Introduction
Osteosarcoma is a common type of primary malignant
bone tumor (1), that occurs mostly
in adolescents (2). Highly
invasive, it easily invades the long-growing metaphysis (3) and it most commonly metastasizes to
bone and the lungs (4). The high
mortality rate of osteosarcoma is related to systemic metastasis
(5). Despite the multimodal
combination of chemotherapy and extensive tumor resection, the
5-year survival rate is only 60-70% (2,6).
Therefore, osteosarcoma requires further study. Gene therapy may be
a potential mode of osteosarcoma treatment (7).
Cell volume regulation has an important role in
various cellular functions, such as cell metabolism, proliferation,
migration and death (8,9). Channel proteins on the cell membrane
regulate the cell volume by controlling the movement of water and
electrolytes. Although channel proteins have been indicated to have
important roles in a variety of physiological processes, the
molecular mechanisms of volume-regulated anion channels (VRACs)
remain to be fully elucidated (10). Leucine-rich repeat-containing 8A
(LRRC8A) is a major molecular determinant of the VRAC current,
which has been proven by previous studies (11,12).
Although the primary function of VRACs is cell
volume regulation, they are considered potential targets for cancer
therapy in view of their important roles in cell proliferation,
migration and apoptosis of both normal and cancer cells (13-15).
LRRC8A has been isolated from and identified in patients with
congenital gammaglobulinemia (16).
It was reported to be involved in inflammation by supporting
TNF-α-induced superoxide production in vascular smooth-muscle cells
(17). In glioblastoma,
downregulation of LRRC8A inhibits proliferation and increases
sensitivity to temozolomide and carmustine (18). In colon cancer, LRRC8A was
determined to be highly expressed and it was indicated to promote
the growth and metastasis of cancer cells (19). In ovarian cancer and alveolar-cell
carcinoma, a decrease in LRRC8A may be a factor affecting cisplatin
resistance (20,21). However, the expression of LRRC8A in
osteosarcoma has remained elusive. Therefore, in the present study,
the expression profile of LRRC8A in osteosarcoma was determined
using tissue microarrays (TMAs) and immunohistochemistry (IHC). The
expression of LRRC8A in the nuclei and cytoplasm of U2OS tumor
cells and MC3T3-E1 osteoblast-like cells was determined using
reverse transcription-quantitative (RT-q)PCR.
Materials and methods
TMAs and pathology
Paraffin-embedded tumor TMAs (cat. no. BO481a) were
purchased from US Biomax, Inc. Specimens on the TMAs were from 40
patients (18 with osteosarcoma, 19 with chondrosarcoma and 3 with
giant-cell tumors of the bone) and 8 healthy subjects (6 of
marginal bone and bone marrow tissue, 2 of cartilage tissue).
Osteosarcoma grades IA-IIB and chondrosarcoma were classified into
lowly differentiated, moderately differentiated and
well-differentiated tissues. There were a total of 96 tissue
samples on the microarray, 2 from each patient.
IHC
TMAs were routinely dewaxed with xylene and hydrated
with an alcohol gradient. They were then subjected to antigen
retrieval using a 0.01 M sodium citrate buffer (pH 6.0, 95˚C for 15
min). Endogenous peroxidase was blocked with 3%
H2O2-methanol for 10 min at room temperature.
The TMAs were then incubated with normal goat serum
(UltraSensitive™ SP; KIT-9720; MAXIM) for 10 min at room
temperature to block non-specific binding. Subsequently, samples
were incubated overnight with ready-to-use primary antibody
(UltraSensitive™ SP; KIT-9720; MAXIM) at 4˚C according to
manufacturer's method. After three washes with PBS, the TMAs were
incubated with biotinylated goat anti-mouse/rabbit immunoglobulin G
secondary antibody (UltraSensitive™ SP; KIT-9720; MAXIM) for 10 min
at room temperature, according to manufacturer's method. Next, the
TMAs were incubated with streptavidin-peroxidase for 10 min at room
temperature. Finally, blots were visualized using diaminobenzidine
(DAB)/ACE (DAB color development kit; MAXIM) for 10 min and the
development was stopped by adding distilled water (according to
manufacturer's method).
Imaging and data analysis
IHC staining images were acquired using an Olympus
BX60 microscope (Olympus Corp.) equipped with a Leica DP70 digital
camera (Leica Microsystems). A pathologist (double-blinded) scored
staining using the previously described four-point system (score
0-3) (22) as follows: Score 3,
dark staining that was easily visible and present in >50% of
cells; score 2, focal areas of dark staining (<50% of cells) or
moderate staining in >50% of cells; score 1, focal moderate
staining in <50% of cells, or pale staining in any proportion of
cells not easily observable at low power; and score 0, none of the
above. A high level of expression was defined as a score of 2-3 and
a low level as a score of 0-1, as described previously.
Cell culture
The osteosarcoma cell line U2OS (Cell Bank of the
Chinese Academy of Sciences) and the osteoblast-like cell line
ME3T3-E1 (Cell Center, Institute of Basic Medicine, Chinese Academy
of Medical Sciences) were cultured in DMEM (Gibco; Thermo Fisher
Scientific, Inc.) containing 10% fetal bovine serum (Gibco; Thermo
Fisher Scientific, Inc.) and double antibodies (1% penicillin and
streptomycin). The medium was changed every 2-3 days and the
culture conditions in a humidified atmosphere (37˚C and 5%
CO2). When the cell confluence reached 80-90%, the cells
were used for the experiments.
RT-qPCR
A Cytoplasmic and Nuclear Ribonucleic Acid (RNA
Purification Kit; (Norgen Biotek Corp.) was used to separate and
extract cytoplasmic and nuclear RNA from U2OS and MC3T3-E1 cells.
They were immediately reverse-transcribed to complementary (c)DNA
using an PrimeScript™ RT reagent kit (cat. no. RR037A; Takara Bio,
Inc.), according to the manufacturer's protocol. The amplification
reaction conditions were 42˚C for 2 min and a hold at 4˚C. RT
conditions were 37˚C for 15 min, 85˚C for 5 sec and a hold at 4˚C.
Subsequently, qPCR was performed by using the kit (PrimeScript™ RT
reagent kit; cat. no. RR037A; Takara Bio, Inc.) on the extracted
cDNA, according to the manufacturer's protocols. SYBR®
Premix Ex Taq™ II (cat. no. RR820A; Takara Bio, Inc.) was used to
perform qPCR. The reaction conditions were as follows: Step 1, 95˚C
for 10 sec; Step 2, 95˚C for 5 sec and 60˚C for 30 sec, the cycle
was repeated for 40 times; and Step 3,95˚C for 15 sec, 60˚C for 60
sec and 95˚C for 15 sec. GAPDH was used as the internal reference
gene. The primers were as follows: GAPDH forward,
5'-GGCACAGTCAAGGCTGAGAATG-3' and reverse,
5'-ATGGTGGTGAAGACGCCAGTA-3'; LRRC8A forward,
5'-TCACAGCCAATAGGATTGAAGC-3' and reverse,
5'-CCTAGCCCAGTGCCAATAAG-3'. Ribozyme-free operation was ensured
throughout the process. The 2-ΔΔCq method was used to
determine the relative expression levels of LRRC8A (23).
Statistical analysis
Data were compared between groups using a
χ2 or Student's t-test. SPSS software version 21.0 (IBM
Corp.) was used for all analyses. All P-values were two-tailed and
P<0.05 was considered to indicate statistical significance.
Results
Subcellular localization of
LRRC8A
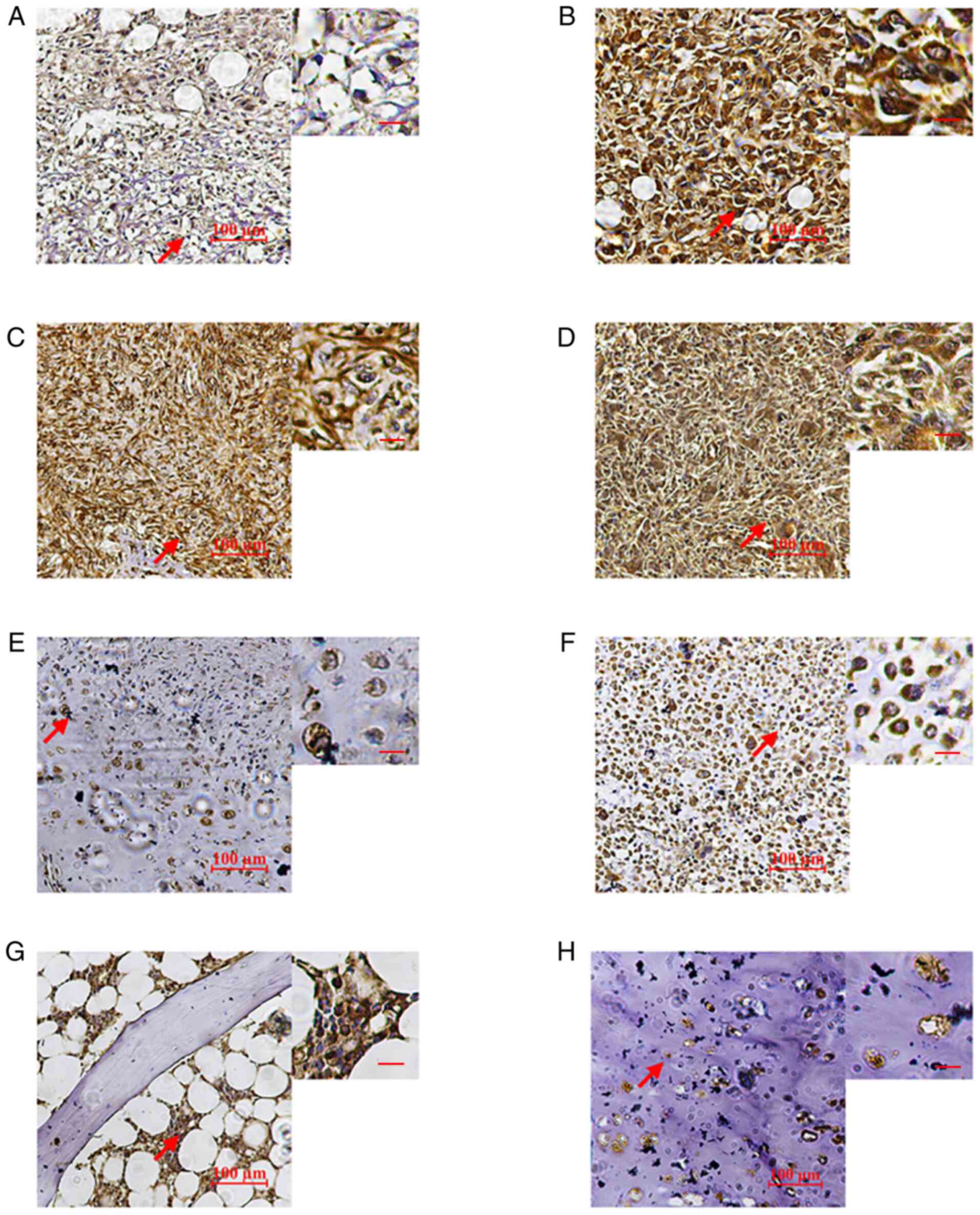
IHC analysis suggested that LRRC8A was present in
the nuclei and cytoplasm of osteosarcoma and giant-cell tumor cells
(Fig. 1A-D). In normal bone
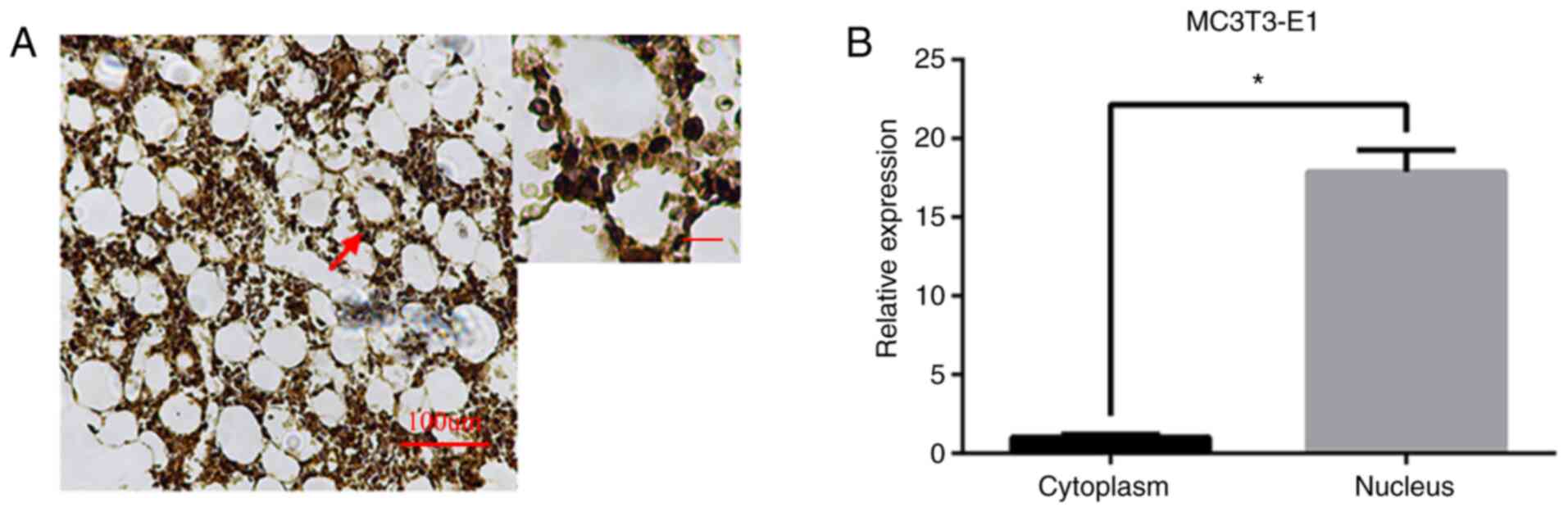
tissues, it was mainly expressed in the nucleus (Figs. 1G and 2). In MC3T3-E1 osteoblasts, the expression
of LRRC8A at the RNA level was mainly in the cytoplasm and the
difference was statistically significant (Fig. 2). In U2OS osteosarcoma cells, the
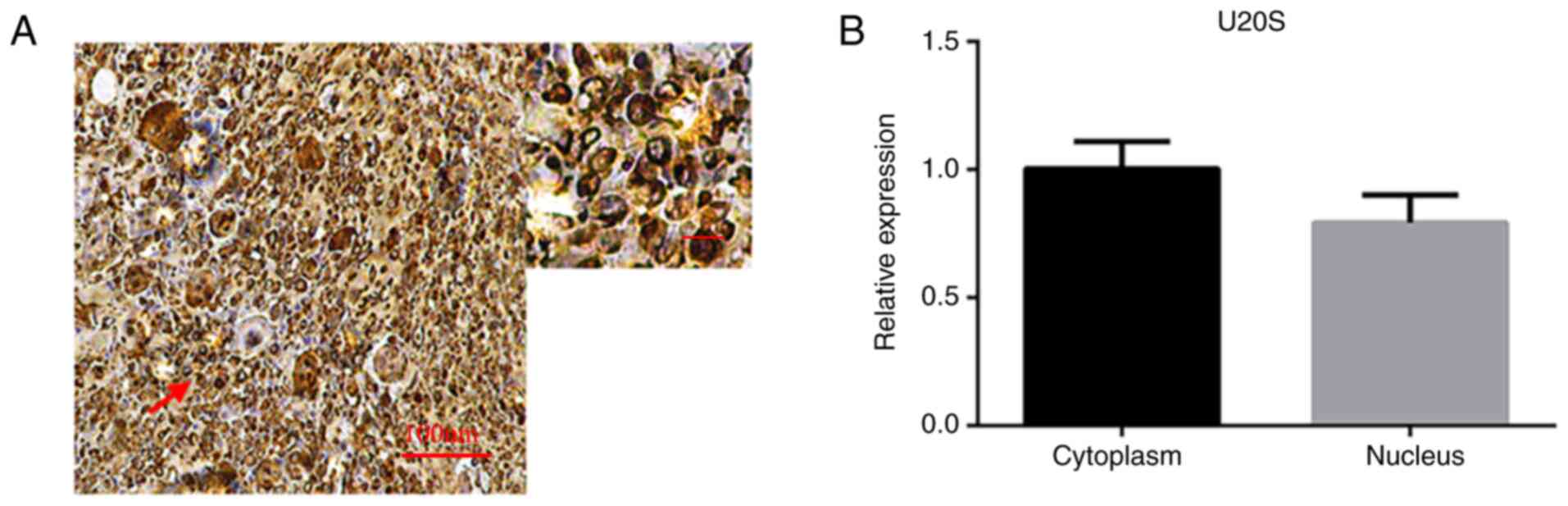
expression of LRRC8A at the RNA level was expressed in the nuclei
and cytoplasm and the difference was not statistically significant
(Fig. 3). The expression level of
LRRC8A was relatively low in chondrosarcoma and cartilage tissues.
However, the expression was slightly higher in poorly
differentiated chondrosarcoma (Fig.
1E, F and H).
Expression of LRRC8A in the cytoplasm
of bone tumors
Levels of LRRC8A immunoreactivity in the cytoplasm
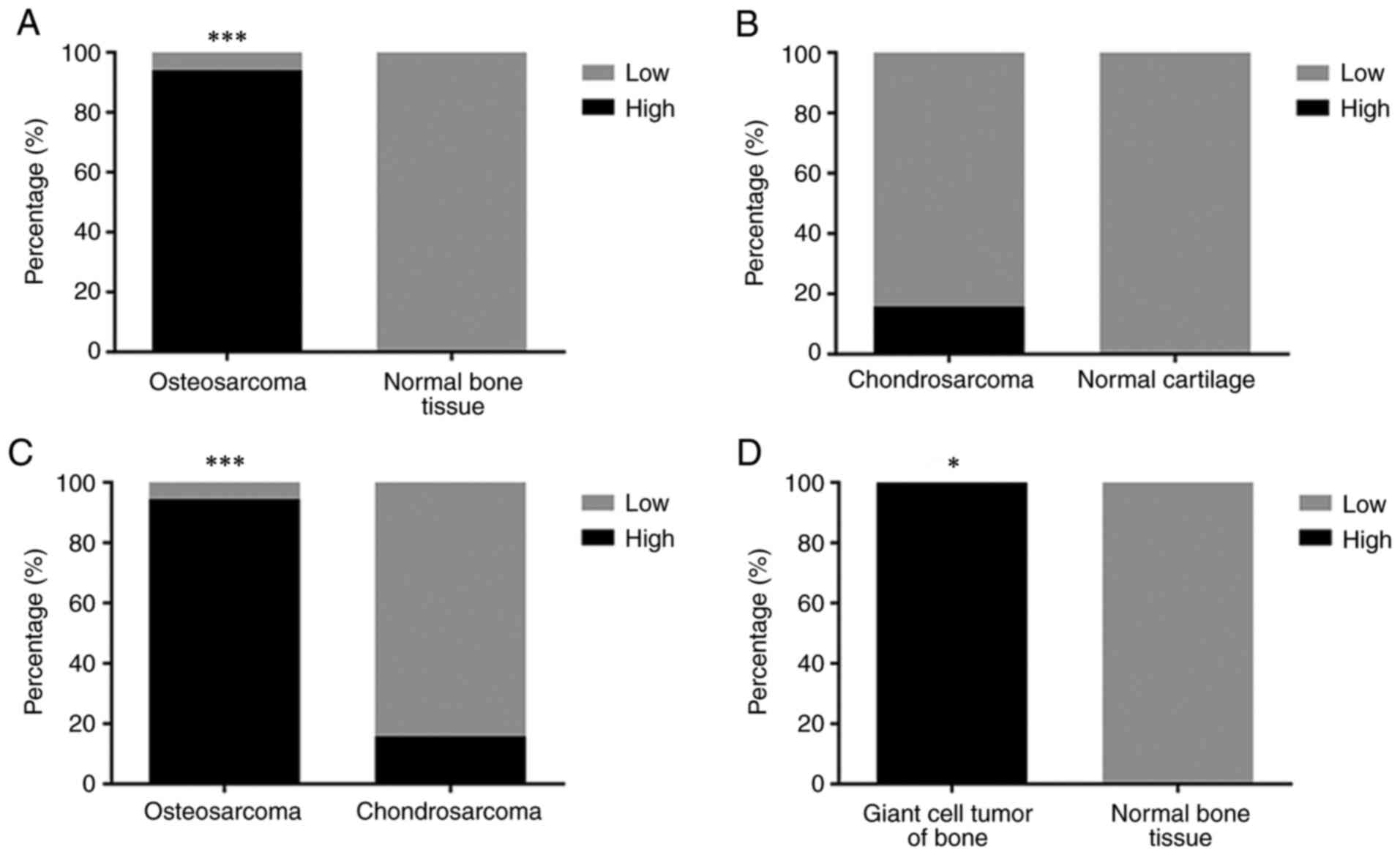
were compared between normal tissues and bone tumors. 18
osteosarcoma tissue samples on the TMA, 94% (17/18) exhibited high
levels of LRRC8A expression in the cytoplasm (score 2-3; Fig. 1A-C), whereas six bone tissue samples
did not. The cytoplasm of osteosarcoma samples had significantly
higher levels of LRRC8A than that of normal bone tissues
(P<0.001; Fig. 4A). Of the 19
chondrosarcoma samples, 16% (3/19) had high levels of LRRC8A
(Fig. 4B); overall, the expression
of LRRC8A in chondrosarcoma tissue did not significantly differ
from normal cartilage tissue (P>0.05; Fig. 4B). Osteosarcoma samples had high
levels of LRRC8A in the cytoplasm, differing significantly from
chondrosarcoma samples with this regard (P<0.001; Fig. 4C). Compared with those of normal
bone tissue, the cytoplasmic LRRC8A levels of giant-cell tumors of
the bone were significantly increase (P<0.05; Fig. 4D). In addition, although the number
of cases with tumor staging was insufficient, it may be observed
from Fig. 1 that a higher grade of
osteosarcoma was paralleled by a higher cytoplasmic expression
level of LRRC8A.
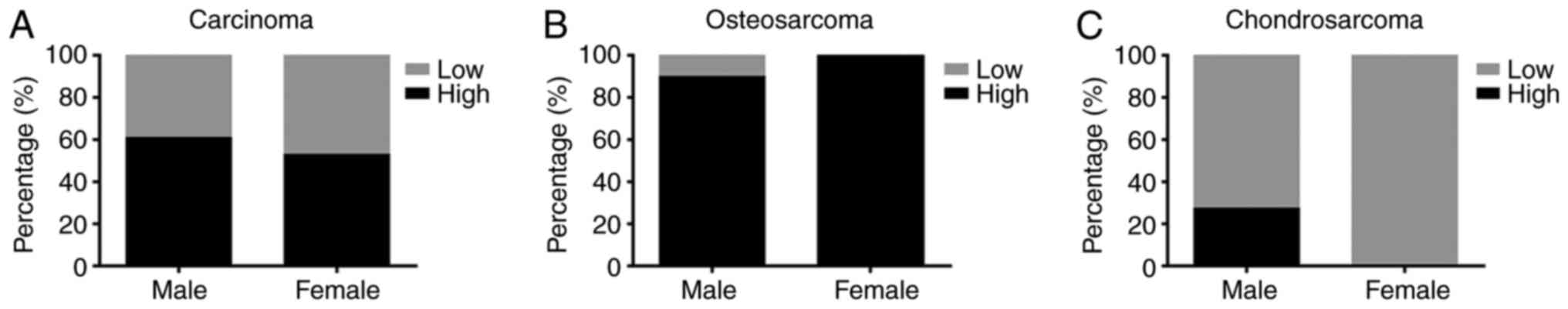
Sex-associated differences in LRRC8A
in bone tumors
The occurrence of bone tumors is sex-dependent;
osteosarcoma and chondrosarcoma occur more frequently in males than
in females (24,25). Therefore, in the present study, the
expression of LRRC8A was compared between bone tumors from males
and those from females. Of the 40 patients with bone tumors,
samples with high cytoplasmic LRRC8A levels accounted for 61%
(14/23) of males and 53% (9/17) of females and there was no
significant difference between sexes (P>0.05; Fig. 5A). Of the 18 patients with
osteosarcoma, only 1 (a male) had low expression of LRRC8A, while
the remainder (9 males and 8 females) had high expression of
LRRC8A, with no sex-related difference (P>0.05; Fig. 5B). Of the 19 patients with
chondrosarcoma, only 3 males had high expression of LRRC8A and the
other 16 patients (8 males, 8 females) had low expression thereof.
Therefore, no sex-related differences in LRRC8A expression were
detected (P>0.05; Fig. 5C).
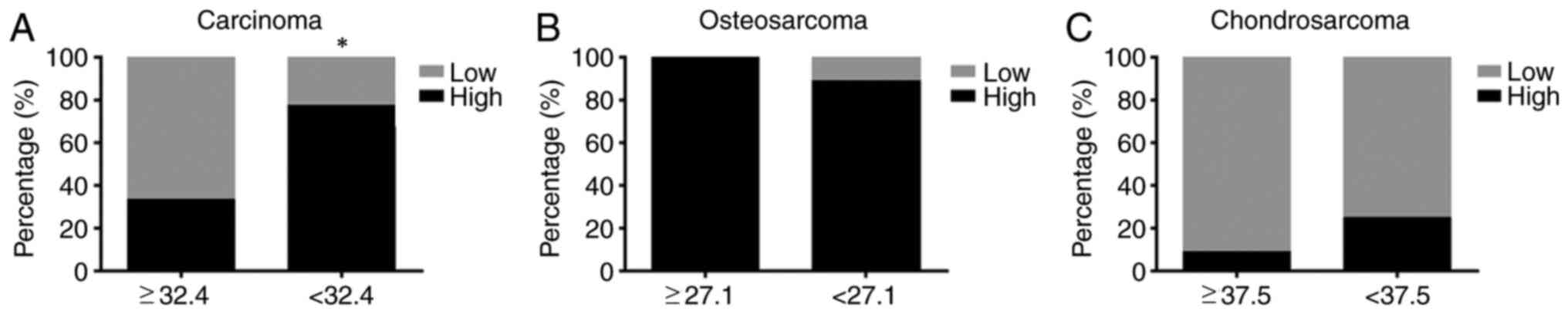
Age-associated differences in LRRC8A
in bone tumors
As the incidence of bone tumors also differs by age
(26); the effect of age on LRRC8A
immunoreactivity in bone tumor tissues was also investigated. Among
all bone tumor patients, the average age was 32.4 years. The
percentage of samples with high cytoplasmic expression of LRRC8A
was 33% (6/18) among those from patients aged ≥32.4 years and 77%
(17/22) among those from patients aged <32.4 years. Statistical
analysis indicated a significant difference between these two age
groups (P<0.05; Fig. 6A). Among
the 18 patients with osteosarcoma, the average age was 27.1 years.
In patients aged ≥27 years, 100% had high expression of LRRC8A and
in the group aged <27 years, 89% had high expression of LRRC8A.
Statistical analysis indicated no significant difference between
these two age groups (P>0.05; Fig.
6B). Among the 19 cases of chondrosarcoma, the average patient
age was 37.5 years. Only one patient aged ≥37.5 years and two
patients aged <37.5 years had high levels of LRRC8A and
statistical analysis indicated no significant difference between
these two age groups (P>0.05; Fig.
6C).
Discussion
The results of the TMA of the present study
indicated that LRRC8A was expressed in the cytoplasm and nuclei of
osteosarcoma cells, while it was mainly expressed in the nuclei of
normal bone tissue cells. To verify this phenomenon, RT-qPCR was
used to analyze the expression of LRRC8A in the nuclei and
cytoplasm of U2OS osteosarcoma cells and MC3T3-E1 osteoblast-like
cells and the results were consistent with the IHC results.
Analysis of the IHC images suggested that a higher degree of
malignancy in osteosarcoma was paralleled by a higher expression of
LRRC8A; furthermore, a lower degree of differentiation in
chondrosarcoma was also associated with higher expression of LRRC8A
(due to the low osteosarcoma grade). The present results also
suggested that in osteosarcoma, the expression of LRRC8A was
independent of sex and age. However, in all bone tumor samples,
younger patients exhibited higher levels of LRRC8A expression
(P<0.05). The results suggested that LRRC8A was increased in the
cytoplasm of osteosarcoma cells as compared with that of normal
bone tissue. The expression of LRRC8A in bone tumor cells was
preliminarily verified at the RNA and protein levels. It was
previously reported that LRRC8A is mainly expressed on the cell
membrane of colon cancer cells (19).
The limitations of the present study were as
follows: The expression of LRRC8A in osteosarcoma and normal bone
tissue at the RNA level was studied using U2OS osteosarcoma cells
and MC3T3-E1 osteoblast-like cell lines. LRRC8A expression was did
not quantitatively analyzed at the protein level in either
osteosarcoma or bone cells. In addition, the expression of LRRC8A
was not quantified in tissues from patients and healthy volunteers
and the use of 2 different cell lines is not sufficient. The
subcellular expression and functions of LRRC8A in osteosarcoma
require further study. For instance, the whole-cell patch-clamp
technique may be used to record the current difference between
osteosarcoma cells and normal bone cells (19). After blocking the LRRC8A chloride
channel with small interfering (si)RNA or
4-(2-Butyl-6,7-dichloro-2-cyclopentylindan-1-on-5-yl) oxybutyric
acid inhibitor, the difference in the change of the chloride
current may be observed in order to investigate the function of the
LRRC8A chloride channel in osteosarcoma. In recent years, the role
of LRRC8A in cancer has been researched. Knockdown of LRRC8A in
glioblastoma reduced cell proliferation and increased the
sensitivity of the cells to temozolomide and carmustine (18). After knockout of LRRC8A in HCT116
colon cancer cells, the chloride current and cell migration were
significantly inhibited and the incidence of tumors in nude mice
was also significantly reduced (19). In cisplatin-insensitive cells,
transient downregulation of LRRC8A reduced p53 activation and
contributed to cisplatin resistance in ovarian and lung cancer
cells (20). A study by Konishi
et al (27) suggested that
LRRC8A is of great significance to the proliferation, survival and
migration of esophageal squamous-cell carcinoma (ESCC) cell lines.
High expression of LRRC8A was determined to be an indicator of poor
prognosis in ESCC (27). Lu et
al (28) indicated that in the
process of cerebrovascular remodeling induced by angiotensin II,
the expression of LRRC8A in human-brain vascular smooth-muscle
cells (HBVSMCs) increases. In addition, siRNA-mediated knockout of
LRRC8A significantly inhibited the proliferation, migration and
invasion of HBVSMCs (28).
The above studies pointed out that high expression
of LRRC8A increases cell proliferation, migration and invasion,
whether in normal or tumor cells. However, completely reducing the
expression of LRRC8A in the cells of animals may negatively affect
the proliferation and migration of normal cells. For instance,
LRRC8A has an important role in the development and function of T
cells (29,30). This said, the location, roles and
mechanisms of LRRC8A in cells remain to be fully elucidated.
Therefore, the subcellular distribution of LRRC8A in cells and the
functions of different parts require to be further studied. Whether
the subcellular distribution and physiological functions of LRRC8A
differ between normal rapidly proliferating cells (such as stem
cells) and tumor cells also requires further study.
In conclusion, the present study suggested that
LRRC8A was more highly expressed in the cytoplasm of osteosarcoma
cells than in that of normal bone cells. The expression was also
associated with the degree of osteosarcoma malignancy. The
subcellular locations and physiological functions of LRRC8A in
normal rapidly proliferating cells (such as stem cells) and in
tumor cells require further study.
Acknowledgements
Not applicable.
Funding
This work was supported by the Natural Science
Foundation of China (grant nos. 81800785, 81572198 and 81772394),
Shenzhen Peacock Project (grant no. KQTD20170331100838136),
Shenzhen Science and Technology Projects (grant nos.
JCYJ20170817172023838, JCYJ20170306092215436,
JCYJ20170412150609690, JCYJ20170413161649437,
JCYJ20170413161800287, SGLH20161209105517753, JCYJ20160301111338144
and JCYJ20150330102720175) and the Fund for High-Level Medical
Discipline Construction of Shenzhen University (grant no.
2016031638).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
NZ and ZD performed the experiments, collected the
results and wrote the manuscript. WL, YZ and JX contributed to data
analysis and manuscript revision. LD and DW conceived the study and
contributed to reviewing/editing the manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by Shenzhen Second People's
Hospital (Shenzhen, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Heare T, Hensley MA and Dell'orfano S:
Bone tumors: Osteosarcoma and Ewing's sarcoma. Curr Opin Pediatr.
21:365–372. 2009.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Mirabello L, Troisi RJ and Savage SA:
International osteosarcoma incidence patterns in children and
adolescents, middle ages and elderly persons. Int J Cancer.
125:229–234. 2009.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Eppert K, Wunder JS, Aneliunas V, Kandel R
and Andrulis IL: von Willebrand factor expression in osteosarcoma
metastasis. Mod Pathol. 18:388–397. 2005.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Laverdiere C, Hoang BH, Yang R, Sowers R,
Qin J, Meyers PA, Huvos AG, Healey JH and Gorlick R: Messenger RNA
expression levels of CXCR4 correlate with metastatic behavior and
outcome in patients with osteosarcoma. Clin Cancer Res.
11:2561–2567. 2005.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Urakawa H, Nishida Y, Nakashima H,
Shimoyama Y, Nakamura S and Ishiguro N: Prognostic value of
indoleamine 2,3-dioxygenase expression in high grade osteosarcoma.
Clin Exp Metastasis. 26:1005–1012. 2009.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bacci G, Ferrari S, Tienghi A, Bertoni F,
Mercuri M, Longhi A, Fiorentini G, Forni C, Bacchini P, Rimondini
S, De Giorgi U and Picci P: A comparison of methods of
loco-regional chemotherapy combined with systemic chemotherapy as
neo-adjuvant treatment of osteosarcoma of the extremity. Eur J Surg
Oncol. 27:98–104. 2001.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tan ML, Choong PF and Dass CR:
Osteosarcoma: Conventional treatment vs. gene therapy. Cancer Biol
Ther. 8:106–117. 2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lang F, Busch GL, Ritter M, Völkl H,
Waldegger S, Gulbins E and Häussinger D: Functional significance of
cell volume regulatory mechanisms. Physiol Rev. 78:247–306.
1998.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Pedersen SF, Hoffmann EK and Novak I: Cell
volume regulation in epithelial physiology and cancer. Front
Physiol. 4(233)2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Pedersen SF, Klausen TK and Nilius B: The
identification of a volume-regulated anion channel: An amazing
Odyssey. Acta Physiol (Oxf). 213:868–881. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Syeda R, Qiu Z, Dubin AE, Murthy SE,
Florendo MN, Mason DE, Mathur J, Cahalan SM, Peters EC, Montal M
and Patapoutian A: LRRC8 proteins form volume-regulated anion
channels that sense ionic strength. Cell. 164:499–511.
2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Trothe J, Ritzmann D, Lang V, Scholz P,
Pul Ü, Kaufmann R, Buerger C and Ertongur-Fauth T: Hypotonic stress
response of human keratinocytes involves LRRC8A as component of
volume-regulated anion channels. Exp Dermatol. 27:1352–1360.
2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Pedersen SF, Okada Y and Nilius B:
Biophysics and physiology of the volume-regulated anion channel
(VRAC)/Volume-sensitive outwardly rectifying anion channel (VSOR).
Pflugers Arch. 468:371–383. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kunzelmann K: Ion channels in regulated
cell death. Cell Mol Life Sci. 73:2387–2403. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Xu B, Jin X, Min L, Li Q, Deng L, Wu H,
Lin G, Chen L, Zhang H, Li C, et al: Chloride channel-3 promotes
tumor metastasis by regulating membrane ruffling and is associated
with poor survival. Oncotarget. 6:2434–2450. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sawada A, Takihara Y, Kim JY,
Matsuda-Hashii Y, Tokimasa S, Fujisaki H, Kubota K, Endo H, Onodera
T, Ohta H, et al: A congenital mutation of the novel gene LRRC8
causes agammaglobulinemia in humans. J Clin Invest. 112:1707–1713.
2003.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Choi H, Ettinger N, Rohrbough J, Dikalova
A, Nguyen HN and Lamb FS: LRRC8A channels support TNFα-induced
superoxide production by Nox1 which is required for receptor
endocytosis. Free Radic Biol Med. 101:413–423. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Rubino S, Bach MD, Schober AL, Lambert IH
and Mongin AA: Downregulation of leucine-rich repeat-containing 8A
limits proliferation and increases sensitivity of glioblastoma to
temozolomide and carmustine. Front Oncol. 8(142)2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhang H, Deng Z, Zhang D, Li H, Zhang L,
Niu J, Zuo W, Fu R, Fan L, Ye JH and She J: High expression of
leucinerich repeatcontaining 8A is indicative of a worse outcome of
colon cancer patients by enhancing cancer cell growth and
metastasis. Oncol Rep. 40:1275–1286. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Sorensen BH, Nielsen D, Thorsteinsdottir
UA, Hoffmann EK and Lambert IH: Downregulation of LRRC8A protects
human ovarian and alveolar carcinoma cells against
cisplatin-induced expression of p53, MDM2, p21Waf1/Cip1, and
caspase-9/-3 activation. Am J Physiol Cell Physioly. 310:C857–C873.
2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sorensen BH, Dam CS, Sturup S and Lambert
IH: Dual role of LRRC8A-containing transporters on cisplatin
resistance in human ovarian cancer cells. J Inorg Biochem.
160:287–295. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Liu C, Zhang Y, Zhang K, Bian C, Zhao Y
and Zhang J: Expression of estrogen receptors, androgen receptor
and steroid receptor coactivator-3 is negatively correlated to the
differentiation of astrocytic tumors. Cancer Epidemiol. 38:291–297.
2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Mirabello L, Troisi RJ and Savage SA:
Osteosarcoma incidence and survival rates from 1973 to 2004: Data
from the Surveillance, Epidemiology, and End Results Program.
Cancer. 115:1531–1543. 2009.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Karpik M and Reszeć J: Low grade
chondrosarcoma-epidemiology, diagnosis, treatment. Ortop Traumatol
Rehabil. 20:65–70. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Estrada-Villaseñor EG, Flores-Carmona JF,
Delgado-Cedillo EA and Rico-Martínez G: Bone tumor frequency in
adults and elderly. Acta Ortop Mex. 22:356–360. 2008.PubMed/NCBI(In Spanish).
|
|
27
|
Konishi T, Shiozaki A, Kosuga T, Kudou M,
Shoda K, Arita T, Konishi H, Komatsu S, Kubota T, Fujiwara H, et
al: LRRC8A expression influences growth of esophageal squamous cell
carcinoma. Am J Pathol. 189:1973–1985. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lu J, Xu F and Zhang J: Inhibition of
angiotensin II-induced cerebrovascular smooth muscle cell
proliferation by LRRC8A downregulation through suppressing PI3K/AKT
activation. Hum Cell. 32:316–325. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Platt CD, Chou J, Houlihan P, Badran YR,
Kumar L, Bainter W, Poliani PL, Perez CJ, Dent SYR, Clapham DE, et
al: Leucine-rich repeat containing 8A.
|
|
30
|
LRRC8A)-dependent volume-regulated anion
channel activity is dispensable for T-cell development and
function. J Allergy Clin Immunol. 140:1651–1659.e1. 2017.PubMed/NCBI View Article : Google Scholar
|