Introduction
Since November 2015, direct-acting antiviral (DAA)
based regimens have been used in Romania for treating patients with
hepatitis C virus (HCV) infection. The patients with liver
cirrhosis were treated during the first year that the protocol was
being implemented in Romania (1,2) and in
the following years non-cirrhotic patients were also included. The
percentage of sustained virologic response (SVR) in patients with
HCV genotype 1 (found in 99% of the HCV infected patients in
Romania) is known to be over 95% (3). There are limited previous data
collected on the regression of fibrosis in patients who have
achieved SVR after interferon-free treatments. A perfect method for
assessing liver fibrosis and its dynamics has not been established
yet. The gold standard for evaluating the liver fibrosis has been
the liver biopsy (LB), but in the recent years, non-invasive
methods, especially the FibroTest, have been used instead (4). However, liver biopsy has significant
limitations: possible complications during the procedure, sometimes
with life-threatening potential (5), difficulties to carry out serial
examinations in order to monitor the dynamic of liver fibrosis,
some patients may be afraid to do the test, and usually the test is
poorly accepted. Small liver samples may not always be sufficient
to estimate the structure of such a large organ (sampling errors
because it only evaluates 1/50,000 of the liver parenchyma).
Moreover, it is considered that fibrosis has a heterogeneous
disposition in the liver (6-8).
The optimal size of liver fragment at liver biopsy seems to be
around 40 mm and the acceptable size is 25 mm. However, it is
difficult to obtain optimal size fragments and Poynard et al
(9) analyzed in 2004 more than
10,000 liver biopsies with 25-35% inadequate sample size.
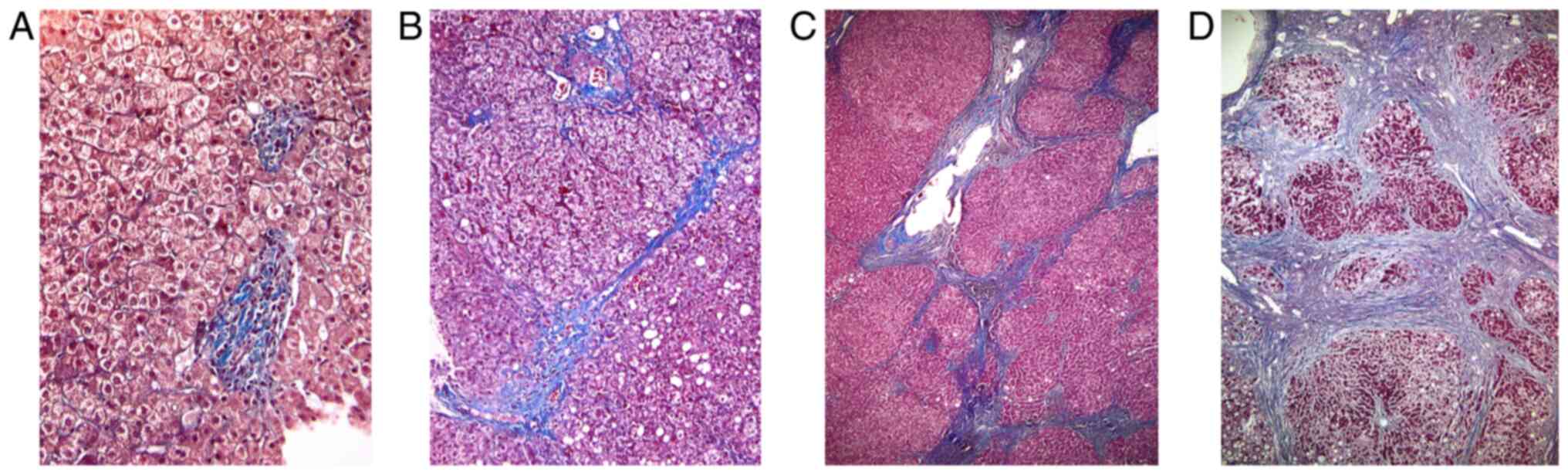
Histopathological aspects from different fibrosis
stages of the liver tissue in patients with chronic hepatitis
obtained through liver biopsy are shown in Fig. 1. Using Masson staining of liver
tissue, stage 1 Ishak fibrosis score (A) corresponds to fibrous
expansion of portal tracts with inflammatory cell infiltration;
stage 3 Ishak fibrosis score (B) consists in fibrous septa which
form occasional bridges between adjacent vascular structures; stage
5 Ishak fibrosis score (C) appears later in the progression of
disease, with numerous bridges and rare parenchymal nodules
completely surrounded by fibrosis; the late stage: Stage 6 Ishak
score-cirrhosis (D) corresponds to the entire tissue being composed
of parenchymal nodules surrounded by fibrosis (Fig. 1).
In Romania, the national guidelines use the
FibroTest as a reference. Other non-invasive, cheaper and faster
methods for evaluating the liver fibrosis in HCV infected patients
have been developed by scientists, among them being the aspartate
aminotransferase to platelet ratio index (APRI) (10) and the Fibrosis-4 (FIB-4) score
(11), but these are rarely used in
daily practice.
A meta-analysis conducted in 2018 by Zubair and
Wajid (12) stresses upon the fact
that the FibroTest (although not a perfect method), has a better
diagnostic accuracy that APRI and FIB-4, but it is more expensive,
not as accessible and as simple as calculating the APRI and FIB-4
scores.
The main objective of this study was to evaluate the
dynamics of APRI and FIB-4 scores in patients with HCV who
registered SVR and to evaluate if they could be useful and less
expensive tools for screening HCV patients for cirrhosis and
monitoring after DAA treatment. We performed ROC curve analysis to
evaluate the diagnostic performance of APRI and FIB-4 scores in
determining the presence of cirrhosis in comparison to
FibroTest.
Materials and methods
Design and ethics
This study is a prospective observational analysis
of HCV patients, both cirrhotic and non-cirrhotic (liver fibrosis
determined by FibroTest), treated with DAA therapies and monitored
in a tertiary-care infectious disease hospital, ‘Prof. Dr. Matei
Balş’ National Institute for Infectious Diseases (Bucharest,
Romania). The study was approved by the Ethics Committee and all
patients signed an informed consent before inclusion in the
analysis.
Patients were enrolled between November 2015 and
January 2020 and included HCV patients who received DAA therapies
for 12 weeks and achieved SVR. According to the National Protocol,
patients were categorized as cirrhotic by having a result at
FibroTest of F3-F4 or F4, each level below and equal to F3 being
considered non-cirrhotic. All the patients included in the study
were evaluated at baseline (before the start of treatment), at the
first visit, at 6-months after the end of treatment (post-EOT), the
2nd visit and at 12-months post-EOT, the 3rd visit. At each visit,
FibroTest, APRI and FIB-4 scores were determined for each patient.
The main group of study consisted of cirrhotic patients (164
patients), as labeled by the National Protocol using the scores of
FibroTest of F3-F4 or F4 (a value of >0.72) and a control group
of HCV non-cirrhotic patients (83 patients ≤F3).
APRI was calculated with the formula: [AST
(IU/l)/AST (Upper Limit of Normal-IU/l)/Platelet count
(109/l)] x100 and the patients were distributed
according to prior determined cut-offs from the medical literature
(<1, 1-2, >2) (13). FIB-4
was determined according to the formula: [Age (years) x AST level
(IU/l)]/[(Platelet count (109/l)x √ALT(IU/l)] and
patients were distributed by previously studied cut-offs (<1.45,
1.45-3.25, >3.25) (14).
Information was gathered regarding the demographic
parameters (age, gender) and complete medical history for all
patients, and at each visit we determined FibroTest and biological
parameters: including complete blood count (CBC), complete
biochemistry analysis and coagulation parameters.
Statistical analysis
The statistical analysis was performed using IBM
SPSS® Statistics version 22 (IBM Corp.). In univariate
analysis, the type of variable distribution was assessed by visual
inspection of histograms, Q-Q plots and the Shapiro-Wilk test. The
central tendency and dispersion for non-Gaussian distributed
variables were expressed as median and interquartile range (IQR).
In multivariate analysis, associations between continuous
non-Gaussian distributed variables were assessed using Kendall's
tau-b (τb) correlation coefficient. Paired t-test was used to
determine the significance of differences between paired continuous
sample data. The Friedman test was also performed for differences
between three groups of continuous data that has marked deviations
from normality. ROC curve analysis was performed to assess the
diagnostic ability of several variables. P<0.05 was considered
to indicate a statistically significant difference.
Results
A total of 251 patients were enrolled in the study,
divided into 2 groups: The cirrhotic patients (164 patients) and
the non-cirrhotic patients (83 patients). The median age for the
cirrhotic group was 62.5 years (35-80); 92 were females (56.1%) and
72 (43.9%) were males. The median age of the non-cirrhotic group
was 63 years (33-85), including 57 (68.7%) females and 26 (31.3%)
males.
At baseline, FibroTest, APRI and FIB-4 scores were
performed for both groups and the results are shown in Table I.
 | Table IBaseline APRI, FIB-4 and FibroTest
scores in cirrhosis vs. non-cirrhosis groups. |
Table I
Baseline APRI, FIB-4 and FibroTest
scores in cirrhosis vs. non-cirrhosis groups.
| Variable | Cirrhosis (164
patients) (F3-F4 and F4) | Non-cirrhosis (83
patients) (F0-F3) | P-value |
|---|
| APRI (n, %) | | | |
|
Median
(IQR) | 1.14 (0.72-2.3) | 0.73 (0.41-1.13) | 0.002 |
|
<1 | 67 (40.85) | 58 (69.88) | |
|
1-2 | 47 (28.66) | 16 (19.28) | |
|
>2 | 50 (30.49) | 9 (10.84) | |
| FIB-4 (n, %) | | | |
|
Median
(IQR) | 3.32 (2.17-5.32) | 2.21 (1.5-2.98) | <0.0001 |
|
<1.35 | 13 (7.93) | 11 (13.25) | |
|
1.35-3.25 | 75 (45.73) | 55 (66.27) | |
|
>3.25 | 86 (52.44) | 17 (20.48) | |
| FibroTest | | | |
|
Median
(IQR) | 0.84 (0.78-0.9) | 0.63 (0.58-0.68) | <0.0001 |
For the second visit (6 months post-EOT) and the
third visit (12 months post-EOT), the same evaluations were
performed and are described in Table
II (for the cirrhotic patients) and Table III (for the non-cirrhotic
patients).
 | Table IIFollow-up FibroTest, APRI and FIB-4
scores for cirrhotic patients. |
Table II
Follow-up FibroTest, APRI and FIB-4
scores for cirrhotic patients.
| | Baseline vs. 6 months
post-EOT | 6 months vs. 12
months post-EOT |
|---|
| Variable | Baseline (164
patients) | 6 months post-EOT (83
patients) | P-value | 6 months post-EOT (83
patients) | 12 months post-EOT
(63 patients) | P-value |
|---|
| APRI (n, %) |
|
Median
(IQR) | 1.14 (0.72-2.3) | 0.428
(0.289-0.685) | <0.001 | 0.428
(0.289-0.685) | 0.423
(0.28-0.635) | 0.739 |
|
<1 | 67 (40.85) | 76 (91.57) | | 76 (91.57) | 54 (85.71) | |
|
1-2 | 47 (28.66) | 7 (8.43) | | 7 (8.43) | 9 (14.29) | |
|
>2 | 50 (30.49) | 0 (0) | | 0 (0) | 0 (0) | |
| FIB-4 (n, %) |
|
Median
(IQR) | 3.32 (2.17-5.32) | 1.987
(1.459-2.9) | <0.001 | 1.987
(1.459-2.9) | 1.958
(1.44-3.14) | 0.913 |
|
<1.35 | 13 (7.93) | 20 (24.1) | | 20 (24.1) | 16 (25.4) | |
|
1.35-3.25 | 75 (45.73) | 50 (60.24) | | 50 (60.24) | 32 (50.8) | |
|
>3.25 | 86 (52.44) | 13 (15.66) | | 13 (15.66) | 15 (23.8) | |
| FibroTest |
|
Median
(IQR) | 0.84 (0.78-0.9) | 0.68 (0.57-0.79) | <0.001 | 0.68 (0.57-0.79) | 0.72 (0.58-0.78) | 0.42 |
 | Table IIIFollow-up FibroTest, APRI and FIB-4
scores for non-cirrhotic patients. |
Table III
Follow-up FibroTest, APRI and FIB-4
scores for non-cirrhotic patients.
| | Baseline vs. 6 months
post-EOT | 6 months vs. 12
months post-EOT |
|---|
| Variable | Baseline (83
patients) | 6 months post-EOT (37
patients) | P-value | 6 months post-EOT (37
patients) | 12 months post-EOT
(21 patients) | P-value |
|---|
| APRI (n, %) |
|
Median
(IQR) | 0.73 (0.41-1.13) | 0.343
(0.23-0.58) | 0.01 | 0.343
(0.23-0.58) | 0.341
(0.27-0.66) | 0.214 |
|
<1 | 58 (69.88) | 32 (86.49) | | 32 (86.49) | 18 (85.71) | |
|
1-2 | 16 (19.28) | 5 (13.51) | | 5 (13.51) | 3 (14.29) | |
|
>2 | 9 (10.84) | 0 (0) | | 0 (0) | 0 (0) | |
| FIB-4 (n, %) |
|
Median
(IQR) | 2.21
(1.5-2.98) | 1.53
(1.18-2.8) | 0.014 | 1.53
(1.18-2.8) | 1.99
(1.48-3.32) | 0.441 |
|
<1.35 | 11 (13.25) | 13 (35.13) | | 13 (35.13) | 4 (19.05) | |
|
1.35-3.25 | 55 (66.27) | 17 (45.95) | | 17 (45.95) | 12 (57.14) | |
|
>3.25 | 17 (20.48) | 7 (18.92) | | 7 (18.92) | 5 (23.81) | |
| FibroTest |
|
Median
(IQR) | 0.63
(0.58-0.68) | 0.53
(0.44-0.62) | <0.001 | 0.53
(0.44-0.62) | 0.6
(0.54-0.69) | 0.011a |
In the cirrhotic group, at baseline, correlations
were made between the FibroTests, APRI and FIB-4 and the results
showed that there was a weak, but statistically significant
correlation between APRI and FibroTest (τ=0.173, P=0.001), as well
as between FIB-4 and FibroTest (τ=0.265, P<0.001). At the 6
months follow-up, APRI no longer correlated with the FibroTest
(τ=0.144, P=0.057), but the FIB-4 score had a weak correlation with
the gold standard of the study (τ=0.256, P=0.001). The same pattern
was observed at 12 months post-EOT, APRI did not correlate with
FibroTest (τ=0.100, P=0.255), but FIB-4 showed significant
correlation (τ=0.200, P=0.023).
For the cirrhotic patients group, Friedman tests
were performed which showed that there was a statistically
significant difference between the baseline and the two follow-up
visits of APRI values (P<0.001), FIB-4 values (P<0.001) and
FibroTest values (P<0.001). The study showed that between
baseline and the 6-month evaluation, there was a statistically
significant difference for APRI (P<0.001, confidence interval
(CI) 95%: 0.982-1.61) and for FIB-4 (P<0.001, 95% CI,
1.43-2.26), but for the next follow-up period (between 6 and 12
months post-EOT) no reduction for these scores (P=0.739 for APRI,
P=0.913 for FIB-4) was observed.
On the contrary, in the non-cirrhotic group, APRI
and FIB-4 did not correlate with the FibroTest at any of the
evaluation times. For APRI the results were: Baseline: τ=0.015,
P=0.841, visit 2: τ=0.104, P=0.402, visit 3: τ=0.005, P=0.974; and
for FIB-4: Baseline: τ=0.041, P=0.589, visit 2: τ=0.107, P=0.384,
visit 3: τ=-0.037, P=0.820.
Friedman tests were performed for this group as well
and they showed that there was a statistically significant
difference between visits in the APRI values (P<0.001), FIB-4
values (P<0.001) and FibroTest values (P=0.02). However, the
statistically significant difference was observed between baseline
and the 6-month visit (P=0.01 for APRI and P=0.014 for FIB-4), but
for the next 6 months no reduction was shown.
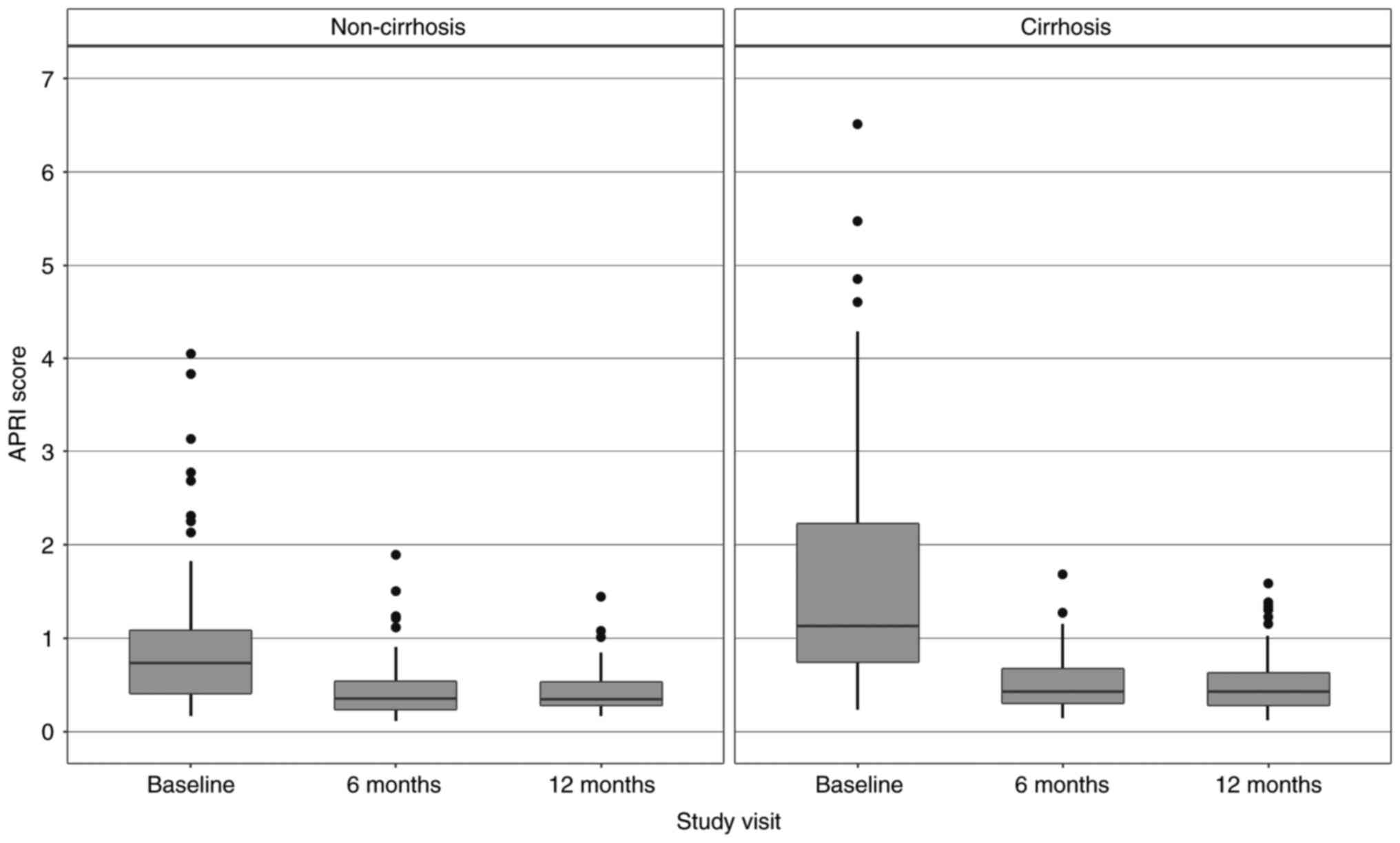
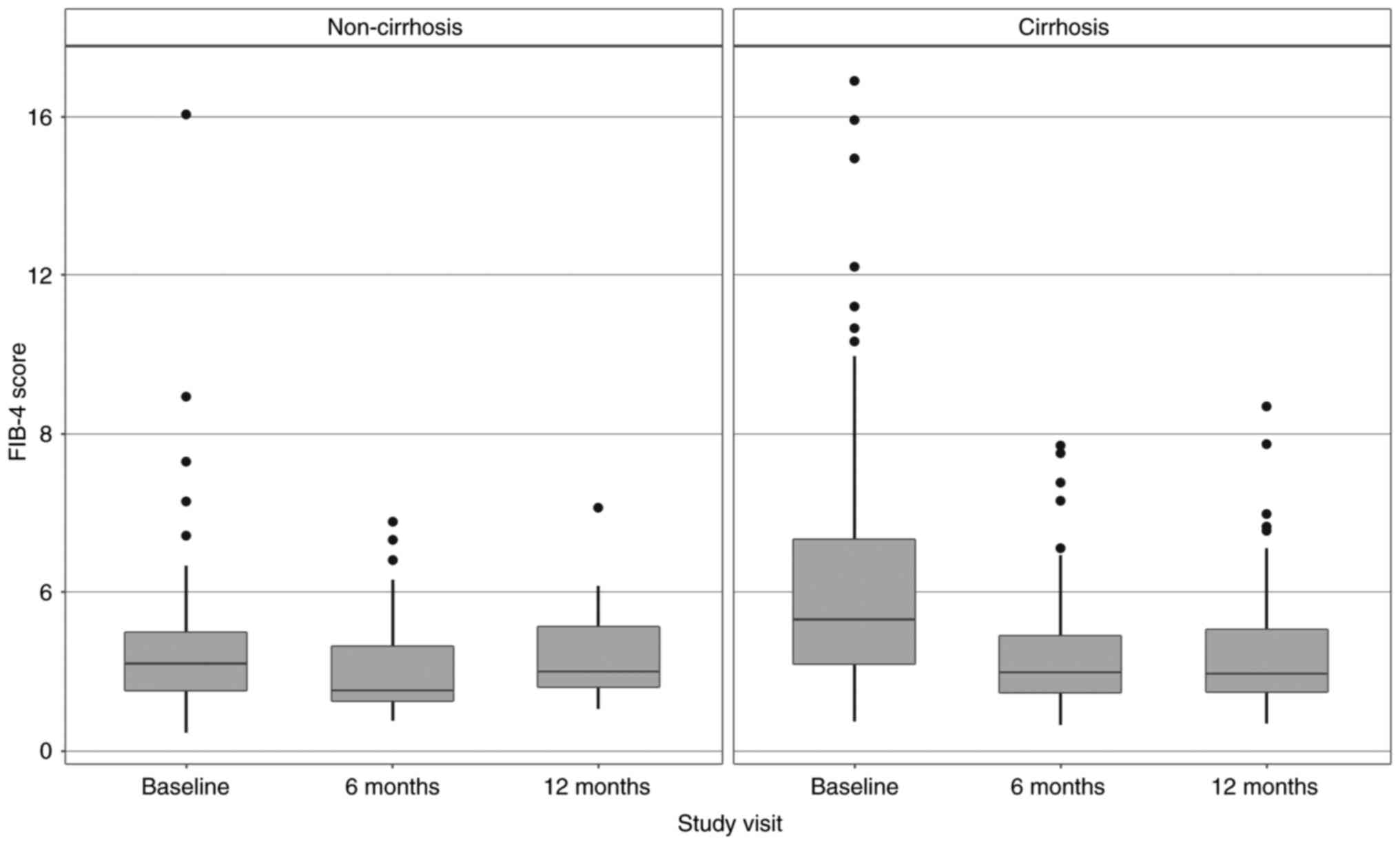
The regression of APRI and FIB-4 scores in the
cirrhotic and non-cirrhotic groups can also be observed in Fig. 2 (for APRI) and Fig. 3 (for FIB-4).
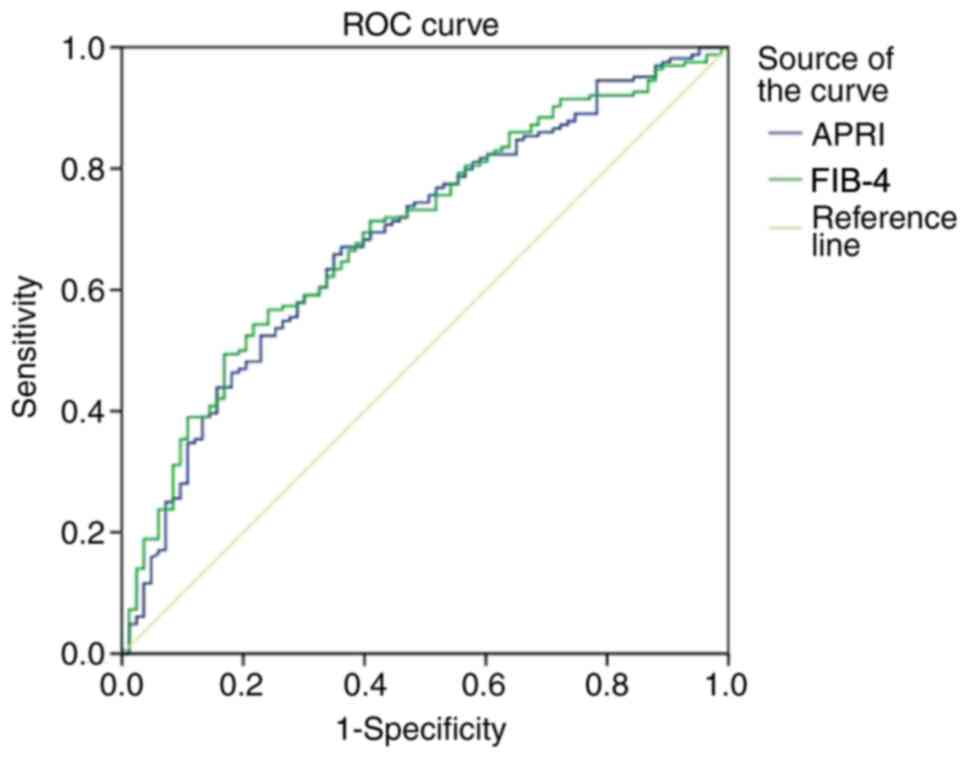
Additionally to these results, we performed the area
under the receiver operating characteristics curve (AUROC),
sensitivities, specificities, positive predictive value (PPV) and
negative predictive value (NPV) for our data in evaluating the
presence of cirrhosis in comparison to the FibroTest for the two
scores: APRI and FIB-4 when differentiating F0-F3 vs. F4. The ROC
curves for APRI and FIB-4 are presented in Fig. 4, and the AUROC of these 2 scores
have values of 0.682 (95% CI, 0.613-0.752) for APRI and 0.693 (95%
CI, 0.625-0.76) for FIB-4 (Fig.
4).
The calculated optimal cut-off value for APRI was
0.867 and for this value, the score had a sensitivity of 68%, a
specificity of 58%, a PPV of 76% and NPV of 48% for predicting
cirrhosis (F4) in comparison to F0-F3. For the FIB-4 score, at a
cut-off of 2.32, the sensitivity was 71%, the specificity was 58%,
the PPV was 76.9% and NPV was 51% for predicting liver cirrhosis in
comparison to F0-F3.
In this study, the sensitivity and specificity of
APRI and FIB-4 were evaluated at previously studied cut-offs in
expressing liver cirrhosis. For APRI, at a cut-off >1, the
sensitivity was 59.1%, the specificity was 69% and for APRI >2,
the sensitivity was 30.5% and the specificity 89.3% for predicting
cirrhosis. At a level of FIB-4 over 3.25, a sensitivity level of
52.4% and a specificity of 78.6% were determined for cirrhosis.
Discussion
Patients with HCV cirrhosis who registered SVR under
DAA therapies need further monitoring in the following years as
liver decompensation and hepatocellular carcinoma (HCC) could
appear despite the viral clearance. In follow-up evaluations of the
liver fibrosis, it could be very difficult to perform LB,
especially since it has been mostly replaced by FibroTest (nowadays
all other tests are being compared to it). Although it is a very
useful tool, with no differences compared with LB regarding its
prognostic value (15), healthcare
workers are trying to find easier and less expensive methods to
evaluate the degree of liver fibrosis, and especially
cirrhosis.
According to the present study, both APRI and FIB-4
scores proved to be useful tools in predicting the presence of
liver cirrhosis before treatment initiation as they can be rapidly
calculated by screening and are less expensive than performing
FibroTest. At lower levels of fibrosis (the non-cirrhotic group),
neither APRI, nor FIB-4 correlated statistically with FibroTest.
That is why these two scores cannot be used to differentiate
between F0-F1, F1-F2 or F2-F3. Also, at the follow-up evaluations,
APRI no longer statistically correlated to the FibroTest, but FIB-4
continued to correlate with FibroTest, which shows that FIB-4 can
be a useful tool for both screening for cirrhosis and for
monitoring the patients after treatment with DAA (sometimes the
patient is not able to pay for the FibroTest, as it is not
mandatory for the patient follow-up after DAA treatment).
In a study published in 2007, Vallet-Pichard et
al (14) reported that FIB-4
had an AUROC of 0.91 in identifying cirrhosis when comparing to the
LB. They also performed a comparison between FIB-4 and FibroTest
which showed a concordance between the two of 92.1% when FIB-4 was
<1.45 and 76% when FIB-4 was >3.25, but for the values
between 1.45 and 3.25 there was no correlation between these
tests.
Another aspect observed in the present study was
that between baseline and the 6-month post-EOT evaluation, there
was an important decrease in the values of APRI and FIB-4, but no
difference regarding the two scores between 6 months post-EOT and
12-month post-EOT could be observed. This decrease may occur as a
result of the normal values of transaminases obtained after
starting the DAA treatment. Similar results were published in 2017
which described a decrease of APRI and FIB-4 scores, along with
decreased transient elastography (TE) results in patients who
achieved SVR after DAA therapies (16).
In a study published in 2012, Tamaki et al
(17) made a comparison between
FIB-4 and repeated liver biopsies in evaluating the progression of
liver fibrosis and concluded that using FIB-4 repeatedly, one could
predict the changes in liver fibrosis every year, without having to
perform LB.
Although there are many studies and systematic
reviews in which the AUROCs for APRI and FIB-4 compared with LB
were higher than 0.8 (classified as good to excellent) when
predicting cirrhosis, we performed the AUROCs, sensitivities and
specificities compared with FibroTest. Our results show lower
values compared with the systematic review of Chou and Wasson
(13), which reported a median
AUROC for APRI of 0.84 (range, 0.54-0.97), for which an optimal
cut-off of 2 had a median sensitivity of 48% (range, 17-76%) and
median specificity of 94% (range, 65-99%). Also, the median AUROC
for FIB-4 was 0.87 (range, 0.83-0.92), with an optimal cut-off of
3.25 for which the median sensitivity was 55% and the median
specificity was 92%.
Houot et al (18) elaborated a systematic review which
concluded that for patients with HCV, information gathered from 18
different studies, APRI had lower AUROC than FibroTest in
describing different degrees of fibrosis, but without any
difference regarding cirrhosis.
Cepeda et al (19) tested APRI and FIB-4 at previously
validated cut-offs (only APRI had a cut-off of >1.5) in
estimating severe liver stiffness by using TE. Their results for
severe stiffness (≥12.3 kPa) show an AUROC for APRI of 0.77
(cut-off 1.5, sensitivity 61% and specificity 80%) and for FIB-4 of
0.8 (cut-off 3.25, sensitivity 62% and specificity 87%). They also
tried to develop new scoring systems that included FIB-4,
gamma-glutamyl transferase (GGT), high-density lipoprotein (HDL),
homeostatic model assessment insulin resistance (HOMA-IR) and body
mass index (BMI), with enhanced accuracy for predicting cirrhosis
and a simplified APRI score that added GGT, BMI and age. This new
APRI score had higher accuracy than the classic APRI (AUROC 0.83,
cut-off 0.22, sensitivity 82% and specificity of 70%), but all new
FIB-4 models outranked APRI (the highest AUROC for FIB-4 best
subset model: 0.87, sensitivity 70% specificity 87%).
Overall, the AUROCs for both APRI and FIB-4
determined in our study were lower than the data described in most
studies, but the difference is that most studies from literature
have evaluated these scores in comparison with LB (20), while our study evaluates them in
comparison to FibroTest. Another explanation could be that the
non-cirrhotic group might not have been as well represented as the
general population of HCV non-cirrhotic patients.
Both APRI and FIB-4 prove to be easy, quick and
inexpensive tools for screening HCV cirrhosis, with moderate
diagnostic performance and FIB-4 can also be useful for monitoring
patients post-EOT. The ideal biomarker (with very high sensitivity,
specificity, specific for liver cells, reliable, useful for
monitoring liver fibrosis and inexpensive) is yet to be
discovered.
Acknowledgements
The follow-up FibroTests were performed with the
help of BioPredictive, both for cirrhotic and non-cirrhotic
patients, with help provided by Dr Mona Munteanu (BioPredictive
Hepatology Research Unit; Hepatology Department, Assistance
Publique-Hôpitaux de Paris, PitiéSalpêtrière Hospital, Paris,
France). This study is part of ‘Carol Davila’ University of
Medicine and Pharmacy doctoral program and will be integrated in
the PhD thesis of author Anca Leuştean.
Funding
This work was partially supported by a grant of
Ministry of Research and Innovation, CNCS-UEFISCDI (project no.
PN-III-P4-ID-PCE-2016-0641) within PNCDI-III. This work was
partially supported by a grant of Romanian Ministry of Research and
Innovation, CCCDI-UEFISCDI (project nο. 61PCCDI⁄2018
PN-III-P1-1.2-PCCDI-2017-0341) within PNCDI-III.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
AL, CP, LN, CT and VA contributed in the conception
and design of the study, data acquisition, analysis and
interpretation of the data, statistical analysis, manuscript
drafting, and critical revision of the manuscript for important
intellectual content. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by The Ethics Committee of
‘Prof. Dr. Matei Balş’ National Institute for Infectious Diseases.
All patients enrolled in the study gave written informed
consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Popescu C, Stratan L, Catană R, Leuștean
A, Dragomirescu C, Badea A, Murariu C, Năstase R, Molagic V, Aramă
V, et al: The efficacy of Ombitasvir-paritaprevir/ritonavir,
dasabuvir and ribavirin in patients with genotype 1 HCV compensated
cirrhosis. The 12th Edition of the Scientific Days of the National
Institute for Infectious Diseases “Prof. Dr. Matei Bals” and the
12th National Infectious Diseases Conference. BMC Infectious
Diseases. 16:31–76. 2016.10.1186/s12879-016-1877-4.
|
|
2
|
Aramă V, Leuştean A, Catană R, Stratan L,
Nastase RM, Molagic V, Radulescu M, Munteanu DI, Tiliscan C, Orfanu
A, et al: The efficacy of OBV/PTV/r + DSV and RBV in a Romanian
cohort with genotype 1 HCV compensated cirrhosis. Poster
presentation at the 26th Annual Conference of APASL, Shanghai,
China. Hepatol Int. 11 (Suppl 1)(S1028 (PP1765))2017.
|
|
3
|
Poordad F, Hezode C, Trinh S1028R, Kowdley
KV, Zeuzem S, Agarwal K, Shiffman ML, Wedemeyer H, Berg T, Yoshida
EM, et al: ABT-450/r-ombitasvir and dasabuvir with ribavirin for
hepatitis C with cirrhosis. N Engl J Med. 370:1973–1982.
2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Poynard T, Imbert-Bismut F, Munteanu M,
Messous D, Myers RP, Thabut D, Ratziu V, Mercadier A, Benhamou Y
and Hainque B: Overview of the diagnostic value of biochemical
markers of liver fibrosis (FibroTest, HCV FibroSure) and necrosis
(ActiTest) in patients with chronic hepatitis C. Comp Hepatol.
3(8)2004.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Cadranel JF, Rufat P and Degos F:
Practices of liver biopsy in France: Results of a prospective
nationwide survey. For the group of epidemiology of the French
Association for the study of the liver (AFEF). Hepatology.
32:477–481. 2000.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Regev A, Berho M, Jeffers LJ, Milikowski
C, Molina EG, Pyrsopoulos NT, Feng ZZ, Reddy KR and Schiff ER:
Sampling error and intraobserver variation in liver biopsy in
patients with chronic HCV infection. Am J Gastroenterol.
97:2614–2618. 2002.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Bedossa P, Dargère D and Paradis V:
Sampling variability of liver fibrosis in chronic hepatitis C.
Hepatology. 38:1449–1457. 2003.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ferraioli G, Tinelli C, Dal Bello B,
Zicchetti M, Lissandrin R, Filice G, Filice C, Above E, Barbarini
G, Brunetti E, et al: Performance of liver stiffness measurements
by transient elastography in chronic hepatitis. World J
Gastroenterol. 19:49–56. 2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Poynard T, Munteanu M, Imbert-Bismut F,
Charlotte F, Thabut D, Le Calvez S, Messous D, Thibault V, Benhamou
Y, Moussalli J and Ratziu V: Prospective analysis of discordant
results between biochemical markers and biopsy in patients with
chronic hepatitis C. Clin Chem. 50:1344–1355. 2004.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wai CT, Greenson JK, Fontana RJ,
Kalbfleisch JD, Marrero JA, Conjeevaram HS and Lok AS: A simple
noninvasive index can predict both significant fibrosis and
cirrhosis in patients with chronic hepatitis C. Hepatology.
38:518–526. 2003.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sterling RK, Lissen E, Clumeck N, Sola R,
Correa MC, Montaner J, S Sulkowski M, Torriani FJ, Dieterich DT,
Thomas DL, et al: Development of a simple noninvasive index to
predict significant fibrosis patients with HIV/HCV coinfection.
Hepatology. 43:1317–1325. 2006.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zubair I and Wajid B: Comparison of APRI,
FIB-4 and fibro test in prediction of fibrosis and cirrhosis in
patients with hepatitis C. 2018 15th International Bhurban
Conference on Applied Sciences and Technology (IBCAST), Islamabad,
pp222-227, 2018.
|
|
13
|
Chou R and Wasson N: Blood tests to
diagnose fibrosis or cirrhosis in patients with chronic hepatitis C
virus infection: A systematic review. Ann Intern Med. 158:807–820.
2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Vallet-Pichard A, Mallet V, Nalpas B,
Verkarre V, Nalpas A, Dhalluin-Venier V, Fontaine H and Pol S:
FIB-4: An inexpensive and accurate marker of fibrosis in HCV
infection. Comparison with liver biopsy and fibrotest. Hepatology.
46:32–36. 2007.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Poynard T, Ngo Y, Perazzo H, Munteanu M,
Lebray P, Moussalli J, Thabut D, Benhamou Y and Ratziu V:
Prognostic value of liver fibrosis biomarkers: A meta-analysis.
Gastroenterol Hepatol. 7:445–454. 2011.PubMed/NCBI
|
|
16
|
Bachofner JA, Valli PV, Kröger A, Bergamin
I, Künzler P, Baserga A, Braun D, Seifert B, Moncsek A, Fehr J, et
al: Direct antiviral agent treatment of chronic hepatitis C results
in rapid regression of transient elastography and fibrosis markers
fibrosis-4 score and aspartate aminotransferase-platelet ratio
index. Liver Int. 37:369–376. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Tamaki N, Kurosaki M, Tanaka K, Suzuki Y,
Hoshioka Y, Kato T, Yasui Y, Hosokawa T, Ueda K, Tsuchiya K, et al:
Noninvasive estimation of fibrosis progression overtime using the
FIB-4 index in chronic hepatitis C. J Viral Hepat. 20:72–76.
2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Houot M, Ngo Y, Munteanu M, Marque S and
Poynard T: Systematic review with meta-analysis: Direct comparisons
of biomarkers for the diagnosis of fibrosis in chronic hepatitis C
and B. Aliment Pharmacol Ther. 43:16–29. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Cepeda JA, Solomon SS, Srikrishnan AK,
Nandagopal P, Balakrishnan P, Kumar MS, Thomas DL, Sulkowski MS and
Mehta SH: Serum fibrosis markers for the diagnosis of liver disease
among people with chronic hepatitis C in Chennai, India. Open Forum
Infect Dis. 3(ofw156)2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Amorim TG, Staub GJ, Lazzarotto C, Silva
AP, Manes J, Ferronato Mda G, Shiozawa MB, Narciso-Schiavon JL,
Dantas-Correa EB and Schiavon Lde L: Validation and comparison of
simple noninvasive models for the prediction of liver fibrosis in
chronic hepatitis C. Ann Hepatol. 11:855–861. 2012.PubMed/NCBI
|