Introduction
Acute kidney injury (AKI), which is one of the most
common major complications in patients admitted to hospital and
especially those admitted to the intensive care unit worldwide
(1,2), is associated with adverse short- and
long-term prognosis and increased medical expenses (3). Despite an increasing heterogeneity in
its causes and clinical features, detecting patients who are at
high risk of AKI may result in earlier diagnosis, avoidance of
potentially nephrotoxic exposures and lower health care costs
(4,5). A number of biomarkers have been
demonstrated to identify patients with an increased risk of AKI and
to predict long-term prognosis (6,7).
However, the clinical utilization of these biomarkers in patients
in the coronary care unit (CCU) is limited.
The prognostic nutritional index (PNI), which is
calculated from the serum albumin concentration and total
lymphocyte count in the peripheral blood, is an index that reflects
chronic inflammation, immune system and nutritional status and
indicates prognostic significance in different patients (8). PNI had been described as a simple and
objective indicator of adverse outcomes not only in chronic
conditions, such as hepatocellular carcinoma (9), chronic heart failure (10) and different cancer types (11), but also in acute illnesses,
including acute coronary syndrome (12), acute heart failure (13) and stroke (14). Furthermore, a previous study
demonstrated an association between PNI and AKI in patients with
normal serum creatinine levels who underwent coronary artery bypass
grafting (15). However, to the
best of our knowledge, no study has investigated the potential
value of PNI for patients in the CCU. The current two-stage
observational study aimed to explore and validate the predictive
value of PNI for the development of AKI and its prognosis in
patients in the CCU.
Materials and methods
Participants. In the current two-stage
observational study, initial data was obtained from the Medical
Information Mart for Intensive Care (MIMIC) III database (16). Access to the database was approved
by the Institutional Review Board of the Massachusetts Institute of
Technology and a waiver for informed consent was granted. All
adults in the database with a first CCU admission and length of
stay ≥48 h were selected (n=6,903). Since MIMIC III is a freely
accessible public database, the age of all patients was calculated
from the date of admission minus the date of birth. However, for
patients >89 years old, the date of birth was set to 300 years
before their first admission, therefore the actual age of those
patients would not be acquired. Moreover, the median age of
patients >89 years old was 91.4 in this database. Subsequently,
these patients were excluded (n=459). Therefore, 6,444 patients
constituted the test cohort. A total of 412 adult patients (age,
66.3±13.1 years; 32.8% male) with duration of hospitalization ≥48 h
who were admitted to the CCU at Zhongnan Hospital of Wuhan
University (Wuhan, China) from January 1, 2014 to June 1, 2015,
were prospectively included in the validation cohort, as reported
in a previous study (17). The
current study was approved by the Ethics Committee of Zhongnan
Hospital of Wuhan University and all patients in the validation
cohort were required to provide written informed consent.
All laboratory results for the patients enrolled in
the test cohort were collected from the MIMICIII database. Since a
patient may undergo a single laboratory test more than once during
their hospital admission, only the initial test results were
included in the final analysis. Patients' baseline characteristics
and acute physiology scores III (APSIII) were calculated using the
SQL code that was available in the MIMIC code repository, as
described in previous studies (18).
PNI value was calculated using the following
equation: 10x serum albumin (g/dl) + 0.005 x total lymphocyte count
(mm3). Estimated glomerular filtration rate (eGFR) was
calculated using the Chronic Kidney Disease Epidemiology
Collaboration (CKD-EPI) equation (19).
The primary outcome was the development of AKI,
which was classified according to the Kidney Disease Improving
Global Outcomes Clinical Practice Guidelines for AKI based on serum
creatinine criteria within 7 days after hospital admission
(20), using the SQL code that was
available in the MIMIC code repository.
The secondary outcomes included in-hospital
mortality and 2-year mortality from admission to the end of the
2-year follow-up period.
Missing data
There were missing data for albumin and lymphocyte
counts: 986 (15.3%) patients had a missing albumin variable, and
467 (7.2%) had a missing lymphocyte count variable in the MIMICIII
database. Additionally, 1,269 (19.7%) patients had a missing PNI
value. Imputation for missing variables was considered if they were
missing in >20% of the patients in the dataset. Predictive mean
matching was used to impute numeric features, logistic regression
was used for binary variables, and Bayesian polytomous regression
was used for factor features.
Statistical analysis
R software (version 3.6.1, http://www.r-project.org) was used for all statistical
analyses. Unpaired two-sample t-test or the Mann-Whitney U test was
used to compare continuous variables across groups and Pearson's
Chi-squared test (χ2) to compare categorical variables
across groups. Spearman's correlation coefficients were calculated
for the correlation between PNI and other biochemical factors.
Adjusted odds ratios (ORs) were calculated for AKI using multiple
logistic regression. The Cox proportional hazards model was used to
estimate the hazard ratio and its associated 95% confidence
interval (95% CI). The selection of covariates was based on known
or clinically relevant risk factors of AKI (21,22).
The clinical model for AKI and prognosis was adjusted for age, sex,
BMI, hypertension, diabetes, CKD, eGFR, hemoglobin, leucocytes,
mean arterial pressure, triglycerides, total cholesterol, serum
potassium and serum sodium. To evaluate the utility of biomarkers
in risk classification, the net reclassification index (NRI) and
integrated discrimination improvement (IDI) was determined, as
described in previous studies (23,24).
Furthermore, a decision curve analysis (DCA) was conducted to
determine the clinical usefulness of PNI values in the test cohort
and validation cohort. P<0.05 was considered to indicate a
statistically significant difference.
Results
Subject characteristics
A total of 6,856 patients (6,444 patients in the
test cohort and 412 patients in the validation cohort) were
analyzed in the current study. There were 1693 (26.3%) patients and
268 (65.0%) cases primarily diagnosed with acute coronary syndrome
in the test cohort and in the validation cohort, respectively.
Patients were split into groups of those with and those without
AKI. In the test cohort, patients who developed AKI were indicated
to be older, exhibited more comorbidities (CKD, hypertension and
diabetes), higher serum levels of leukocyte, serum albumin, PNI and
higher APSIII scores, and lower levels of mean arterial pressure
(MAP), serum creatinine, eGFR, hemoglobin and serum albumin
compared with those who did not develop AKI. However, the sex
distribution, BMI, lymphocyte counts, serum sodium, total
cholesterol and triglycerides were comparable between the two
groups. Patients enrolled in the validation cohort also exhibited
characteristics similar to those in the test cohort except for
leukocyte numbers (Table I).
 | Table ICharacteristics of the CCU patients
on admission. |
Table I
Characteristics of the CCU patients
on admission.
| | Test set
(n=6,444) | Validation set
(n=412) |
|---|
| Characteristic | No AKI
(n=1,987) | AKI (n=4,457) | P-value | No AKI (n=282) | AKI (n=130) | P-value |
|---|
| Age, year | 63.6±13.6 | 68.6±15.2 | <0.001 | 65.5±13.1 | 68.2±13.1 | <0.001 |
| Sex, male, n
(%) | 762 (38.3) | 1,845 (38.0) | 0.851 | 93 (33.0) | 42 (32.3) | 0.893 |
| BMI,
kg/m2 | 27.9±4.1 | 28.0±6.3 | 0.652 | 23.8±3.5 | 23.7±3.3.2 | 0.870 |
| Primary diagnosis,
n (%) | | | | | | |
|
Acute
coronary syndrome | 503 (25.3) | 1,190 (26.7) | 0.243 | 179 (63.5) | 89 (68.5) | 0.325 |
| Preexisting
diseases, n (%) | | | | | | |
|
CKD | 207 (10.5) | 1,247 (28.0) | <0.001 | 17 (6.0) | 42 (32.3) | 0.001 |
|
Hypertension | 1,089 (54.8) | 2,669 (59.9) | <0.001 | 118 (41.8) | 90 (69.2) | <0.001 |
|
Diabetes | 405 (20.4) | 1,140 (25.6) | <0.001 | 79 (28.0) | 63 (48.5) | <0.001 |
| Biochemical
data | | | | | | |
|
MAP,
mmHg | 58.4±16.7 | 56.3±12.7 | <0.001 | 94.6±13.0 | 87.8±13.4 | <0.001 |
|
Leukocyte,
x109/l | 12.0±4.9 | 13.2±5.0 | <0.001 | 8.9±2.8 | 9.2±2.3 | 0.550 |
|
Lymphocyte
count, x109/l | 1.6±0.6 | 1.5±0.8 | 0.565 | 1.5±0.78 | 1.4±0.5 | 0.178 |
|
Hemoglobin,
g/l | 127.6±31.9 | 109.6±22.3 | <0.001 | 130.0±18.6 | 109.1±23.4 | <0.001 |
|
eGFR,
ml/min/1.73 m2 | 72.9±17.7 | 50.3±10.4 | <0.001 | 86.3±10.7 | 57.1±15.3 | <0.001 |
|
Serum
albumin, g/l | 34.2±2.6 | 31.9±2.6 | <0.001 | 38.1±4.1 | 35.7±5.0 | <0.001 |
|
Serum
creatinine, umol/l | 84.2±19.2 | 120.1±18.1 | <0.001 | 70.9±15.4 | 108.4±12.9 | <0.001 |
|
PNI | 56.6±15.0 | 46.6±20.6 | <0.001 | 48.9±5.3 | 41.4±6.0 | <0.001 |
|
Total
cholesterol, mmol/l | 3.9±1.2 | 4.0±1.0 | 0.740 | 4.2±0.9 | 4.0±1.0 | 0.085 |
|
Triglycerides,
mmol/l | 1.2±0.6 | 1.1±0.5 | 0.364 | 1.7±1.3 | 1.5±1.2 | 0.243 |
| APACHEII,
points | - | - | - | 6.8±1.8 | 10.4±2.2 | <0.001 |
|
Serum
sodium, mmol/l | 139.5±3.6 | 139.5±4.4 | 0.537 | 138±4.8 | 137±5.7 | 0.450 |
|
Serum
potassium, mmol/l | 4.5±0.8 | 4.7±0.9 | <0.001 | 3.9±0.5 | 4.1±0.7 | 0.001 |
|
APSIII,
points | 32.5±10.1 | 47.1±19.3 | <0.001 | - | - | - |
|
LOS,
days | 7.1±2.6 | 12.0±4.5 | <0.001 | 11.0±5.6 | 16.2±9.9 | <0.001 |
|
Hospital
mortality | 445 (22.4) | 1,747 (39.2) | <0.001 | 17 (6.0) | 44 (33.8) | <0.001 |
|
2-year
mortality | 696 (35.0) | 2,454 (55.1) | <0.001 | 42 (14.9) | 70 (53.8) | <0.001 |
Correlation between PNI and other
parameters
The Spearman correlation coefficients indicated that
PNI values were significantly positively correlated with leukocyte
counts (r=0.053), MAP levels (r=0.057), eGFR (r=0.047), hemoglobin
levels (r=0.051) and BMI (r=0.032). Baseline PNI values were
negatively correlated with age (r=-0.103) and APSIII scores
(r=-0.077). However, no correlations between PNI and triglyceride
or total cholesterol were observed (Table II).
 | Table IICorrelations between baseline PNI and
selected clinical parameters. |
Table II
Correlations between baseline PNI and
selected clinical parameters.
| | PNI |
|---|
| Variables | r-value | P-value |
|---|
| Age, years | -0.103 | <0.001 |
| Hemoglobin,
g/l | 0.051 | <0.001 |
| BMI,
kg/m2 | 0.032 | 0.021 |
| Leucocyte,
x109/l | 0.053 | <0.001 |
| Total cholesterol,
mg/dl | -0.009 | 0.697 |
| Triglycerides,
mg/dl | -0.012 | 0.705 |
| eGFR, ml/min/1.73
m2 | 0.047 | <0.001 |
| MAP, mmHg | 0.057 | <0.001 |
| APSIII, points | -0.077 | <0.001 |
PNI as a predictor of the primary
endpoint
In the test cohort, AKI occurred in a total of 4,457
patients (69.2%) within 7 days after admission (Table I). The PNI value was lower in the
patients with AKI than in the non-AKI patients (Table I), which was supported by the
results of the multivariate analyses. PNI value was associated with
incident AKI in patients with or without preexisting chronic kidney
disease (CKD) after adjustment for clinical variables in the test
cohort. The highest quartile of PNI was associated with an
increased risk for AKI by 1.8-fold compared with the lowest
quartile in all participants (Table
III). When PNI was analyzed as a continuous variable, higher
PNI values were also associated with the development of AKI
(OR=0.982; 95% CI, 0.975-0.988; P<0.001; data not shown) in a
multivariable model. Moreover, lower PNI values group (<48.8)
had a higher risk of AKI by 1.3-fold compared with the higher PNI
group after adjusting for other risk factors (data not shown).
 | Table IIIMultivariate logistic regression
analyses of PNI as a predictor for AKI in the test cohort. |
Table III
Multivariate logistic regression
analyses of PNI as a predictor for AKI in the test cohort.
| A, All study
participants (n=6,444) |
|---|
| PNI on
admission | Unadjusted OR | Adjusted
ORa | 95% CI | P-value |
|---|
| Quartile 1
(>61.0) | 1.0 (ref.) | 1.0 (ref.) | | |
| Quartile 2
(42.6-61.0) | 1.652 | 1.414 | 1.170-1.708 | <0.001 |
| Quartile 3
(34.1-42.5) | 1.799 | 1.447 | 1.195-1.753 | <0.001 |
| Quartile 4
(<34.0) | 1.897 | 1.765 | 1.457-2.137 | <0.001 |
| B, Patients without
preexisting CKD (n=4,990) |
| PNI on
admission | Unadjusted OR | Adjusted
ORa | 95% CI | P-value |
| Quartile 1
(>53.5) | 1.0 (ref.) | 1.0 (ref.) | | |
| Quartile 2
(43.1-53.5) | 2.229 | 1.817 | 1.484-2.226 | <0.001 |
| Quartile 3
(34.0-43.0) | 2.398 | 1.852 | 1.484-2.226 | <0.001 |
| Quartile 4
(<34.0) | 3.084 | 2.262 | 1.820-2.811 | <0.001 |
| C, Patients with
preexisting CKD (n=1,454) |
| PNI on
admission | Unadjusted OR | Adjusted
ORa | 95% CI | P-value |
| Quartile 1
(>55.0) | 1.0 (ref.) | 1.0 (ref.) | | |
| Quartile 2
(41.6-55.0) | 2.394 | 2.215 | 1.358-3.613 | 0.001 |
| Quartile 3
(33.0-41.5) | 2.457 | 2.389 | 1.469-3.883 | <0.001 |
| Quartile 4
(<33.0) | 2.492 | 2.506 | 1.528-4.110 | <0.001 |
Performance of PNI for predicting AKI
in subgroup analyses
For the prediction of AKI, the area under the
receiver operating characteristic curve (AUC) of PNI on admission
for all participants in the test set was 0.755. A cutoff of 48.8
yielded good specificity (81.8%) and sensitivity (63.5%; Fig. 1A). The AUCs of PNI in subgroups with
and without preexisting CKD were greater compared with APSIII
scores, eGFR and the clinical model (Fig. 1B and C; all P<0.05). To further validate the
predictive value of PNI for AKI, the AUC for predicting AKI was
analyzed in an independent validation cohort recruited from
Zhongnan Hospital of Wuhan University. As presented in Fig. 1D, PNI was validated in predicting
AKI in the validation cohort (AUC=0.738).
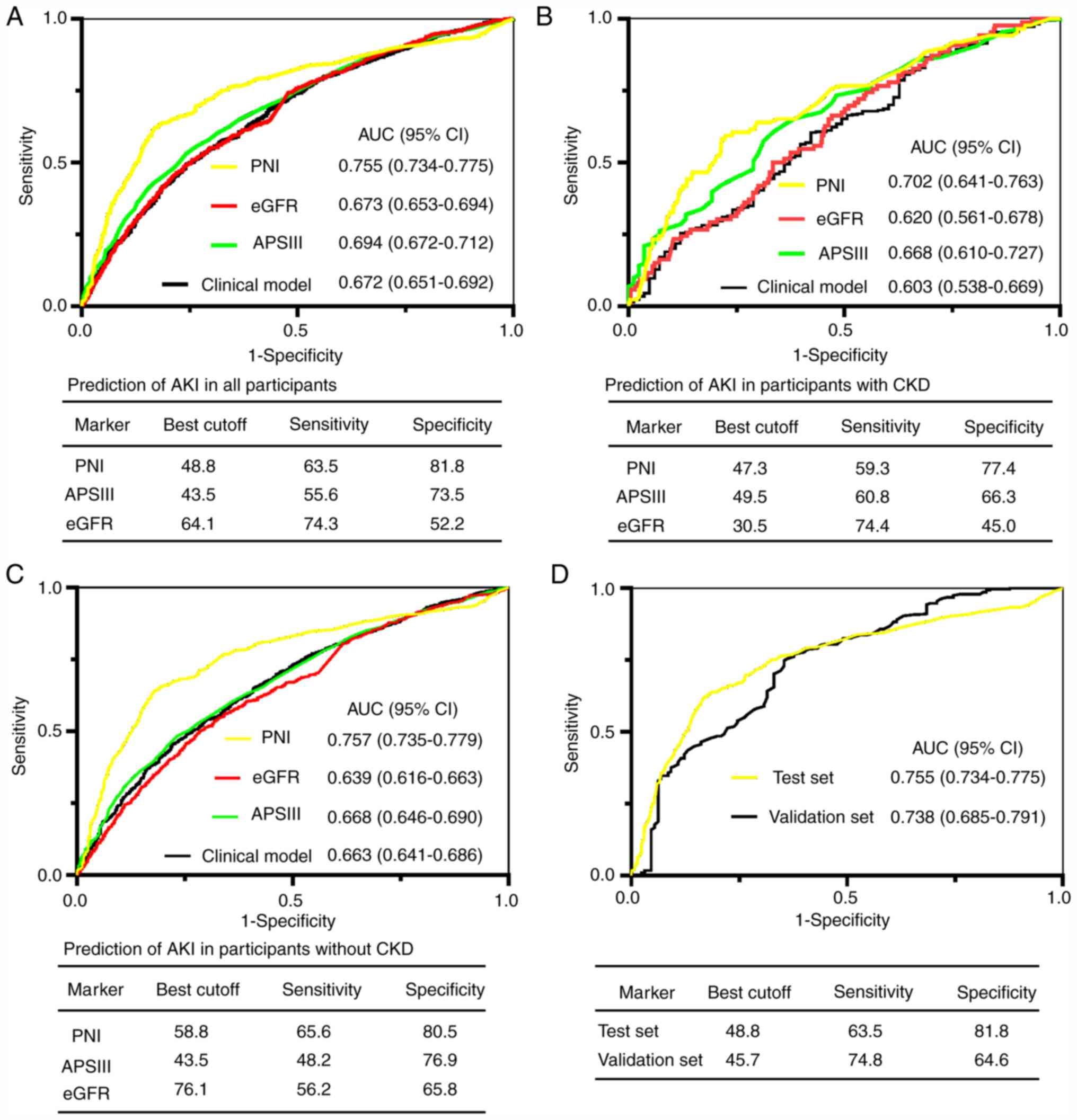 | Figure 1ROC analyses for predicting AKI.
(A-C) PNI, eGFR, APSIII and clinical model for predicting AKI in
(A) all participants, (B) in patients with preexisting CKD, and (C)
in patients without preexisting CKD. (D) ROC analysis for the test
and validation sets. ROC, receiver operator characteristic; AKI,
acute kidney injury; PNI, prognostic nutritional index; eGFR,
Estimated glomerular filtration rate; APSIII, acute physiology
scores III; CKD, chronic kidney disease; CI, confidence interval;
AUC, area under the curve. |
PNI as a predictor for secondary
endpoints
Of the 6,444 participants in the test cohort, 2,219
(34.4%) suffered mortality within 2 years after admission. Lower
PNI levels were associated with a higher risk of in-hospital
mortality [hazard ratio (HR)=0.978 (test cohort) and 0.882
(validation cohort)] and 2-year mortality [HR=0.984 (test cohort)
and 0.906 (validation cohort); Table
IV]. Other parameters, including APACHEII score, eGFR, MAP and
age, associated with a higher risk of in-hospital mortality and
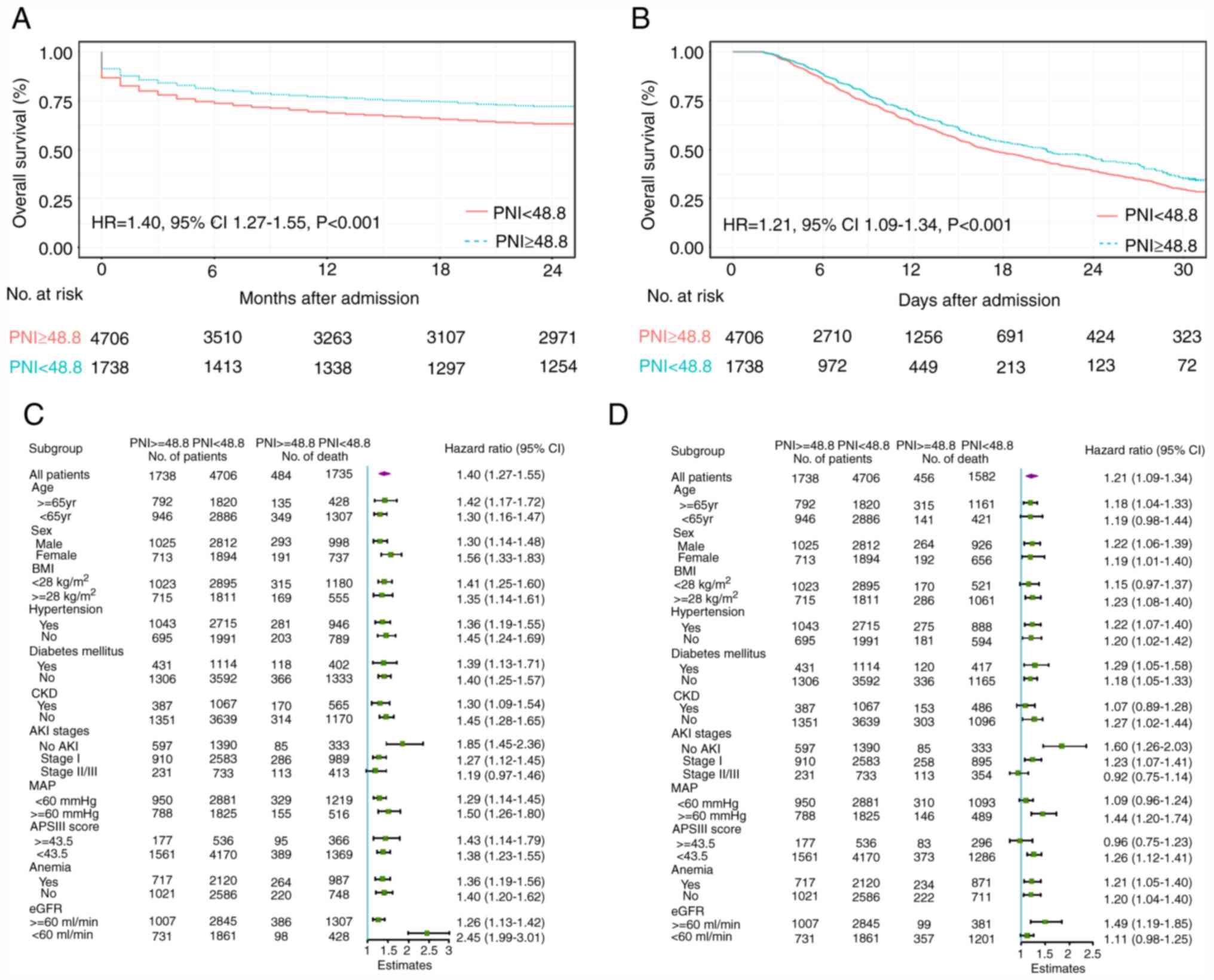
2-year mortality are also presented in Table IV. Moreover, a PNI level <48.8
on admission was associated with a significantly increased
probability of in-hospital mortality (HR=1.40; 95% CI: 1.27-1.55)
and 2-year mortality (HR=1.21; 95% CI, 1.09-1.34; P<0.001) over
the 2-year follow-up period in both cohorts (Fig. 2A and B). In the pre-specified subgroup analysis,
patients with PNI <48.8 had a higher risk of mortality than
those with PNI ≥48.8 in all subgroups except for AKI stages
(Fig. 2C and D). Furthermore, a lower level of PNI value
(PNI <48.8) on admission was associated with a significantly
increased risk of sepsis (OR=1.53, 95% CI, 1.32-1.77; P<0.001)
and acute respiratory distress syndrome (ARDS) (OR=1.52, 95% CI,
1.04-2.22; P=0.029) after adjusting for other clinical risk factors
(data not shown).
 | Table IVMultivariate Cox regression analyses:
Predictors of hospital mortality and 2-year mortality in test and
validation sets. |
Table IV
Multivariate Cox regression analyses:
Predictors of hospital mortality and 2-year mortality in test and
validation sets.
| A, in-hospital
mortality |
|---|
| | Test set | Validation set |
|---|
| Parameter | Adjusted HR (95%
CI) | P-value | Adjusted HR (95%
CI) | P-value |
|---|
| APACHEII score | - | - | 1.121
(1.040-1.208) | 0.003 |
| PNI | 0.978
(0.975-0.981) | <0.001 | 0.882
(0.845-0.921) | <0.001 |
| MAP, mmHg | 0.977
(0.964-0.989) | <0.001 | 0.986
(0.974-0.999) | 0.034 |
| APSIII score | 1.003
(1.001-1.006) | 0.016 | - | - |
| eGFR, ml/min | 0.994
(0.992-0.996) | <0.001 | 0.982
(0.955-1.010) | 0.210 |
| B, 2-year
mortality |
| | Test set | Validation set |
| Parameter | Adjusted HR (95%
CI) | P-value | Adjusted HR (95%
CI) | P-value |
| APACHEII score | - | - | 1.145
(1.080-1.214) | <0.001 |
| PNI | 0.984
(0.983-0.986) | <0.001 | 0.906
(0.877-0.936) | <0.001 |
| MAP, mmHg | 0.974
(0.965-0.982) | <0.001 | 0.983
(0.973-0.993) | 0.001 |
| APSIII score | 1.021
(1.017-1.024) | <0.001 | - | - |
| eGFR, ml/min | 0.997
(0.995-0.999) | 0.006 | 1.001
(0.988-1.014) | 0.855 |
| Age, years | 1.014
(1.012-1.016) | <0.001 | 1.002
(0.980-1.025) | 0.857 |
| Preexisting
CKD | 1.223
(1.098-1.363) | <0.001 | 1.008
(0.548-1.856) | 0.979 |
| Hypertension | 1.255
(1.155-1.365) | <0.001 | 1.166
(0.762-1.783) | 0.480 |
Effect of PNI on risk reclassification
of AKI and mortality
To determine whether PNI materially improved risk
reclassification, NRI and IDI were used in the test cohort. As
presented in Table V, the addition
of PNI significantly improved the risk reclassification (as
measured using NRI and IDI) of AKI and mortality compared to the
APSIII score and clinical model alone.
 | Table VNRI and IDI analyses for risk
reclassification of AKI and mortality in test cohort. |
Table V
NRI and IDI analyses for risk
reclassification of AKI and mortality in test cohort.
| A, AKI |
|---|
| | AUC | IDI | NRIa |
|---|
| Outcome | Biomarker | Biomarker+ clinical
model | Clinical
modelb |
P-valuec | Value (95% CI) | P-value | Value (95% CI) | P-value |
|---|
| PNI | 0.755 | 0.787 | 0.672 | 0.003 | 0.044
(0.020-0.117) | <0.001 | 0.169
(0.086-0.382) | <0.001 |
| APSIII | 0.694 | 0.730 | | 0.279 | 0.021
(0.017-0.076) | <0.001 | 0.014
(-0.071-0.101) | 0.737 |
| PNI+APSIII | 0.784 | 0.801 | | <0.001 | 0.712
(0.047-0.158) | <0.001 | 0.199
(0.120-0.408) | <0.001 |
| B, In hospital
mortality |
| | AUC | IDI | NRIa |
| Outcome | Biomarker | Biomarker+ clinical
model | Clinical
modelb |
P-valuec | Value (95% CI) | P-value | Value (95% CI) | P-value |
| PNI | 0.737 | 0.788 | 0.663 | <0.001 | 0.015
(-0.011-0.253) | 0.066 | 0.177
(-0.030-0.238) | 0.134 |
| APSIII | 0.686 | 0.716 | | 0.001 | 0.037
(-0.030-0.140) | 0.294 | 0.068
(-0.150-0.332) | 0.420 |
| PNI+APSIII | 0.779 | 0.804 | | <0.001 | 0.124
(0.022-0.244) | 0.028 | 0.192
(0.007-0.404) | 0.046 |
| C, 2-year
mortality |
| | AUC | IDI | NRIa |
| Outcome | Biomarker | Biomarker+ clinical
model | Clinical
modelb |
P-valuec | Value (95% CI) | P-value | Value (95% CI) | P-value |
| PNI | 0.735 | 0.780 | 0.683 | <0.001 | 0.047
(0.021-0.137) | 0.002 | 0.123
(-0.009-0.356) | 0.058 |
| APSIII | 0.684 | 0.711 | | 0.003 | 0.024
(0.003-0.103) | 0.024 | 0.095
(-0.068-0.286) | 0.236 |
| PNI+APSIII | 0.777 | 0.797 | | <0.001 | 0.072
(0.033-0.158) | 0.002 | 0.213
(0.037-0.387) | 0.026 |
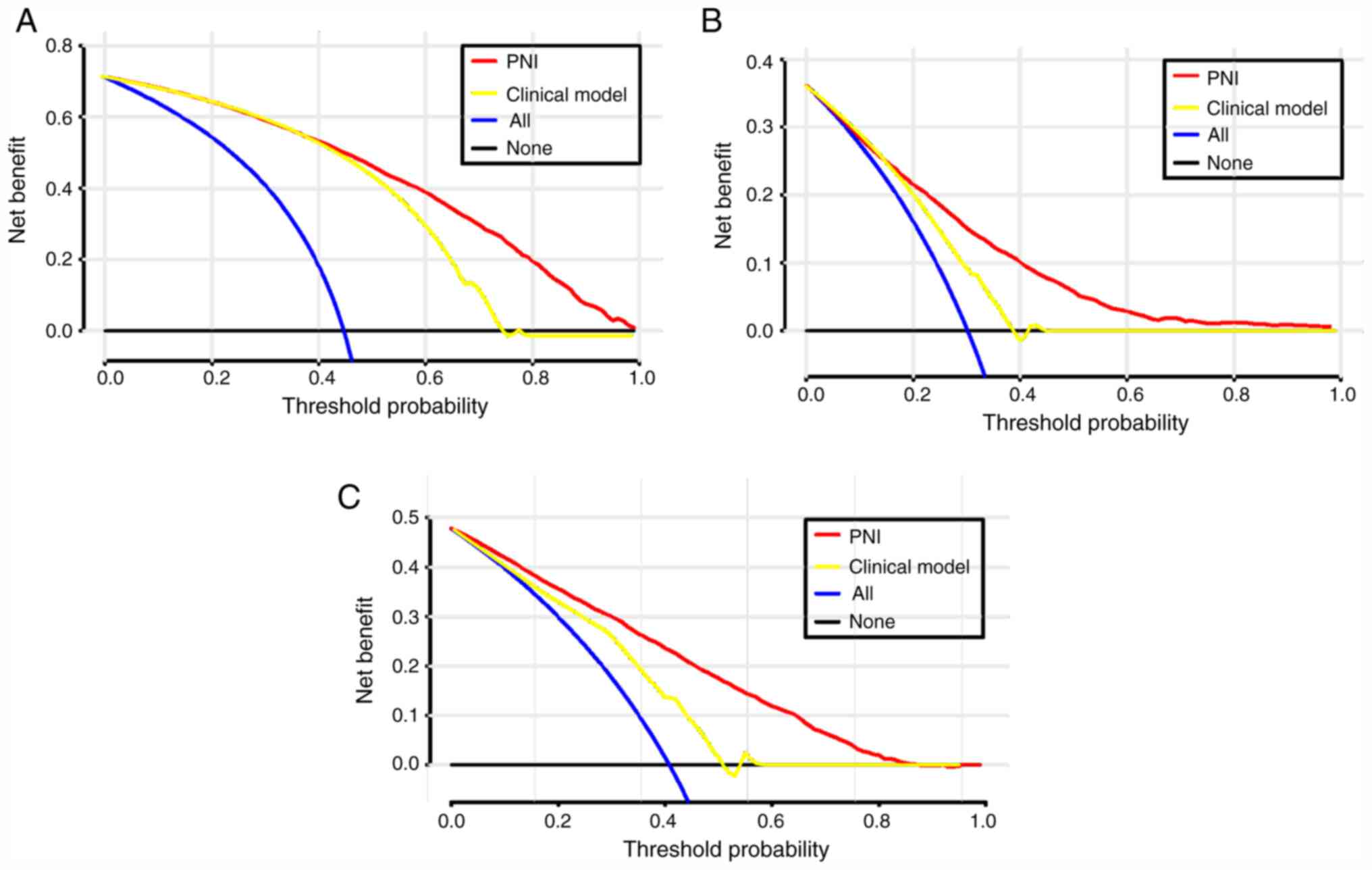
Clinical usefulness of PNI
To evaluate the clinical use of PNI, a DCA was
introduced. According to the DCA, when the threshold probability
for a patient was within the range of 0-100%, the PNI added more
net benefit than the ‘treat all’ or ‘treat none’ strategies both in
the test cohort and in the validation cohort (Figs. 3 and S1).
Discussion
In the test cohort of 6,444 patients, it was
revealed that PNI measured on the first day of admission was an
independent predictor of AKI in patients in the CCU. The best
cutoff value of 48.8 was a good threshold for the risk of mortality
in almost all subgroups. The performance of PNI was superior to
that of the APSIII scores and the clinical model in the test
cohort. Furthermore, the risk reclassification, as measured by the
NRI and IDI, was significantly improved through the addition of the
APSIII score to the clinical model. The predictive value of PNI was
further demonstrated in the validation cohort of 412 CCU patients.
These data suggest that the PNI may be a good predictor for
identifying patients at high risk of AKI and mortality in the
CCU.
To the best of our knowledge, only two studies have
assessed the predictive value of PNI for AKI risk. Dolapoglu et
al (15) conducted a
retrospective study of 336 consecutive patients with normal serum
creatinine levels who underwent coronary artery bypass grafting.
The aforementioned study concluded that PNI was independently
predictive of AKI in a multivariate logistic regression (OR=0.83;
95% CI, 0.78-0.88). A similar conclusion was drawn in another
retrospective study of 423 patients following donor liver
transplantation (25). However, to
the best of our knowledge, the current study is the first to
investigate the association between PNI levels and AKI in a public
database, demonstrating that each decrease of a score of 1 in PNI
led to a 1.8% risk of AKI. The results were also independently
verified in the hospital cohort.
The association between PNI and CKD has been
demonstrated in previous studies. Hori et al (26) revealed an association between
decreased PNI value (<54) and lower postoperative renal function
after 12 months of donor nephrectomy in a retrospective
observational study of 75 living kidney donors. The aforementioned
study further concluded that the decreased PNI value independently
predicted the development of CKD G3b (OR=0.3; 95% CI, 0.1-0.8).
Similarly, a positive correlation between PNI value and eGFR
(r=0.047) was indicated in the current study based on Spearman's
correlation coefficient. Furthermore, a previous study that
investigated the impact of PNI on cardiovascular disease (CVD)
mortality in patients with incident peritoneal dialysis concluded
that PNI may be a better predictor of CVD mortality than hemoglobin
and leukocytes (27). A similar
association was also indicated in patients undergoing continuous
ambulatory peritoneal dialysis (CAPD). Cai et al (28) conducted a retrospective cohort study
of 1,501 patients with CAPD and found a robust and consistent
association between lower PNI value (<45) and overall mortality
or CVD mortality that was independent of other common risk factors
(HR=1.82; 95% CI, 1.36-2.43; HR=1.63; 95% CI, 1.06-2.51).
Additionally, PNI was a prognostic factor in patients undergoing
kidney transplantation and in patients undergoing peritoneal
dialysis (25,29). The results of the current study
similarly suggested that PNI admission was a significant
determinant of mortality, indicating that each decrease by a score
of 1 in PNI led to a 2.2% increase in the risk of in-hospital
mortality and 1.6% increase in the risk of 2-year mortality.
Up to 80% of hospitalized patients are malnourished
worldwide (30), especially
critically ill patients, as they may lose 10-25% of their body
protein content within 10 days after admission (31). Serum albumin, which is a common
indicator of nutritional status, was an independent predictor for
the development of postoperative AKI and mortality in a
retrospective study of 2,339 patients who underwent aneurysm
clipping surgery (32).
Additionally, a previous study demonstrated that nutritional
intervention may improve clinical outcomes with shorter hospital
stays and lower costs (33). In the
current study, PNI was correlated with the known indicators of
nutritional status, including BMI and hemoglobin. Furthermore,
patients in the CCU are often in a heightened proinflammatory
state, which can significantly worsen nutritional status (34), and AKI is known to be associated
with intrarenal and systemic inflammation (35). Consequently, PNI, combined serum
albumin and total lymphocyte count, which represent nutritional
status and chronic inflammation (36,37),
may be indicated for risk stratification and clinical management
for patients in the CCU. In the present study, it was demonstrated
that PNI, which is clinically and easily available, was an
independent predictor for the development of AKI and prognosis in
patients in the CCU. In the subgroup analyses, PNI <48.8
exhibited a higher risk of 2-year mortality in almost all
subgroups. These findings were then further validated in the
hospital cohort. Moreover, in the current study, the AUCs of PNI
for AKI were 0.755 in the test cohort and 0.738 in the validation
cohort, which were consistent with previous models for AKI in
different populations (38,39).
The present study had a few limitations. There was a
sizeable number of missing data related to PNI and given the
possible selection bias, the affected patients were not excluded,
which may have led to oversights in the analysis. Additionally, PNI
values were calculated on the first day of admission but changes in
PNI were not assessed in the patients during their hospital stay
and their 2-year follow-up. The initial results also may not
represent patient situation, and the maximum or minimum results may
have been more suitably compared with the initial results. However,
for prediction models, the initial results may be more appropriate
to predict outcomes as clinicians are able to rapidly classify the
risk of AKI and prognosis. Moreover, some of the data requires
further analysis as MAP was much higher in the validation cohort
compared with the test cohort, which may reflect organ perfusion.
In previous studies, a target MBP up to 80-95 mmHg has been
associated with favorable outcomes, including a reduction in renal
failure and mortality for critically ill patients (40). Lower MAP has also been indicated to
be an independent predictor for AKI in a previous study (41). In the present study, patient blood
pressure was collected upon CCU admission and a lower MAP may
indicate worse organ perfusion, which can lead to a higher
incidence of AKI. Finally, some factors such as troponin, brain
natriuretic peptide and the usage of drugs and markers of
inflammation were not assessed in the clinical model. Therefore,
further studies are required to validate the results of the current
study.
In conclusion, the current study demonstrated that
PNI values could serve as an early predictor for the development of
AKI and mortality in patient in CCU, and PNI on admission exhibited
good predictive performance and may be a useful clinical marker
that can be used for estimating long-term survival in these
patients. These results were validated in a hospital cohort. If the
results are further confirmed in future studies, given that PNI is
a readily available and cost-effective parameter, its use as an
index to stratify the risk of AKI and mortality is suggested to be
promising.
Supplementary Material
DCA for PNI value and clinical model
to detect its clinical usefulness in the validation cohort. (A) The
DCA of PNI and clinical model for the development of AKI; (B) the
DCA of PNI and clinical model for in-hospital mortality; (C) the
DCA of PNI and clinical model for 2-year mortality. DCA, Decision
curves analysis; PNI, prognostic nutritional index.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YH contributed to the conception and design of this
work, acquisition of data, analysis and interpretation of the data,
and drafting of the discussion, QC, HW, YY and YX contributed to
interpretation of the data and drafting of the discussion. QZ and
XL are the guarantors of this work, contributed to the conception
and design of this work, and have full access to all of the data in
the current study. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Access to the MIMIC-III database for research was
approved by the Institutional Review Boards of the Massachusetts
Institute of Technology (Cambridge, MA, USA) and the Beth Israel
Deaconess Medical Center, and was granted a waiver of informed
consent. Due to the HIPAA-compliant deidentification in the
MIMIC-III database, the institutional IRB requirement was waived.
The current study was performed in accordance with the ethical
standards laid down in the 1964 Declaration of Helsinki and its
later amendments. Furthermore, the current study was also approved
by the Ethics Committee of Zhongnan Hospital of Wuhan University.
All patients in the validation cohort were required to provide
written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bellomo R, Kellum JA, Ronco C, Wald R,
Martensson J, Maiden M, Bagshaw SM, Glassford NJ, Lankadeva Y,
Vaara ST and Schneider A: Acute kidney injury in sepsis. Intensive
Care Med. 43:816–828. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Santos RPD, Carvalho ARS, Peres LAB, Ronco
C and Macedo E: An epidemiologic overview of acute kidney injury in
intensive care units. Rev Assoc Med Bras (1992). 65:1094–1101.
2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Abd ElHafeez S, Tripepi G, Quinn R, Naga
Y, Abdelmonem S, AbdelHady M, Liu P, James M, Zoccali C and Ravani
P: Risk, predictors, and outcomes of acute kidney injury in
patients admitted to intensive care units in Egypt. Sci Rep.
7(17163)2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hobson C, Ozrazgat-Baslanti T, Kuxhausen
A, Thottakkara P, Efron PA, Moore FA, Moldawer LL, Segal MS and
Bihorac A: Cost and mortality associated with postoperative acute
kidney injury. Ann Surg. 261:1207–1214. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Collister D, Pannu N, Ye F, James M,
Hemmelgarn B, Chui B, Manns B and Klarenbach S: Alberta Kidney
Disease Network. Health care costs associated with AKI. Clin J Am
Soc Nephrol. 12:1733–1743. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Greenberg JH, Zappitelli M, Jia Y,
Thiessen-Philbrook HR, de Fontnouvelle CA, Wilson FP, Coca S,
Devarajan P and Parikh CR: Biomarkers of AKI progression after
pediatric cardiac surgery. J Am Soc Nephrol. 29:1549–1556.
2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
da Rocha EP, Yokota LG, Sampaio BM,
Cardoso Eid KZ, Dias DB, de Freitas FM, Balbi AL and Ponce D:
Urinary neutrophil gelatinase-associated lipocalin is excellent
predictor of acute kidney injury in septic elderly patients. Aging
Dis. 9:182–191. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Hong X, Cui B, Wang M, Yang Z, Wang L and
Xu Q: Systemic immune-inflammation index, based on platelet counts
and neutrophil-lymphocyte ratio, is useful for predicting prognosis
in small cell lung cancer. Tohoku J Exp Med. 236:297–304.
2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wang D, Hu X, Xiao L, Long G, Yao L, Wang
Z and Zhou L: Prognostic nutritional index and systemic
immune-inflammation index predict the prognosis of patients with
HCC. J Gastrointest Surg: Feb 5, 2020 (Epub ahead of print).
|
|
10
|
Zencirkiran Agus H and Kahraman S:
Prognostic nutritional index predicts one-year outcome in heart
failure with preserved ejection fraction. Acta Cardiol. 75:450–455.
2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Salati M, Filippi R, Vivaldi C, Caputo F,
Leone F, Salani F, Cerma K, Aglietta M, Fornaro L, Sperti E, et al:
The prognostic nutritional index predicts survival and response to
first-line chemotherapy in advanced biliary cancer. Liver Int.
40:704–711. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Cheng Y, Li H, Li D, Liang L, Jia Y, Zou
L, Li F, Zhu X, Qian H, He N, et al: Prognostic nutritional index
may not be a good prognostic indicator for acute myocardial
infarction. Sci Rep. 9(14717)2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Cheng YL, Sung SH, Cheng HM, Hsu PF, Guo
CY, Yu WC and Chen CH: Prognostic nutritional index and the risk of
mortality in patients with acute heart failure. J Am Heart Assoc.
6(e004876)2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Scrutinio D, Lanzillo B, Guida P,
Passantino A, Spaccavento S and Battista P: Association between
malnutrition and outcomes in patients with severe ischemic stroke
undergoing rehabilitation. Arch Phys Med Rehabil. 101:852–860.
2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Dolapoglu A, Avci E, Kiris T and Bugra O:
The predictive value of the prognostic nutritional index for
postoperative acute kidney injury in patients undergoing on-pump
coronary bypass surgery. J Cardiothorac Surg. 14(74)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Johnson AE, Pollard TJ, Shen L, Lehman LW,
Feng M, Ghassemi M, Moody B, Szolovits P, Celi LA and Mark RG:
MIMIC-III, a freely accessible critical care database. Sci Data.
3(160035)2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hu Y, Liu H, Fu S, Wan J and Li X: Red
blood cell distribution width is an independent predictor of AKI
and mortality in patients in the coronary care unit. Kidney Blood
Press Res. 42:1193–1204. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Singh P, Pathak S and Sharma RM: A
comparison of acute physiology and chronic health evaluation III
and simplified acute physiology score II in predicting sepsis
outcome in intensive care unit. Anesth Essays Res. 12:592–597.
2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Schwandt A, Denkinger M, Fasching P,
Pfeifer M, Wagner C, Weiland J, Zeyfang A and Holl RW: Comparison
of MDRD, CKD-EPI, and cockcroft-gault equation in relation to
measured glomerular filtration rate among a large cohort with
diabetes. J Diabetes Complications. 31:1376–1383. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kellum JA and Lameire N: Diagnosis,
evaluation, and management of acute kidney injury: A KDIGO summary
(Part 1). Crit Care. 17(204)2013.PubMed/NCBI View
Article : Google Scholar
|
|
21
|
Singbartl K and Kellum JA: AKI in the ICU:
Definition, epidemiology, risk stratification, and outcomes. Kidney
Int. 81:819–825. 2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhang Y, Jiang L, Wang B and Xi X:
Epidemiological characteristics of and risk factors for patients
with postoperative acute kidney injury: A multicenter prospective
study in 30 Chinese intensive care units. Int Urol Nephrol.
50:1319–1328. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Pencina MJ, D'Agostino RB Sr, D'Agostino
RB Jr and Vasan RS: Evaluating the added predictive ability of a
new marker: From area under the ROC curve to reclassification and
beyond. Stat Med. 27:157–172, 207-212. 2008.PubMed/NCBI View
Article : Google Scholar
|
|
24
|
Cook NR: Statistical evaluation of
prognostic versus diagnostic models: Beyond the ROC curve. Clin
Chem. 54:17–23. 2008.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Min JY, Woo A, Chae MS, Hong SH, Park CS,
Choi JH and Chung HS: Predictive impact of modified-prognostic
nutritional index for acute kidney injury within 1-week after
living donor liver transplantation. Int J Med Sci. 17:82–88.
2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hori S, Miyake M, Morizawa Y, Nakai Y,
Onishi K, Iida K, Gotoh D, Anai S, Torimoto K, Aoki K, et al:
Impact of preoperative abdominal visceral adipose tissue area and
nutritional status on renal function after donor nephrectomy in
Japanese living donors for renal transplantation. Ann Transplant.
23:364–376. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Peng F, Chen W, Zhou W, Li P, Niu H, Chen
Y, Zhu Y and Long H: Low prognostic nutritional index associated
with cardiovascular disease mortality in incident peritoneal
dialysis patients. Int Urol Nephrol. 49:1095–1101. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Cai L, Yu J, Yu J, Peng Y, Ullah H, Yi C,
Lin J, Yang X and Yu X: Prognostic value of inflammation-based
prognostic scores on outcome in patients undergoing continuous
ambulatory peritoneal dialysis. BMC Nephrol. 19(297)2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Chen KH, Wu CH, Hsu CW, Chen YM, Weng SM,
Yang CW and Hung CC: Protein nutrition index as a function of
patient survival rate in peritoneal dialysis. Kidney Blood Press
Res. 33:174–180. 2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hartz LLK, Stroup BM, Bibelnieks TA,
Shockey C and Ney DM: ThedaCare nutrition risk screen improves the
identification of non-intensive care unit patients at risk for
malnutrition compared with the nutrition risk screen 2002. JPEN J
Parenter Enteral Nutr. 43:70–80. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Puthucheary ZA, Rawal J, McPhail M,
Connolly B, Ratnayake G, Chan P, Hopkinson NS, Phadke R, Dew T,
Sidhu PS, et al: Acute skeletal muscle wasting in critical illness.
JAMA. 310:1591–1600. 2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Bang JY, Kim SO, Kim SG, Song JG, Kang J,
Kim JW and Ha S: Impact of the serum albumin level on acute kidney
injury after cerebral artery aneurysm clipping. PLoS One.
13(e206731)2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Piggott KD, Liu A, Monczka J, Fakioglu H,
Narasimhulu SS, Pourmoghadam K and DeCampli W: Inadequate
preoperative nutrition might be associated with acute kidney injury
and greater illness severity postoperatively. J Thorac Cardiovasc
Surg. 155:2104–2109. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
White JV, Guenter P, Jensen G, Malone A
and Schofield M: Academy of Nutrition and Dietetics Malnutrition
Work Group; A.S.P.E.N. Malnutrition Task Force; A.S.P.E.N. Board of
Directors. Consensus statement of the academy of nutrition and
Dietetics/American society for parenteral and enteral nutrition:
Characteristics recommended for the identification and
documentation of adult malnutrition (undernutrition). J Acad Nutr
Diet. 112:730–738. 2012.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Rabb H, Griffin MD, McKay DB, Swaminathan
S, Pickkers P, Rosner MH, Kellum JA and Ronco C: Acute Dialysis
Quality Initiative Consensus XIII Work Group. Inflammation in AKI:
Current understanding, key questions, and knowledge gaps. J Am Soc
Nephrol. 27:371–379. 2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhang H, Tao Y, Wang Z and Lu J:
Evaluation of nutritional status and prognostic impact assessed by
the prognostic nutritional index in children with chronic kidney
disease. Medicine (Baltimore). 98(e16713)2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Itami Y, Miyake M, Tatsumi Y, Gotoh D,
Hori S, Morizawa Y, Iida K, Ohnishi K, Nakai Y, Inoue T, et al:
Preoperative predictive factors focused on inflammation-,
nutrition-, and muscle-status in patients with upper urinary tract
urothelial carcinoma undergoing nephroureterectomy. Int J Clin
Oncol. 24:533–545. 2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Kalisvaart M, Schlegel A, Umbro I, de Haan
JE, Polak WG, IJzermans JN, Mirza DF, Perera MTP, Isaac JR,
Ferguson J, et al: The AKI prediction Score: A new prediction model
for acute kidney injury after liver transplantation. HPB (Oxford).
21:1707–1717. 2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Patidar KR, Xu C, Shamseddeen H, Cheng YW,
Ghabril MS, Mukthinuthalapati VVPK, Fricker ZP, Akinyeye S, Nephew
LD, Desai AP, et al: Development and validation of a model to
predict acute kidney injury in hospitalized patients with
cirrhosis. Clin Transl Gastroenterol. 10(e00075)2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Asfar P, Meziani F, Hamel JF, Grelon F,
Megarbane B, Anguel N, Mira JP, Dequin PF, Gergaud S, Weiss N, et
al: High versus low blood-pressure target in patients with septic
shock. N Engl J Med. 370:1583–1593. 2014.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Kwon HM, Moon YJ, Jung KW, Jeong HW, Park
YS, Jun IG, Song JG and Hwang GS: Low mean arterial blood pressure
is independently associated with postoperative acute kidney injury
after living donor liver transplantation: A propensity score
weighing analysis. Ann Transplant. 23:236–245. 2018.PubMed/NCBI View Article : Google Scholar
|