Introduction
The side effects of chemotherapy often pose an
important problem that both patients and medical personnel are
confronted with during treatment. The characteristics of cytotoxic
agents manifest both systemically and locally, in various degrees
of severity with an incidence of up to 100% of chemotherapy cases
(1). It is well known that one of
the most severe side effects is myelosuppression and the cascade of
events generated by it, such as neutropenia, leucopenia,
thrombocytopenia and even pancytopenia (2). The resulted immunosuppression
significantly increases the risk of infectious complications that
have the potential to interfere with chemotherapy outcome and
results and threaten the life of the patient (3).
The primary target of chemotherapy drugs are
malignant cells; however, considering the lack of cell specificity,
these substances interfere with some of the normal tissues as well.
The specific tissues affected by chemotherapy present a high
turnover cellular rate in the basal layer, including digestive
tract mucosa, bone marrow, hair follicle tissue, respiratory tract
mucosa and oral soft tissues. The result within the oral cavity is
the appearance of mucositis, infections, dysgeusia, hyposialia or
xerostomia, and an increased probability of hemorrhage (4).
The periodontal disease represents a multi-factorial
condition affecting the supporting tissues of teeth that, in the
absence of treatment, leads to a progressive decrease of
attachment, tooth mobility and ultimately, tooth loss (5). The bone destruction that occurs is
highly correlated with the level of periodontal inflammation and
can also be influenced by the interactions between the periodontal
pathogenic bacteria, host immune response as well as other factors
including infections, chemotherapy, immune suppression (6).
The aim of the present study was to evaluate the
effects of two antiseptic, antimicrobial and antifungal products on
oral cavity and periodontal tissues in oncologic patients during
chemotherapy.
Patients and methods
The study was conducted on 50 subjects with ages
ranging between 48 and 60 years old, between November 2015 and
October 2016 at the Oncology Clinic of ‘Victoria’ Hospital in Iaşi,
Romania. The present study was approved by the Ethics Committee of
‘Grigore T. Popa’ University of Medicine and Pharmacy (Iasi,
Romania). All patients included in the sample population signed an
informed consent prior to being accepted to take part in this
study. The total number of subjects included in the present study
consisted of 22 females and 28 males, thus 44% were women and 56%
were men, the distribution being similar regarding the sex
(Table I). Most patients were from
urban areas (90%) and only a small percentage were from rural areas
(10%).
 | Table ISubject distribution in groups
according to the oral antimicrobial, antiseptic and antifungal
substance used. |
Table I
Subject distribution in groups
according to the oral antimicrobial, antiseptic and antifungal
substance used.
| Substance | Absolute
frequency | Percentage
frequency |
|---|
| Placebo
(control) | 12 | 24.0 |
| Oral rinse | 22 | 44.0 |
| Oral coating | 16 | 32.0 |
| Total | 50 | 100.0 |
Most patients were retired and only 20% were still
working or unemployed. The chemotherapy administered to the
patients was comprised of cisplatin, oxaliplatin and gemcitabine,
the highest frequency of antineoplastic drugs being cisplatin
(n=26, 52%), followed by oxaliplatin (n=17, 34%) and gemcitabine
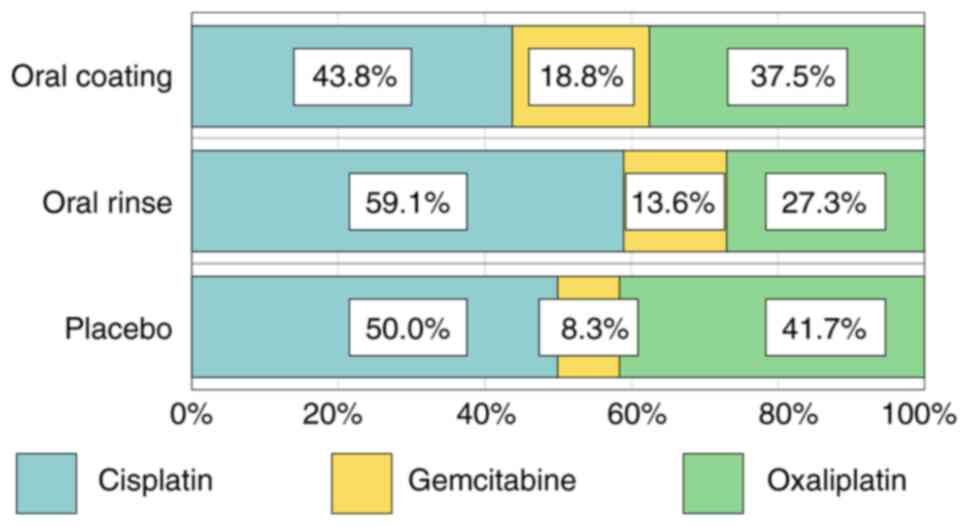
(n=7, 14%) (Fig. 1).
The patients included in the present study suffered
from systemic cancer, were undergoing chemotherapy and had a form
of periodontal disease. In order to avoid compromising the
relevance and validity of the results, the following exclusion
criteria were considered: i) tobacco smokers; ii) patients with
infectious and/or inflammatory disease that may have affected the
periodontal status, with the exception of systemic cancer; iii)
patients that had had periodontal treatment in the previous 6
months; iv) patients that had had antibiotherapy or
anti-inflammatory treatment in the previous 3 months, with the
exception of chemotherapy; vi) patients that used antiseptic oral
rinses or medical toothpaste.
All subjects were randomly split into three groups:
i) controls, which included chemotherapy patients that did not use
any active substance throughout the present study; ii) group A,
which included chemotherapy patients that used oral rinses with
cetrimide mouthwash three times a day; iii) group B, that included
chemotherapy patients that used mouth coating with a pharmacy-made
compound two times a day.
The clinical examination considered several
elements: probing depth (PD), clinical attachment loss (CAL),
dental mobility (M), plaque index and periodontal disease index
(PDI) In addition, pathological probing depths higher than 3 mm on
teeth with no gingival recessions were considered. The clinical
examination took place at two time-points: T0, before beginning to
use the active substances and T1, after 14 days of antiseptic,
antimicrobial and antifungal substance usage.
The two substances evaluated in the present study
were Citrolin oral rinse and an oral coating recipe developed at
the pharmaceutical laboratory BabyFarm, Ltd. Citrolin is an oral
rinsing solution that contains 25 mg cetrimide, 3 mg lidocaine and
excipients per 100 ml of product and is administered in the form of
oral rinses 15 ml per rinse, three times a day. The oral coating
substance was developed in collaboration with BabyFarm, Ltd.
laboratory and its composition contains neomycin, nystatin,
metronidazole, sodium bicarbonate, vitamin A, xylene 2% and oleum
helianthi.
The three groups were evaluated based on oral
hygiene and periodontal status before the commencement of oral
rinse use and oral coating and 14 days after use. None of the
patients declared any side effects after using the two compounds
included in the present study.
The statistical analysis of the data included in the
present study consisted of descriptive statistics, one Sample
t-test and a paired Sample t-test using the SPSS software version
21.0 (IBM Corporation). P<0.05 was considered to indicate a
statistically significant difference.
Results
The paired Sample t-test revealed a high statistical
significance of improvements for group A that used Citrolin oral
rinse, the positive modification of all parameters being
statistically significant (P<0.05), with the exception of dental
mobility, as revealed in Table
II.
 | Table IIStatistical significance analysis for
group A, between time-point T0 and T1. |
Table II
Statistical significance analysis for
group A, between time-point T0 and T1.
| | Group A, Citrolin
oral rinse T0-T1 |
|---|
| Analyzed indices | Mean difference | t | P-value |
|---|
| Silness-Loe Plaque
index | 0.092 | 2.358 | 0.028 |
| PDI | 0.104 | 2.097 | 0.048 |
| PBI | 0.165 | 3.578 | 0.002 |
| Mean dental
mobility | -0.045 | -1.821 | 0.083 |
| Mean PD | 0.145 | 4.661 | <0.001 |
| Mean CAL | 0.161 | 3.409 | 0.003 |
The results obtained for the controls revealed an
increase in the values of all analyzed indices and periodontal
disease progression. The modifications of values for these indices
had statistical significance with the exception of the average
tooth mobility value that had no statistical significance (P=0.077)
(Table III). Similarly, group B
that used oral coating did not exhibit any improvement of the
indices evaluated (data not shown).
 | Table IIIStatistical significance analysis for
controls between time-points T0 and T1. |
Table III
Statistical significance analysis for
controls between time-points T0 and T1.
| | Placebo group
T0-T1 |
|---|
| Quantified
indices | Mean Difference | t | P-value |
|---|
| Silness-Loe Plaque
Index | -0.164 | -2.680 | 0.021 |
| PDI | -0.472 | -4.513 | 0.001 |
| PBI | -0.301 | -7.473 | <0.001 |
| Mean tooth
mobility | -0.080 | -1.948 | 0.077 |
| Mean PD | -0.479 | -4.823 | 0.001 |
| Mean CAL | -1.183 | -3.467 | 0.005 |
Moreover, CAL values were different for each of the
three chemotherapy agents included in the study between T0 and T1.
The average value of CAL was increased in patients treated with
oxaliplatin (mean difference=-0.239) and cisplatin (mean
difference=-0.19) (data not shown).
Discussion
The efficiency of antineoplastic treatment with
platinum-based drugs (cisplatin, oxaliplatin) has been demonstrated
multiple times in the past (7,8),
although what does sometimes limit their dosage is their potential
side effects. Patients treated with one of these chemotherapy
agents may develop up to 40 specific adverse reactions. The most
important and frequent effect is nephrotoxicity in the case of
cisplatin administration and neurotoxicity in the case of
oxaliplatin alongside the well-known myelosuppressive effects
(1).
Ideally, periodontal disease should be assessed and
treated before the beginning of chemotherapy, bearing in mind that
a pre-chemotherapy evaluation and maintaining good oral hygiene has
been demonstrated to be efficient in preventing oral and systemic
complications during anti-neoplasic treatment (9). Frequent erythematous lesions,
ulcerations or candidiasis can occur in the oral cavity during
chemotherapy (3). Moreover,
modifications of periodontal parameters can be observed through an
increase in the quantity of oral bacterial plaque, an exacerbation
of gingival inflammatory signs and even modifications of the
bacterial community composition at oral and periodontal levels
(10).
The use of antimicrobial and antiseptic substances
is efficient in plaque reduction and improving periodontal
parameters (11). Cetrimide, the
active substance in Citrolin, is an antiseptic with multiple
quaternary ammonium salts that has a bactericidal effect on a wide
spectrum of gram-positive and gram-negative bacteria (12). Its action consists of affecting the
permeability of the bacterial cellular membrane. It is used in a
high number of pharmaceutical compounds with the role to decrease
the level of gingival pain and increase oral hygiene (13). It is sometimes used in products that
also contain chlorhexidine gluconate (14); however, in the present study,
cetrimide was the only active substance in the oral rinse to avoid
errors in the results. The use of cetrimide can eliminate bacterial
plaque to a great extent, some authors claiming that it has an even
higher antimicrobial effect than that of chlorhexidine (15). The effects of cetrimide were also
demonstrated to be efficient in preventing carious lesions; a
concentration of 0.2% cetrimide used as oral rinse for a minute had
the capacity to destroy Streptococcus mutans in a proportion
of >99% (16).
Conversely, presently, there are available
substances with topic application that contain either only
metronidazole, or neomycin and prednisolone (17). In the present study, we selected to
introduce a new compound with topical administration that contained
neomycin and metronidazole with the aim of evaluating its
periodontal efficiency. This combination of drugs has been used in
the past, but in association with general surgery of the digestive
tract. The pre-operatory administration was revealed to be an
efficient combination of antibiotics that leads to a significant
reduction of post-operatory infections (18,19).
The most important modifications of the Silness-Loe
plaque index were observed in the subjects of group A that used
oral rinses with cetrimide. The values of the plaque index were
decreased after 14 days of using cetrimide and consequently
improved the level of oral hygiene. Conversely, higher values in T1
compared to T0 in the control group (2.002 vs. 1.838) were
obtained, thus manifesting an increase of 0.164 between the two
evaluations. The values of the plaque index in group B were also
increased after 14 days (T1=2.055 vs. T0=1.996) (data not
shown).
Another fact for consideration is the medullar
modifications that occur during chemotherapy that lead to
thrombocytopenia, which can be translated into a pronounced
tendency to gingival bleeding in the oral cavity, especially in the
conditions of a pre-existing periodontal disease (20). In the study, the PBI exhibited a
decrease in value for group A after the 14 days of oral rinsing,
signifying an improvement in the periodontal inflammatory status
(T1=2.120 vs. T0=2.285; difference =0.165). Then again, controls
had higher values of the bleeding index in T1 (T1=2.338 vs.
T0=2.037) and group B exhibited similar values at both evaluations
(T1=2.206 vs. T0=2.238) (data not shown). These results reflect the
modifications obtained for the level of oral hygiene, creating an
association between the level of oral hygiene and level of bleeding
at the periodontal level.
The average PD obtained in the present study
revealed considerable differences between the controls and patients
that used antimicrobial/antiseptic/antifungal substances. The
highest improvements were observed in group A, which used cetrimide
oral rinses. This may be explained by the fact that cetrimide has a
higher salivary retention rate than chlorhexidine immediately after
the rinse is performed, but decreases more significantly at 4 h
than chlorhexidine (21), which is
why the patients were recommended to perform the action of rinsing
more often than they would otherwise in order to maintain an
optimal concentration in the saliva and at the periodontal
level.
Oral mucositis and periodontal disease progression
are important modifiers for the level of quality of life of
patients undergoing chemotherapy and negatively impact the
affective state of patients (22),
as we have shown in a previous study (23). The present study offers more options
regarding the secondary means of oral hygiene that oncology
patients can use in order to prevent the progression of periodontal
disease and obtain an improved periodontal status during
chemotherapy, thus improving their experience during chemotherapy
and obtaining an improvement in their level of quality of life
(24-26).
It can thus be concluded that cetrimide oral rinses
were demonstrated to be the most efficient secondary means of oral
hygiene assessed in the present study. Cetrimide oral rinse
decreased the level of bacterial plaque and gingival bleeding and
it was efficient in preventing the progression of periodontal
disease in patients undergoing chemotherapy.
The present results offer new perspectives regarding
a reliable alternative to the contemporary-used secondary means of
oral hygiene for oncologic patients undergoing chemotherapy. Thus,
the periodontal status of these particular patients can be better
controlled and their quality of life can be significantly
improved.
Acknowledgements
Professional editing, linguistic and technical
assistance was performed by Mrs. Irina Radu, Individual Service
Provider, certified translator in Medicine and Pharmacy.
Funding
The study was partially funded by the ‘Grigore T.
Popa’ University of Medicine and Pharmacy Iași-Romania, during the
PhD studies of Dr Diana Cristala Kappenberg-Niţescu.
Availability of data and materials
The data used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DCKN, IL, IM, SM and SMS designed the study. AG and
MT contributed substantially in drafting the work and revising it
critically for important intellectual content. LP, IAS and AM
contributed to the data analysis and data interpretation and edited
the final form of the manuscript. All authors read and approved the
final manuscript.
Ethical approval and consent to
participate
The present study was approved by the Ethics
Committee of ‘Grigore T. Popa’ University of Medicine and Pharmacy
(Iasi, Romania). All protocols were in accordance with the
provisions of the Declaration of Helsinki. All patients included in
the sample populations signed an informed consent prior to being
accepted to take part in this study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Oun R, Moussa YE and Wheate NJ: The side
effects of platinum-based chemotherapy drugs: A review for
chemists. Dalton Trans. 47:6645–6653. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
American Cancer Society: After diagnosis:
A guide for patients and families, 2012.
|
|
3
|
Napeñas JJ, Brennan MT, Bahrani-Mougeot
FK, Fox PC and Lockhart PB: Relationship between mucositis and
changes in oral microflora during cancer chemotherapy. Oral Surg
Oral Med Oral Pathol Oral Radiol Endod. 103:48–59. 2007.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Poulopoulos A, Papadopoulos P and
Andreadis D: Chemotherapy: Oral side effects and dental
interventions -a review of the literature. Stomatological Dis Sci.
1:35–49. 2017.
|
|
5
|
Luchian I, Nanu S, Martu I, Martu A,
Nichitean G, Kappennerg-Nitescu DC, Gurău G, Stefanescu V, Pasarin
L, Tatarciuc M and Solomon SM: The influence of the composite resin
material on the clinical working time in fiberglass reinforced
periodontal splints. Mater Plast. 57:316–320. 2020.
|
|
6
|
Teodorescu AC, Martu I, Teslaru S,
Kappenberg-Nitescu DC, Goriuc A, Luchian I, Martu MA, Solomon SM
and Mârțu S: Assessment of salivary levels of RANKL and OPG in
aggressive versus chronic periodontitis. J Immunol Res.
2019(6195258)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhou C, Ren S, Zhou S, Zhang L, Su C,
Zhang Z, Deng Q and Zhang J: Predictive effects of ERCC1 and XRCC3
SNP on efficacy of platinum-based chemotherapy in advanced NSCLC
patients. Jpn J Clin Oncol. 40:954–960. 2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Park SY, Lee JG, Kim J, Byun GE, Bae MK,
Lee CY, Kim DJ and Chung KY: Efficacy of platinum-based adjuvant
chemotherapy in T2aN0 stage IB non-small cell lung cancer. J
Cardiothorac Surg. 8(151)2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Chambers MS, Toth BB, Martin JW, Fleming
TJ and Lemon JC: Oral and dental management of the cancer patient:
Prevention and treatment of complications. Support Care Cancer.
3:168–175. 1995.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Jensen SB, Mouridsen HT, Bergmann OJ,
Reibel J, Brünner N and Nauntofte B: Oral mucosal lesions,
microbial changes, and taste disturbances induced by adjuvant
chemotherapy in breast cancer patients. Oral Surg Oral Med Oral
Pathol Oral Radiol Endod. 106:217–226. 2008.PubMed/NCBI View Article : Google Scholar
|
|
11
|
DePaola LG, Overholser CD, Meiller TF,
Minah GE and Niehaus C: Chemotherapeutic inhibition of
supragingival dental plaque and gingivitis development. J Clin
Periodontol. 16:311–315. 1989.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Engebretsen KA, Hald M, Johansen JD and
Thyssen JP: Allergic contact dermatitis caused by an antiseptic
containing cetrimide. Contact Dermatitis. 72:60–61. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Elzanfaly ES, Bassuoni YF, Essam HAM and
Zaazaa HE: Ion selective membrane electrodes for determination of
cetrimide in pure form and in pharmaceutical formulations. Anal
Bioanal Electrochem. 7:401–414. 2015.
|
|
14
|
Dostie S, Alkadi LT, Owen G, Bi J, Shen Y,
Haapasalo M and Larjava HS: Chemotherapeutic decontamination of
dental implants colonized by mature multispecies oral biofilm. J
Clin Periodontol. 44:403–409. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Guerreiro-Tanomaru JM, Nascimento CA,
Faria-Júnior NB, Graeff MS, Watanabe E and Tanomaru-Filho M:
Antibiofilm activity of irrigating solutions associated with
cetrimide. Confocal laser scanning microscopy. Int Endod J.
47:1058–1063. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ruiz-Linares M, Ferrer-Luque CM,
Arias-Moliz T, de Castro P, Aguado B and Baca P: Antimicrobial
activity of alexidine, chlorhexidine and cetrimide against
Streptococcus mutans biofilm. Ann Clin Microbiol Antimicrob.
13(41)2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Moisei M, Pasarin L, Solomon S, Oanta C,
Tatarciuc D, Ursarescu I and Martu S: The role of antibiotherapy in
the oral rehabilitation of the periodontal affected patient. Rom J
Oral Rehabil. 7:107–112. 2015.
|
|
18
|
Vallance S, Jones B, Arabi Y and Keighley
MR: Importance of adding neomycin to metronidazole for bowel
preparation. J R Soc Med. 73:238–240. 1980.PubMed/NCBI
|
|
19
|
Espin-Basany E, Sanchez-Garcia JL,
Lopez-Cano M, Lozoya-Trujillo R, Medarde-Ferrer M, Armadans-Gil L,
Alemany-Vilches L and Armengol-Carrasco M: Prospective, randomised
study on antibiotic prophylaxis in colorectal surgery. Is it really
necessary to use oral antibiotics? Int J Colorectal Dis.
20:542–546. 2005.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Rapone B, Nardi GM, DI Venere D, Pettini
F, Grassi FR and Corsalini M: Oral hygiene in patients with oral
cancer undergoing chemotherapy and/or radiotherapy after prosthesis
rehabilitation: Protocol proposal. Oral Implantol (Rome). 9 (Suppl
1/2016 to N 4/2016):S90–S97. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Bonesvoll P and Gjermo P: A comparison
between chlorhexidine and some quaternary ammonium compounds with
regard to retention, salivary concentration and plaque-inhibiting
effect in the human mouth after mouth rinses. Arch Oral Biol.
23:289–294. 1978.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Dodd MJ, Dibble S, Miaskowski C, Paul S,
Cho M, MacPhail L, Greenspan D and Shiba G: A comparison of the
affective state and quality of life of chemotherapy patients who do
and do not develop chemotherapy-induced oral mucositis. J Pain
Symptom Manage. 21:498–505. 2001.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Nitescu DCK, Constantin M, Oanta C, Martu
I, Volovat SR and Martu S: Evaluation of cumulative effects of
chemotherapy and bevacizumab (Avastin®) in oncological
patients with periodontal disease. Rev Chim (Bucharest).
68:549–552. 2017.
|
|
24
|
Calenic B, Greabu M, Caruntu C, Nicolescu
MI, Moraru L, Surdu-Bob CC, Badulescu M, Anghel A, Logofatu C and
Boda D: Oral keratinocyte stem cells behavior on diamond like
carbon films. Rom Biotechnol Lett. 21:11914–11922. 2016.
|
|
25
|
Boda D: Cellomics as integrative omics for
cancer. Curr Proteomics. 10:237–245. 2013.
|
|
26
|
Neagu M, Constantin C, Tanase C and Boda
D: Patented biomarker panels in early detection of cancer. Recent
Pat Biomark. 1:10–24. 2011.
|