Introduction
Assisted reproductive technology has been widely
used to treat patients with infertility complications (1,2).
Clinical ovulation induction, ovulation collection and
transplantation techniques for assisted reproduction have been
optimized, which has improved pregnancy rates and successful
deliveries (3). However, negative
outcomes associated with assisted reproductive technology are
associated with the effects of certain primary diseases, including
severe endometriosis, polycystic ovary syndrome and old-age, which
lead to a significant decline in the quality of eggs and embryos,
and the clinical pregnancy rate (4). Oxidative stress damage in the
follicular microenvironment contributes to the decline of oocyte
quality in these primary diseases (5). Various gynecological diseases, such as
endometriosis, polycystic ovary syndrome and reproductive aging,
are common hormonal reproductive disorders leading to infertility,
in which oxidative stress serves a crucial role (6).
Granulosa cells (GCs) are a type of ovarian cell
that exist outside oocytes and in follicle walls. Their metabolic
activity serves an important role in the quality of the oocyte)
(7). GCs are involved in follicular
development, oocyte maturation and atresia (7). The role of GCs in the process of
follicular differentiation is vital, resulting in optimal
conditions for oocyte development, ovulation, fertilization and
embryo implantation (7).
Reactive oxygen species (ROS) are oxygen-derived
molecules that include superoxide anions,
H2O2 and hydroxyl radicals (8). ROS causes oxidative stress, damage to
oocytes and damage to luteinized GCs (9). Increased ROS concentrations in GC
reduces the number of retrieved oocytes (10) and in follicular fluid this
correlates with poor oocyte and embryo quality (11). SOD is an antioxidant enzyme that
acts as a scavenger in oxygen-free radical production, while MDA is
produced by lipid peroxidation and can be used as a marker of
oxidative stress and injury (12).
In addition, GSH is a cellular antioxidant that protects cellular
proteins from oxidative stress (12).
Salvia miltiorrhiza is a traditional Chinese
herbal medicine (13). In Shen
Nong's Herbal Classic, the dried roots or rhizomes of Salvia
miltiorrhiza Bge are described as a medicine to promote blood
circulation and regulate menstruation (14). There are multiple potential
biological effects of Salvia miltiorrhiza including
antioxidation, anti-inflammation, inhibition of apoptosis and
protective effects on organs such as the liver (15,16).
Salvia miltiorrhiza solutions are the aqueous extracts of
the dried roots or rhizomes of Salvia miltiorrhiza Bge
(17). The major chemical
components of the water-soluble fractions are salvianic acid A,
salvianolic acid B and lithospermic acids (14). The concentrations of salvianic acid
A and salvianolic acid B in Salvia miltiorrhiza solution are
2.15 and 1.01 mg/ml respectively, which have been previously
detected by high-performance liquid chromatography-UV (18). Salvia miltiorrhiza solution,
as a blood-activating drug, has achieved good clinical results in
improving placental circulation in patients with recurrent
abortion, as well as improving prognosis in the in vitro
fertilization and embryo transfer cycle to treat infertility
(19). In addition, previous
studies have demonstrated that salvianic acid A and salvianolic
acid B attenuated damage induced by oxidative stress (20,21).
Treatments with salvianic acid A and salvianolic acid B reduce
platelet-derived growth factor-induced ROS formation in rat hepatic
stellate cells, possibly via the inhibition of nicotinamide adenine
dinucleotide phosphate oxidase and are also effective against
hepatic fibrosis in thioacetamide-intoxicated rats in vivo
(22). Salvianic acids prevent
acute doxorubicin cardiotoxicity in mice via the suppression of
oxidative stress (23).
Furthermore, mixed aqueous extracts of Salvia miltiorrhiza
inhibit hypertension via the inhibition of vascular remodeling and
oxidative stress in spontaneously hypertensive rats (20). However, to the best of our
knowledge, the effect of salvianic acid A and salvianolic acid B on
ovarian granulose cells has not been previously reported.
Therefore, the aims of the present study were to investigate the
protective effect of Salvia miltiorrhiza solution and its
active compounds in H2O2-induced cell damage
of the human ovarian granulosa tumor cells (KGN), and to identify
the associated underlying mechanisms.
Materials and methods
Cell culture and treatment
KGN cells (cat. no. bncc37610; Beijing Beina
Chuanglian Biotechnology Research Institute) were cultured in
RPMI-1640 medium (Beijing Solarbio Science & Technology Co.,
Ltd.) supplemented with 10% FBS (Gibco; Thermo Fisher Scientific,
Inc.) and antibiotics (100 IU/ml penicillin G; 100 mg/ml
streptomycin; Beijing Solarbio Science & Technology Co., Ltd.)
in a humidified incubator at 37˚C with 5% CO2. The
H2O2 group consisted of KGN cells grown to
80% confluence, which were then treated with 200 µM
H2O2 for 24 h at room temperature. The
treatment group consisted of KGN cells grown to 80% confluence that
were pretreated with Salvia miltiorrhiza solution (0.2, 1
and 5%), salvianic acid A (3, 10 and 30 µM; Beijing SLF Chemical
Research Institute) and salvianolic acid B (3, 10 and 30 µM;
Nanjing Spring and Autumn Biological Engineering Co., Ltd.) for 4 h
at 37˚C, followed by treatment with 200 µM
H2O2 for 24 h at 37˚C (24). Salvia miltiorrhiza solution
(1.5 g/ml) was purchased from Shineway Pharmaceutical Co., Ltd.
(cat. no. Z13020777). Stock solutions of Salvia miltiorrhiza
solution (0.2, 1 and 5%), salvianic acid A and salvianolic acid B
(3, 10 and 30 µM) were dissolved in 0.1% DMSO and stored at -40˚C.
All solutions were freshly prepared from stock solutions prior to
each experiment and the final concentration of DMSO was
<0.1%.
Cell Counting Kit-8 (CCK-8) assay
A CCK-8 assay kit (cat. no. EP328-500t; Beijing
Zoman Biotechnology Co., Ltd.) was used to investigate the
viability of KGN cells. The CCK-8 assay was performed according to
the manufacturer's instructions. KGN cells were cultured in 96-well
plates at a density of 5,000 cells/well. When grown to 80%
confluence, cells were treated with drugs for 24 h at 37˚C. Cells
treated with 0.1% DMSO served as the control group. Then, 10 µl
CCK-8 solution was added to each well for 2 h at 37˚C. Absorbance
was measured at 450 nm using a microplate reader. Cell viability
was expressed as the percentage of the drug group to the control
group (100%). Data represents the mean of three independent
experiments.
Malondialdehyde (MDA), superoxide
dismutase (SOD), glutathione (GSH) and tumor necrosis factor-α
(TNF-α) analysis
Following Salvia miltiorrhiza solution,
salvianic acid A and salvianolic acid B treatment, KGN cells were
collected and expressions of MDA and GSH were detected, along with
SOD activity. MDA concentration was measured using a lipid
peroxidation MDA assay kit (cat. no. A003-1; Nanjing Jiancheng
Bio-Engineering Institute Co., Ltd.) and SOD activity was measured
with a SOD assay kit (cat. no. A001-3; Nanjing Jiancheng
Bio-Engineering Institute Co., Ltd.). GSH concentration was
measured with a GSH assay kit (cat. no. A006-2; Nanjing Jiancheng
Bio-Engineering Institute Co., Ltd.). The cell culture supernatant
was obtained by centrifugation at 326.5 x g. TNF-α concentration in
the cell culture supernatant was measured with a TNF-α ELISA kit
(cat. no. ARG80120; Arigo Biolaboratories), according to the
manufacturer's protocol.
Western blot analysis
Following cell treatment as described above, KGN
cells were harvested and cells were lysed using cell lysis buffer
RIPA (cat. no. 89900; Thermo Fisher Scientific, Inc.). Total
protein concentration was quantified using a Pierce bicinchoninic
acid protein assay kit (cat. no. 23227; Thermo Fisher Scientific,
Inc). A total of 90 µg protein was used for analysis. Protein
electrophoresis was performed using 12% SDS-PAGE and a PVDF
membrane that was blocked by 5% non-fat milk at room temperature
for 1 h. Western blotting was used to detect the protein expression
of cleaved caspase-3 and cleaved caspase-9 in KGN cells. Primary
antibodies included: Anti-cleaved-caspase-3 (1:1,000; cat. no.
9664; Cell Signaling Technology, Inc.), anti-cleaved-caspase-9
polyclonal (1:1,000; cat. no. A2636; ABclonal Biotech Co., Ltd.)
and anti-β-actin monoclonal (1:10,000; cat. no. AC026; ABclonal
Biotech Co., Ltd.). The secondary antibodies used were goat
anti-rabbit immunoglobulin G-horseradish peroxidase (1:5,000; cat.
no. 5220-0336; Kirkegaard & Perry Laboratories; Seracare).
Protein bands were developed using an EZ-ECL kit (cat. no.
20-500-120; Biological Industries) and analyzed with a Tanon 2500
chemiluminescence imaging system (Tanon Science and Technology Co.,
Ltd.).
Immunocytochemistry
KGN cells were seeded in 96-well plates at a density
of 5x103 cells/well on glass coverslips in a
24-multiwell plate. Drug treatment was administered 15 h after
cells were seeded. The cellular detection and localization of TNF-α
(TNF-α rabbit polyclonal antibody; cat. no. bs-2081R; BIOSS; 1:100)
was determined using an immunocytochemistry assay. Cells were fixed
in 4% paraformaldehyde overnight at 4˚C. Fixed cells were then
washed three times with PBS and subsequently incubated with 0.3%
triton at room temperature for 10 min. Samples were then blocked
with 10% goat serum (cat. no. 04-009-1A; Biological Industries) for
1 h at room temperature and incubated with primary antibody against
TNF-α overnight at 4˚C. Cells were washed three times with PBS and
incubated with secondary antibody (goat anti rabbit/mouse
horseradish peroxidase kit; cat. no. SP-9000; ZSGB BIO) for 1 h at
37˚C. After washing three times with PBS, slides were incubated
with 3,3'-diaminobenzidine tetrahydrochloride (Beijing Zhongshan
Jinqiao Biotechnology Co., Ltd.) for 1 min at room temperature .and
immediately washed with water after color development. Slides were
then counter-stained with 0.2% hematoxylin for 5 min at room
temperature. Slides were mounted with 1% hydrochloric acid Alcohol
and then observed under a light microscope, at 400x
magnification.
Statistical analysis
Data were analyzed using Origin 9.1 software
(OriginLab Corp.) and presented as the mean ± SEM. Each n value
represented data from one culture well. Unless otherwise indicated,
n=8-12 culture wells were used for each group in each experiment,
with repetitions conducted using ≥3 independent dissections for
each experiment. Statistical analyses were performed using one-way
ANOVA followed by Bonferroni's post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effects of Salvia miltiorrhiza
solution and its active compounds on the viability of KGN
cells
In Shen Nong's Herbal Classic, the dried roots or
rhizomes of Salvia miltiorrhiza Bge are described as a
medicine to promote blood circulation and regulate menstruation
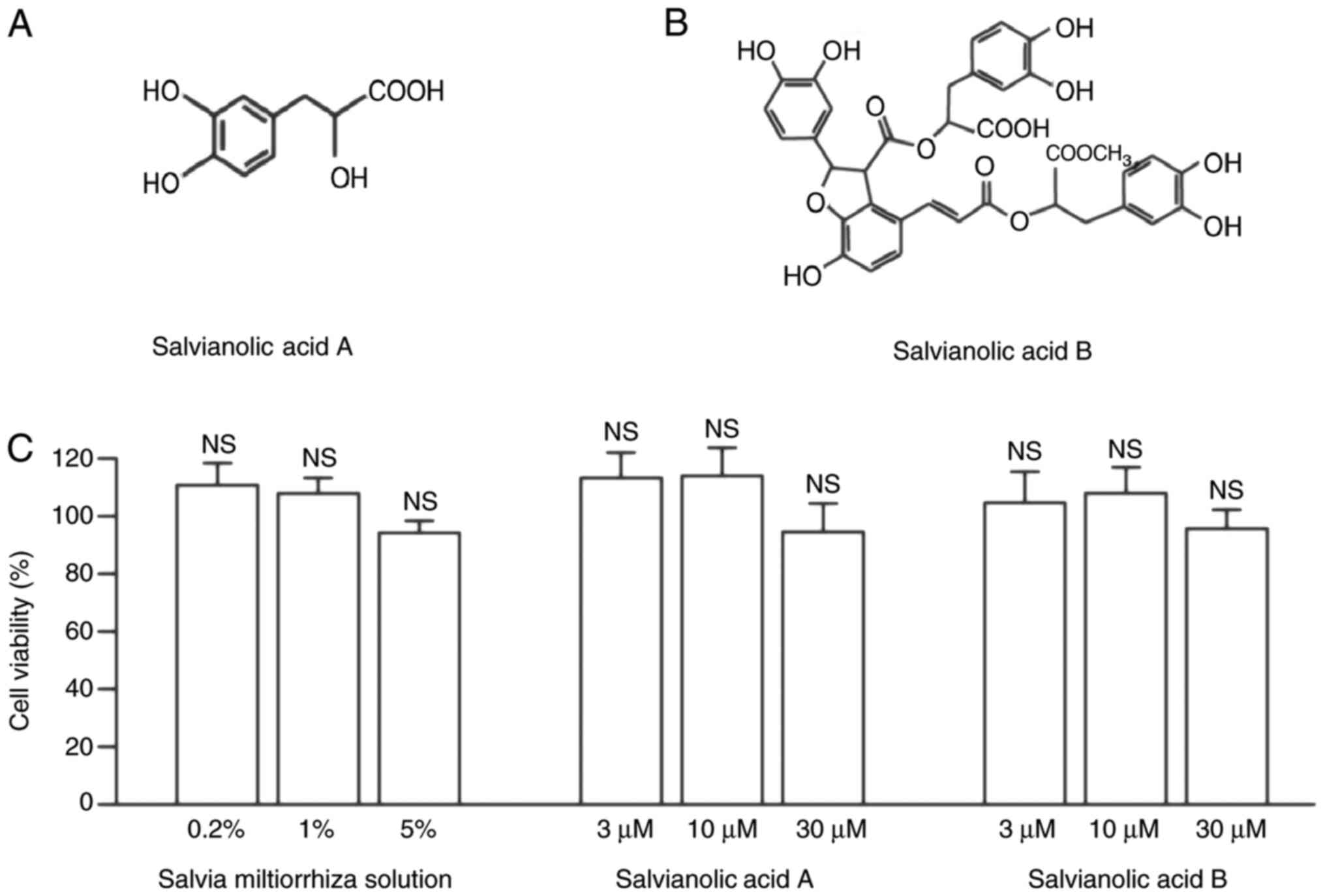
(14). Salvianic acid A (Fig. 1A) and salvianolic acid B (Fig. 1B) are the main water-soluble
compounds in Salvia miltiorrhiza solution (18).
The present study investigated the effects of Salvia
miltiorrhiza solution and its active compounds, salvianic acid A
and salvianolic acid B, on the viability of KGN cells by performing
a CCK-8 assay (Fig. 1C). KGN cells
were treated with Salvia miltiorrhiza solution or its main
active compounds for 24 h. As presented in Fig. 1C, neither Salvia miltiorrhiza
solution (0.2, 1 and 5%), salvianic acid A or salvianolic acid B
(3, 10 and 30 µM) affected the viability of KGN cells when compared
with the control group (100%; Fig.
1C). Therefore, the present results indicated that Salvia
miltiorrhiza solution, salvianic acid A and salvianolic acid B
exerted no toxic effects on granulosa cells.
Salvia miltiorrhiza solution,
salvianic acid A and salvianolic acid B suppress
H2O2-induced oxidative stress in KGN
cells
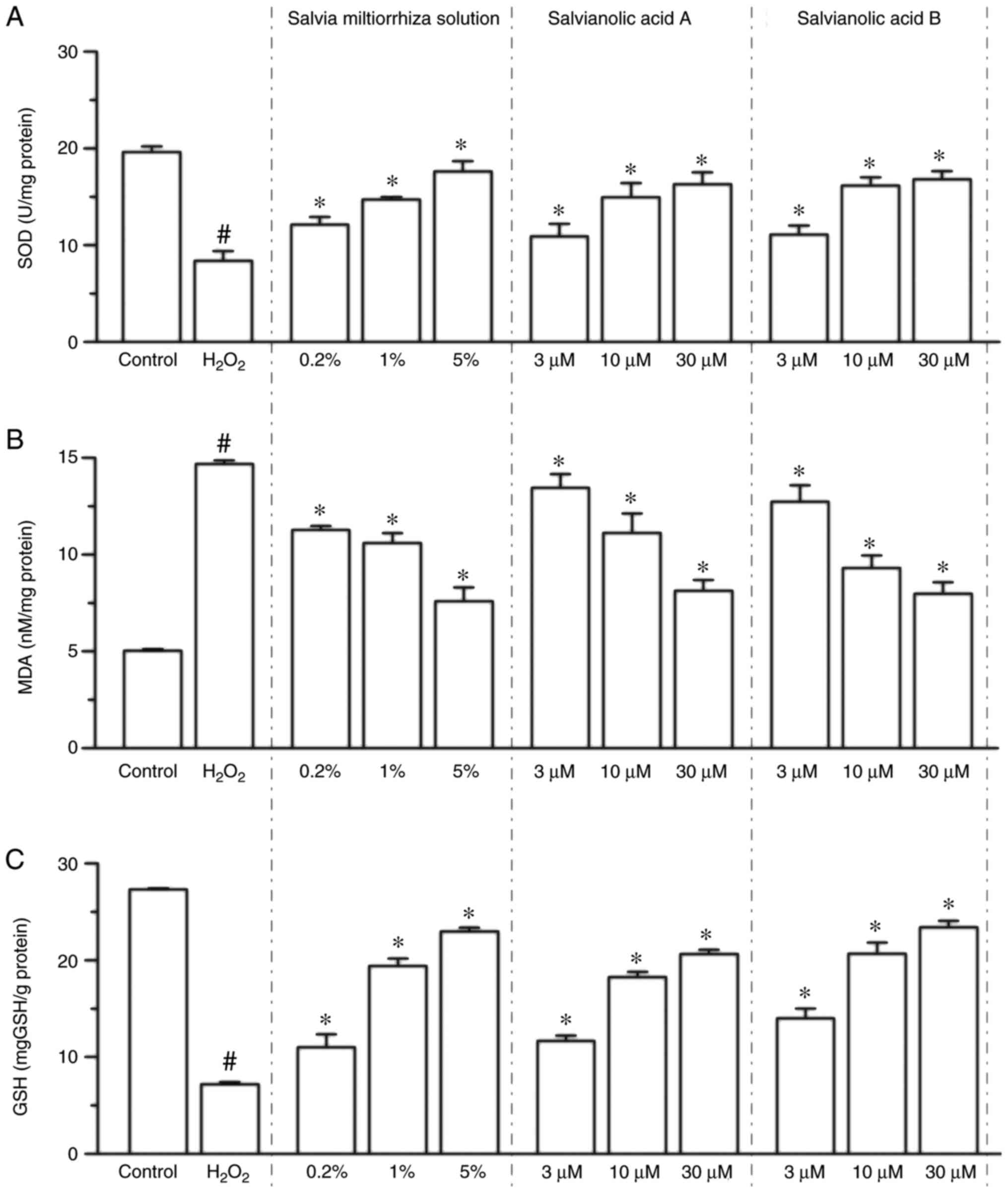
Compared with the control group, the expression of
MDA was increased 1.92-fold in the
H2O2-treated group (P<0.05 vs. control
group; Fig. 2B), while Salvia
miltiorrhiza solution (0.2, 1 and 5%), salvianic acid A or
salvianolic acid B (3, 10 and 30 µM) treatment significantly
inhibited the H2O2-induced increase of MDA
levels in a dose-dependent manner (P<0.05; Fig. 2B). It was also revealed that 200 µM
H2O2 treatment significantly decreased SOD
activity by 57.4% (P<0.05 vs. control group; Fig. 2A), while Salvia miltiorrhiza
solution (0.2, 1 and 5%), salvianic acid A and salvianolic acid B
(3, 10 and 30 µM) significantly attenuated the suppression of SOD
activity compared with the H2O2 group
(P<0.05; Fig. 2A). In addition,
GSH activities were significantly decreased in the
H2O2-treated group (P<0.05 vs. control
group; Fig. 2C), while Salvia
miltiorrhiza solution, salvianic acid A and salvianolic acid B
significantly attenuated the suppression of GSH activity compared
with the H2O2 group (P<0.05; Fig. 2C). Collectively, the present results
indicated that Salvia miltiorrhiza solution, as well as
salvianic acid A and salvianolic acid B, possess antioxidant
properties and increase the resistance of KGN cells to
oxidation.
Salvia miltiorrhiza solution,
salvianic acid A and salvianolic acid B suppresses
H2O2-induced cleaved caspase-3 and -9 protein
expression in KGN cells
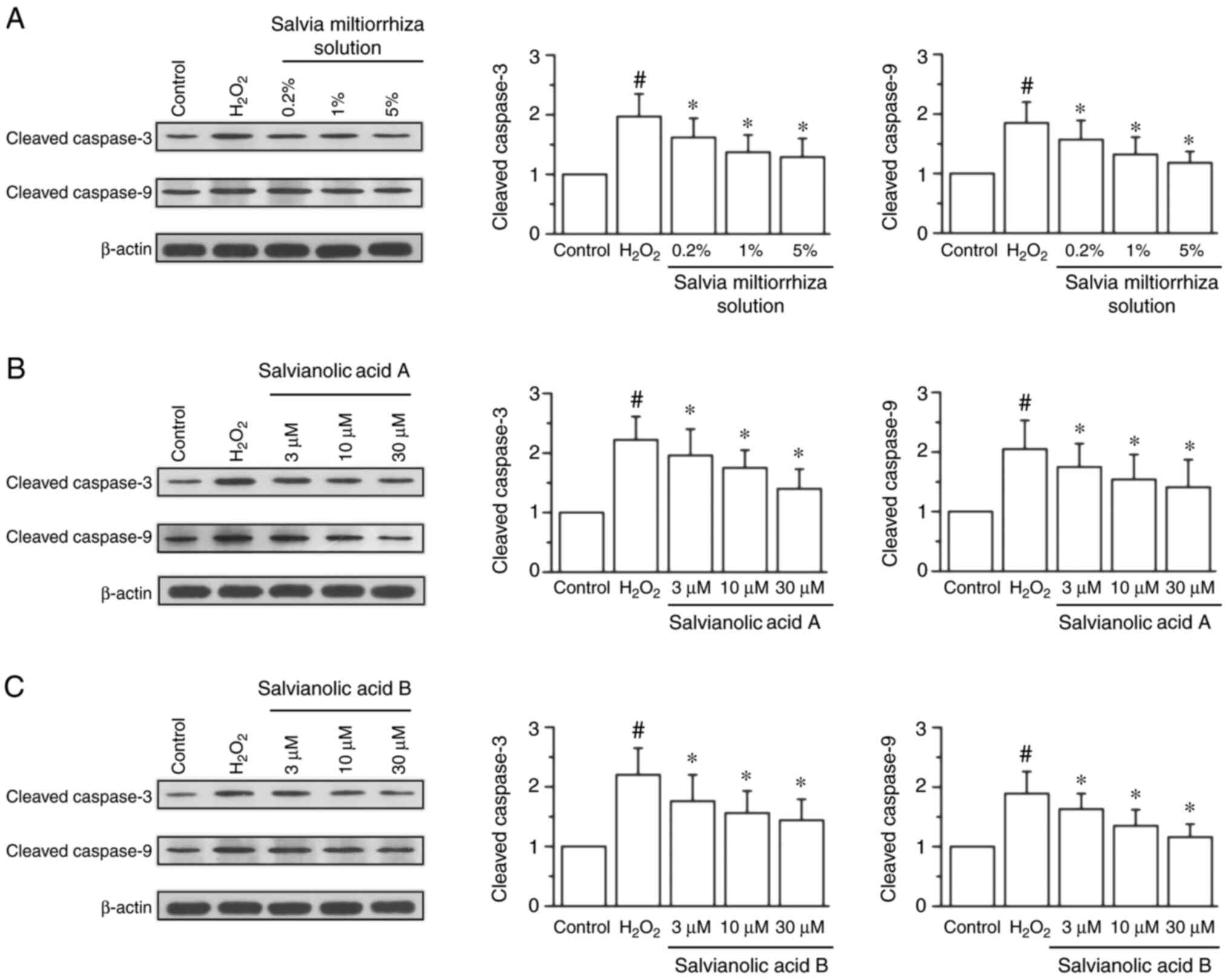
Western blotting data demonstrated that treatment
with 200 µM H2O2 significantly increased the
protein expression of cleaved caspase-3 by 0.97-fold and cleaved
caspase-9 by 0.85-fold compared with the control group (P<0.05;
Fig. 3). Treatment with Salvia
miltiorrhiza solution significantly attenuated the increased
expression of cleaved caspase-3 and cleaved caspase-9 compared with
the H2O2 group (P<0.05; Fig. 3A-C). In addition, salvianic acid A
(Fig. 3D-F) and salvianolic acid B
(Fig. 3G-I) significantly reversed
the increased expression of cleaved caspase-3 and cleaved caspase-9
induced by H2O2.
Salvia miltiorrhiza solution,
salvianic acid A and salvianolic acid B suppresses the
H2O2-induced increased expression of TNF-α in
KGN cells
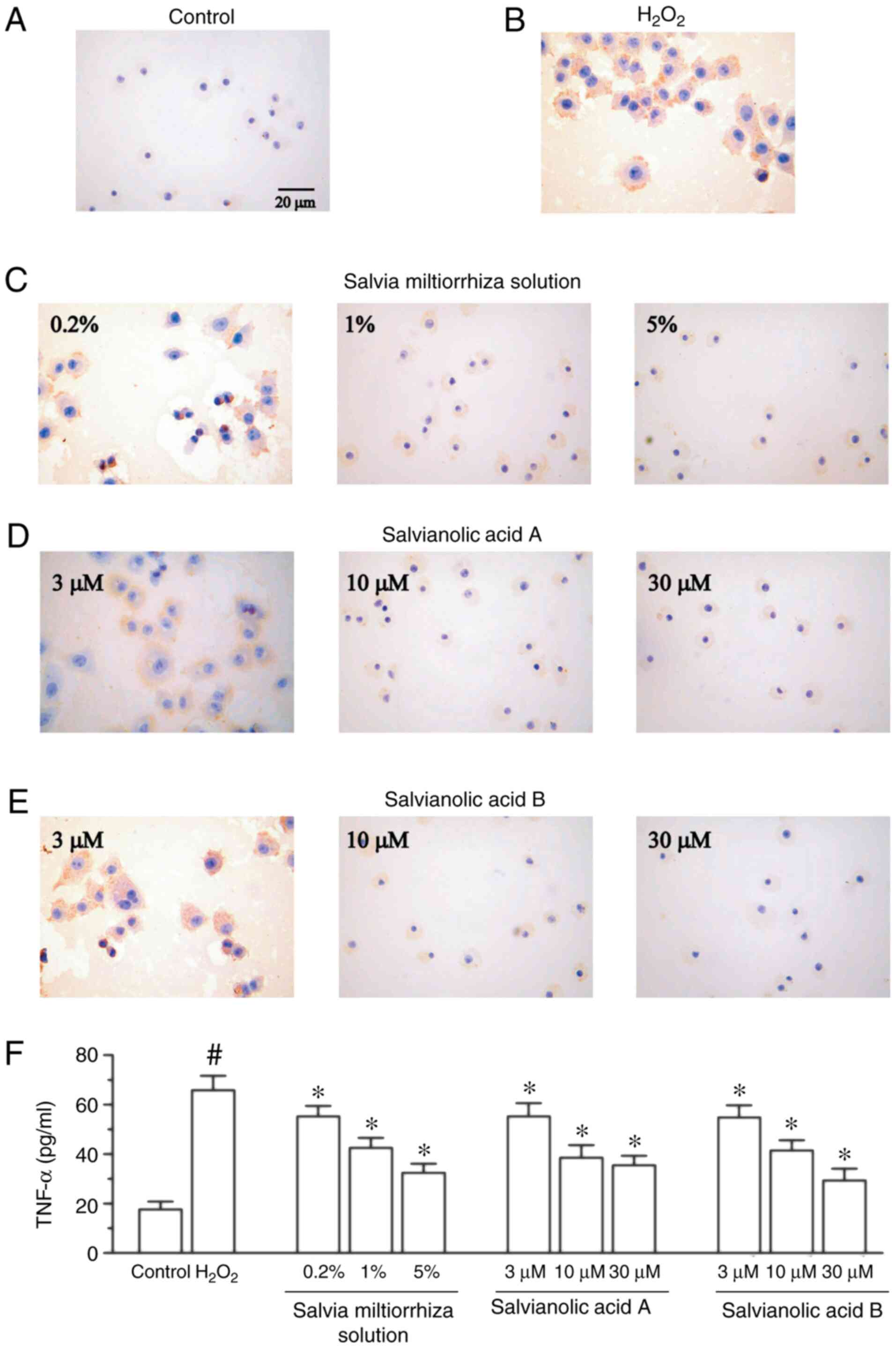
To investigate whether Salvia miltiorrhiza
inhibits the increased protein expression of
H2O2-induced TNF-α, KGN cells were pretreated
with Salvia miltiorrhiza solution or its main active
compounds for 4 h, followed by the addition of 200 µM
H2O2 for 24 h. As presented in Fig. 4B, treatment with 200 µM of
H2O2 significantly enhanced the protein
expression of TNF-α in KGN cells compared with the control group
(Fig. 4A). It was also revealed
that Salvia miltiorrhiza solution, in a
concentration-dependent manner, significantly decreased the
H2O2-induced upregulation of TNF-α (Fig. 4C). Furthermore, salvianic acid A and
salvianolic acid B significantly attenuated TNF-α protein
upregulation compared with the H2O2 group
(Fig. 4D and E). The present study also investigated the
release of TNF-α, and demonstrated that Salvia miltiorrhiza
solution, salvianic acid A and salvianolic acid B significantly
attenuated TNF-α release induced by H2O2
(Fig. 4F).
Discussion
The present results indicated that the survival of
granulosa cells was not affected by different concentrations of
Salvia miltiorrhiza solution and its main water-soluble
compounds. In addition, it was revealed that these compounds
exerted no toxic effects on granulosa cells and significantly
attenuated the H2O2-induced increase of MDA
levels and H2O2-induced decrease of SOD and
GSH activity. These compounds also reduced the
H2O2-induced increased expression of cleaved
caspase-3, cleaved caspase-9 and TNF-α. Therefore, the present
results indicated that Salvia miltiorrhiza solution and its
main water-soluble compounds ameliorated KGN cell damage induced by
H2O2, suggesting that these may protect
against oxidative stress in the granulosa cells around oocytes.
Thus, Salvia miltiorrhiza may facilitate the treatment of
infertility.
Oxidative stress has been implicated in many
reproductive disorders including endometriosis, polycystic ovarian
syndrome, infertility and aging (25). The degradation of oocyte quality
caused by oxidative stress injury is the key factor leading to
infertility and adverse fertility outcomes (26). ROS serves an important role in the
induction of meiosis in the oocyte and high levels of ROS have been
revealed to impair oocyte maturation (27). A previous study indicated that
oocyte quality may be affected by an increase in ROS, which was
associated with advancing maternal age (25). The clearance of oxidative stress
products from follicular fluid is decreased in older women
(25). In addition, glutathione,
transferase and catalase in ovarian follicular fluid are
significantly decreased in older women undergoing in vitro
fertilization (25). Therefore,
oxidative stress induces granulosa cell discordant function and
influences oocyte quality (28).
The antioxidant effect of Salvia miltiorrhiza
has been extensively studied. Salvianic acid A reduces liver
fibrosis by regulating the caspase-3/cleaved caspase-3 signaling
pathway (29). Salvianolic acid B
promotes anticonvulsant and anti-apoptotic effects in a
pentylenetetrazole-induced seizure model by activitating the
AKT/cAMP response element-binding protein/brain-derived
neurotrophic factor signaling pathways, including the inhibition of
cleaved caspase-3 overexpression (30). Furthermore, TNF serves a crucial
role in the amplification of luteolytic signals, mediating vascular
regression, and promoting apoptosis and necroptosis of luteal cells
(31). Furthermore, it has been
demonstrated that salvianolic acid B inhibited TNF-α to extenuate
cholestatic liver injury in vivo and in vitro,
indicating that the anticholestatic effects of salvianolic acid B
may be associated with the inhibition of inflammation and the
maintenance of bile acid homeostasis (32). These previous studies indicate that
salvianic acid A and B serve an important role in the progression
of oxidative stress and apoptosis via caspase and TNF-α (33). However, the molecular mechanism
underlying the anti-oxidative effects of Salvia miltiorrhiza
in KGN cells is not fully understood. In the present study,
Salvia miltiorrhiza solution, salvianic acid A and
salvianolic acid B significantly attenuated the
H2O2-induced increase of MDA levels, and the
H2O2-induced suppression of SOD and GSH
activities in KGN cells. These compounds also attenuated the
increased expression of cleaved caspase-3, cleaved caspase-9 and
TNF-α. However, further research is required to investigate the
effects of Salvia miltiorrhiza on ovary function using in
vivo studies, such as the effects on theca cells, angiogenesis
and ovarian functions. Furthermore, the effects of Salvia
miltiorrhiza on the follicular microenvironment, the
endometrium in infertility and the regulation of the pelvic
microenvironment require further investigation.
Salvia miltiorrhiza solution and its main
water-soluble compounds, salvianic acid A and salvianolic acid B,
may ameliorate oxidative stress damage in
H2O2-induced KGN cells by suppressing the
overexpression of cleaved caspase-3, cleaved caspase-9 and TNF-α.
Thus, Salvia miltiorrhiza may facilitate the treatment of
infertility.
Acknowledgements
The current study was supported by the Research
Foundation of Administration of Traditional Chinese Medicine of
Hebei Province (grant no. 2016111).
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL designed the experiments and performed western
blot experiments. YL drafted the manuscript. LK performed oxidative
stress experiments. LK and ZQ collected the data. ZQ and XG
performed cell culture and CCK-8 experiments. HQ performed cell
culture and revised the final manuscript critically for important
intellectual content. HL designed the experiments and performed
ELISA experiments. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mu Z, Sa Y, Sun Z and Yi Y: Ovulation
induction with high progesterone levels may be more suitable for
elderly patients with low ovarian response. J Gynecol Obstet Hum
Reprod: Dec 3, 2019 (Epub ahead of print).
|
|
2
|
Benaglia L, Somigliana E, Santi G,
Scarduelli C, Ragni G and Fedele L: IVF and endometriosis-related
symptom progression: Insights from a prospective study. Hum Reprod.
26:2368–2372. 2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Jin B, Niu Z, Xu B, Chen Q and Zhang A:
Comparison of clinical outcomes among dual ovarian stimulation,
mild stimulation and luteal phase stimulation protocols in women
with poor ovarian response. Gynecol Endocrinol. 34:694–697.
2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Nasiri N, Moini A, Eftekhari-Yazdi P,
Karimian L, Salman-Yazdi R, Zolfaghari Z and Arabipoor A: Abdominal
obesity can induce both systemic and follicular fluid oxidative
stress independent from polycystic ovary syndrome. Eur J Obstet
Gynecol Reprod Biol. 184:112–116. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Karuputhula NB, Chattopadhyay R,
Chakravarty B and Chaudhury K: Oxidative status in granulosa cells
of infertile women undergoing IVF. Syst Biol Reprod Med. 59:91–98.
2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Visioli F and Hagen TM: Antioxidants to
enhance fertility: Role of eNOS and potential benefits. Pharmacol
Res. 64:431–437. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Attaran M, Pasqualotto E, Falcone T,
Goldberg JM, Miller KF, Agarwal A and Sharma RK: The effect of
follicular fluid reactive oxygen species on the outcome of in vitro
fertilization. Int J Fertil Womens Med. 45:314–320. 2000.PubMed/NCBI
|
|
8
|
Combelles CM, Gupta S and Agarwal A: Could
oxidative stress influence the in-vitro maturation of oocytes?
Reprod Biomed Online. 18:864–880. 2009.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Luderer U: Ovarian toxicity from reactive
oxygen species. Vitam Horm. 94:99–127. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lai Q, Xiang W, Li Q, Zhang H, Li Y, Zhu
G, Xiong C and Jin L: Oxidative stress in granulosa cells
contributes to poor oocyte quality and IVF-ET outcomes in women
with polycystic ovary syndrome. Front Med. 12:518–524.
2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Liang Y, Tian QH, Mu YX and Du HL: Effects
of Cangfu Congxian Decoction on oxidative stress in polycystic
ovary syndrome patients. Zhongguo Zhong Xi Yi Jie He Za Zhi.
36:685–689. 2016.PubMed/NCBI(In Chinese).
|
|
12
|
Zhao Y, Kong GY, Pei WM, Zhou B, Zhang QQ
and Pan BB: Dexmedetomidine alleviates hepatic injury via the
inhibition of oxidative stress and activation of the Nrf2/HO-1
signaling pathway. Eur Cytokine Netw. 30:88–97. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Chen HY, Lin YH, Wu JC, Chen YC, Yang SH,
Chen JL and Chen TJ: Prescription patterns of Chinese herbal
products for menopausal syndrome: Analysis of a nationwide
prescription database. J Ethnopharmacol. 137:1261–1266.
2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhang JP, Zhang YY, Zhang Y, Gao YG, Ma
JJ, Wang N, Wang JY, Xie Y, Zhang FH and Chu L: Salvia
miltiorrhiza (Danshen) injection ameliorates iron
overload-induced cardiac damage in mice. Planta Med. 79:744–752.
2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Liu J, Yang CF, Lee BL, Shen HM, Ang SG
and Ong CN: Effect of Salvia miltiorrhiza on aflatoxin
B1-induced oxidative stress in cultured rat hepatocytes. Free Radic
Res. 31:559–568. 1999.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Tian LL, Wang XJ, Sun YN, Li CR, Xing YL,
Zhao HB, Duan M, Zhou Z and Wang SQ: Salvianolic acid B, an
antioxidant from Salvia miltiorrhiza, prevents
6-hydroxydopamine induced apoptosis in SH-SY5Y cells. Int J Biochem
Cell Biol. 40:409–422. 2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lu P, Xing Y, Xue Z, Ma Z, Zhang B, Peng
H, Zhou QT, Liu H, Liu Z and Li J: Pharmacokinetics of salvianolic
acid B, rosmarinic acid And Danshensu in rat after pulmonary
administration of Salvia miltiorrhiza polyphenolic acid
solution. Biomed Chromatogr. 33(e4561)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Gao Y, Zhang K, Zhu F, Wu Z, Chu X, Zhang
X, Zhang Y, Zhang J and Chu L: Salvia miltiorrhiza (Danshen)
inhibits L-type calcium current and attenuates calcium transient
and contractility in rat ventricular myocytes. J Ethnopharmacol.
158 (Pt A):397–403. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yao DN, Chen WY and Xiao Y: Exploration
into rules of combined Chinese and Western medical treatment on
immune infertility. Zhongguo Zhong Xi Yi Jie He Za Zhi. 30317–319.
(333)2010.PubMed/NCBI(In Chinese).
|
|
20
|
Zhang J, An SJ, Fu JQ, Liu P, Shao TM, Li
M, Li X, Jiao Z and Chai XQ: Mixed aqueous extract of Salvia
miltiorrhiza reduces blood pressure through inhibition of
vascular remodelling and oxidative stress in spontaneously
hypertensive rats. Cell Physiol Biochem. 40:347–360.
2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yang X, Yao W, Li Q, Liu H, Shi H, Gao Y
and Xu L: Mechanism of Tang Luo Ning effect on attenuating of
oxidative stress in sciatic nerve of STZ-induced diabetic rats. J
Ethnopharmacol. 174:1–10. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Tsai MK, Lin YL and Huang YT: Effects of
salvianolic acids on oxidative stress and hepatic fibrosis in rats.
Toxicol Appl Pharmacol. 242:155–164. 2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Jiang B, Zhang L, Li M, Wu W, Yang M, Wang
J and Guo DA: Salvianolic acids prevent acute doxorubicin
cardiotoxicity in mice through suppression of oxidative stress.
Food Chem Toxicol. 46:1510–1515. 2008.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Cunha MP, Lieberknecht V, Ramos-Hryb AB,
Olescowicz G, Ludka FK, Tasca CI, Gabilan NH and Rodrigues AL:
Creatine affords protection against glutamate-induced nitrosative
and oxidative stress. Neurochem Int. 95:4–14. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Carbone MC, Tatone C, Delle Monache S,
Marci R, Caserta D, Colonna R and Amicarelli F: Antioxidant
enzymatic defences in human follicular fluid: Characterization and
age-dependent changes. Mol Hum Reprod. 9:639–643. 2003.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Štšepetova J, Baranova J, Simm J, Parm Ü,
Rööp T, Sokmann S, Korrovits P, Jaagura M, Rosenstein K, Salumets
A, et al: The complex microbiome from native semen to embryo
culture environment in human in vitro fertilization procedure.
Reprod Biol Endocrinol. 18(3)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Behrman HR, Kodaman PH, Preston SL and Gao
S: Oxidative stress and the ovary. J Soc Gynecol Investig. 8 (1
Suppl Proceedings):S40–S42. 2001.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Tripathi A, Pandey V, Sahu AN, Singh A and
Dubey PK: Di-(2-ethylhexyl) phthalate (DEHP) inhibits
steroidogenesis and induces mitochondria-ROS mediated apoptosis in
rat ovarian granulosa cells. Toxicol Res (Camb). 8:381–394.
2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wang R, Song F, Li S, Wu B, Gu Y and Yuan
Y: Salvianolic acid A attenuates CCl4-induced liver fibrosis by
regulating the PI3K/AKT/mTOR, Bcl-2/Bax and caspase-3/cleaved
caspase-3 signaling pathways. Drug Des Devel Ther. 13:1889–1900.
2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yu X, Guan Q, Wang Y, Shen H, Zhai L, Lu X
and Jin Y: Anticonvulsant and anti-apoptosis effects of salvianolic
acid B on pentylenetetrazole-kindled rats via AKT/CREB/BDNF
signaling. Epilepsy Res. 154:90–96. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Galvão AM, Szóstek AZ, Skarzynski DJ and
Ferreira-Dias GM: Role of tumor necrosis factor-α, interferon-γ and
Fas-ligand on in vitro nitric oxide activity in the corpus luteum.
Cytokine. 64:18–21. 2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Li S, Wang R, Wu B, Wang Y, Song F, Gu Y
and Yuan Y: Salvianolic acid B protects against ANIT-induced
cholestatic liver injury through regulating bile acid transporters
and enzymes, and NF-κB/IκB and MAPK pathways. Naunyn Schmiedebergs
Arch Pharmacol. 392:1169–1180. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Katary MA, Abdelsayed R, Alhashim A,
Abdelhasib M and Elmarakby AA: Salvianolic acid B slows the
progression of breast cancer cell growth via enhancement of
apoptosis and reduction of oxidative stress, inflammation, and
angiogenesis. Int J Mol Sci. 20(E5653)2019.PubMed/NCBI View Article : Google Scholar
|