Introduction
Ischemic heart disease is a serious condition due to
its high morbidity and mortality, which is responsible for ~40%
deaths associated with cardiovascular diseases worldwide (1). At present, timely restoration of blood
flow is a generally accepted strategy that is effective for
improving ischemic heart injury (2,3).
However, reperfusion may trigger several adverse responses,
including calcium overload, oxidative-nitrosylation stress,
endoplasmic reticulum stress, mitochondrial dysfunction and
inflammation, leading to cell death and heart dysfunction (2,3).
Therefore, myocardial ischemia/reperfusion injury is an essential
clinical problem worldwide that has attracted increasing attention
from clinicians. An increasing number of studies have focused on
strategies for alleviating this condition.
A number of studies have previously demonstrated
that cardiomyocyte autophagy is increased in both acute
ischemia-reperfusion (4-6)
and chronic ischemia models (7,8).
Autophagy is increasingly recognized as a potential target for the
treatment of myocardial ischemia-reperfusion (9,10) and
is generally activated by oxidative stress, hypoxia and starvation,
which is physiologically a cell defense and adaption mechanism to
promote cell survival (11).
However, excessive and uncontrolled autophagy can result in cell
death (12). Therefore, maintaining
autophagy at a reasonable level is essential for determining cell
fate. Tert-butylhydroperoxide (t-BHP) is an organic peroxide
compound that is used widely as an alternative to
H2O2 in oxidative stress studies to mimic
cellular oxidative damage (13-15).
In addition to the increased production of reactive oxygen species
(ROS), t-BHP-induced cell death can also result from the activation
of autophagy (16,17). Therefore, inhibition of autophagy
may protect against t-BHP-induced H9c2 cardiomyocyte death
(15).
Traditional Chinese medicine provide an important
platform for the discovery of novel therapeutic agents. Danshen and
Chuanxiong are two common traditional Chinese medicines that have
been used widely for myocardial ischemia therapy (18,19).
Danshensu (DSS) and tetramethypyrazin (TMP) are the major active
components present within Danshen and Chuanxiong, which exert
various pharmacological properties on the cardiovascular system
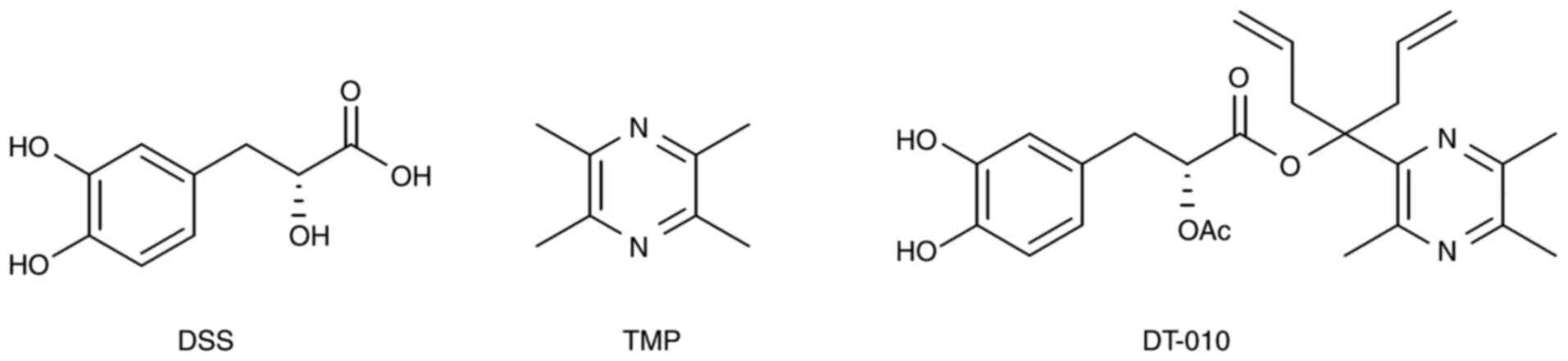
(18,19). In particular, compound DT-010
(Fig. 1) is a patented compound
derived from DSS and TMP that was previously synthesized (20). A previous study demonstrated the
protective activities of DT-010 against myocardial ischemia and
reperfusion injury in a rat model, where DT-010 treatment reduced
the infarct size (20). The
cardioprotective effect of DT-010 is more potent compared with that
of DSS and TMP (21). This previous
study also revealed that DT-010 markedly increased cell viability
and suppressed cell apoptosis in t-BHP-treated H9c2 cells via
activation of the peroxisome proliferator-activated receptor γ
coactivator (PGC)-1/nuclear factor erythroid 2-related factor 2
(Nrf2)/transcription factor A and the mitochondrial/heme
oxygenase-1 (HO-1) signaling pathway (20). In addition, DT-010 has also been
reported to prevent doxorubicin-induced cardiotoxicity by
inhibiting apoptosis, ROS generation and autophagosome formation
(21).
It was speculated that autophagy may also be
involved in the protective effects of DT-010 against myocardial
ischemia-reperfusion injury. Therefore, the present study
established a t-BHP-induced oxidative injured cell model using H9c2
cardiomyocytes and evaluated the effect of DT-010 on autophagy and
its underlying regulatory signaling mechanism.
Materials and methods
Materials
t-BHP, acridine orange (AO), monodansylcadaverine
(MDC), MTT, rapamycin and hydroxy-chloroquine were obtained from
Sigma-Aldrich, Merck KGaA. Hoechst 33342 was obtained from
Molecular Probes, Thermo Fisher Scientific, Inc. The primary
antibody against microtubule-associated protein 1A/1B-light chain 3
(LC3; cat. no. L8918) was purchased from Sigma-Aldrich, Merck KGaA.
Primary antibodies against β-actin (cat. no. 4967), p62 (cat. no.
8025), mTOR (cat. no. 2983), 5'-AMP-activated protein kinase (AMPK;
cat. no. 5831), unc-51 like autophagy activating kinase 1 (Ulk1;
cat. no. 8054), phosphorylated (p)-mTOR (cat. no. 5536), p-AMPK
(cat. no. 2535) and p-Ulk1 (cat. no. 14202) were obtained from Cell
Signaling Technology, Inc. All primary antibodies were rabbit
anti-rat antibodies (dilution, 1:1,000). Anti-rabbit IgG,
horseradish peroxidase-conjugated antibody (cat. no. 7074) was
purchased from Cell Signaling Technology, Inc. Monomeric red
fluorescent protein (mRFP)-green fluorescent protein (GFP)-LC3
adenovirus (cat. no. HB-AP21000001; https://www.hanbio.net/cn/services/item/7) was
purchased from Hanbio Biotechnology Co., Ltd. The lactate
dehydrogenase (LDH) kit (cat. no. C0016) was obtained from Beyotime
Institute of Biotechnology. DMEM and FBS were obtained from Thermo
Fisher Scientific, Inc. The purity of DT-010 was >95% (22).
Cell culture
The rat myocyte cell line H9C2 was obtained from The
Cell Bank of Type Culture Collection of Chinese Academy of
Sciences. H9c2 cardiomyocytes were maintained in DMEM containing
10% FBS. A humidified atmosphere containing 5% CO2 at
37˚C was required for cell growth and cell treatment. Cells were
passaged or treated when they reached ~80% confluence.
MTT and LDH measurement
Cell survival was detected using MTT and LDH kits.
To assess cytotoxicity, cells were seeded into 96-well plates at a
density of 1x104 cells/well and cultured at 37˚C. After
24 h, DT-010 was added alone for 1 h at 37˚C. The concentration of
DT-010 was set based on a previous study (20), which showed that 300 µM DT-010
treatment alone may exert cytotoxicity. Therefore, a dose range of
DT-010 between 10 and 100 µM was chosen for the present study.
Cell viability was measured using MTT assay. To
detect the effects of DT-010 on t-BHP-induced injury, DT-010 was
first added and the cells were incubated for 1 h at 37˚C.
Subsequently, cell supernatant was removed, and the cells were
washed twice with Hanks' balanced salt solution (HBSS) to avoid
drug interaction. After 6 h exposure to 300 µM t-BHP at 37˚C, the
cell supernatant was then removed before the cells were incubated
with 100 µl 0.5 mg/ml MTT in the dark at 37˚C for 4 h. The crystals
were dissolved by DMSO and the optical density at 570 nm was
measured in each well. Cell viability was determined as previously
described (20).
The activity of LDH in the cell supernatant was
measured using the LDH kit according to the manufacturer's
protocol.
For autophagy agonist and blocker treatment, cells
were first pretreated with the autophagy agonist rapamycin (500 nM)
(23) or the autophagy blocker
hydroxy-chloroquine (50 µM) (24,25)
for 1 h before being treated with DT-010 for 1 h and t-BHP for a
further 6 h. All treatments were performed at 37˚C. In between each
treatment, cells were washed twice with HBSS to avoid drug
interaction.
Cell apoptosis analysis
Hoechst 33342 nuclear staining was performed to
assess cell apoptosis detection as described previously (20). Following DT-010 treatment for 1 h,
the cell supernatant was removed, and cells were washed twice with
HBSS. Subsequently, the cells were treated with 300 µM t-BHP for
another 2 h at 37˚C. H9c2 cells were subsequently dyed with Hoechst
33342 at a final concentration of 5 µg/ml at 37˚C in the dark for
20 min and then observed under an inverted fluorescence microscope
(magnification, x400). After Hoechst staining, normal cells stain
dark blue with homogeneous fluorescence intensity whereas apoptotic
cells are characterized by shrunken and irregular nuclear shapes,
in addition to broken and condensed chromatin. Cell apoptosis
measurement was repeated three times independently, with 6-8 fields
of view in each group randomly photographed. The apoptosis rate was
calculated using ImageJ software version 1.44p (bundled with Java
v1.6.0_20; National Institutes of Health) using the following
formula: Apoptotic rate=(number of apoptotic cells/number of total
cells) x100%.
Autophagic flow analysis
Autophagic flow was evaluated using the mRFP-GFP-LC3
assay. H9c2 cells were first seeded onto the laser confocal culture
dishes at a density of 5x103 cells/dish. After reaching
30% confluence, cells were transfected with the mRFP-GFP-LC3
adenovirus (1.58x1010 pfu/ml) at 1,500 MOI for 36 h.
After another 48 h of culture in complete DMEM, the cells were
treated with DT-010 for 1 h and 300 µM t-BHP for 2 h at 37˚C.
Finally, cells were fixed with 4% paraformaldehyde at room
temperature for 15 min and then observed under a laser confocal
microscope (magnification, x630). Autophagic flux was visualized
and analyzed via fluorescence imaging of autophagosomes and
autolysosomes. Yellow (GFP+/mRFP+) and red
(GFP-/mRFP+) puncta indicate the presence of
autophagosomes and autolysosomes, respectively.
AO and MDC staining
AO and MDC staining are commonly used to measure
autophagosome vesicle formation (26,27).
After 1 h treatment with 30 µM DT-010 and another 2 h 300 µM t-BHP
treatment at 37˚C, cells were gently rinsed with HBSS and stained
with 1 µM AO and 50 nM MDC for 10 min in the incubator at 37˚C.
Cells were observed under an inverted fluorescence microscope
(magnification, x200), where six-eight fields of view were randomly
imaged in each group.
Western blotting
Proteins were extracted from H9c2 cells using RIPA
lysis buffer (Beyotime Institute of Biotechnology). Proteins were
quantified with a BCA protein assay kit (Beyotime Institute of
Biotechnology) and 30 µg denatured protein from each sample was
subjected to 10 or 12% SDS-PAGE. Proteins were then transferred
onto PVDF membranes. Following blocking of the membrane with 5%
skimmed milk at room temperature for 2 h, immunoblotting was
performed by incubation with specific primary antibodies (1:1,000)
at 4˚C overnight. After washing, horseradish peroxidase-labeled
secondary antibodies (1:2,000) were added and the membranes were
incubated at room temperature for 2 h. An ECL detection kit (cat.
no. P0018S; Beyotime Institute of Biotechnology) was utilized for
protein detection. The optical density of the protein bands was
analyzed using the Carestream MI SE system (4,000 MM PRO system;
Carestream Health, Inc.).
Statistical analysis
Results are presented as the mean ± SD of at least
three experimental repeats. All data were analyzed using GraphPad
Prism software (version 6.01; GraphPad Software, Inc.). Significant
differences were determined using one-way ANOVA followed by Tukey's
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
DT-010 protects cardiomyocytes against
oxidative stress damage
H9c2 cardiomyocytes were first exposed to t-BHP to
mimic ischemia-reperfusion-induced cellular oxidative stress
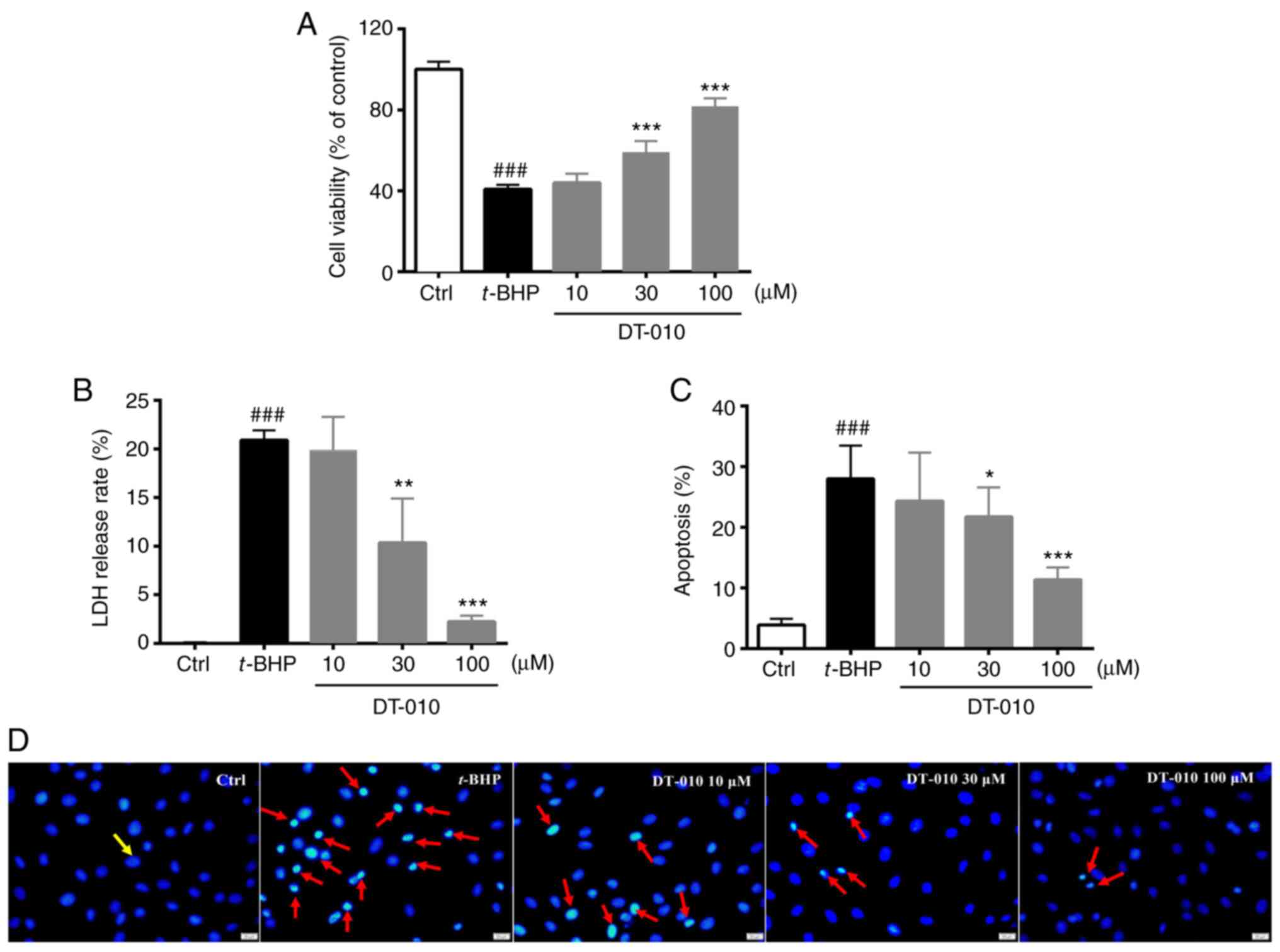
damage. DT-010 alone did not induce cytotoxicity in H9c2 cells
(Figs. 2A, B and S1).
Compared with cells in the control group, t-BHP exposure
significantly decreased cell viability whilst significantly
increasing LDH release (Fig. 2A and
B). DT-010 pretreatment
dose-dependently restored cell viability and reduced LDH release
compared with cells in the t-BHP-only group (Fig. 2A and B), suggesting a cardioprotective effect of
DT-010 which was observed at 30 and 100 µM. Subsequently, Hoechst
33342 staining was performed for cell apoptosis detection. Normal
cells were stained dark blue with homogeneous fluorescence
intensity whereas apoptotic cells were characterized by shrunken
and irregular nuclear shapes, coupled with condensed chromatin
(Fig. 2C and D). The level of apoptosis in H9c2 cells
increased significantly following t-BHP exposure compared with that
in the control group, but was significantly lower in cells treated
with DT-010. This observation suggests a protective effect of
DT-010 against apoptosis induced by t-BHP (Fig. 2C and D).
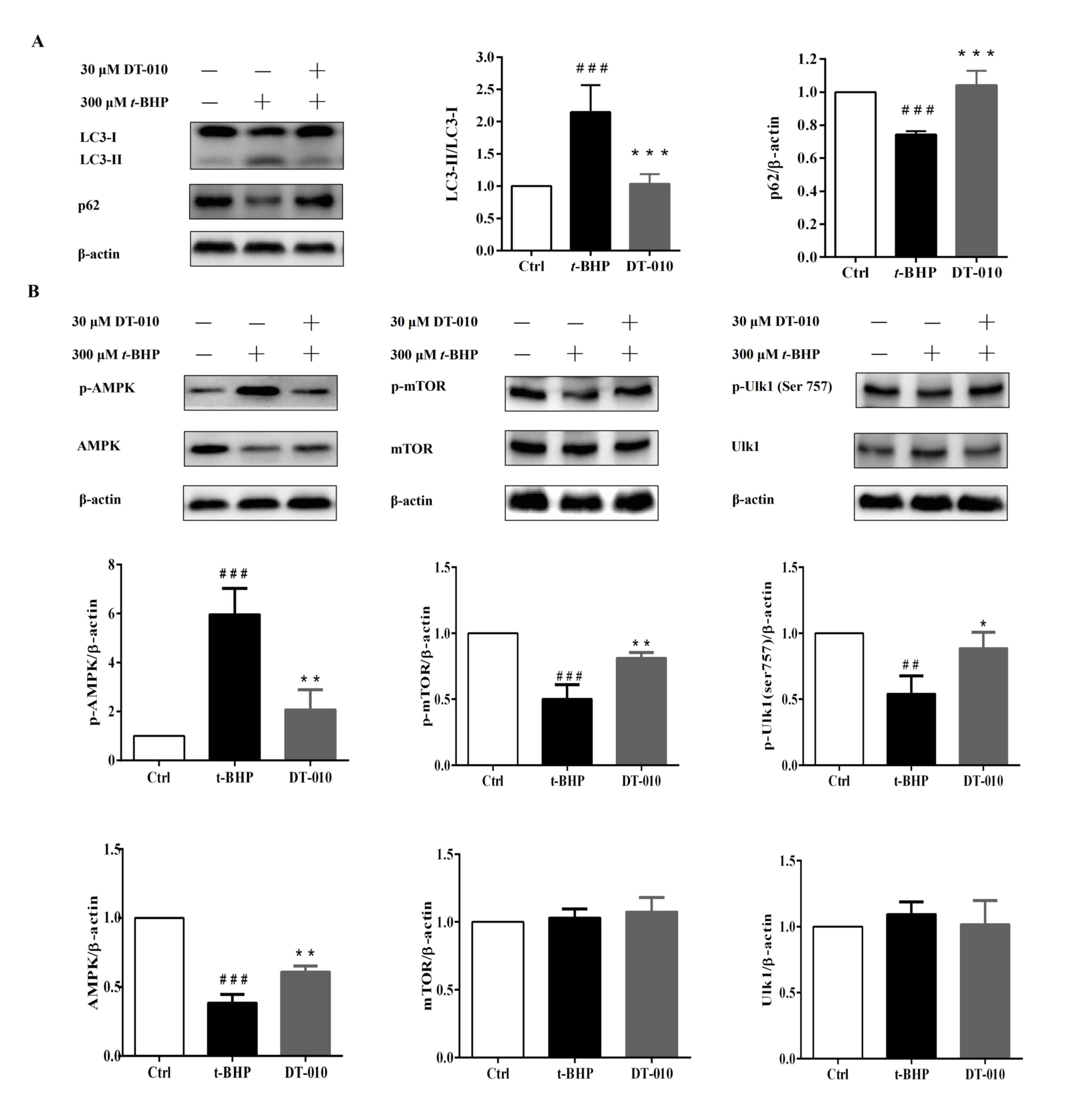
DT-010 inhibits oxidative
stress-induced autophagy in cardiomyocytes
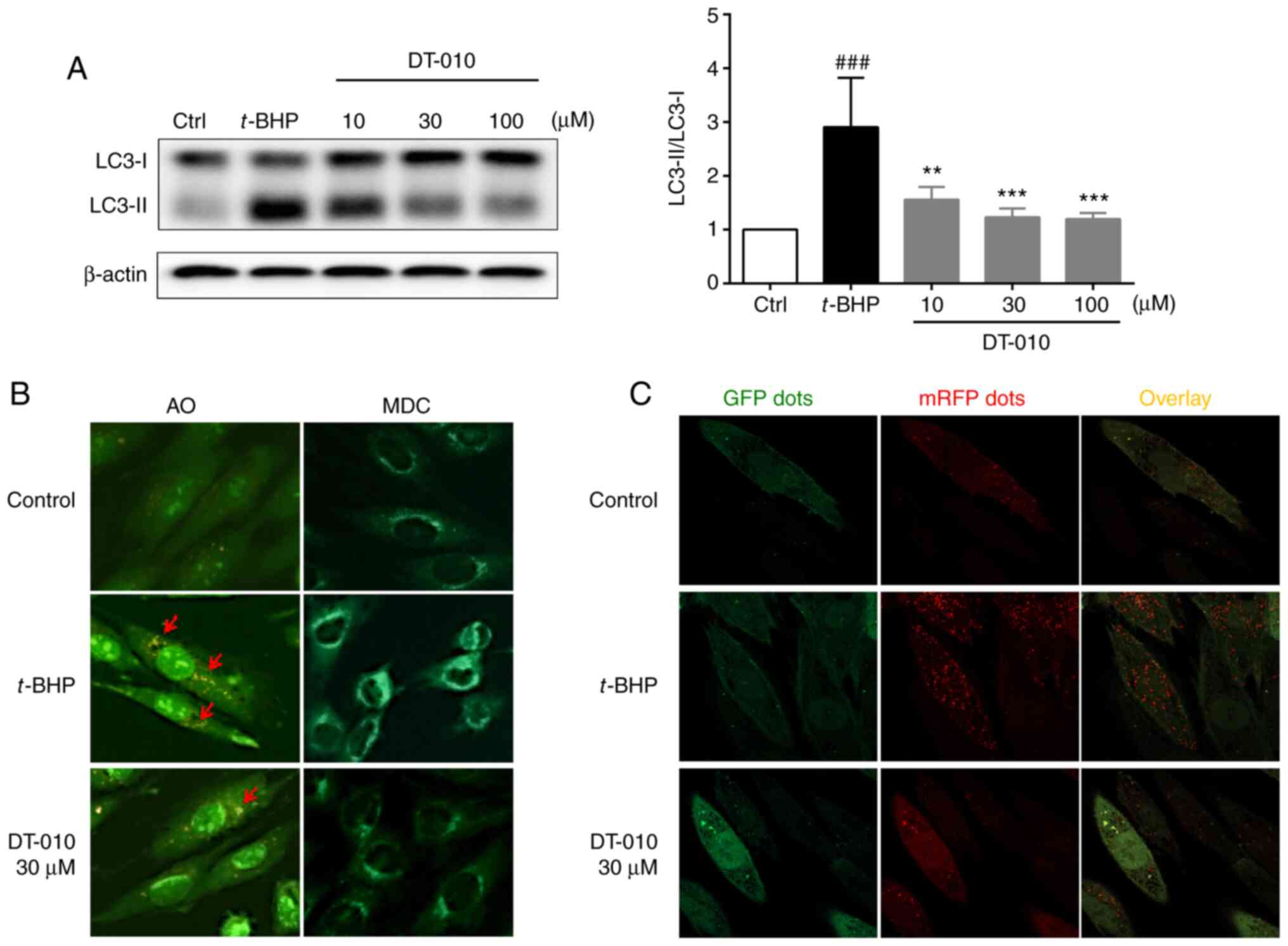
LC3 protein is a hallmark protein of autophagy
induction (26). When autophagy is
initiated, LC3-I, the cytosolic form of LC3 is converted into the
autophagosomal membrane LC3-II form via enzymatic digestion
(26). Therefore, increased
LC3-II/LC3-I ratios can be used to indicate autophagosome formation
(26). t-BHP treatment
significantly increased the LC3-II/LC3-I ratio compared with cells
in the control group, which was significantly prevented by DT-010
pretreatment (Fig. 3A), suggesting
that DT-010 can inhibit oxidative stress-induced autophagy in
cardiomyocytes. Furthermore, AO and MDC staining demonstrated that
DT-010 may inhibit autophagy induced by oxidative stress (Fig. 3B). AO and MDC are two commonly used
specific agents for the assessment of autophagosomes, acidic
endosomes and lysosomes. Acidic vesicular organelles including
autophagolysosomes can be stained by AO, while MDC represents
widely used fluorescent probes that preferentially accumulate in
autophagic vacuoles (26-28).
Following t-BHP treatment, the number of orange-red AO stained
particles and the extent of MDC green fluorescence were observed to
be increased. By contrast, DT-010 pre-treatment markedly reduced
the formation of autophagosome vesicles as indicated by AO and MDC
staining.
Autophagic flux is characterized by sequential
conversion of autophagosomes into autolysosomes (26). The number of autolysosomes (red
puncta; GFP-/mRFP+) were found to be markedly
increased in cells in the t-BHP-treated group compared with that in
the control group, while autophagosomes (yellow puncta;
GFP+/mRFP+) exhibited no difference between
the two groups. However, in cells pre-treated with DT-010, the
numbers of autolysosomes (GFP-/mRFP+) were
decreased compared with that in the t-BHP-treated group (Fig. 3C). Therefore, these data suggest
that DT-010 may inhibit autophagic flow induced by t-BHP in
cardiomyocytes.
DT-010 prevents oxidative damage
induced by t-BHP by inhibiting of autophagy
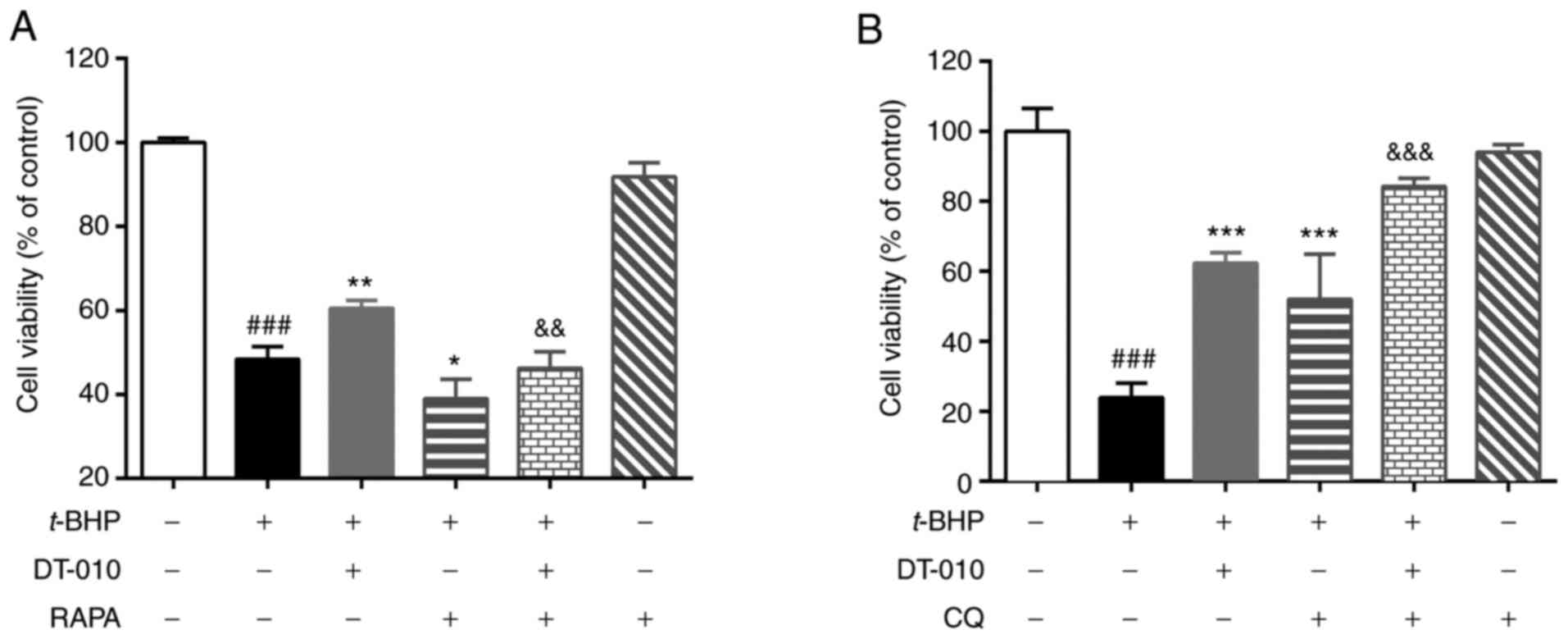
MTT assay results revealed that t-BHP treatment
significantly decreased cell viability (Fig. 4). Treatment with the autophagy
agonist rapamycin treatment significantly aggravated cell injury
induced by t-BHP further (Fig.
4A), whilst autophagy blocker chloroquine treatment
significantly attenuated t-BHP-induced cell injury (Fig. 4B). These data indicate that
autophagy may be involved in t-BHP-induced cardiomyocyte damage.
DT-010 pretreatment significantly prevented t-BHP-induced cell
damage (Fig. 4), which was
partially but significantly abolished by rapamycin (Fig. 4A) and significantly improved by
hydroxy-chloroquine treatment (Fig.
4B). These results suggest that the cardioprotective effects of
DT-010 could be mediated by inhibiting autophagy.
DT-010 attenuates autophagy via the
AMPK-mTOR-Ulk1 signaling pathway
t-BHP treatment increased the LC3-II/LC3-I ratio
whilst downregulating the expression levels of p62 compared with
those in cells in the control group (Fig. 5). However, DT-010 pretreatment
significantly reduced the LC3-II/LC3-I ratio and significantly
increased the expression levels of p62 compared with those in the
t-BHP group (Fig. 5A).
AMPK-mTOR-Ulk1/2 has been previously reported to be one of the
signaling pathways that regulate autophagy (29-32).
Therefore, the present study further measured the expression levels
of key components of the AMPK/mTOR/Ulk1 signaling pathway. t-BHP
treatment significantly increased the levels of AMPK
phosphorylation whilst downregulating the expression of total AMPK.
By contrast, AMPK phosphorylation was significantly decreased
whereas the total expression of AMPK was significantly increased in
cells pre-treated with DT-010 compared with those in cells treated
with t-BHP alone (Fig. 5B). The
expression of total mTOR and Ulk1 proteins were not observed to be
altered after t-BHP treatment or DT-010 pretreatment (Fig. 5B). However, t-BHP treatment
significantly decreased the levels of mTOR and Ulk1 phosphorylation
compared with cells in the control group (Fig. 5B), both of which were significantly
prevented by DT-010 pretreatment (Fig.
5B). These results suggested that DT-010 may inhibit
t-BHP-induced intracellular autophagy by inhibiting the
AMPK-mTOR-Ulk1 signaling pathway.
Discussion
Based on the previously reported properties of DSS
and TMP on the cardiovascular system, a large number of DSS and TMP
conjugates was synthesized previously, following which their
cardiovascular effects were screened in different models in
vivo and in vitro (21).
Previous structure-effect studies demonstrated that DT-010 is a
compound that constitute DSS and TMP linked via an ester bond
(22) and displayed better
cardioprotective effects compared with DSS, TMP or a combination of
the two (20-22,33-35).
However, the mechanism underlying the cardioprotective effects of
DT-010 remain unclear. Therefore, the present study explored the
involvement of autophagy in the cardioprotective effect of DT-010
against oxidative stress injury. DT-010 may facilitate
cardiomyocyte survival following t-BHP insult, implicating the
cardioprotective effects of DT-010 reported in a previous study
(20). DT-010 may be considered as
a candidate for myocardial ischemia and reperfusion injury
treatment.
Autophagy has been considered to be an important
target for treating ischemia-reperfusion injury (5). A large number of studies have
previously revealed that myocardial ischemia and reperfusion causes
mass generation of ROS in cells, which triggers autophagy (5,36,37).
t-BHP is commonly used as an oxidative stress inducer due to its
ability of producing free radicals (15). The present study demonstrated that
t-BHP exposure stimulated autophagy formation in a manner that was
associated with marked increases in the LC3-II/LC3-I ratio and
autophagic flux. Furthermore, the expression of p62, an
autophagy-specific metabolic substrate, was decreased following
t-BHP treatment. DT-010 pretreatment was found to inhibit
t-BHP-induced autophagy. Subsequently, rapamycin treatment prior to
t-BHP exposure was observed to aggravate t-BHP-mediated cell damage
and autophagy. By contrast, hydroxy-chloroquine treatment
attenuated cell damage and autophagy induced by t-BHP treatment,
suggesting that t-BHP induced H9c2 cell damage by activating
autophagy. DT-010 attenuated t-BHP-induced cardiomyocyte injury by
at least in part inhibiting autophagy, which was partially but
significantly abolished by rapamycin and significantly improved by
hydroxy-chloroquine treatment. At present, different physiological
consequences of autophagy have been observed in myocardial ischemia
reperfusion injury. Short-term and moderate activation of autophagy
may confer beneficial effects by degrading dysfunctional or damaged
proteins and organelles to promote cell survival, whilst a
persistent elevation of autophagy may promote cell death (38-42).
The duration and degree of ischemia and reperfusion are essential
for the modulation of autophagy as a pharmacological strategy
(43,44). Therefore, a number of studies have
reported that activation of autophagy could mitigate cardiac
ischemia/reperfusion injury (45-47).
However, other studies have also reported that inhibition of
autophagy could alleviate cardiac ischemia/reperfusion injury
(42,48,49),
which were consistent with results from the present study. In the
future, the optimal time course of DT-010 treatment following
myocardial ischemia and reperfusion injury in animal models should
be investigated, such that more data is required prior to its
proposed clinical use.
The regulation of signal transduction molecules and
pathways involved in autophagy is highly complex. The
AMPK-mTOR-Ulk1/2 pathway is an important signaling pathway that
regulates autophagy (29-32,50-54).
AMPK is a pivotal energy sensor for maintaining metabolic
homeostasis, whereby AMPK is activated in cells suffering from
ischemia and reperfusion during starvation (29). t-BHP-induced ROS accumulation causes
oxidative stress and dysfunction in mitochondria and results in an
energy crisis (16,55,56),
which can lead to the activation of AMPK. AMPK activation may
result in the inactivation of mTOR and activation of Ulk1 via the
phosphorylation of the Ser317 and Ser777 residues, thus promoting
autophagy (57). Phosphorylation of
Ulk1 Ser757 prevents Ulk1 activation and inhibits autophagy
(57). It was previously reported
that AMPK inhibitor Compound C could suppress AMPK/mTOR-mediated
autophagy in a rat myocardial infarction model (58). The present study revealed that the
levels of p-AMPK were increased whereas total AMPK protein
expression was inhibited after t-BHP treatment. Following DT-010
pre-treatment, phosphorylation of AMPK was decreased, whilst total
expression of AMPK was enhanced compared with cells treated with
t-BHP alone. A previous study reported that the expression of total
AMPK protein can be altered by various factors, such as
menadione-induced oxidative stress (59). In the present study, it was
therefore speculated that the expression of total AMPK protein may
be inhibited by severe oxidative stress. However, the underlying
mechanism of this phenomenon need to be investigated further. In
addition, it was also found that the levels of p-mTOR and p-Ulk1
(Ser757) were decreased in cells in the t-BHP group, which could be
prevented by DT-010 pre-treatment. Rapamycin treatment attenuated
the cardioprotective effects of DT-010 against t-BHP-induced
toxicity in H9c2 cells. This observation suggested that DT-010
stimulated mTOR activity, leading to the suppression of autophagy
initiation. A previous study revealed that DT-010 markedly elevated
Akt phosphorylation, followed by the reduction in AMPK
phosphorylation (inhibition) and subsequent phosphorylation and
activation of mTOR within 10 min after treatment (21). Therefore, it can be concluded that
DT-010 may inhibit t-BHP-induced autophagy in cardiomyocytes by
inhibiting the AMPK-mTOR-Ulk1 signaling pathway, thereby preventing
t-BHP-induced cardiomyocyte injury. Previous studies also revealed
the potent antioxidative effects of DT-010 (20,22).
DT-010 was found to eliminate a number of free radical species
(•O2-, •OH and ONOO-) induced by
t-BHP and increases the expression levels of cellular redox-related
proteins, including PGC-1α, Nrf2, and HO-1(20). Since oxidative stress caused by ROS
is a potent inducer of autophagy (16), scavenging of ROS and alleviation of
oxidative stress by DT-010 could also contribute to its inhibitory
effects on t-BHP-induced autophagy.
In summary, DT-010 was found to protect
cardiomyocytes against oxidative stress injury by inhibiting
autophagy through the AMPK-mTOR-Ulk1 signaling pathway. The
findings of the present study provided evidence for the use of
autophagy regulators in therapeutic strategies for myocardial
ischemia and reperfusion injury. DT-010 may be a promising
candidate for myocardial ischemia-reperfusion injury therapy.
Supplementary Material
DT-010 alone does not induce
cytotoxicity in H9c2 cells. H9c2 cells were treated with DT-010 for
1 h. Cell viability was detected using MTT assay. Data are
presented as the mean ± SD from six experimental repeats.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 81703509),
and Science and Technology Planning Project of Guangdong Province,
China (grant no. 2015A020211015).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request. Compound DT-010 is available with the agreement of the
corresponding author.
Authors' contributions
LW and LS conceived, designed and supervised the
whole study. JL, CX and HH performed the experiments, and further
analyzed and interpreted the data. YW, PY, YS, and LX provided some
professional help during the experiment. CX, LW, YW and LS wrote
and revised the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
Yuqiang Wang, Pei Yu, Luchen Shan and Yewei Sun are
inventors of the patent covering DT-010 (patent no. CN105294666A)
and have financial interest in Changzhou Magpie Pharmaceuticals,
Inc. (Changzhou, China), which is developing DT-010 as a
therapeutic agent.
References
|
1
|
Mendis S, Puska P and Norrving B: World
Health Organization, World Heart Federation: Global atlas on
cardiovascular disease prevention and control. Mendis S and Puska P
(eds). WHO, Geneva, pp1-155, 2011.
|
|
2
|
Kalogeris T, Baines CP, Krenz M and
Korthuis RJ: Ischemia/reperfusion. Compr Physiol. 7:113–170.
2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Li T, Su Y, Yu X, Mouniir DSA, Masau JF,
Wei X and Yang J: Trop2 guarantees cardioprotective effects of
cortical bone-derived stem cells on myocardial ischemia/reperfusion
injury. Cell Transplant. 27:1256–1268. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Matsui Y, Takagi H, Qu X, Abdellatif M,
Sakoda H, Asano T, Levine B and Sadoshima J: Distinct roles of
autophagy in the heart during ischemia and reperfusion: Roles of
AMP-activated protein kinase and Beclin 1 in mediating autophagy.
Circ Res. 100:914–922. 2007.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hamacher-Brady A, Brady NR, Logue S, Sayen
MR, Jinno M, Kirshenbaum L, Gottlieb RA and Gustafsson AB: Response
to myocardial ischemia/reperfusion injury involves Bnip3 and
autophagy. Cell Death Differ. 14:146–157. 2007.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Valentim L, Laurence KM, Townsend PA,
Carroll CJ, Soond S, Scarabelli TM, Knight RA, Latchman DS and
Stephanou A: Urocortin inhibits Beclin1-mediated autophagic cell
death in cardiac myocytes exposed to ischaemia/reperfusion injury.
J Mol Cell Cardiol. 40:846–852. 2006.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yan L, Sadoshima J, Vatner DE and Vatner
SF: Autophagy: A novel protective mechanism in chronic ischemia.
Cell Cycle. 5:1175–1177. 2006.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yan L, Vatner DE, Kim SJ, Ge H, Masurekar
M, Massover WH, Yang G, Matsui Y, Sadoshima J and Vatner SF:
Autophagy in chronically ischemic myocardium. Proc Natl Acad Sci
USA. 102:13807–13812. 2005.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Li X, Huang Q, Wang M, Yan X, Song X, Ma
R, Jiang R, Zhao D and Sun L: Compound K inhibits
autophagy-mediated apoptosis through activation of the PI3K-Akt
signaling pathway thus protecting against ischemia/reperfusion
injury. Cell Physiol Biochem. 47:2589–2601. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Przyklenk K, Dong Y, Undyala VV and
Whittaker P: Autophagy as a therapeutic target for
ischaemia/reperfusion injury? Concepts, controversies, and
challenges. Cardiovasc Res. 94:197–205. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Araujo TF, Cordeiro AV, Vasconcelos DAA,
Vitzel KF and Silva VRR: The role of cathepsin B in autophagy
during obesity: A systematic review. Life Sci. 209:274–281.
2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ravikumar B, Sarkar S, Davies JE, Futter
M, Garcia-Arencibia M, Green-Thompson ZW, Jimenez-Sanchez M,
Korolchuk VI, Lichtenberg M, Luo S, et al: Regulation of mammalian
autophagy in physiology and pathophysiology. Physiol Rev.
90:1383–1435. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhao W, Feng H, Sun W, Liu K, Lu JJ and
Chen X: Tert-butyl hydroperoxide (t-BHP) induced apoptosis and
necroptosis in endothelial cells: Roles of NOX4 and mitochondrion.
Redox Biol. 11:524–534. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhao Y, Zhang F, Zhao X, Yuan W, Zhang J
and Wang Y: Shenmai injection protects mitochondria from oxidative
injury in myocardial cells and its mechanism. Zhejiang Da Xue Xue
Bao Yi Xue Ban. 47:507–513. 2018.PubMed/NCBI(In Chinese).
|
|
15
|
Silva JP, Sardao VA, Coutinho OP and
Olveira PJ: Nitrogen compounds prevent h9c2 myoblast oxidative
stress-induced mitochondrial dysfunction and cell death. Cardiovasc
Toxicol. 10:51–65. 2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lu D, Zhu LH, Shu XM, Zhang CJ, Zhao JY,
Qi RB, Wang HD and Lu DX: Ginsenoside Rg1 relieves tert-Butyl
hydroperoxide-induced cell impairment in mouse microglial BV2
cells. J Asian Nat Prod Res. 17:930–945. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Li Z, Jiang T, Lu Q, Xu K, He J, Xie L,
Chen Z, Zheng Z, Ye L, Xu k, et al: Berberine attenuated the
cytotoxicity induced by t-BHP via inhibiting oxidative stress and
mitochondria dysfunction in PC-12 cells. Cell Mol Neurobiol.
40:587–602. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Fan G, Yu J, Asare PF, Wang L, Zhang H,
Zhang B, Zhu Y and Gao X: Danshensu alleviates cardiac
ischaemia/reperfusion injury by inhibiting autophagy and apoptosis
via activation of mTOR signalling. J Cell Mol Med. 20:1908–1919.
2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhigang P, Huishan P, Guangchun J and
Xiangshan L: An experimental study of ligustrazine on ischemic
myocardial protection and scavenging oxygen free radicals. Chin
Wild Plant Resou. 5(21)2000.
|
|
20
|
Zhang X, Hu H, Luo J, Deng H, Yu P, Zhang
Z, Zhang G, Shan L and Wang Y: A novel
danshensu-tetramethylpyrazine conjugate DT-010 provides
cardioprotection through the PGC-1α/Nrf2/HO-1 pathway. Biol Pharm
Bull. 40:1490–1498. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Tang F, Zhou X, Wang L, Shan L, Li C, Zhou
H, Lee SM and Hoi MP: A novel compound DT-010 protects against
doxorubicin-induced cardiotoxicity in zebrafish and H9c2 cells by
inhibiting reactive oxygen species-mediated apoptotic and
autophagic pathways. Eur J Pharmacol. 820:86–96. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wang Y, Zhang X, Xu C, Zhang G, Zhang Z,
Yu P, Shan L, Sun Y and Wang Y: Synthesis and biological evaluation
of danshensu and tetramethylpyrazine conjugates as cardioprotective
agents. Chem Pharm Bull (Tokyo). 65:381–388. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Shi X, Zhu H, Zhang Y, Zhou M, Tang D and
Zhang H: XuefuZhuyu decoction protected cardiomyocytes against
hypoxia/reoxygenation injury by inhibiting autophagy. BMC
Complement Altern Med. 17(325)2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zuo Y, Zhang J, Cheng X, Li J, Yang Z, Liu
X, Gu E and Zhang Y: Enhanced autophagic flux contributes to
cardioprotection of remifentanil postconditioning after
hypoxia/reoxygenation injury in H9c2 cardiomyocytes. Biochem
Biophys Res Commun. 514:953–959. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhao M, Sun L, Yu XJ, Miao Y, Liu JJ, Wang
H, Ren J and Zang WJ: Acetylcholine mediates AMPK-dependent
autophagic cytoprotection in H9c2 cells during
hypoxia/reoxygenation injury. Cell Physiol Biochem. 32:601–613.
2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Mizushima N: Methods for monitoring
autophagy. Int J Biochem Cell Biol. 36:2491–2502. 2004.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chang CH, Lee CY, Lu CC, Tsai FJ, Hsu YM,
Tsao JW, Juan YN, Chiu HY, Yang JS and Wang CC: Resveratrol-induced
autophagy and apoptosis in cisplatin-resistant human oral cancer
CAR cells: A key role of AMPK and Akt/mTOR signaling. Int J Oncol.
50:873–882. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Liu F, Gao S, Yang Y, Zhao X, Fan Y, Ma W,
Yang D, Yang A and Yu Y: Curcumin induced autophagy anticancer
effects on human lung adenocarcinoma cell line A549. Oncol Lett.
14:2775–2782. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Alers S, Löffler AS, Wesselborg S and
Stork B: Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy:
Cross talk, shortcuts, and feedbacks. Mol Cell Biol. 32:2–11.
2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ansari MY, Ahmad N and Haqqi TM: Butein
activates autophagy through AMPK/TSC2/ULK1/mTOR pathway to inhibit
IL-6 expression in IL-1β stimulated human chondrocytes. Cell
Physiol Biochem. 49:932–946. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhang Y and Miao JM: Ginkgolide K promotes
astrocyte proliferation and migration after oxygen-glucose
deprivation via inducing protective autophagy through the
AMPK/mTOR/ULK1 signaling pathway. Eur J Pharmacol. 832:96–103.
2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Cai ZY, Yang B, Shi YX, Zhang WL, Liu F,
Zhao W and Yang MW: High glucose downregulates the effects of
autophagy on osteoclastogenesis via the AMPK/mTOR/ULK1 pathway.
Biochem Biophys Res Commun. 503:428–435. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Cui G, Shan L, Hung M, Lei S, Choi I,
Zhang Z, Yu P, Hoi P, Wang Y and Lee SM: A novel Danshensu
derivative confers cardioprotection via PI3K/Akt and Nrf2 pathways.
Int J Cardiol. 168:1349–1359. 2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Cui Q, Chen Y, Zhang M, Shan L, Sun Y, Yu
P, Zhang G, Wang D, Zhao Z, Xu Q, et al: Design, synthesis, and
preliminary cardioprotective effect evaluation of danshensu
derivatives. Chem Biol Drug Des. 84:282–291. 2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Xu CJ, Deng HX, Chen HY, Cui QB, Shan LC,
Yu P, Sun YW and Wang YQ: Design, synthesis and biological
evaluations of novel conjugates of Danshensu, tetramethylpyrazine
and hydrogen sulfide donors as cardioprotective agents. Asian J
Chem. 28:2555–2561. 2016.
|
|
36
|
Lemasters JJ, Nieminen AL, Qian T, Trost
LC, Elmore SP, Nishimura Y, Crowe RA, Cascio WE, Bradham CA,
Brenner DA and Herman B: The mitochondrial permeability transition
in cell death: A common mechanism in necrosis, apoptosis and
autophagy. Biochim Biophys Acta. 1366:177–196. 1998.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Huang KY, Wang JN, Zhou YY, Wu SZ, Tao LY,
Peng YP, Que JQ, Xue YJ and Ji KT: Antithrombin III alleviates
myocardial ischemia/reperfusion injury by inhibiting excessive
autophagy in a phosphoinositide 3-kinase/Akt-dependent manner.
Front Pharmacol. 10(516)2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Zhang YW, Shi J, Li YJ and Wei L:
Cardiomyocyte death in doxorubicin-induced cardiotoxicity. Arch
Immunol Ther Exp (Warsz). 57:435–445. 2009.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Wang D, Yu W, Liu Y, Zhong G, Zhao Z, Yan
X and Liu Q: Roles of autophagy in ischemic heart diseases and the
modulatory effects of Chinese herbal medicine. Am J Chin Med.
45:1401–1419. 2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Hao M, Zhu S, Hu L, Zhu H, Wu X and Li Q:
Myocardial ischemic postconditioning promotes autophagy against
ischemia reperfusion injury via the activation of the
nNOS/AMPK/mTOR pathway. Int J Mol Sci. 18(614)2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Gao C, Wang R, Li B, Guo Y, Yin T, Xia Y,
Zhang F, Lian K, Liu Y, Wang H, et al: TXNIP/Redd1 signalling and
excessive autophagy: A novel mechanism of myocardial
ischaemia/reperfusion injury in mice. Cardiovasc Res. 116:645–657.
2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Li X, Hu X, Wang J, Xu W, Yi C, Ma R and
Jiang H: Inhibition of autophagy via activation of PI3K/Akt/mTOR
pathway contributes to the protection of hesperidin against
myocardial ischemia/reperfusion injury. Int J Mol Med.
42:1917–1924. 2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Yang SS, Liu YB, Yu JB, Fan Y, Tang SY,
Duan WT, Wang Z, Gan RT and Yu B: Rapamycin protects heart from
ischemia/reperfusion injury independent of autophagy by activating
PI3 kinase-Akt pathway and mitochondria K(ATP) channel. Pharmazie.
65:760–765. 2010.PubMed/NCBI
|
|
44
|
Xu Q, Li X, Lu Y, Shen L, Zhang J, Cao S,
Huang X, Bin J and Liao Y: Pharmacological modulation of autophagy
to protect cardiomyocytes according to the time windows of
ischaemia/reperfusion. Br J Pharmacol. 172:3072–3085.
2015.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Qu X, Chen X, Shi Q, Wang X, Wang D and
Yang L: Resveratrol alleviates ischemia/reperfusion injury of
diabetic myocardium via inducing autophagy. Exp Ther Med.
18:2719–2725. 2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Yang J, He J, Ismail M, Tweeten S, Zeng F,
Gao L, Ballinger S, Young M, Prabhu SD, Rowe GC, et al: HDAC
inhibition induces autophagy and mitochondrial biogenesis to
maintain mitochondrial homeostasis during cardiac
ischemia/reperfusion injury. J Mol Cell Cardiol. 130:36–48.
2019.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Wang F, Pulinilkunnil T, Flibotte S,
Nislow C, Vlodavsky I, Hussein B and Rodrigues B: Heparanase
protects the heart against chemical or ischemia/reperfusion injury.
J Mol Cell Cardiol. 131:29–40. 2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Wang G, Dai G, Song J, Zhu M, Liu Y, Hou
X, Ke Z, Zhou Y, Qiu H, Wang F, et al: Lactone component from
ligusticum chuanxiong alleviates myocardial ischemia injury through
inhibiting autophagy. Front Pharmacol. 9(301)2018.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Wu S, Chang G, Gao L, Jiang D, Wang L, Li
G, Luo X, Qin S, Guo X and Zhang D: Trimetazidine protects against
myocardial ischemia/reperfusion injury by inhibiting excessive
autophagy. J Mol Med (Berl). 96:791–806. 2018.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Xi X, Zou C, Ye Z, Huang Y, Chen T and Hu
H: Pioglitazone protects tubular cells against
hypoxia/reoxygenation injury through enhancing autophagy via
AMPK-mTOR signaling pathway. Eur J Pharmacol.
863(172695)2019.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Yang Y, Li N, Chen T, Zhang C, Liu L, Qi Y
and Bu P: Trimetazidine ameliorates sunitinib-induced
cardiotoxicity in mice via the AMPK/mTOR/autophagy pathway. Pharm
Biol. 57:625–631. 2019.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Dong Y, Chen H, Gao J, Liu Y, Li J and
Wang J: Molecular machinery and interplay of apoptosis and
autophagy in coronary heart disease. J Mol Cell Cardiol. 136:27–41.
2019.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Yan J, Yan JY, Wang YX, Ling YN, Song XD,
Wang SY, Liu HQ, Liu QC, Zhang Y, Yang PZ, et al:
Spermidine-enhanced autophagic flux improves cardiac dysfunction
following myocardial infarction by targeting the AMPK/mTOR
signalling pathway. Br J Pharmacol. 176:3126–3142. 2019.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Wang L, Yuan D, Zheng J, Wu X, Wang J, Liu
X, He Y, Zhang C, Liu C, Wang T and Zhou Z: Chikusetsu saponin IVa
attenuates isoprenaline-induced myocardial fibrosis in mice through
activation autophagy mediated by AMPK/mTOR/ULK1 signaling.
Phytomedicine. 58(152764)2019.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Rabinovitc RC, Samborska B, Faubert B, Ma
EH, Gravel SP, Andrzejewski S, Raissi TC, Pause A, St-Pierre J and
Jones RG: AMPK maintains cellular metabolic homeostasis through
regulation of mitochondrial reactive oxygen species. Cell Rep.
21:1–9. 2017.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Zhang T, Liu J, Tong Q and Lin L: SIRT3
acts as a positive autophagy regulator to promote lipid
mobilization in adipocytes via activating AMPK. Int J Mol Sci.
21(372)2020.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Kim J, Kundu M, Viollet B and Guan KL:
AMPK and mTOR regulate autophagy through direct phosphorylation of
Ulk1. Nat Cell Biol. 13:132–141. 2011.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Ren PH, Zhang ZM, Wang P, Zhu HP and Li
ZQ: Yangxinkang tablet protects against cardiac dysfunction and
remodelling after myocardial infarction in rats through inhibition
of AMPK/mTOR-mediated autophagy. Pharm Biol. 58:321–327.
2020.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Jin Q, Jhun BS, Lee SH, Lee J, Pi Y, Cho
YH, Baik HH and Kang I: Differential regulation of
phosphatidylinositol 3-kinase/Akt, mitogen-activated protein
kinase, and AMP-activated protein kinase pathways during
menadione-induced oxidative stress in the kidney of young and old
rats. Biochem Biophys Res Commun. 315:555–561. 2004.PubMed/NCBI View Article : Google Scholar
|