Introduction
Rheumatoid arthritis is a chronic inflammatory
disease caused by the dysregulation of the autoimmune system, which
severely threatens the individual's health (1). Approximately 1% of the population
suffers from rheumatoid arthritis worldwide, placing a heavy burden
on economy and health (2). Chronic
inflammation in rheumatoid arthritis leads to synovial hyperplasia
and serious damage in the joints and cartilage (3). Rheumatoid arthritis is confirmed as a
progressive disease and is also able to induce numerous
implications on osteoporosis, myocarditis, neuropathy and pleurisy
(4-6).
Genetic and environmental factors are considered as the major
contributors to rheumatoid arthritis (7,8).
Furthermore, the exact mechanism of the pathogenesis and
progression of rheumatoid arthritis remains unknown (9). The major characteristic of rheumatoid
arthritis is synovitis (10).
Normal synovium mainly consists of fibroblast-like synoviocytes and
acts as a nutrient supplier for articular cartilage and protector
of joint structures or adjacent tissues (10). The number of synovial cells are
significantly increased in rheumatoid arthritis and are capable of
causing destruction in the joint via the release of
pro-inflammatory factors (11,12).
In addition, synovitis is also involved in osteoarthritis. During
osteoarthritis, stromal vascularization, fibrosis and hyperplasia
occur in the synovium (13).
Synovitis is closely associated with dysfunction and damage of the
joint, as well as pain, and also contributes to the degeneration of
cartilage in osteoarthritis (14).
Therefore, it is important to find an effective therapeutic agent
for synovitis and elucidate its mechanism, which will be beneficial
for both the treatment of rheumatoid arthritis and
osteoarthritis.
Icariin (ICA) as a bioactive monomer extracted from
Epimedium in the Berberidaceae family, which exerts
anticancer, antiaging, neuroprotective and anti-inflammation
effects (15-18).
ICA is confirmed as a promising agent for the treatment of
rheumatoid arthritis and osteoarthritis in several previous
studies. ICA was reported to inhibit osteoarthritis via suppressing
pyroptosis mediated by NLRP3(19).
ICA repressed the death and angiogenesis of chondrocytes in a rat
osteoarthritis model via the modulation of the TDP-43 signaling
pathway (20), enhanced chondrocyte
viability via promoting anaerobic glycolysis and the expression of
hypoxia-inducible factor-1α (21),
exerted an immune-suppressive role in rheumatoid arthritis
(22) and ameliorated rheumatoid
arthritis in in a murine model of rheumatoid arthritis (23). However, the mechanism of ICA in
rheumatoid arthritis and osteoarthritis is not fully elucidated,
and clarifying the concrete mechanism is beneficial for developing
more therapeutic drugs based on this compound.
Synovitis, as the crucial pathological process in
rheumatoid arthritis and osteoarthritis, is a vital research topic.
During synovitis, oxidative stress and lipid peroxidation are all
involved and are confirmed as vital contributors to the
pathological progress (24-26).
Moreover, oxidative stress and lipid peroxidation are two major
causes for ferroptosis, which is an iron-dependent, non-apoptotic
form of cell death, characterized by the intracellular accumulation
of reactive oxygen species. Thus, it is speculated that ferroptosis
is a crucial process for injury in synovitis (27). Simultaneously, ICA has a regulatory
role on iron metabolism (28).
Therefore, the treatment effects of ICA may be related to the
modulation of ferroptosis. In the present study, LPS-induced
synovitis served as the cell model and the association between ICA
and ferroptosis was investigated in the cell model of
synovitis.
Materials and methods
Cell culture and treatment
Human synoviocytes (HUM-CELL-0060; Wuhan PriCells
Biomedical Technology Co., Ltd.) were cultured in DMEM (Thermo
Fisher Scientific, Inc.) supplemented with 1%
streptomycin-penicillin, 2 mM L-glutamine and 10% FBS (Gibco;
Thermo Fisher Scientific, Inc.) in an incubator with 5%
CO2 at 37˚C. Cells at >80% confluence were treated
with LPS (1 µg/ml; Sigma-Aldrich; Merck KGaA) for 24 h to establish
a synovitis cell model as previously described (29). The cells were treated with LPS for
24 h, followed by pretreatment with 2 (low), 5 (mid) or 10 µM
(high) ICA for 24 h. Cells with no treatment served as the control
group. Next, in order to further investigate the potential
mechanism of action of ICA, RSL3 was used to induce ferroptosis in
synoviocyte. We pre-treated synoviocyte with RSL3 (1 µM, cat. no.
HY-100218A, MedChemExpress, China) for 8 h. After that, cells were
incubated with ICA for 24 h and used for further experiments.
Measurement of cell death
Following LPS and ICA treatment, cells were digested
by trypsin (0.25%) and resuspended in binding buffer. Subsequently,
the cells were incubated with Annexin V-FITC for 15 min at 37˚C in
the dark. Subsequently, propidium iodide was added into the cells
for 2 min at 37˚C before measurement. Flow cytometry (BD FACSCanto
II; BD Biosciences) was used for cell death measurement. The
apoptotic cells were analyzed using a FlowJo™ v10.7 software
(Becton-Dickinson & Company). The levels of cell death were
calculated using the formula: Cell death ratio=Q1 + Q2 + Q3.
Quantification of malondialdehyde
(MDA), iron and glutathione peroxidase 4 (GPX4) activity
levels
The levels of MDA, iron and GPX4 activity were
determined using their corresponding kits. MDA was detected via
using the Lipid Peroxidation (MDA) Assay kit (cat. no. ab118970;
Abcam), iron was determined by using the Iron Assay kit (cat. no.
ab83366; Abcam) and GPX4 was detected using the GPX activity
detection kit (cat. no. BC1195; Beijing Solarbio Science &
Technology Co., Ltd.). All detection processes were performed
according to the manufacturer's protocol.
Western blotting
The cells were harvested after treatment and,
following lysis in NP40 buffer (Beijing Solarbio Science &
Technology Co., Ltd.), the supernatant was centrifuged at 4˚C at
12,000 x g for 15 min for collecting the samples. The BCA protein
Assay kit (cat. no. P0012S; Beyotime Institute of Biotechnology)
was used to quantify protein samples. Subsequently, separation of
the total protein was performed on 10% SDS-PAGE. Next, the proteins
were transferred to polyvinyl difluoride membranes (Bio-Rad
Laboratories, Inc.). Following blocking with 5% skim milk at room
temperature for 1 h, the membranes were incubated with primary
antibodies against GPX4 (1:10,000, cat. no. ab125066; Abcam),
cystine/glutamate transporter (1:10,000, SLC7A11; cat. no. ab37185;
Abcam), 4F2 cell-surface antigen heavy chain (1:1,000, SLC3A2L;
cat. no. ab215952; Abcam), transferrin receptor protein (1:1,000,
TFR; cat. no. ab84036; Abcam), nuclear factor erythroid 2-related
factor 2 (1:500, Nrf2; cat. no. ab62352; Abcam), nuclear receptor
coactivator 4 (1:100, NCOA4; cat. no. ab62495; Abcam) and GADPH
(1:1,000, cat. no. ab8245; Abcam) overnight at 4˚C. Subsequently,
HRP-conjugated secondary antibody (HRP goat anti-rabbit, 1:20,000,
cat. no. ab97051; HRP goat anti-mouse, cat. no. ab205719, 1:10,000)
was incubated with the membranes at room temperature for 2 h. ECL
Plus Western Blotting Substrate (Pierce; Thermo Fisher Scientific,
Inc.) was used to visualize protein bands, and ImageJ software
(version 1.52v; National Institutes of Health) was used for
quantification.
Cell Counting Kit-8 (CCK-8) assay
Cell viability was determined using a CCK-8 assay.
The cells were cultured in 96-well plates at a density of
1x105 cells/well. After treatment, CCK-8 reagent (10 µl;
Beyotime Institute of Biotechnology) was added to the cells. After
incubation for 2 h, the absorbance of cells at a wavelength of 450
nm was determined in the different study groups using a microplate
reader (Omega Bio-Tek, Inc.).
Statistical analysis
Data were processed using SPSS 18.0 software (SPSS,
Inc.) and GraphPad Prism 8.0 (GraphPad Software, Inc.) and shown as
the mean ± SD. One-way ANOVA with Tukey's test was used for
evaluating significant differences between groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
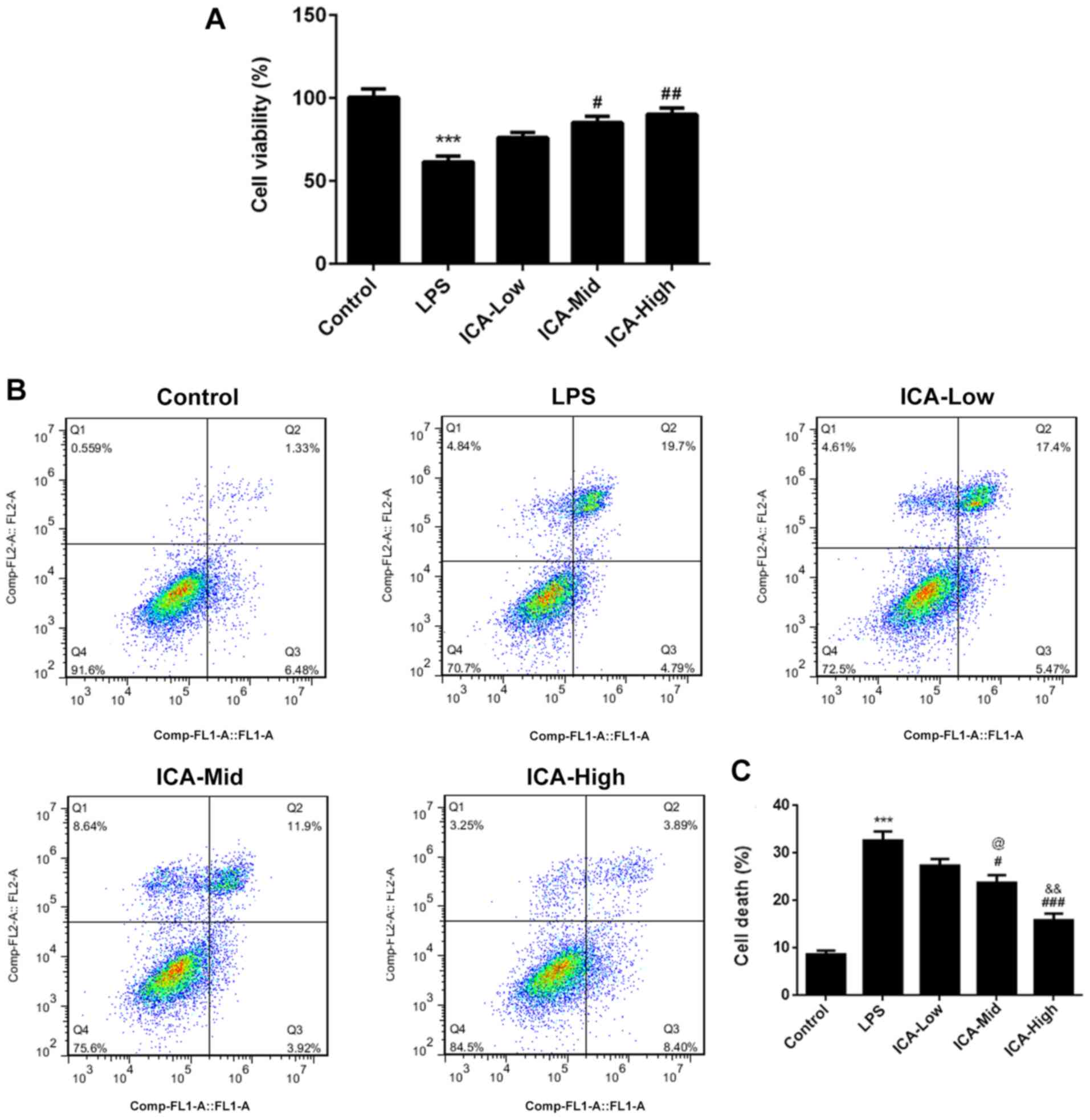
ICA attenuates cell death and
increases cell viability in LPS-induced synoviocytes
In the LPS group, cell viability significantly
decreased (Fig. 1A) and cell death
significantly increased compared with the control group (Fig. 1B and C), suggesting that the synovitis cell
model was successfully established. Simultaneously, LPS-induced
cell death was decreased by ICA and LPS-induced cell viability was
increased by ICA in a concentration-dependent manner, suggesting
that ICA has protective effects in LPS-induced synoviocytes.
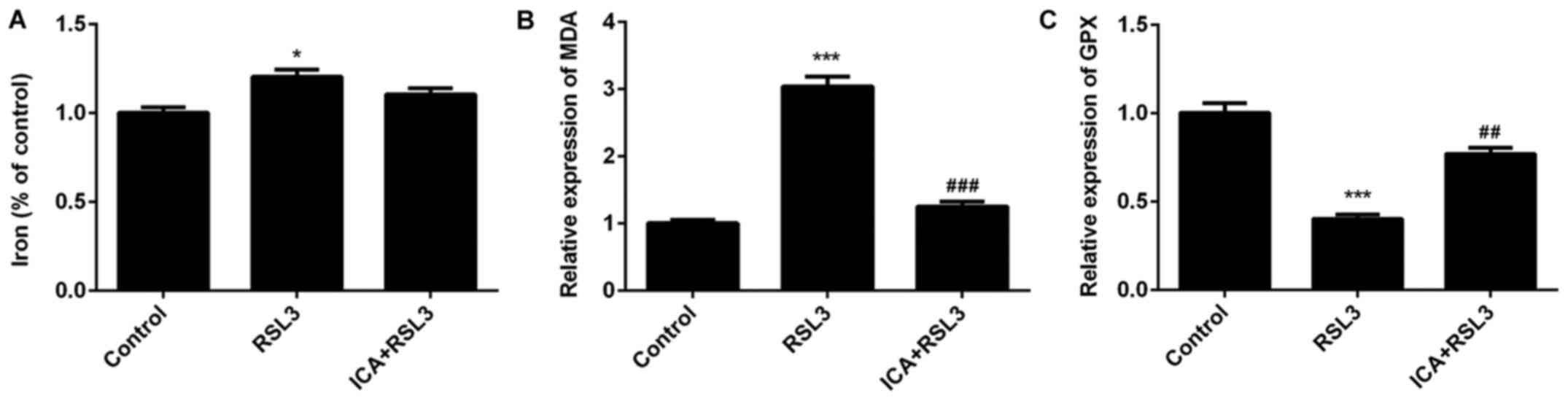
ICA reduces MDA levels and iron
content and increases GPX levels in a concentration-dependent
manner in LPS-induced synoviocytes
Lipid peroxidation is a type of oxidative stress and
is a vital process in rheumatoid arthritis and osteoarthritis
(30,31). MDA, as the common biomarker of lipid
peroxidation (32), was detected in
the present study. There was a significant increase in MDA levels
in the LPS group compared with the control (Fig. 2A), suggesting that lipid
peroxidation levels are high in the synovitis cell model. MDA
levels induced by LPS was reduced by MDA in a
concentration-dependent manner, indicating that ICA has attenuative
effects on lipid peroxidation in LPS-induced synoviocytes. Iron was
confirmed as the stimulator of oxidative stress and iron overload
(33,34). In the present study, the iron
content was significantly increased in the LPS group compared with
the control group. The iron content induced by LPS was reduced by
ICA in a concentration-dependent manner (Fig. 2B), demonstrating that ICA could
attenuate the iron deposit in LPS-induced synoviocytes. GPX, an
antioxidant enzyme (35), was also
detected in the present study. GPX levels were significantly
reduced by LPS compared with the control group. The levels of GPX
induced by LPS was further increased by ICA in a
concentration-dependent manner. These results suggested that ICA
exerted antioxidant effects by reducing MDA levels and iron content
and increasing GPX levels in LPS-induced synoviocytes.
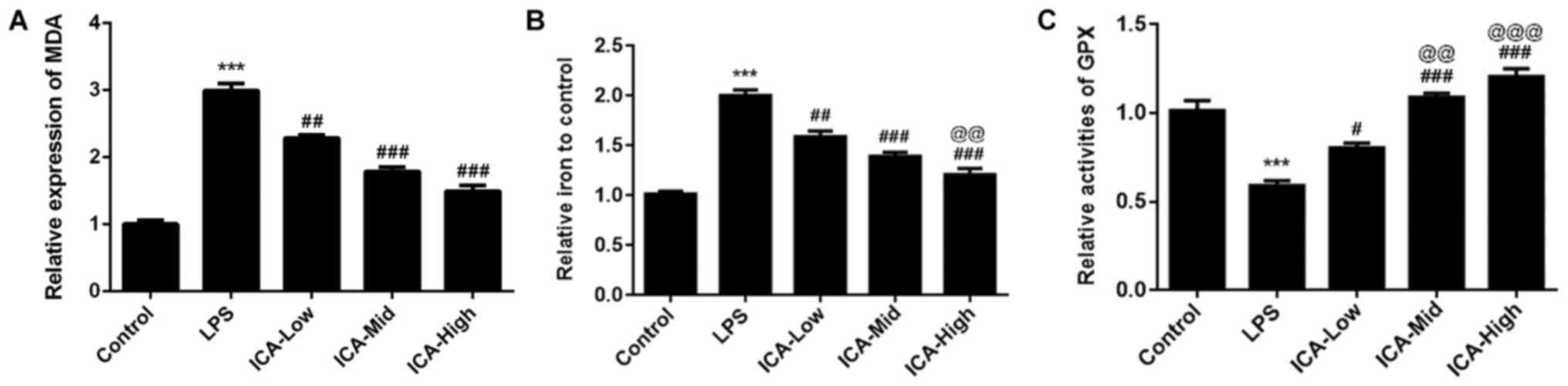 | Figure 2Effects of ICA on the levels of MDA,
iron content and GPX. The levels of (A) MDA, (B) iron content and
(C) GPX in the study groups. Experimental data were obtained from
five independent experiments and shown as the mean ± standard
deviation. @@P<0.01 and @@@P<0.001 vs. ICA-Low;
***P<0.001 vs. control; #P<0.05,
##P<0.01 and ###P<0.001 vs. LPS. ICA,
icariin; ICA-Low, 2 µM ICA; ICA-Mid, 5 µM ICA; ICA-High, 10 µM ICA;
LPS, lipopolysaccharide; MDA, malondialdehyde; GPX, glutathione
peroxidase. |
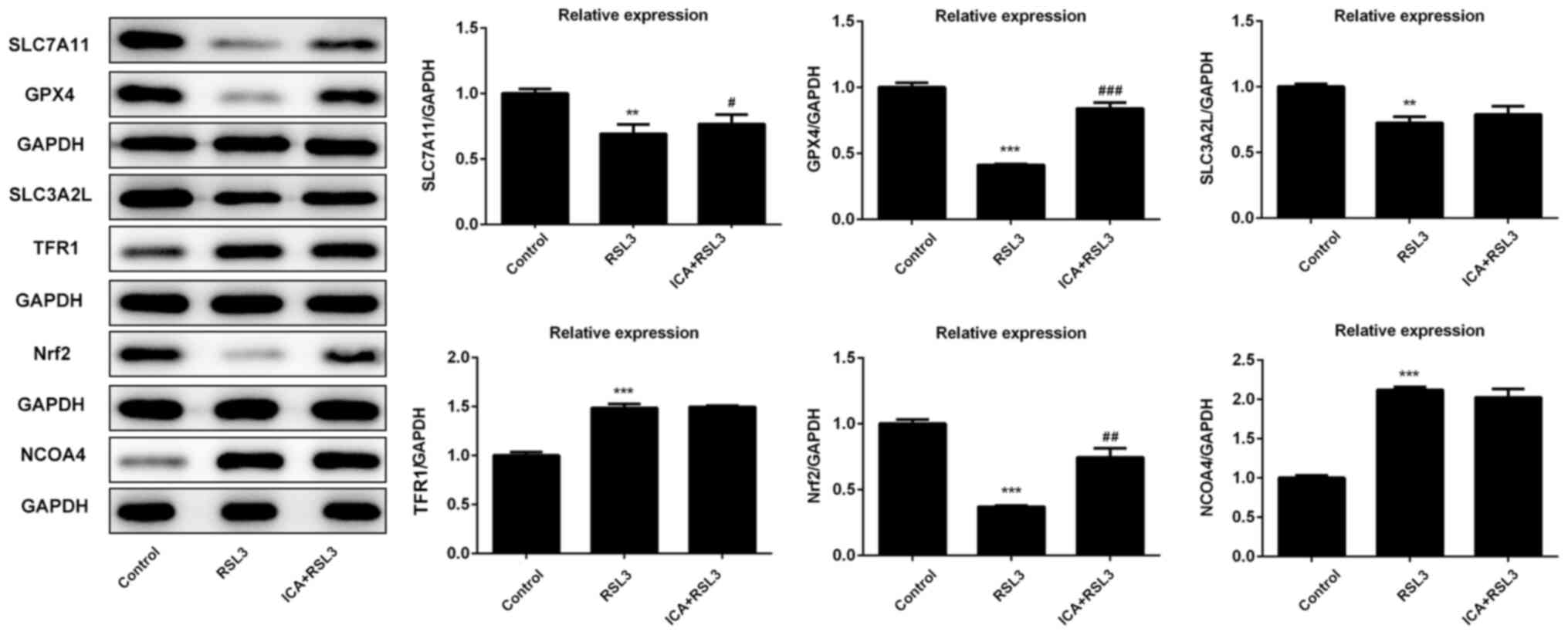
ICA regulates the relative expressions
level of ferroptosis proteins in LPS-induced synoviocytes
Lipid peroxidation and iron metabolism disorder,
which are the major inducers of ferroptosis (36), both occurred in LPS-induced
synoviocytes. Considering the attenuative effects of ICA on iron
content and lipid peroxidation levels, it was speculated that there
may be an association between ferroptosis and ICA. Xc-/GPX4, which
is capable of suppressing lipid peroxidation, is a vital pathway
for the inhibition of ferroptosis (37). In the current study, GPX4, SLC7A11
and SLC3A2L expression were all significantly decreased by LPS
compared with controls, and this effect was further inhibited by
ICA (Fig. 3), confirming that ICA
inhibited ferroptosis via the activation of Xc-/GPX4. Pathways that
are associated with iron metabolism were also investigated in the
present study. TFR1, a vital importer for iron transport into the
cells (38), was significantly
upregulated by LPS compared with the control group, which is
consistent with the high levels of iron content in the LPS group.
The TFR1 levels induced by LPS was decreased by ICA in a
concentration-dependent manner, suggesting that inhibition of TFR1
is one of the avenues for the inhibitory effect of ICA on
ferroptosis in LPS-induced synoviocytes. Nrf2 acts as a crucial
factor for inhibition of ferroptosis (39), which was significantly reduced by
LPS and further elevated by ICA in a concentration-dependent manner
in LPS-induced cells. NCOA4 acts as mediator of ferritinophagy,
resulting in iron accumulation and ferroptosis (40). As shown by the present data, NCOA4
levels were significantly upregulated in the LPS group compared
with controls. Following ICA treatment, the NCOA4 levels induced by
LPS gradually decreased upon increasing concentrations of ICA.
Collectively, ICA inhibited ferroptosis via the activation of
Xc-/GPX4 and Nrf2, and inhibition of TFR1 and NCOA4.
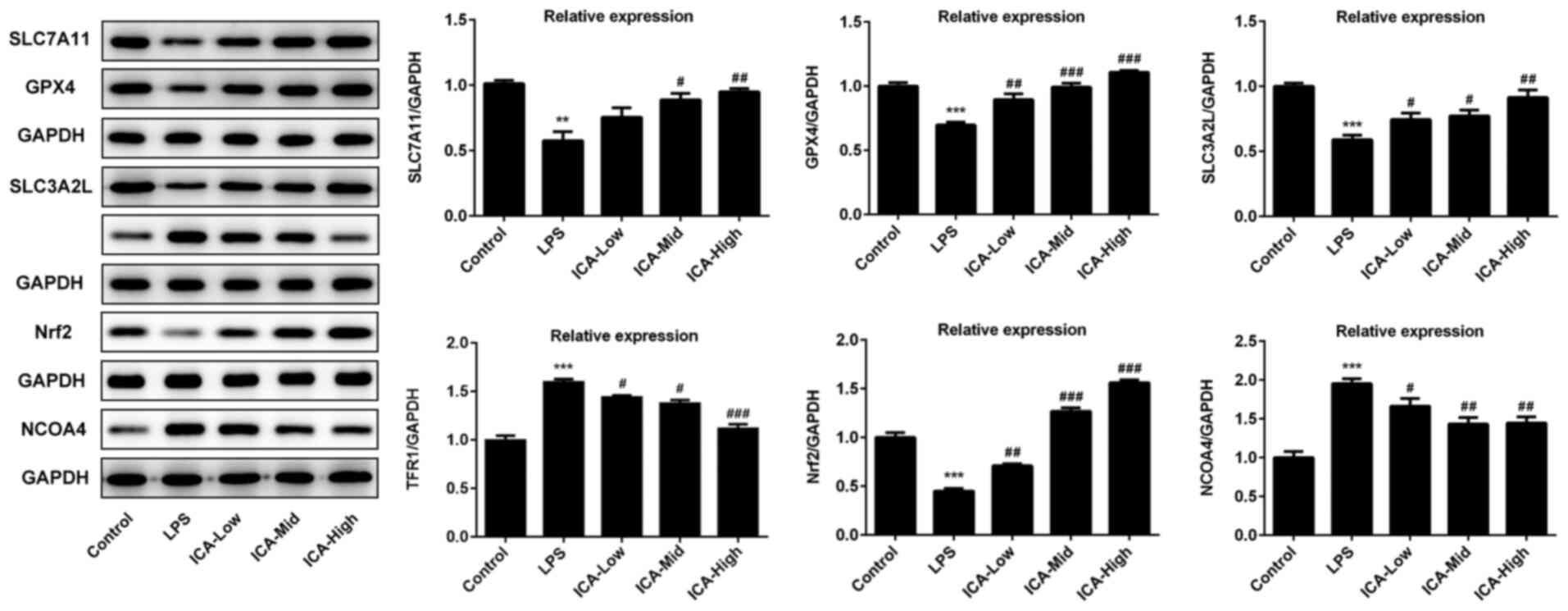 | Figure 3Effects of ICA on the levels of
ferroptosis-associated proteins. The levels of proteins associated
with ferroptosis in the study groups. The representative blot of
five independent experiments with similar results are expressed as
the mean ± standard deviation. ***P<0.001 vs.
control; **P<0.01 vs. control; #P<0.05,
##P<0.01 and ###P<0.001 vs. LPS. ICA,
icariin; ICA-Low, 2 µM ICA; ICA-Mid, 5 µM ICA; ICA-High, 10 µM ICA;
LPS, lipopolysaccharide; SLC7A111, cystine/glutamate transporter;
GPX, glutathione peroxidase; SLC3A2L, 4F2 cell-surface antigen
heavy chain; TFR1, transferrin receptor protein 1; Nrf2, nuclear
factor erythroid 2-related factor 2; NCOA4, nuclear receptor
coactivator 4. |
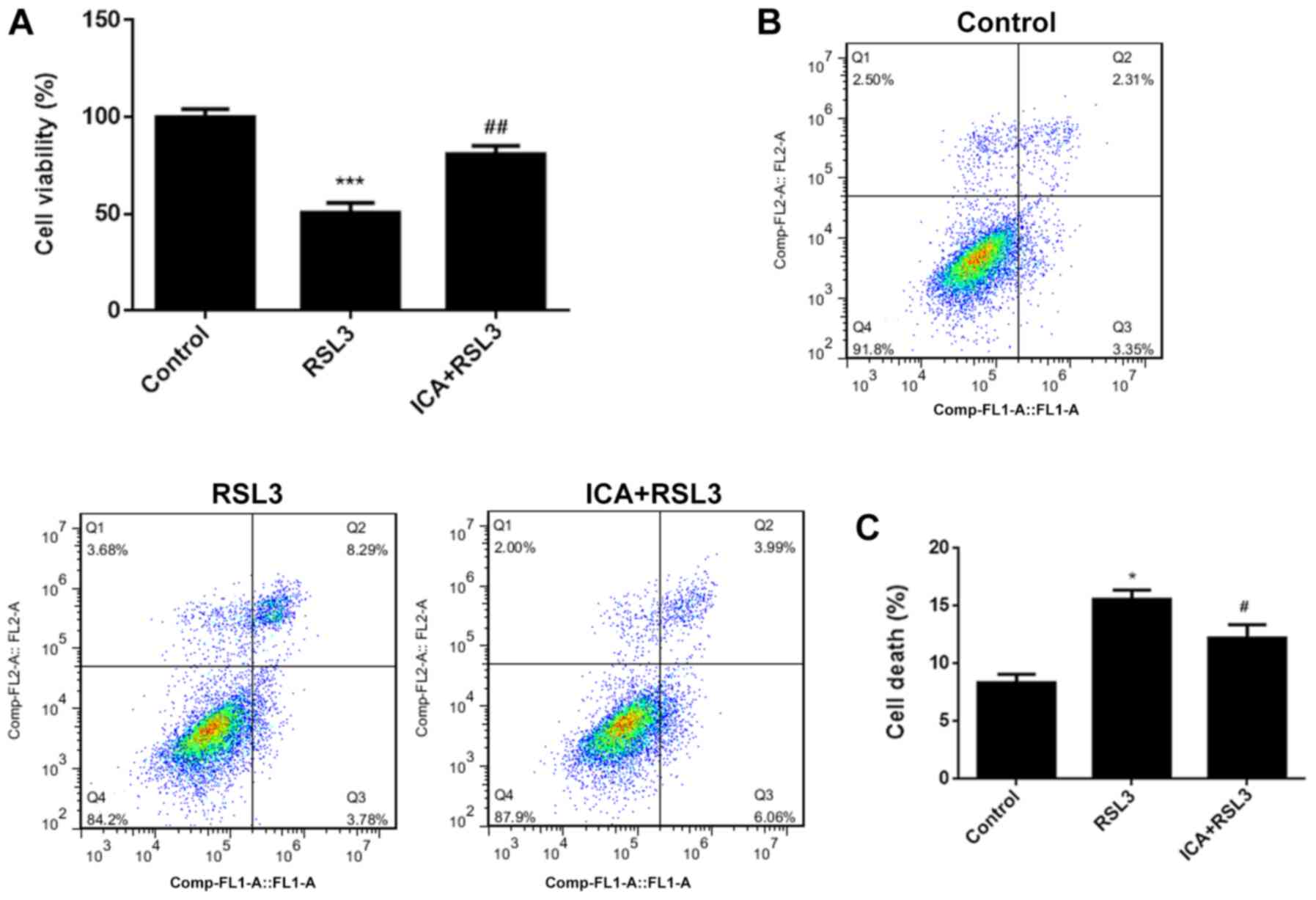
ICA attenuates cell death via
inhibition of ferroptosis by activating the Xc-/GPX4 axis
In order to further investigate the underlying
mechanism, the effects of ICA after blockage of Xc-/GPX4 axis with
RSL3 was further assessed. RSL3 is known to be a type of
ferroptosis activator (41). As
shown by the data, the cell viability was significantly reduced and
cell death was increased in the RSL3 group compared with the
controls (Fig. 4A-C), confirming
that marked ferroptotic cell death was observed after RSL3
induction. Iron and MDA content significantly increased and GPX
levels significantly decreased in the RSL3 group compared with the
ICA + RSL3 group (Fig. 5A-C). Thus,
the effects of RSL on lipid peroxidation and iron content were
counteracted by ICA and the effects of ICA in cells were achieved
via activation of the Xc-/GPX4 axis. The expression of
ferroptosis-associated proteins was also investigated. Compared
with the ICA + RSL3 group, Xc-/GPX4-associated proteins (SLC7A11,
GPX4 and SLC3A2L) and Nrf2 expression were significantly reduced by
RSL3, demonstrating that Xc-/GPX4 and Nrf2 were activated by ICA
(Fig. 6). Following the blockage of
the Xc-/GPX4 axis, there was almost no difference in the levels of
NCOA4 and TFR1 between the ICA + RSL3 group and RSL3 group,
suggesting that NCOA4 and TFR1 were not the major players in the
mechanism of ICA. All these results support that the Xc-/GPX4 axis
is involved in the effects of ICA via the modulation of
ferroptosis.
Discussion
ICA has been demonstrated to serve a vital role in
both rheumatoid arthritis and osteoarthritis and reported to act as
a regulator of gene expression and cellular functions in the
synoviocytes of osteoarthritis (19,42).
ICA reduces the number of Th17 cells, resulting in repression of
the generation of IL-1 in rheumatoid arthritis (23). Synovitis is the vital pathological
process for arthritis and osteoarthritis (13). The mechanism and effects of ICA in
synovitis is still not fully elaborated. In the present study, it
was found that ICA protected synoviocytes from death via the
modulation of ferroptosis.
Ferroptosis was confirmed to be involved in several
pathological processes. Ferroptosis is confirmed as the novel
mechanism of anticancer agents, such as non-small cell lung cancer
and breast cancer (43). Edaravone
functions in amyotrophic lateral sclerosis via the inhibition of
ferroptosis (44). Ferroptosis,
which contributes to the progress of ulcerative colitis, is a
promising treatment target for ulcerative colitis (45). However, whether ferroptosis is
involved in osteoarthritis or rheumatoid arthritis is still
unknown. Lipid peroxidation and abnormal iron metabolism, as
critical stimulators for ferroptosis, are involved in
osteoarthritis and rheumatoid arthritis (46-50).
Thus, it was postulated that ferroptosis may be also be involved in
synovitis, which is the vital process of osteoarthritis and
rheumatoid.
As ferroptosis is a form of inducing cell death, the
cell viability was evaluated in the present study. It was found
that the cell viability was significantly reduced in the cell model
of LPS-induced synoviocytes, which is further confirmed by cell
death observed in the LPS group. The cell death induced by LPS was
significantly reduced by ICA at a high concentration. The lipid
peroxidation and iron content levels were detected to determine
whether the cell death in the LPS group was induced by ferroptosis.
MDA, as a common indicator of lipid peroxidation, is increased in
synoviocytes with osteoarthritis and rheumatoid arthritis (51,52).
Simultaneously, GPX, an anti-oxidant agent, is decreased in
osteoarthritis and rheumatoid arthritis (53,54).
Consistent with previous studies, MDA levels were increased and GPX
levels were decreased in the LPS group. Furthermore, it was found
that ICA reversed the effects on MDA and GPX levels induced by LPS
in a concentration-dependent manner. Abnormal iron metabolism is
another contributor of ferroptosis and iron deposits are found in
osteoarthritis and rheumatoid arthritis (50,55,56).
In accordance with previous studies, it was found that the iron
content was increased in the LPS group. In addition, ICA reduced
the iron content induced by LPS. Collectively, the data
demonstrated that ferroptosis may be involved in synovitis and ICA
exerted inhibitory effects on ferroptosis.
To further confirm the results observed,
ferroptosis-associated proteins were detected in the present study.
The Xc-/GPX4 axisregulated lipid ROS levels and its inhibition
contributed to ferroptosis (57).
In the present study, the levels of GPX4, SLC7A11 and SLC3A2L were
decreased in the LPS group and this effect was inhibited by ICA,
suggesting that ferroptosis was involved in synovitis and ICA
inhibited ferroptosis. TFR1 and NCOA4, as contributors of
ferroptosis were increased by LPS, and Nrf2 was reduced by LPS,
confirming that ferroptosis was involved in synovitis. Furthermore,
the effects of LPS on the levels of TFR1, NCOA4 and Nrf2 were all
alleviated by ICA via a concentration-dependent manner, further
supporting that ICA has suppressive effects on ferroptosis.
RSL3, a GPX4 inhibitor (58), was used to investigate whether the
Xc-/GPX4 axis was the main avenue for the inhibitory effects of ICA
on ferroptosis. As expected, cell viability was decreased and cell
death was increased by RSL3, suggesting that following inhibition
of the Xc-/GPX4 axis, ferroptosis was enhanced. Lipid peroxidation
and iron content were elevated by RSL3. Moreover, the
aforementioned effects of RSL3 were reversed by ICA, confirming
that ICA exerted inhibitory effects on ferroptosis via activation
of the Xc-/GPX4 axis. GPX4, SLC7A11 and SLC3A2L were all reduced by
RSL3 and this effect was partly reversed by ICA, strongly
supporting that the protective effects of ICA on cell viability was
mediated via activation of the Xc-/GPX4 axis, which protects
against ferroptosis. ICA has no effects on the levels of TFR1,
NCOA4 and SLC3A2L induced by RSL3, indicating that ICA exerted
anti-ferroptosis mainly depending on SLC7A11-/GPX4 axis. Thus, the
results confirm that the Xc-/GPX4 axis is a mediator for the
protective effects of ICA in synovitis.
To the best of our knowledge, the present study
first identified that ferroptosis was involved in synovitis and ICA
exerted protective effects via inhibition of ferroptosis and by the
activation of the Xc-/GPX4 axis in synovitis, providing a new
strategy for the treatment of synovitis. However, the ferroptosis
regulation of ICA via Xc-/GPX4 axis in osteoarthritis needs to be
further confirmed through in vivo study. Determining how
other ferroptosis activator or inhibitors affect ferroptosis of
osteoarthritis would be helpful to further understand the mechanism
of ICA in ferroptosis.
Acknowledgements
Not applicable.
Funding
Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HL and RZ conceived and designed the study,
collected, analysed and interpreted the data, and revised the
manuscript. HL wrote the manuscript. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Smolen JS, Aletaha D and McInnes IB:
Rheumatoid arthritis. Lancet. 388:2023–2038. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Safiri S, Kolahi AA, Hoy D, Smith E,
Bettampadi D, Mansournia MA, Almasi-Hashiani A, Ashrafi-Asgarabad
A, Moradi-Lakeh M, Qorbani M, et al: Global, regional and national
burden of rheumatoid arthritis 1990-2017: A systematic analysis of
the global burden of disease study 2017. Ann Rheum Dis.
78:1463–1471. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Smolen JS, Aletaha D, Barton A, Burmester
GR, Emery P, Firestein GS, Kavanaugh A, McInnes IB, Solomon DH,
Strand V and Yamamoto K: Rheumatoid arthritis. Nat Rev Dis Primers.
4(18001)2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Orsolini G, Fassio A, Rossini M, Adami G,
Giollo A, Caimmi C, Idolazzi L, Viapiana O and Gatti D: Effects of
biological and targeted synthetic DMARDs on bone loss in rheumatoid
arthritis. Pharmacol Res. 147(104354)2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lazzerini PE, Capecchi PL and Laghi-Pasini
F: Systemic inflammation and arrhythmic risk: Lessons from
rheumatoid arthritis. Eur Heart J. 38:1717–1727. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Li Y, Jiang L, Zhang Z, Li H, Jiang L,
Wang L and Li Z: Clinical characteristics of rheumatoid arthritis
patients with peripheral neuropathy and potential related risk
factors. Clin Rheumatol. 38:2099–2107. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Scott DL, Wolfe F and Huizinga TWJ:
Rheumatoid arthritis. Lancet. 376:1094–1108. 2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lundberg K, Bengtsson C, Kharlamova N,
Reed E, Jiang X, Kallberg H, Pollak-Dorocic I, Israelsson L, Kessel
C, Padyukov L, et al: Genetic and environmental determinants for
disease risk in subsets of rheumatoid arthritis defined by the
anticitrullinated protein/peptide antibody fine specificity
profile. Ann Rheum Dis. 72:652–658. 2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
McInnes IB and Schett G: The pathogenesis
of rheumatoid arthritis. N Engl J Med. 365:2205–2219.
2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Falconer J, Murphy AN, Young SP, Clark AR,
Tiziani S, Guma M and Buckley CD: Review: Synovial cell metabolism
and chronic inflammation in rheumatoid arthritis. Arthritis
Rheumatol. 70:984–999. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Chen X, Zhang L, Song Q and Chen Z:
MicroRNA-216b regulates cell proliferation, invasion and cycle
progression via interaction with cyclin T2 in gastric cancer.
Anticancer Drugs. 31:623–631. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Bragg R, Gilbert W, Elmansi AM, Isales CM,
Hamrick MW, Hill WD and Fulzele S: Stromal cell-derived factor-1 as
a potential therapeutic target for osteoarthritis and rheumatoid
arthritis. Ther Adv Chronic Dis.
10(2040622319882531)2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Mathiessen A and Conaghan PG: Synovitis in
osteoarthritis: Current understanding with therapeutic
implications. Arthritis Res Ther. 19(18)2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Scanzello CR and Goldring SR: The role of
synovitis in osteoarthritis pathogenesis. Bone. 51:249–257.
2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Tan HL, Chan KG, Pusparajah P, Saokaew S,
Duangjai A, Lee LH and Goh BH: Anti-cancer properties of the
naturally occurring aphrodisiacs: Icariin and its derivatives.
Front Pharmacol. 7(191)2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhang B, Wang G, He J, Yang Q, Li D, Li J
and Zhang F: Icariin attenuates neuroinflammation and exerts
dopamine neuroprotection via an Nrf2-dependent manner. J
Neuroinflammation. 16(92)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zheng Y, Zhu G, He J, Wang G, Li D and
Zhang F: Icariin targets Nrf2 signaling to inhibit
microglia-mediated neuroinflammation. Int Immunopharmacol.
73:304–311. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wang GQ, Li DD, Huang C, Lu DS, Zhang C,
Zhou SY, Liu J and Zhang F: Icariin reduces dopaminergic neuronal
loss and microglia-mediated inflammation in vivo and in vitro.
Front Mol Neurosci. 10(441)2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zu Y, Mu Y, Li Q, Zhang ST and Yan HJ:
Icariin alleviates osteoarthritis by inhibiting NLRP3-mediated
pyroptosis. J Orthop Surg Res. 14(307)2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Huang H, Zhang ZF, Qin FW, Tang W, Liu DH,
Wu PY and Jiao F: Icariin inhibits chondrocyte apoptosis and
angiogenesis by regulating the TDP-43 signaling pathway. Mol Genet
Genomic Med. 7(e00586)2019.PubMed/NCBI View
Article : Google Scholar
|
|
21
|
Wang P, Xiong X, Zhang J, Qin S, Wang W
and Liu Z: Icariin increases chondrocyte vitality by promoting
hypoxia-inducible factor-1α expression and anaerobic glycolysis.
Knee. 27:18–25. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Shen R and Wang JH: The effect of icariin
on immunity and its potential application. Am J Clin Exp Immunol.
7:50–56. 2018.PubMed/NCBI
|
|
23
|
Chi L, Gao W, Shu X and Lu X: A natural
flavonoid glucoside, icariin, regulates Th17 and alleviates
rheumatoid arthritis in a murine model. Mediators Inflamm.
2014(392062)2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Cheleschi S, Gallo I, Barbarino M,
Giannotti S, Mondanelli N, Giordano A, Tenti S and Fioravanti A:
MicroRNA mediate visfatin and resistin induction of oxidative
stress in human osteoarthritic synovial fibroblasts via NF-κB
pathway. Int J Mol Sci. 20(5200)2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Franz A, Joseph L, Mayer C, Harmsen JF,
Schrumpf H, Frobel J, Ostapczuk MS, Krauspe R and Zilkens C: The
role of oxidative and nitrosative stress in the pathology of
osteoarthritis: Novel candidate biomarkers for quantification of
degenerative changes in the knee joint. Orthop Rev (Pavia).
10(7460)2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ostalowska A, Birkner E, Wiecha M,
Kasperczyk S, Kasperczyk A, Kapolka D and Zon-Giebel A: Lipid
peroxidation and antioxidant enzymes in synovial fluid of patients
with primary and secondary osteoarthritis of the knee joint.
Osteoarthritis Cartilage. 14:139–145. 2006.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Xie Y, Hou W, Song X, Yu Y, Huang J, Sun
X, Kang R and Tang D: Ferroptosis: Process and function. Cell Death
Differ. 23:369–379. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhang M, Liu J, Guo W, Liu X, Liu S and
Yin H: Icariin regulates systemic iron metabolism by increasing
hepatic hepcidin expression through Stat3 and Smad1/5/8 signaling.
Int J Mol Med. 37:1379–1388. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yin S, Wang P, Xing R, Zhao L, Li X, Zhang
L and Xiao Y: Transient receptor potential ankyrin 1 (TRPA1)
mediates lipopolysaccharide (LPS)-induced inflammatory responses in
primary human osteoarthritic fibroblast-like synoviocytes.
Inflammation. 41:700–709. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Tsikas D: Assessment of lipid peroxidation
by measuring malondialdehyde (MDA) and relatives in biological
samples: Analytical and biological challenges. Anal Biochem.
524:13–30. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Srivastava NK, Sharma S, Sinha N, Mandal
SK and Sharma D: Abnormal lipid metabolism in a rat model of
arthritis: One possible pathway. Mol Cell Biochem. 448:107–124.
2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Luczaj W, Jarocka-Karpinska I, Sierakowski
S, Andrisic L, Zarkovic N and Skrzydlewska E: Lipid peroxidation in
Rheumatoid arthritis; consequences and monitoring. Free Radic Biol
Med. 75 (Suppl 1)(S49)2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Winyard PG, Blake DR, Chirico S,
Gutteridge JM and Lunec J: Mechanism of exacerbation of rheumatoid
synovitis by total-dose iron-dextran infusion: In-vivo
demonstration of iron-promoted oxidant stress. Lancet. 1:69–72.
1987.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Morris CJ, Wainwright AC, Steven MM and
Blake DR: The nature of iron deposits in haemophilic synovitis. An
immunohistochemical, ultrastructural and X-ray microanalytical
study. Virchows Arch A Pathol Anat Histopathol. 404:75–85.
1984.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Zhang C, Zhang W, Shi R, Tang B and Xie S:
Coix lachryma-jobi extract ameliorates inflammation and oxidative
stress in a complete Freund's adjuvant-induced rheumatoid arthritis
model. Pharm Biol. 57:792–798. 2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Yang L, Wang H, Yang X, Wu Q, An P, Jin X,
Liu W, Huang X, Li Y, Yan S, et al: Auranofin mitigates systemic
iron overload and induces ferroptosis via distinct mechanisms.
Signal Transduct Target Ther. 5(138)2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Yang WS and Stockwell BR: Ferroptosis:
Death by lipid peroxidation. Trends Cell Biol. 26:165–176.
2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Kawabata H: Transferrin and transferrin
receptors update. Free Radic Biol Med. 133:46–54. 2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Dodson M, Castro-Portuguez R and Zhang DD:
NRF2 plays a critical role in mitigating lipid peroxidation and
ferroptosis. Redox Biol. 23(101107)2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Zhou B, Liu J, Kang R, Klionsky DJ,
Kroemer G and Tang D: Ferroptosis is a type of autophagy-dependent
cell death. Semin Cancer Biol. 66:89–100. 2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Imai H, Matsuoka M, Kumagai T, Sakamoto T
and Koumura T: Lipid peroxidation-dependent cell death regulated by
GPx4 and ferroptosis. Curr Top Microbiol Immunol. 403:143–170.
2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Pan L, Zhang Y, Chen N and Yang L: Icariin
regulates cellular functions and gene expression of osteoarthritis
patient-derived human fibroblast-like synoviocytes. Int J Mol Sci.
18(2656)2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Su Y, Zhao B, Zhou L, Zhang Z, Shen Y, Lv
H, AlQudsy LH and Shang P: Ferroptosis, a novel pharmacological
mechanism of anti-cancer drugs. Cancer Lett. 483:127–136.
2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Spasić S, Nikolić-Kokić A, Miletić S,
Oreščanin-Dušić Z, Spasić MB, Blagojević D and Stević Z: Edaravone
may prevent ferroptosis in ALS. Curr Drug Targets. 21:776–780.
2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Xu M, Tao J, Yang Y, Tan S, Liu H, Jiang
J, Zheng F and Wu B: Ferroptosis involves in intestinal epithelial
cell death in ulcerative colitis. Cell Death Dis.
11(86)2020.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Łuczaj W, Gindzienska-Sieskiewicz E,
Jarocka-Karpowicz I, Andrisic L, Sierakowski S, Zarkovic N, Waeg G
and Skrzydlewska E: The onset of lipid peroxidation in rheumatoid
arthritis: Consequences and monitoring. Free Radic Res. 50:304–313.
2016.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Olszewska-Slonina DM, Jung S, Olszewski
KJ, Cwynar A and Drewa G: Evaluation of selected parameters of
lipid peroxidation and paraoxonase activity in blood of patients
with joint osteoarthritis. Protein Pept Lett. 25:853–861.
2018.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Morris CJ, Blake DR, Wainwright AC and
Steven MM: Relationship between iron deposits and tissue damage in
the synovium: An ultrastructural study. Ann Rheum Dis. 45:21–26.
1986.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Ogilvie-Harris DJ and Fornaiser VL:
Synovial iron deposition in osteoarthritis and rheumatoid
arthritis. J Rheumatol. 7:30–36. 1980.PubMed/NCBI
|
|
50
|
Bennett RM, Williams ED, Lewis SM and Holt
PJ: Synovial iron deposition in rheumatoid arthritis. Arthritis
Rheum. 16:298–304. 1973.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Grigolo B, Roseti L, Fiorini M and
Facchini A: Enhanced lipid peroxidation in synoviocytes from
patients with osteoarthritis. J Rheumatol. 30:345–347.
2003.PubMed/NCBI
|
|
52
|
Grönwall C, Amara K, Hardt U,
Krishnamurthy A, Steen J, Engström M, Sun M, Ytterberg AJ, Zubarev
RA, Scheel-Toellner D, et al: Autoreactivity to
malondialdehyde-modifications in rheumatoid arthritis is linked to
disease activity and synovial pathogenesis. J Autoimmun. 84:29–45.
2017.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Surapneni KM and Chandrasada Gopan VS:
Lipid peroxidation and antioxidant status in patients with
rheumatoid arthritis. Indian J Clin Biochem. 23:41–44.
2008.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Sutipornpalangkul W, Morales NP,
Charoencholvanich K and Harnroongroj T: Lipid peroxidation,
glutathione, vitamin E, and antioxidant enzymes in synovial fluid
from patients with osteoarthritis. Int J Rheum Dis. 12:324–328.
2009.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Fritz P, Saal JG, Wicherek C, König A,
Laschner W and Rautenstrauch H: Quantitative photometrical
assessment of iron deposits in synovial membranes in different
joint diseases. Rheumatol Int. 15:211–216. 1996.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Bennett RM: Synovial iron deposition in
osteoarthritis and rheumatoid arthritis. J Rheumatol.
7(583)1980.PubMed/NCBI
|
|
57
|
Cao JY and Dixon SJ: Mechanisms of
ferroptosis. Cell Mol Life Sci. 73:2195–2209. 2016.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Shin D, Kim EH, Lee J and Roh JL: Nrf2
inhibition reverses resistance to GPX4 inhibitor-induced
ferroptosis in head and neck cancer. Free Radic Biol Med.
129:454–462. 2018.PubMed/NCBI View Article : Google Scholar
|