Introduction
Parkinson's disease (PD) is a progressive
neurodegenerative disease associated with aging, which is
characterized by the selective loss of nigrostriatal dopaminergic
neurons (1-3).
PD is the second most common neurodegenerative disease in the world
with a prevalence that is estimated to reach between 8.7 and 9.3
million by 2030(4). To date, the
pathophysiology of PD remains to be fully elucidated, though
studies indicate that oxidative stress may be one of the mechanisms
contributing to PD (5). There is
currently no cure for PD; thus, further research into the
development of novel treatment strategies is critical (6). Increasing evidence has demonstrated
that oxidative stress has an important role in the events
contributing to the degeneration of dopaminergic neurons (7), and that redox reactions are a possible
source of oxidative stress in nigral dopaminergic neurons (8). Glutathione (GSH) is a ubiquitous thiol
tripeptide that protects against oxidative stress-induced damage by
neutralizing reactive oxygen species (5). GSH deficiency has been identified as
an early event in the progression of PD (9). Therefore, supplementing GSH may
effectively improve the symptoms of PD. In recent years, a number
of clinical trials have sought to investigate the effects of GSH
treatment for PD (10-12).
Regrettably, the sample size of these studies was small and the
clinical evidence is insufficient (10-12).
To the best of our knowledge, no previous meta-analyses have
assessed the efficacy and safety of GSH in patients with PD. Hence,
in the present study, a meta-analysis was performed with the aim of
providing medical evidence-based support for GSH treatment in these
patients.
Materials and methods
Search strategy
To identify eligible studies, a primary search was
conducted using electronic databases (PubMed, Cochrane Library,
OvidSP, Web of Science, China Science and Technology Journal
Database, Chinese National Knowledge Infrastructure and China
Wanfang Standards Database) from the inception dates to October
1st, 2019, using the keywords ‘glutathione’ or ‘GSH’ and
‘Parkinson’ or ‘Parkinson's disease’ or ‘PD’. Specific retrieval
strategies were adjusted according to different databases. The
procedure was concluded by: i) The perusal of the reference
sections of all relevant studies; ii) a manual search for GSH in
key journals and abstracts from the major annual meetings in the
field of PD; and iii) contact with experts to request unpublished
data. The primary search was completed by independent investigators
(HLW and JZ) and any discrepancies were resolved by consultation
with an investigator (YZC) not involved in the initial
procedure.
Inclusion criteria
The inclusion criteria for the present study were as
follows: i) Participants were clinically diagnosed with PD; ii) GSH
was administered as an intervention treatment; iii) patients
treated with GSH were directly compared with a non-GSH or placebo
group; iv) outcomes were determined using the Unified Parkinson's
Disease Rating Scale (UPDRS) and/or GSH peroxidase (GSH-Px) and/or
related adverse events (AEs); and v) the study was a published
randomized controlled trial (RCT).
Exclusion criteria
Articles fulfilling the following criteria were
excluded from the present study: i) Randomized trials without a
placebo or control group; ii) studies lacking original data; and
iii) abstracts, conference papers, letters or comments.
Quality assessment
The risk of bias in the included studies was
assessed by two independent reviewers (WHL and JZ) using the
Cochrane Handbook for Systematic Reviews of Interventions (13). Bias was evaluated in the following
seven domains: i) Random sequence generation; ii) allocation
concealment; iii) blinding of participants and personnel; iv)
blinding of outcome assessment; v) incomplete outcome data; vi)
selective outcome reporting; and vii) other bias, of which random
sequence generation, blinding of participants and personnel, and
blinding of outcomes assessment were of most interest. Any
disagreements were resolved by discussion among all of the
reviewers. The risk of bias in each domain was rated as low,
unclear or high, according to methods used to ensure the
minimization of each form of bias. Using the following methods,
individual studies were categorized as having low, high or unclear
risk of bias: i) Low risk of bias (plausible bias unlikely to
markedly alter the findings) if the risk of bias was low in all
domains; ii) unclear risk of bias (plausible bias that raises
certain doubt about the results) if the risk of bias was unclear in
one or more domains; or iii) high risk of bias (plausible bias that
seriously weakens confidence in the results) if a high risk of bias
was present in one or more domains. Any disagreements were resolved
through a discussion within the entire review team.
Data extraction
Data were extracted by two independent reviewers
(WHL and YPL) using a predefined data extraction method.
Disagreements were resolved by discussion or consensus with a third
independent author (CYZ). The extracted data included the first
author, study characteristics (i.e. year, duration and design),
participant characteristics (i.e. age, sample size and systemic
therapy) and outcomes (UPDRS/GSH-Px/related AEs). For studies with
insufficient information, the reviewers contacted the corresponding
authors where possible to acquire the data.
Statistical analysis
When conditions permitted, the study was divided
into three arms based on the administered dose of GSH used to
obtain the two-arm data (300 mg/d groups vs. control groups, and
600 mg/d groups vs. control groups). Dichotomous data were analyzed
using the risk ratios (RRs) with 95% confidence intervals (CIs).
When the result unit, measurement method or measurement time was
inconsistent, continuous outcome measurements were analyzed using
standard mean differences (SMDs) with 95% CIs; 95% CIs were
calculated using the inverse variance (IV) statistical method.
I-square (I2) statistics and the Q test were performed
to assess the impact of study heterogeneity on the results of the
meta-analysis. According to the Cochrane review guidelines
(13), if severe heterogeneity was
present at P<0.1 or I2>50%, the random-effects
model was chosen; otherwise, the fixed-effects model was used.
Subgroup analyses were performed according to GSH dose.
Results
Search results
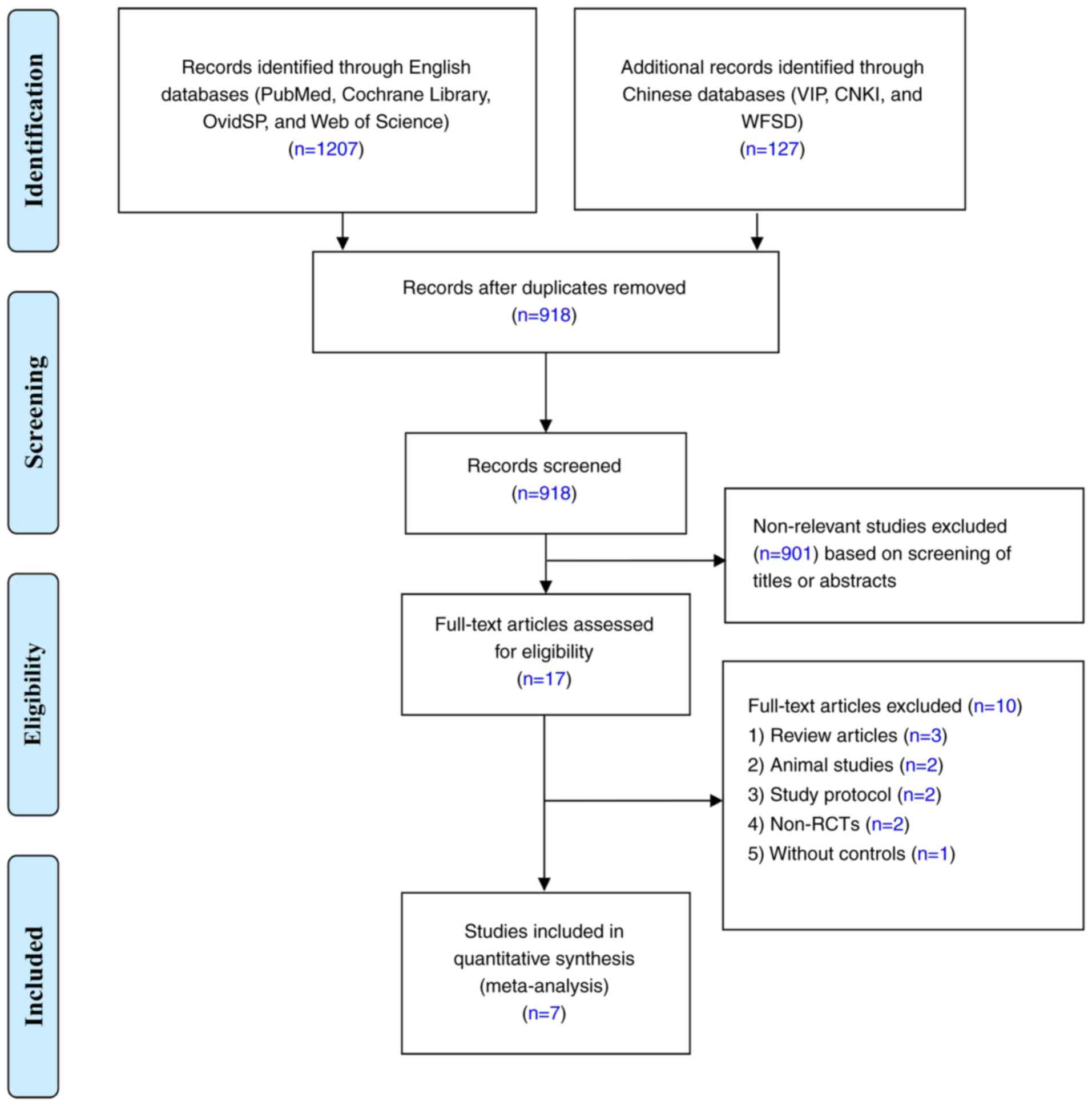
According to the aforementioned retrieval strategy,
a total of 1,334 related articles were initially retrieved and 918
studies were retained after 416 papers with duplicate data were
excluded. Of the identified articles, 901 that did not meet the
inclusion criteria were excluded after reading the title and
abstract. Of the remaining 17 studies (which were evaluated for
applicability by reading the full text), a further 10 were omitted
per the exclusion criteria, leaving a total of 7 included studies
(10-12,14-17).
A flow diagram of the screening process is depicted in Fig. 1. The age distribution of the
patients within these studies was 43-84 years and the included
studies were published between 2003 and 2019. The studies primarily
reported on the outcomes of UPDRS, GSH-Px and related AEs. The
specific basic characteristics of the included studies are listed
in Table I.
 | Table ICharacteristics of the included trials
and participants. |
Table I
Characteristics of the included trials
and participants.
| First author
(year) | Design | Follow-up | Age (years)
GSH/Control | Participants
(males/females) GSH/Control | Intervention Route,
dose, frequency | Outcomes | (Refs.) |
|---|
| Hauser (2009) | RCT | 4 w | 62.6+7.9/
65.9+12.6 | (5/5)/(6/4) | Intravenous push,
1400 mg, Qd | A; B; C; E | (10) |
| Mischley (2017) | RCT | 3 m | 60.9+11/ 60.9+11 | 11; 14/14 | Intranasal
administration, 300 mg or 600 mg, Qd | A; B; C | (11) |
| Mischley (2015) | RCT | 3 m | - | 10; 10/10 | Intranasal
administration, 300 mg or 600 mg, Qd | A; B; C; E | (12) |
| Bao (2018) | RCT | 4 m | 64.6+8.2/
65.1+9.6 |
(56/44)/(55/45) | Intravenous drip,
600 mg, Bid | A; B; C; D | (14) |
| Bao (2003) | RCT | 6 w | 61.41+9.68/
58.87+7.94 |
(14/16)/(16/14) | Intravenous drip,
600 mg, Bid | D | (15) |
| Hu (2019) | RCT | 21 d | 66.8+6.9/
70.7+7 |
(17/15)/(18/13) | Intravenous drip,
1200-1400 mg, Qd | A; B; C; D; E | (16) |
| Zhang (2005) | RCT | 4 m | 56+4.5/ 57+4.9 | (12/7)/(11/8) | Intravenous drip,
600 mg, Bid | D | (17) |
Quality assessment
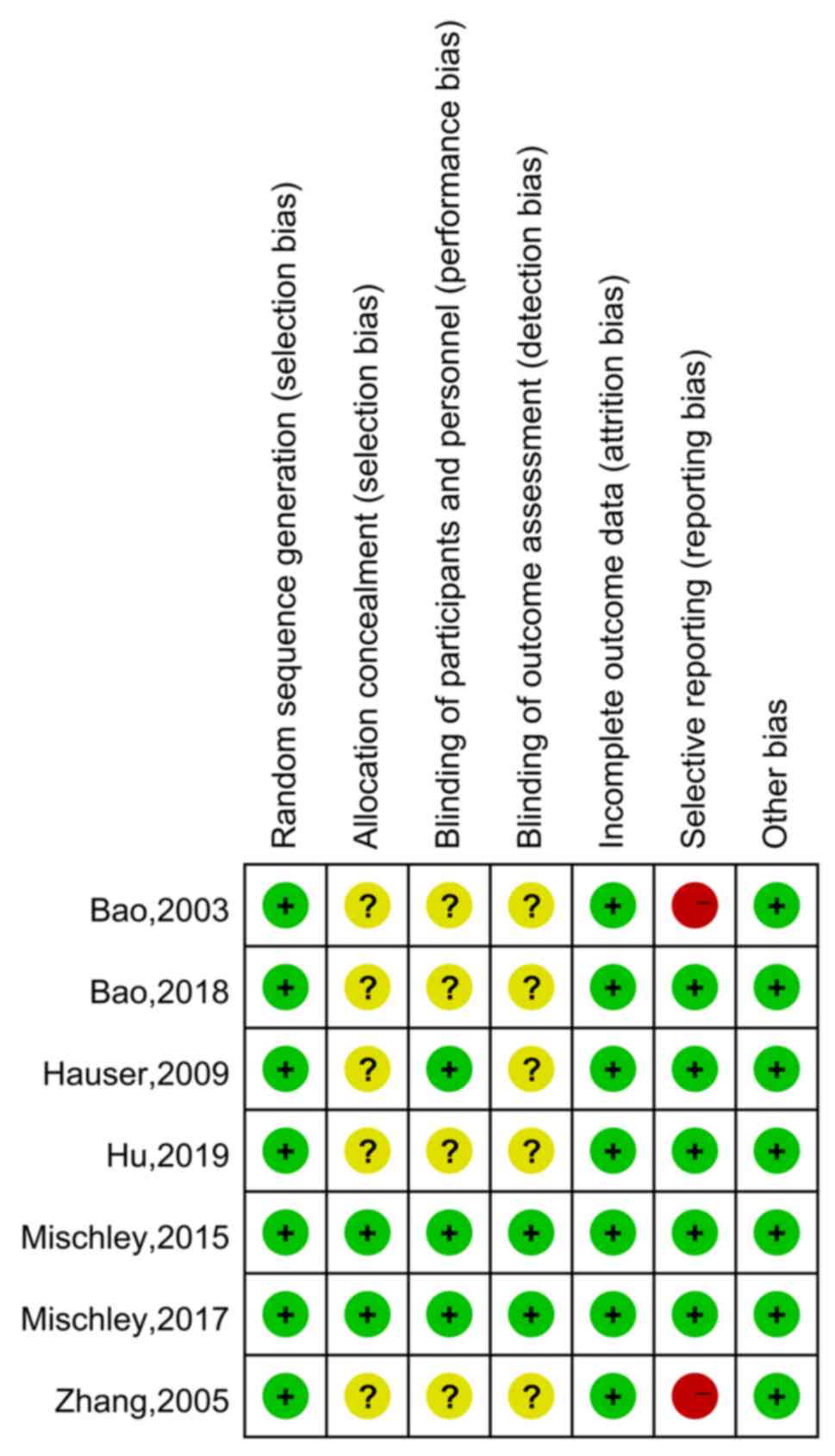
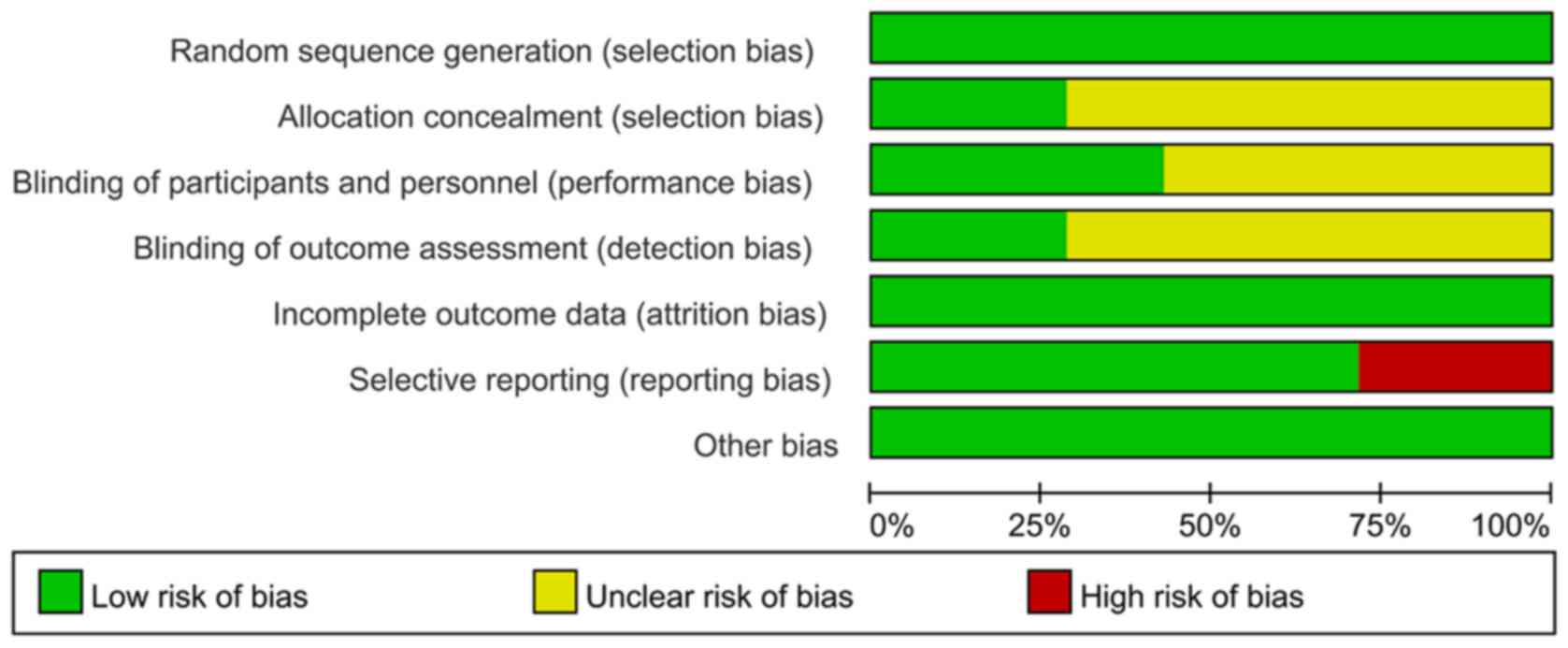
The quality of the included RCTs was assessed
according to the Cochrane Handbook (Figs. 2 and 3) (13).
In the category random sequence generation, the seven studies had a
low risk. There were two articles with sufficient allocation
concealment, while the allocation and concealment schemes of the
other five articles were not clear. Furthermore, performance bias
of three studies were low-risk and four articles were unclear.
There were two papers with low detection bias, while another five
articles were rated as unclear with regard to this bias. In terms
of incomplete data, seven articles were all rated as having low
risk, and the risk of selective reporting was low in five articles
and was high in two studies. There were seven studies with a low
risk of other bias. In conclusion, the overall quality of the seven
included studies was moderate.
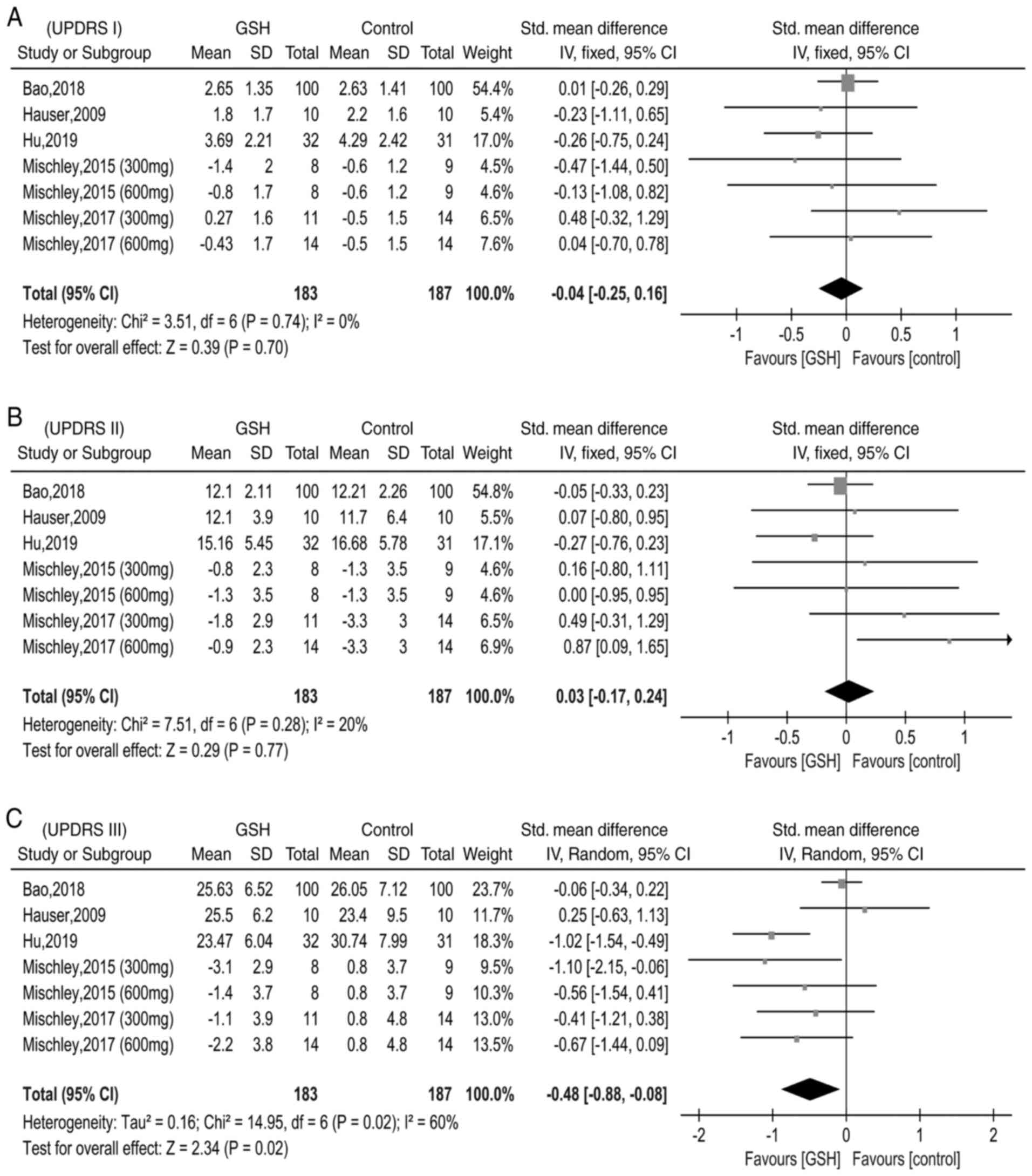
Pooled results UPDRS
There were five studies reporting data on UPDRS I,
II and III (10-12,14,16).
Due to differences in data type (end value and end value minus
baseline value), the SMD was applied to determine differences in
the UPDRS I, II and III scores between the GSH and control groups.
The heterogeneity test did not reveal any differences between
studies reporting data regarding the UPDRS I (χ2=3.51,
I2=0%, P=0.74); thus, the fixed-effects model was used
(Fig. 4A). In addition,
heterogeneity between the studies that reported data on UPDRS Ⅱ was
low (χ2=7.51, I2=20%, P=0.28) and thus, the
fixed-effects model was applied once again (Fig. 4B). However, the heterogeneity test
indicated moderate differences between studies reporting data on
UPDRS Ⅲ (χ2=14.95, I2=60%, P=0.02), and
therefore, the random-effects model was used (Fig. 4C). The pooled SMD was -0.04 (95%
CI=-0.25-0.16, P=0.70) for UPDRS I, 0.03 (95% CI=-0.17-0.24,
P=0.77) for UPDRS Ⅱ and -0.48 [95% CI=-(0.88-0.08), P=0.02] for
UPDRS Ⅲ. These pooled results demonstrated that, compared with the
control groups, GSH may slightly improve the motor scores of
patients with PD.
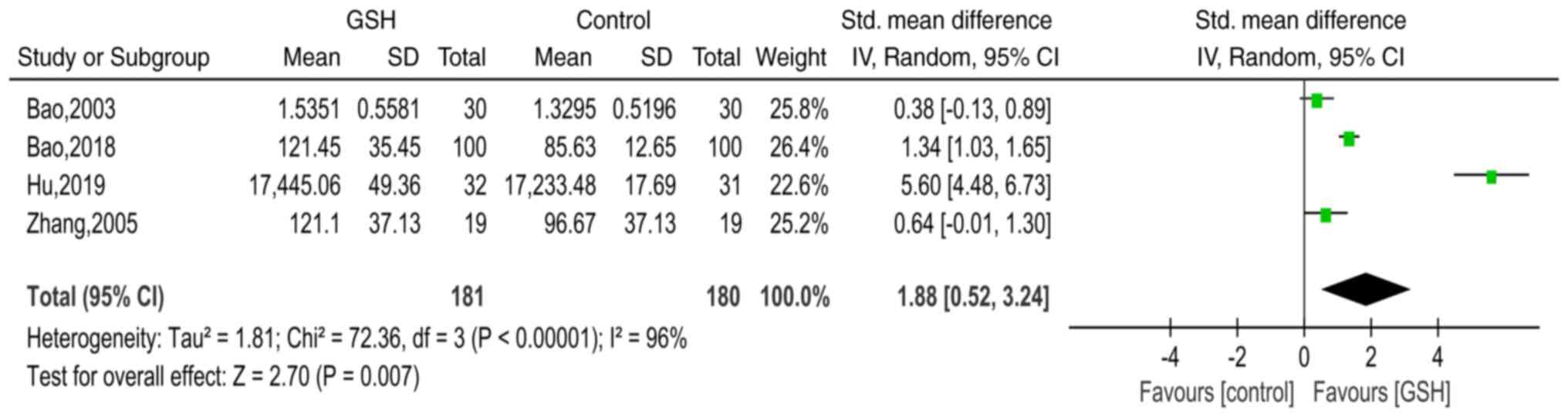
GSH-Px
In total, four studies (14-17)
presented GSH-Px data for the GSH and control groups. The SMD was
used to estimate differences in GSH-Px between the two groups. The
results of the pooled SMD are presented in Fig. 5. There was significant heterogeneity
among the studies (χ2=72.36, I2=96%,
P<0.00001) and thus, the random-effects model was used. The
pooled SMD was 1.88 (95% CI=0.52-3.24, P=0.007), indicating that
compared with the control groups, serum GSH-Px levels were
significantly higher in the GSH groups.
AEs
There were three studies reporting on the incidence
of gastrointestinal reactions (10,12,16);
two papers containing data on dizziness or headache (10,16);
two articles reporting on involuntary movement (10,16);
two papers on labored breathing (10,12);
two articles presenting strep throat-associated data (10,12);
and two studies reported on insomnia (10,16) in
the GSH and control groups (Table
II). The heterogeneity test revealed no differences between the
studies (separately, I2=0, 0, 0, 41, 41 and 0%,
respectively); thus, the fixed-effects model was applied.
Separately, the pooled RRs were 0.78 (95% CI=0.28-2.14, P=0.62),
0.99 (95% CI=0.28-3.49, P=0.99), 0.33 (95% CI=0.44-2.99, P=0.32),
1.59 (95% CI=0.29-8.59, P=0.19), 1.59 (95% CI=0.29-8.59, P=0.59)
and 1.64 (95% CI=0.23-11.74, P=0.62). These pooled results of AEs
demonstrate that the use of GSH appears to be safe.
 | Table IIMeta-analysis of adverse effects
compared between the GSH and control group. |
Table II
Meta-analysis of adverse effects
compared between the GSH and control group.
| | Pooled results |
|---|
| Adverse effect | RR | 95%CI | P-value | I2
(%) | P-value | n-value |
|---|
| Gastrointestinal
reaction | 0.78 | [0.28, 2.14] | 0.62 | 0 | 0.49 | 7 |
| Dizziness or
headache | 0.99 | [0.28, 3.49] | 0.99 | 0 | 0.43 | 4 |
| Involuntary
movement | 0.33 | [0.44, 2.99] | 0.32 | 0 | 0.99 | 4 |
| Labored
breathing | 1.59 | [0.29, 8.59] | 0.19 | 41 | 0.19 | 5 |
| Strep throat | 1.59 | [0.29, 8.59] | 0.59 | 41 | 0.19 | 5 |
| Insomnia | 1.64 | [0.23, 11.74] | 0.62 | 0 | 0.59 | 4 |
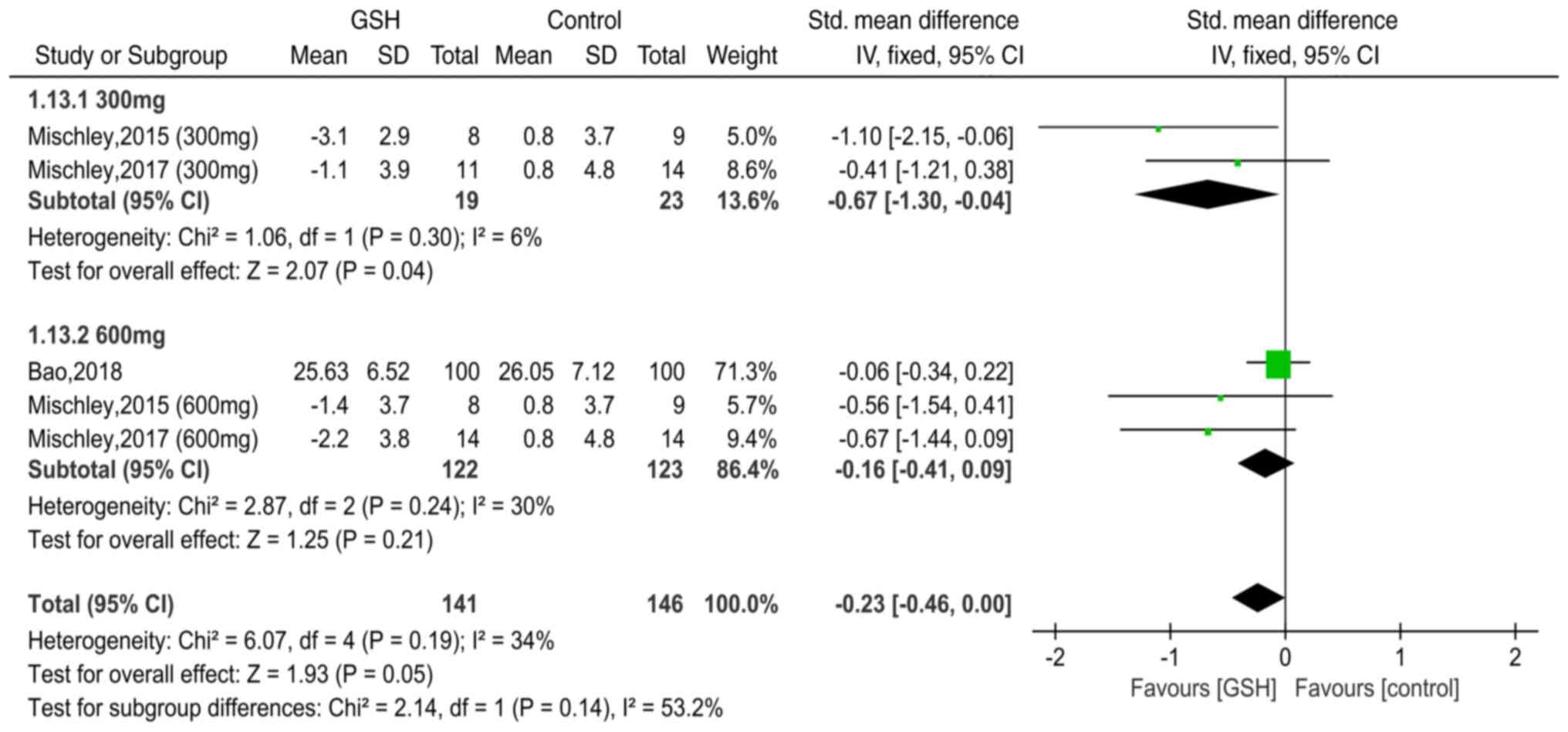
Subgroup analysis: Influence of GSH
dosage on UPDRS Ⅲ
In total, two studies (11,12)
reported data of UPDRS III with the use of GSH (300 mg/d) for PD
and two papers (11,12) included data on the use of GSH (600
mg/d) (Fig. 6). The heterogeneity
test indicated minimal differences between these studies
(individually, χ2=1.06, I2 =6%, P=0.30; and
χ2=2.87, I2=30%, P=0.24, respectively), and
therefore, the fixed-effects model was used. The pooled SMDs were
-0.67 [95% CI=-(1.30-0.04), P=0.04] and -0.16 (95% CI=-0.41-0.09,
P=0.21), respectively, suggesting that the dose (300 vs. 600 mg)
was an influencing factor for UPDRS III. Therefore, it was
conservatively hypothesized that in patients with PD, a 300-mg dose
of GSH may be more effective than a 600-mg/d dose.
Discussion
To the best of our knowledge, the present study was
the first meta-analysis to evaluate the efficacy and safety of GSH
for the treatment of PD. The study provided medical evidence-based
support for the effectiveness and safety of GSH. The results of the
meta-analysis were as follows: i) GSH does not have the potential
to improve mentality, behavior, mood or the ability to perform
daily activities, but has the ability to slightly improve motor
function in patients with PD; ii) compared with the control groups,
serum GSH-Px levels were significantly higher in the GSH groups,
though there was notable heterogeneity between the studies
(I2=96%); iii) GSH appears to be safe and, compared with
the control groups, does not increase the rate of AEs; and iv) the
dose of GSH (300 vs. 600 mg/d) may be one of the factors
influencing motor function in patients with PD.
GSH (an antioxidant) is a tripeptide formed by the
dehydration condensation of cysteine, glycine and glutamic acid
(11,18). The tripeptide participates in redox
reactions, which reduce damage to nerve cells caused by oxygen free
radicals (11). Although most
individuals synthesize sufficient GSH to maintain a redox balance,
this is not the case in patients with PD or other neurodegenerative
diseases, which has been demonstrated to be associated with GSH
consumption (11).
Several studies have indicated that significant GSH
depletion (30-50%) is associated with an increased proportion of
oxidized GSH in post-mortem PD substantia nigra tissues (19-21).
Furthermore, a clinical study by Mischley et al (22) demonstrated that the whole-blood GSH
concentration is negatively correlated with the clinical severity
of PD. Furthermore, in vitro experiments have suggested that
increased depletion of GSH results in selective impairment of
mitochondrial complex I activity (23). To a certain extent, GSH replacement
may provide symptomatic benefits to patients with PD by preventing
mitochondrial dysfunction and thus reducing the impairment of
dopaminergic function (10). In
light of this, a series of clinical studies have been performed. In
RCTs by Hauser et al (10),
21 subjects were randomly assigned to the GSH (n=11) and control
(n=10) groups, which demonstrated that GSH is safe for use in
patients with PD. However, there is currently no evidence to
suggest that GSH is able to effectively improve the symptoms of PD,
which may be the result of the study sample being too small
(10). In addition, Mischley et
al (11,12) also performed RCT studies, though the
sample sizes of these studies were also small. In the present
study, pooling data from Chinese and English studies revealed that
GSH may mildly improve motor function in patients with PD. The
results of several animal and clinical trials support these
findings (24-26).
However, although the present study provides preliminary medical
evidence-based data on clinical studies, the effectiveness and
safety of GSH supplementation requires further clarification.
Pooling the results of previous studies suggested
that GSH-Px is positively associated with GSH levels. GSH
non-enzymatically reacts with toxic free radicals and also acts as
an electron donor in the reduction of peroxides catalyzed by GSH-Px
(27). The resultant oxidized GSH
is then being processed by GSH reductase and thus, GSH is recycled
(27). GSH-Px has a major role in
the recycling of GSH, which is supported by the fact that
GSH-Px-knockout mice challenged with toxins (such as
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) exhibited greater
dopamine depletion compared with age-matched control mice (28). Although the underlying mechanisms
remain elusive, elevated GSH-Px concentrations appeared to be
beneficial in alleviating the AEs of PD treatment.
In addition, several studies (10,12,16)
have reported data surrounding gastrointestinal reactions,
dizziness or headache, involuntary movement, labored breathing,
strep throat and/or insomnia. The pooled results of these studies
revealed that the therapeutic dose of GSH is safe. Further patient
studies also indicated that when GSH was repeatedly administered at
doses of up to 5 g per day, both orally or intravenously, no
toxicity was observed (29,30).
In the present study, a subgroup analysis was used
to identify the source of heterogeneity surrounding UPDRS Ⅲ and to
perform in-depth data mining. Subgroup analysis suggested that 300
mg/day GSH was more effective than 600 mg/d. However, it is worth
noting that 300 mg/day can not yet be confirmed as the optimal
dose, because it is not known whether there are other possible
optimal doses (the present study only compared the difference
between 600 and 300 mg/day). In addition, due to just a few studies
with a relatively low population size reporting these data,
particularly for 300 mg/day, only two studies published by the same
group with only 19 patients treated with GSH in total were
included. Therefore, it was conservatively hypothesized that the
administration of 300 mg/d or other doses of GSH warrants further
investigation in future studies. Such research should be actively
pursued in the future; animal experiments provide good evidence
that GSH is not only safe but also potentially effective, though
findings in humans require further clarification. The dose
differences may be a cause of heterogeneity among studies.
Furthermore, other confounding factors may also be a source of
heterogeneity (such as ethnicity, sex, age, conventional treatment
protocol, route of administration, course of disease and degree of
disease severity).
The present meta-analysis has several limitations:
i) Only seven articles comprising 450 patients were included and
the quality of these articles was variable; ii) only studies
published in the English and Chinese languages were included, which
may have resulted in potential language bias; iii) due to data
limitations, subgroup analysis by ethnicity, sex, age, course of
disease and disease severity ere not performed; and iv) the pooled
results warrant further clarification.
In conclusion, despite the limitations of the
present study (which may have influenced these results), it was
concluded that GSH may slightly improve the motor scores of
patients with PD, though not at the expense of increased AEs.
Furthermore, the GSH dosage may influence the efficacy. However,
these conclusions warrant further investigation in the future.
Acknowledgements
Not applicable.
Funding
The work was supported by grants from the Six
Talents Summit Training in Jiangsu Province (grant no. wsw-246),
the Provincial Discipline Leader Category B (grant no.
YZ201418501), the Jiangsu Province '13th Five-Year Plan' Special
Fund for Science, Education and Health (grant no. RCC201807), the
Jiangsu Province Key Experiments of Basic and Clinical Translation
of Non-coding RNA (grant no. 201902) and the Jiangsu Province
Natural Science Foundation (grant no. BK20190241).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HLW, JZ, YPL, LD and YZC contributed to the
interpretation of the data and writing of the paper. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Panicker N, Kanthasamy A and Kanthasamy
AG: Fyn amplifies NLRP3 inflammasome signaling in Parkinson's
disease. Aging (Albany NY). 11:5871–5873. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Grespi F and Melino G: P73 and age-related
diseases: Is there any link with Parkinson disease? Aging (Albany
NY). 4:923–931. 2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Tamano H, Nishio R, Morioka H and Takeda
A: Extracellular Zn2+ influx into nigral dopaminergic
neurons plays a key role for pathogenesis of
6-hydroxydopamine-induced Parkinson's disease in rats. Mol
Neurobiol. 56:435–443. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Dorsey ER, Constantinescu R, Thompson JP,
Biglan KM, Holloway RG, Kieburtz K, Marshall FJ, Ravina BM,
Schifitto G, Siderowf A and Tanner CM: Projected number of people
with Parkinson disease in the most populous nations, 2005 through
2030. Neurology. 68:384–386. 2007.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Smeyne M and Smeyne RJ: Glutathione
metabolism and Parkinson's disease. Free Radic Biol Med. 62:13–25.
2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Soukup SF, Vanhauwaert R and Verstreken P:
Parkinson's disease: Convergence on synaptic homeostasis. EMBO J.
37(e98960)2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Gaki GS and Papavassiliou AG: Oxidative
stress-induced signaling pathways implicated in the pathogenesis of
Parkinson's disease. Neuromolecular Med. 16:217–230.
2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Bisaglia M, Soriano ME, Arduini I, Mammi S
and Bubacco L: Molecular characterization of dopamine-derived
quinones reactivity toward NADH and glutathione: Implications for
mitochondrial dysfunction in Parkinson disease. Biochim Biophys
Acta. 1802:699–706. 2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Díaz-Hung ML, Yglesias-Rivera A,
Hernández-Zimbrón LF, Orozco-Suárez S, Ruiz-Fuentes JL, Díaz-García
A, León-Martínez R, Blanco-Lezcano L, Pavón-Fuentes N and
Lorigados-Pedre L: Transient glutathione depletion in the
substantia nigra compacta is associated with neuroinflammation in
rats. Neuroscience. 335:207–220. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Hauser RA, Lyons KE, McClain T, Carter S
and Perlmutter D: Randomized, double-blind, pilot evaluation of
intravenous glutathione in Parkinson's disease. Mov Disord.
24:979–983. 2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Mischley LK, Lau RC, Shankland EG, Wilbur
TK and Padowski JM: Phase IIb study of intranasal glutathione in
Parkinson's disease. J Parkinsons Dis. 7:289–299. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Mischley LK, Leverenz JB, Lau RC, Polissar
NL, Neradilek MB, Samii A and Standish LJ: A randomized,
double-blind phase I/IIa study of intranasal glutathione in
Parkinson's disease. Mov Disord. 30:1696–1701. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Higgins JPT and Green S (eds): Cochrane
Handbook for Systematic Reviews of Interventions, version 5.1.0
(updated March 2011). The Cochrane Collaboration, 2011. urihttp://training.cochrane.org/handbooksimplehttp://training.cochrane.org/handbook.
|
|
14
|
Bao H: Clinical effect of reduced
glutathione on Parkinson's disease. Chin J Clin Ration Drug Use.
11:44–45. 2018.(In Chinese).
|
|
15
|
Bao Y, Wang H, Chen H, Zhang B, Wang X, Xu
G, Tong J, Wang Y and Yang X: An observation of 30 cases of
Parkinson's disease treated with glutathiono. Anhui Med
Pharmaceutical J. 7:22–24. 2003.(In Chinese).
|
|
16
|
Hu Y and Yang W: Clinical study of reduced
glutathione in the treatment of Parkinsons disease. Chin J Pract
Nervous Dis. 22:720–724. 2019.(In Chinese).
|
|
17
|
Zhang Y, Cao X, Hu H and Sun S:
Therapeutic effect of reduced glutathione for Parkinson disease.
Chin J Rehabil. 20:29–30. 2005.(In Chinese).
|
|
18
|
Aquilano K, Baldelli S and Ciriolo MR:
Glutathione: New roles inredox signaling for an old antioxidant.
Front Pharmacol. 5(196)2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Fitzmaurice PS, Ang L, Guttman M, Rajput
AH, Furukawa Y and Kish SJ: Nigral glutathione deficiency is not
specific for idiopathic Parkinson's disease. Mov Disord.
18:969–976. 2003.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Pearce RK, Owen A, Daniel S, Jenner P and
Marsden CD: Alterations in the distribution of glutathione in the
substantia nigra in Parkinson's disease. J Neural Transm (Vienna).
104:661–677. 1997.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Liddell JR and White AR: Nexus between
mitochondrial function, iron, copper and glutathione in Parkinson's
disease. eurochem Int. 117:126–138. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Mischley LK, Standish LJ, Weiss NS,
Padowski JM, Kavanagh TJ, White CC and Rosenfeld ME: Glutathione as
a biomarker in Parkinson's disease: Associations with aging and
disease severity. Oxid Med Cell Longev.
2016(9409363)2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Chinta SJ and Andersen JK: Reversible
inhibition of mitochondrial complex I activity following chronic
dopaminergic glutathione depletion in vitro: Implications for
Parkinson's disease. Free Radic Biol Med. 41:1442–1448.
2006.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Sechi G, Deeden MG, Bua G, Satta WM,
Deiana GA, Pes GM and Rosati G: Reduced intraveous glutathione in
the treatment of ealy Parkinson's disease. Prog
Neuropsychopharmacol Biol Psychiatry. 20:1159–1170. 1996.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Mischley LK: Glutathione deficiency in
Parkinson's disease: Intranasal administration as a method of
augmentation. J Orthomol Med. 26:32–36. 2011.
|
|
26
|
Zhou GH, Pao YC and Lu JM: The effect of
glutathione on oxidation stress of Parkinson's disease rat. Chin J
Behav Med Sci. 13:267–268. 2004.(In Chinese).
|
|
27
|
Bharath S, Hsu M, Kaur D, Rajagopalan S
and Andersen JK: Glutathione,iron and Parkinson's disease. Biochem
Pharmacol. 64:1037–1048. 2002.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Klivenyi P, Andreassen OA, Ferrante RJ,
Dedeoglu A, Mueller G, Lancelot E, Bogdanov M, Andersen JK, Jiang D
and Beal MF: Mice deficient in cellular glutathione peroxidase show
increased vulnerability to malonate, 3-nitropropionic acid, and
1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine. J Neurosci. 20:1–7.
2000.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Dalhoff K, Ranek L, Mantoni M and Poulsen
HE: Glutathione treatment of hepatocellular carcinoma. Liver.
12:342–343. 1992.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Tedeschi M, De Cesare A, Oriana S, Perego
P, Silva A, Venturino P and Zunino F: The role of glutathione in
combination with cisplatin in the treatment of ovarian cancer.
Cancer Treat Rev. 18:253–259. 1991.PubMed/NCBI View Article : Google Scholar
|