systemic safety profile for bevacizumab and
ranibizumab as intravitreal pharmacotherapies for common retinal
conditions in routine clinical practice (23). In addition, bevacizumab is available
at low cost and it may have a greater potential for clinical use.
Regrettably, a large number of patients experienced ocular adverse
events in Shanghai in September 2010 after treatment with
bevacizumab (Avastin; Genentech) (24,25).
Therefore, the application of bevacizumab in China is limited by
policy implications (26).
The advantage of the present study is that changes
in patients with nAMD after anti-VEGF injections were closely
observed, but the disadvantages are that the observation time was
short, the number of cases was small and changes in the condition
of recurrent refractory nAMD were not observed. Therefore, further
studies with an increased number of patients will be performed in
the future to avoid the inclusion of two eyes from the same
patient, thereby making the results more credible.
In conclusion, nAMD is a severe, blinding eye
disease and its incidence is increasing year by year, but the
available clinical treatments have no significant long-term effect,
so it is urgent to develop novel drugs and methods that are
efficient alongside these current treatments. OCT-A, a non-invasive
imaging technique that allows for the qualitative and quantitative
analysis of macular lesions, may provide an effective biomarker and
guide the evaluation, treatment and monitoring of patients with
nAMD.
Acknowledgements
Not applicable.
Funding
This study was funded by the National Natural
Science Foundation of China (grant no. 81770939).
Availability of data and materials
The datasets used and/or analyzed during the current
study available from the corresponding author on reasonable
request.
Authors' contributions
JZ, QP and FW made substantial contributions to
conception and design. JZ, QP and TS acquired the data. JZ and MW
analyzed and interpreted the data. JZ and QP involved in drafting
the manuscript. FW gave final approval for the version to be
published. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All procedures performed in studies involving human
participants were in accordance with the ethical standards of the
Ethics Committee of Shanghai 10th People's Hospital (Shanghai,
China) and with the Declaration of Helsinki from 1964 and its later
amendments or comparable ethical standards. Approval was obtained
from the Ethics Committee of Shanghai 10th People's Hospital
(Shanghai, China; no. SHSY-IEC-4.0/19-41/01). Prior written
informed consent was obtained from all participants included in the
study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ambati J, Ambati BK, Yoo SH, Ianchulev S
and Adamis AP: Age-related macular degeneration: Etiology,
pathogenesis, and therapeutic strategies. Surv Ophthalmol.
48:257–293. 2003.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Anderson DH, Mullins RF, Hageman GS and
Johnson LV: A role for local inflammation in the formation of
drusen in the aging eye. Am J Ophthalmol. 134:411–431.
2002.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Jager RD, Mieler WF and Miller JW:
Age-related macular degeneration. N Engl J Med. 358:2606–2617.
2008.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ardeljan C, Ardeljan D, Abu-Asab M and
Chan CC: Inflammation and cell death in age-related macular
degeneration: An immunopathological and ultrastructural model. J
Clin Med. 3:1542–1560. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Cho Y, Cao X, Shen D, Tuo J, Parver LM,
Rickles FR and Chan CC: Evidence for enhanced tissue factor
expression in age-related macular degeneration. Lab Investig.
91:519–526. 2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
MacHalińska A, Kłos P, Safranow K,
Dziedziejko V, Rudnicki M, Paczkowska E, Karczewicz D and
Machaliński B: Neural stem/progenitor cells circulating in
peripheral blood of patients with neovascular form of AMD: A novel
view on pathophysiology. Graefes Arch Clin Exp Ophthalmol.
249:1785–1794. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Faridi A, Jia Y, Gao SS, Huang D and
Bhavsar KV: Sensitivity and Speci fi city of OCT angiography to
detect choroidal neovascularization. Ophthalmol Retin. 1:294–303.
2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Schneider EW and Fowler SC: Optical
coherence tomography angiography in the management of age-related
macular degeneration. Curr Opin Ophthalmol. 29:217–225.
2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lupidi M, Cerquaglia A, Chhablani J, Fiore
T, Singh SR, Cardillo Piccolino F, Corbucci R, Coscas F, Coscas G
and Cagini C: Optical coherence tomography angiography in
age-related macular degeneration: The game changer. Eur J
Ophthalmol. 28:349–357. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kaszubski PA, Ben Ami T, Saade C, Nabati
C, Kumar V, Santos AR, Silva R, Cachulo ML, Cunha-Vaz JG and Smith
RT: Changes in reticular pseudodrusen area in eyes that progressed
from early to late age-related macular degeneration. Int
Ophthalmol. 38:503–511. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Aggarwal K, Agarwal A, Sharma A, Sharma K
and Gupta V: OCTA Study Group. Detection of type 1 choroidal
neovascular membranes using optical coherence tomography
angiography in tubercular posterior uveitis. Retina. 39:1595–1606.
2018.
|
|
12
|
Nesper PL, Soetikno BT, Treister AD and
Fawzi AA: Volume-rendered projection-resolved OCT angiography: 3D
lesion complexity is associated with therapy response in wet
age-related macular degeneration. Investig Ophthalmol Vis Sci.
59:1944–1952. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Pilotto E, Frizziero L, Daniele AR,
Convento E, Longhin E, Guidolin F, Parrozzani R, Cavarzeran F and
Midena E: Early OCT angiography changes of type 1 CNV in exudative
AMD treated with anti-VEGF. Br J Ophthalmol. 103:67–71.
2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Fauser S, Schwabecker V and Muether PS:
Suppression of intraocular vascular endothelial growth factor
during aflibercept treatment of age-related macular degeneration.
Am J Ophthalmol. 158:532–536. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Heier JS, Brown DM, Chong V, Korobelnik
JF, Kaiser PK, Nguyen QD, Kirchhof B, Ho A, Ogura Y, Yancopoulos
GD, et al: Intravitreal aflibercept (VEGF trap-eye) in wet
age-related macular degeneration. Ophthalmology. 119:2537–2548.
2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Liu F, Ding X, Yang Y, Li J, Tang M, Yuan
M, Hu A, Zhan Z, Li Z and Lu L: Aqueous humor cytokine profiling in
patients with wet AMD. Mol Vis. 22:352–361. 2016.PubMed/NCBI
|
|
17
|
Nowak JZ: Age-related macular degeneration
(AMD): Pathogenesis and therapy. Pharmacol Rep. 58:353–363.
2006.PubMed/NCBI
|
|
18
|
Hollyfield JG, Bonilha VL, Rayborn ME,
Yang X, Shadrach KG, Lu L, Ufret RL, Salomon RG and Perez VL:
Oxidative damage-induced inflammation initiates age-related macular
degeneration. Nat Med. 14:194–198. 2008.PubMed/NCBI View
Article : Google Scholar
|
|
19
|
Chen M and Xu H: Parainflammation, chronic
inflammation, and age-related macular degeneration. J Leukoc Biol.
98:713–725. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Xu H, Chen M and Forrester JV:
Para-inflammation in the aging retina. Prog Retin Eye Res.
28:348–368. 2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
CATT Research Group. Martin DF, Maguire
MG, Ying GS, Grunwald JE, Fine SL and Jaffe GJ: Ranibizumab and
bevacizumab for neovascular age-related macular degeneration. N
Engl J Med. 364:1897–1908. 2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Comparison of Age-related Macular
Degeneration Treatments Trials (CATT) Research Group; Writing
Committee. Martin DF, Maguire MG, Fine SL, Ying GS, Jaffe GJ,
Grunwald JE, Toth C, Redford M and Ferris FL III. Ranibizumab and
bevacizumab for treatment of neovascular age-related macular
degeneration. Ophthalmology. 127 (Suppl):S135–S145. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Shah ND and Barkmeier AJ: Risk of systemic
adverse events following intravitreal bevacizumab, ranibizumab, and
aflibercept in routine clinical practice. Ophthalmology: Aug 8,
2020 2020 (Epub ahead of print).
|
|
24
|
Sun X, Xu X and Zhang X: Counterfeit
bevacizumab and endophthalmitis. N Engl J Med. 365:378–379.
2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wang F, Yu S, Liu K, Chen FE, Song Z,
Zhang X, Xu X and Sun X: Acute intraocular inflammation caused by
endotoxin after intravitreal injection of counterfeit bevacizumab
in Shanghai China. Ophthalmology. 120:355–361. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Huang HB, Pan Y and Liu T: Shanghai eye
treatment outbreak: Bevacizumab therapy for AMD in China. Clin Exp
Optom. 96:106–108. 2013.PubMed/NCBI View Article : Google Scholar
|