Introduction
Interleukin 32 (IL-32) is an intracellular
pluripotent cytokine produced by epithelial cells, monocytes, T
lymphocytes and natural killer cells, and is involved in
inflammation and cancer development (1). The gene encoding IL-32 is located on
the chromosome 16p13.3, organized into eight exons, and it consists
of nine splice variants (1). Many
inflammatory diseases such as rheumatoid arthritis (2), acute lung injury (3), HIV infection (4), tuberculosis (5), and inflammatory bowel disease
(1), are believed to be associated
with IL-32.
An association between IL-32 promoter SNP rs28372698
(A/T), giving a higher IL-32γ gene expression, and thyroid
carcinoma has been reported (6).
Other studies have demonstrated that this SNP is associated with an
increased risk of gastric cancer (7) and endometrial cancer (8). The SNP rs28372698 has also been
associated with susceptibility to systemic lupus erythematosus
(9) and lung cancer (10). Damen et al (11) reported a possible protective role
against cardiovascular disease by the variant rs4786370 in the gene
of IL-32. Thus, previous reports indicate that polymorphisms in the
gene of IL-32 are important in disease development.
Other studies have focused on levels of serum or
plasma of IL-32 between patients and healthy controls. Elevated
levels of serum IL-32 in patients with rheumatoid arthritis
(2), tuberculosis (5), gastric cancer (12) and heart failure after myocardial
infarction (13) have been
reported.
The aim of the present study was to explore possible
associations between IL-32 SNP rs28372698 and the plasma levels of
IL-32 in an elderly group of community-living persons in the
south-east of Sweden who were all part of a longitudinal
epidemiological study focusing on cardiovascular risk factors with
a follow-up period of more than seven years.
Materials and methods
Patient population
An elderly population consisting of 486 individuals
(males, 247; females, 239) with a mean age of 77.0 years (range, 18
years) living in a municipality in the south-east of Sweden were
included in this study. They had all been part of a longitudinal
epidemiological study focusing on cardiovascular risk factors
(14). The participants in that
study were invited to participate in the present sub study
conducted from 2003 through 2005. All those living in the
municipality within a specific age interval were invited to
participate in the longitudinal project in order to minimize bias
in the selection process. The population that agreed to participate
delivered blood samples, and underwent echocardiographic
examinations and an electrocardiogram (ECG). The New York Heart
Association functional class [NYHA Class-a functional evaluation
where no limitation of activity equates to class I, and symptoms at
rest are rated as class IV (15)]
was determined by the including physician based on the patient
information. The mortality information was obtained from autopsy
reports or from the National Board of Health and Welfare in Sweden,
which registers all deaths. All participants gave their written
informed consent, and the study was conducted in accordance with
the principles of the Declaration of Helsinki. The study protocol
was approved by the Regional Ethical Review Board of Linköping,
Sweden (Dnr 95044).
Co-morbidity
The following definitions have been used in this
study. Hypertension (HT) was defined as a blood pressure of more
than 140/90 mm Hg measured in the right arm with the patient in the
supine position after at least 30 min of rest. Hypertension was
also assumed if the participant had previously been diagnosed with
hypertension and was receiving antihypertensive medication. IHD was
defined as a history of angina pectoris/myocardial infarction or
ECG-verified myocardial infarction. Heart failure was defined as a
previous diagnosis with on-going treatment, or symptoms/signs of
heart failure and objective demonstration of impaired cardiac
function on echocardiography. Cardiovascular death was defined as
death caused by fatal arrhythmias, myocardial infarction, heart
failure, or cerebrovascular insult. Diabetes mellitus was defined
as a previous diagnosis with on-going treatment, or a fasting blood
glucose ≥7 mmol/l measured on a single occasion.
Echocardiographic examinations
Echocardiography examinations were performed using
an Accuson XP-128c ultrasound system with the patient in a supine,
left position. Values for systolic function expressed as left
ventricular ejection fraction (EF), were categorized into four
classes, with interclass limits of 30, 40 and 50%. Normal systolic
function was defined as EF ≥50%. Thus, only the systolic function
was evaluated.
Determination of IL-32 expression in
plasma
All blood samples were obtained at the start of the
study, while the patients were at rest in a supine position, and
all samples were collected in pre-chilled plastic Vacutainer tubes
(Terumo EDTA K-3). Plasma was prepared by centrifugation at 3,000 x
g for 10 min at 4˚C. All samples were stored at -70˚C until
analysis. None of the samples were thawed more than twice.
Plasma IL-32 levels were measured using a
commercially available enzyme-linked immunosorbent assay (ELISA)
kit (R&D Systems) following the manufacturer's protocol.
According to the product manual, the kit recognizes human IL-32α,
IL-32β and IL-32γ. The plasma IL-32 protein concentration from the
patients and control subjects was expressed as pg/ml and all
measurements including plasma and standard solutions for IL-32
standard curves were performed in duplicate.
Genotype determination
Genomic DNA was isolated from all blood samples
using QiaAmp DNA Kit (Qiagen). A TaqMan SNP genotype assay was used
for analysis of the IL-32 rs28372698 (ID C-64281225-10) (Applied
Biosystems). Ten nanograms of DNA was mixed with TaqMan Genotyping
Master Mix (Applied Biosystems) and was amplified using the 7500
Fast Real-Time Polymerase Chain Reaction (PCR) system (Applied
Biosystems). Amplification was performed using an initial cycle at
50˚C for 2 min, followed by one cycle at 95˚C for 10 min and
finally 40 cycles at 95˚C for 15 sec and at 60˚C for 1 min. The
manual calling option in the allelic discrimination application ABI
PRISM 7500 SDS software, (version 1.3.1; Applied Biosystems) was
used to assign the genotypes.
Statistical methods
Descriptive data are presented as percentages or
mean and standard deviation (SD). Comparative analyses were
performed using a Student's unpaired two-sided t-test, whereas the
chi-square test was used for discrete variables. Both univariate
and multivariate Cox proportional hazard regression analyses were
used to analyze and illustrate the risk of mortality during the
follow-up period, where both all-cause mortality and cardiovascular
mortality were analyzed. Kaplan-Meier graphs were used to
illustrate cardiovascular mortality as a function of follow-up
time. Censored patients were those who were still alive at the end
of the study period or who had died of causes other than
cardiovascular disease. Completed patients comprised those who had
died due to cardiovascular disease. In the multivariate
multivariable regression models, adjustments were made for the
following co-variates: IHD, diabetes, ACE inhibitors/Angiotensin
receptor inhibitors, beta blockers, diuretics, EF <40%, Hb
<120 g/l. The variables were chosen because they are well known
to influence the risk of cardiovascular mortality.
In the Cox regressions, the A/A genotype of
rs28372698 SNP was evaluated against the A/T and the T/T genotypes.
A P-value <0.05 was considered statistically significant. All
data were analyzed using standard software packages (Statistica v.
13.2; Statsoft Inc.).
Results
Study population
The population evaluated consisted of almost equal
numbers of males vs. females (247 vs. 239). Of the 486
participants, 112 (23.0%) had an IHD, and as a result, 36 (7.4%)
had heart failure. In the population, 107 (22.0%) were classified
as having diabetes, and 386 (79.4%) had HT. As a result of the
diseases, 171 (35.2%) were on treatment with beta blockers and 125
(25.7%) were on ACE inhibitors or angiotensin II receptor
antagonists. Thus, the population was representative of an elderly
Western population. The distribution of clinical variables in the
three genotypes is presented in Table
I.
 | Table IBasal characteristics of the study
population divided into genotypes. |
Table I
Basal characteristics of the study
population divided into genotypes.
| | Total population |
|---|
| Variables | A/A | A/T | T/T | P-value |
|---|
| N, (% of the total
population) | 61 (12.6) | 226 (46.5) | 199 (40.9) | |
| Age, mean (SD) | 76.7 (3.4) | 77.4 (3.6) | 76.8 (3.2) | - |
| HT, n (%) | 43 (70.5) | 168 (74.3) | 157 (78.9) | 0.33 |
| IHD, n (%) | 13 (21.3) | 56 (24.8) | 43 (21.6) | 0.67 |
| Diabetes, n (%) | 10 (16.4) | 51 (22.6) | 46 (23.1) | 0.52 |
| NYHA III, n (%) | 14 (23.0) | 44 (19.5) | 39 (19.6) | 0.82 |
| AF, n (%) | 8 (13.1) | 23 (10.2) | 18 (9.0) | 0.65 |
| EF <40%, n
(%) | 5 (8.2) | 20 (8.8) | 11 (5.5) | 0.41 |
| ACEI/ARB, n (%) | 15 (24.6) | 59 (36.1) | 51 (25.6) | 0.97 |
| BB, n (%) | 21 (34.4) | 82 (36.3) | 68 (34.2) | 0.89 |
| Diuretics, n (%) | 16 (26.2) | 93 (41.2) | 66 (33.2) | 0.07 |
| Hb <120 g/l, n
(%) | 12 (19.7) | 21 (9.3) | 21 (10.6) | 0.07 |
IL-32 levels in plasma
The level of IL-32 in plasma has been evaluated
according to quartiles. The distribution of several clinical
variables in the two extreme quartiles, the 1st and 4th quartiles,
is presented in Table II. The
table revealed that no skew distribution regarding clinical
variables could be seen in the two quartiles, and no significant
difference could be noted regarding the clinical variables between
them. The median concentration of IL-32 in the total population was
5782 pg/ml (range, 69-937920). The CV mortality was evaluated in
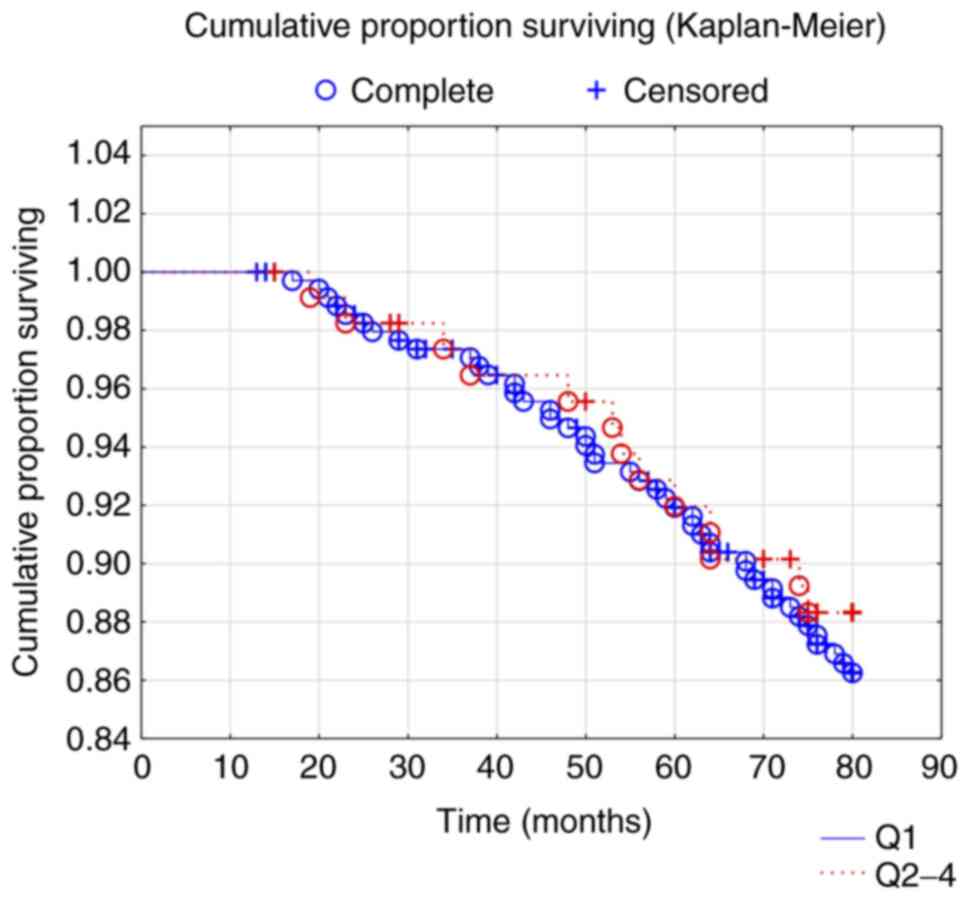
the 1st vs. the 4th quartiles' expression of IL-32 (Fig. 1). No difference in CV mortality
between the two groups could be found (Z=0.50; P=0.61). There were
no associations between IL-32 SNP variants and plasma IL-32 levels
(data not shown).
 | Table IIDistribution of clinical variables
between 1st and 4th quartile of plasma levels of IL-32. |
Table II
Distribution of clinical variables
between 1st and 4th quartile of plasma levels of IL-32.
| Variables | Q1 <549 pg/ml | Q4 >84,633
pg/ml | P-value |
|---|
| Males, n | 63 | 59 | N/A |
| Females, n | 52 | 56 | N/A |
| Peripherial edema, n
(%) | 3 (2.6) | 5 (4.3) | N/A |
| Rales, n (%) | 18 (15.7) | 17 (14.8) | 0.85 |
| Atrial fibrillation,
n (%) | 15 (13.0) | 6 (5.2) | 0.04 |
| ACEI/ARB, n (%) | 30 (28.1) | 29 (25.2) | 0.88 |
| Beta blockers, n
(%) | 42 (36.5) | 49 (42.6) | 0.34 |
| Diuretics, n (%) | 47 (40.9) | 38 (33.0) | 0.22 |
| EF <40%, n
(%) | 5 (4.3) | 10 (8.7) | 0.18 |
| All-cause mortality,
n (%) | 21 (18.3) | 26 (22.6) | 0.41 |
| CV-mortality, n
(%) | 13 (11.3) | 11 (9.6) | 0.67 |
Mortality and genotypes
The mean follow-up time of the population was 7.1
years, and during that time 140/486 (28.8%) suffered all-cause
mortality, and 87/486 (17.9%) suffered CV mortality. From the
distribution of the three genotypes in the study population, it
could be seen that the A/A genotype was about ¼ of the other two
genotypes (A/T and T/T) (Table I).
In an examination of whether different risk groups represented in
this study population had different distributions of the three
genotypes, those with HT, those with IHD and finally those with
diabetes were evaluated. No significant differences in the
distribution of the three genotypes compared with the total study
population could be seen. Evaluating all-cause mortality in the
study population with regard to the three genotypes, a significant
difference could be seen where that A/A genotype had the lowest
survival regarding all-cause mortality (χ2: 6.65;
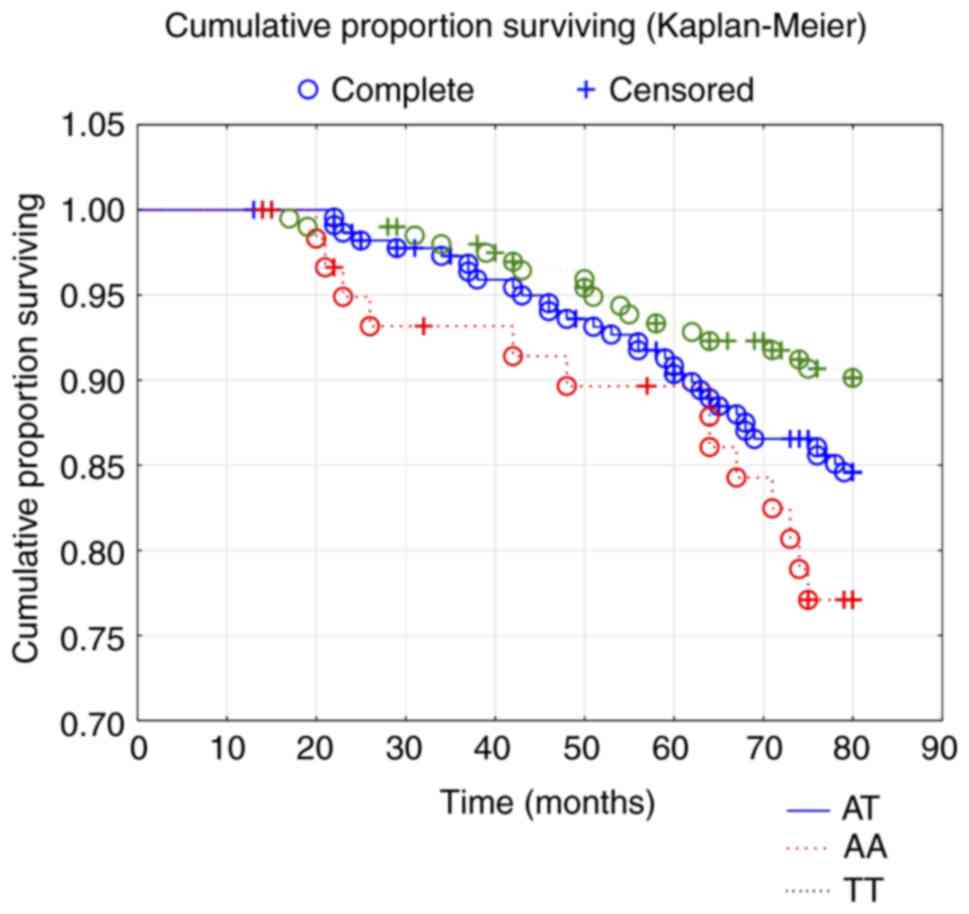
P=0.036). In order to validate the result above, a Kaplan-Meier
evaluation was performed which showed significant differences
(χ2: 6.51; P=0.039) between the three genotypes
(Fig. 2). From the graph it can be
seen that the A/A genotype had the lowest survival from CV
mortality during the follow-up time of 80 months. Regarding CV
mortality, a significantly higher mortality could be found in the
A/A genotype compared to the A/T and the T/T genotypes
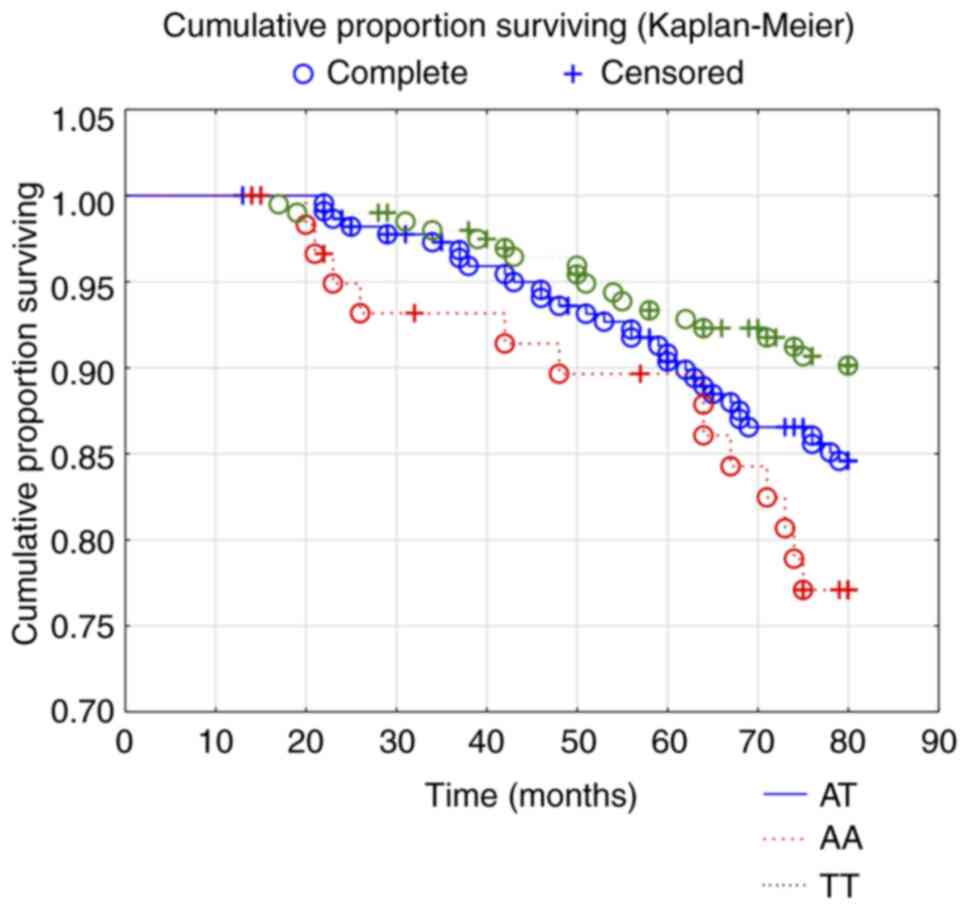
(χ2: 5.52; P=0.02). To validate the obtained results, a
Kaplan-Meier analysis was performed, as above, which also showed
significantly lower survival from CV mortality in the A/A genotype,
compared to the other two genotypes (χ2: 6.50; P=0.039),
(Fig. 3). Through a second
validation a risk evaluation was applied. From that the A/A
genotype could be demonstrated to have a 1.8-fold increased risk of
all-cause mortality, and an almost two-fold increased risk of CV
mortality when including several well-known clinical variables that
influence mortality risk (Table
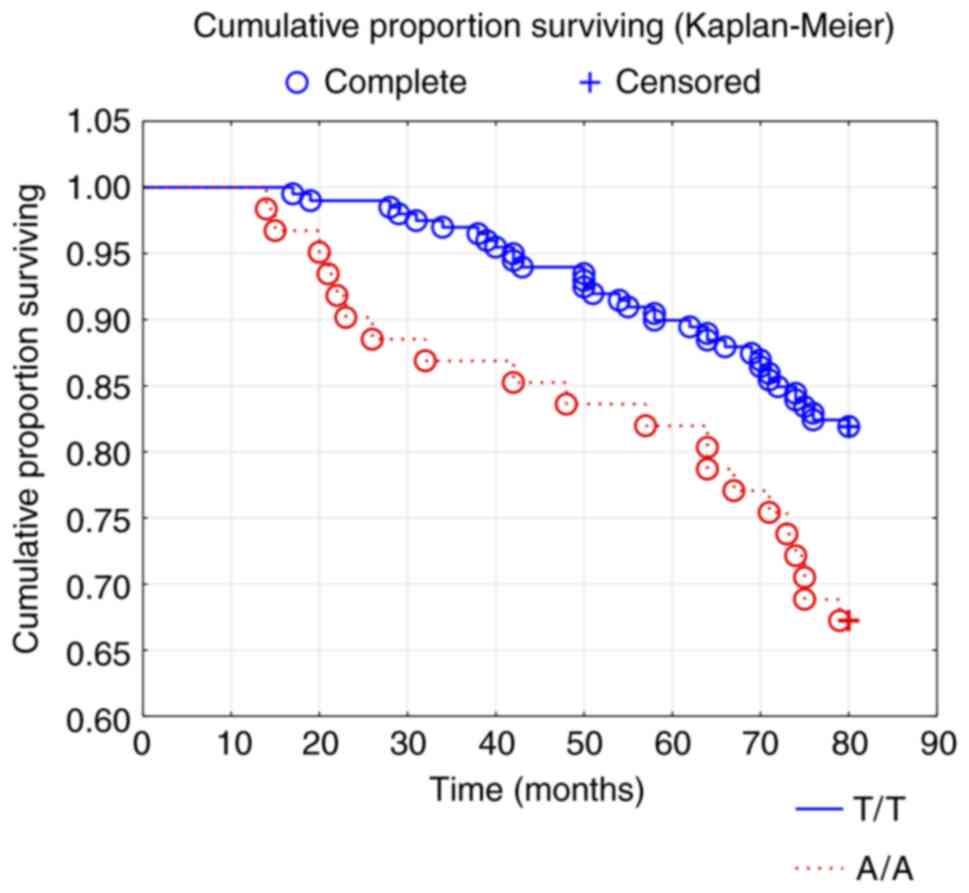
III). When analyzing the two genotypes that have the highest
difference in mortality, that is A/A and the T/T genotypes, a
significant difference in all-cause mortality (χ2: 5.97;
P=0.015) (Fig. 4), as well as in
cardiovascular mortality could be seen (χ2: 5.99;
P=0.014).
 | Table IIICox proportional hazard regression
analysis evaluating risk of all-cause- and cardiovascular mortality
in the study population regarding rs28372698 of IL-32 during a
follow-up period of 7.1 years. |
Table III
Cox proportional hazard regression
analysis evaluating risk of all-cause- and cardiovascular mortality
in the study population regarding rs28372698 of IL-32 during a
follow-up period of 7.1 years.
| | All-cause
mortality | Cardiovascular
mortality |
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| IHD | 1.61 | 1.04-2.49 | 0.03 | 1.75 | 1.00-3.07 | 0.05 |
| Diabetes | 1.65 | 1.07-2.53 | 0.2 | 1.65 | 0.94-2.87 | 0.08 |
| ACEI/ARB | 0.77 | 0.49-1.23 | 0.27 | 0.81 | 0.44-1.47 | 0.49 |
| Beta blockers | 0.96 | 0.63-1.46 | 0.84 | 0.88 | 0.51-1.54 | 0.66 |
| Diuretics | 1.21 | 0.80-1.82 | 0.37 | 1.07 | 0.62-1.85 | 0.80 |
| EF <40% | 2.13 | 1.19-3.80 | 0.01 | 2.25 | 1.07-4.76 | 0.03 |
| Hb <120 g/l | 1.10 | 0.63-1.91 | 0.74 | 1.50 | 0.79-2.85 | 0.22 |
| Rs28372698, IL-32,
A/A | 1.84 | 1.13-3.00 | 0.01 | 1.96 | 1.06-3.63 | 0.03 |
Discussion
As health resources are limited, the need for
instruments to identify those at high or low risk of cardiovascular
complications or mortality is increasing. This report demonstrates
an association between the A/A genotype of the IL-32 SNP rs28372698
and increased risk of both all-cause, and cardiovascular mortality
in an elderly community-living population, with a follow-up time of
90 months.
In the PubMed database only six reports could be
found regarding rs28372698 totally, and just a few regarding
genotypes of the SNP and mortality. Wang et al (9) reported an increased susceptibility of
SLE in the T/T genotype; however, the role of this polymorphism
regarding the function of IL-32 is unknown due to the lack of
information in the literature. Regarding information on
cardiovascular risks and polymorphisms of IL-32, the situation is
the same.
As IL-32 is a cytokine that is involved in
inflammatory states, the increased mortality risk might not be
surprising. However, this relation between IL-32 and mortality risk
has not been reported previously. That there is an intimate
association between inflammation and ischemic heart disease is
well-known in the literature (16-18).
There are also an increasing number of reports indicating that
increased oxidative stress is seen when the vascular system is
diseased (19,20). Also, endothelial dysfunction, one of
the early indicators of cardiovascular injury, is reported to be
associated with ischemic heart disease (21,22).
The association between IL-32 and cardiovascular risk is even more
closely associated with states of chronic inflammation, something
that seems logical (23). One of
the important factors might be the fact that IL-32 promotes
angiogenesis (24). In the
literature, there are reports on the genetic polymorphisms of
IL-32, and risk. Therefore, as the IL-32 is produced in many of the
cell systems involved in inflammation, it is not surprising that
this biomarker has an association with disease states that have
inflammatory components. There are reports indicating that the
plasma concentration of IL-32 is positively associated with
prognosis in heart failure after myocardial infarction (13). In our population we could not see a
corresponding association, which might be explained by the fact
that the population was elderly, and thus had already been exposed
to cardiovascular risk factors for a long time, and that the
follow-up was proportionally short.
There are reports that have focused on the genetic
polymorphisms of IL-32. Shamoun at al. reported no relation to
survival for IL-32 SNP rs 28372698 in patients with colorectal
cancer (25). Gonzalez-Hormazabal
et al (7) reported an
interesting interaction between SNP IL-8 rs4073 and SNP IL-32
rs28372698 and a proposed increased risk for gastric cancer for the
allele A of rs4073. In our evaluation we were able to present data
indicating significant increased mortality, both all-cause, and
cardiovascular mortality, in the A/A genotype of rs28372698.
However, the size of our study population was limited, so there is
uncertainty regarding the result. Because of this, we applied a
validation process including two different evaluations
(Kaplan-Meier evaluation, and Cox proportional hazard regressions
evaluation). Both validations presented the same result, indicating
that there might be an increased risk of all-cause and
cardiovascular mortality among those with the A/A genotype.
However, it is important to stress that the population evaluated
were elderly, and thus, those with the highest risk had probably
already died, and so the present study population might be
characterized as a group of persons with slightly lower risk. That
there was still a significant risk for the participants could be
seen by the mortality figures during the 90 months of
follow-up.
The important question to answer therefore is why
the A/A genotype showed a higher risk, as compared to the A/T and
the T/T genotypes. This SNP is associated with altered gene
expression of IL-32 g which could affect the inflammatory condition
in the patients and also the outcome. It cannot be excluded that
the investigated polymorphism may be in linkage disequilibrium with
other polymorphisms that modulate the susceptibility to the
outcome. However, as already mentioned, the lack of reports of
rs28372698 severely restricts the possibility to make comparisons
with other reports. Thus, we argue that evaluation of the genotypes
of the cytokine IL-32 might give the clinician important knowledge
on which of the everyday patients should receive more intense
follow-up, with increased prevention measures.
As the results presented are from a community-based
study, the majority of patients had discrete or no cardiovascular
symptoms. Therefore, those with cardiovascular disease were in a
minority and thus the size of the group with cardiovascular disease
was small, resulting in wide confidence intervals in risk
evaluations, making their interpretation uncertain. However, it
could be argued that a message regarding cardiovascular risk was
still found. As the message regarding risk of all-cause mortality
points in the same direction, it could be interpreted as
strengthening the obtained results. The study population was an
elderly one, and therefore it was not possible to extrapolate the
obtained results into other age groups without uncertainty. As the
groups with certain genotypes were small, the results should be
interpreted with caution, and should be regarded as
hypothesis-generating.
In the present study the SNP rs28372698 of IL-32 was
evaluated regarding all-cause and cardiovascular mortality in an
elderly community-living population during a follow-up period of
7.1 years. No difference in mortality could be seen that depended
on the plasma concentration of IL-32. However, on evaluating the
three genotypes, A/A, A/T and T/T, significantly higher all-cause
and cardiovascular mortality could be found in the A/A genotype.
This result was replicated in both Kaplan-Meier evaluations and in
risk evaluations according to Cox proportional hazard regression
analyses. The results are interesting and could give important
information in order to better handle patients during a risk
stratification at a time with restricted health resources. However,
the size of the groups was small and the results should be regarded
as hypothesis-generating, so more research is needed.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
County Council of Östergötland, University of Linköping, Linköping,
Sweden, and the Swedish Heart and Lung Foundation.
Availability of data and materials
The datasets generated and/or analyzed during the
current study are not publicly available due to that under Swedish
Law, the authors cannot share the data underlying this study and
cannot do any further research than what is specified in the
ethical permissions application. For inquires on the data,
researchers should first reach out to the owner of the database,
the University of Linköping. Please reach out to the corresponding
author with requests and for assistance with data requests. If the
university approves the request, researchers can submit an
application to the Regional Ethical Review Board for the specific
research question that the researcher wants to examine.
Authors' contributions
UA conceived and designed the experiments, performed
the experiments, analyzed the data and wrote the manuscript. DW
conceived and designed the experiments, performed the experiments,
analyzed the data, contributed reagents/material/analysis tools. LS
performed the experiments and contributed
reagents/material/analysis tools. JID conceived and designed the
experiments, performed the experiments, contributed
reagents/material/analysis tools and wrote the manuscript.
Ethical approval and consent to
participate
The current study was conducted in accordance with
the Declaration of Helsinki principles. The study protocol was
approved by the Regional Ethical Review Board of Linköping, Sweden
(Dnr 95044).
Patient consent for publication
All participants gave their written informed consent
to participate and to allow data being published, and the.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hong JT, Son DJ, Lee CK, Yoon DY, Lee DH,
Yoon DY, Lee DH and Park MH: Interleukin 32, inflammation and
cancer. Pharmacol Ther. 174:127–137. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Gui M, Zhang H, Zhong K, Li Y, Sun J and
Wang L: Clinical significance of interleukin-32 expression in
patients with rheumatoid arthritis. Asian Pac J Allergy Immunol.
31:73–78. 2013.PubMed/NCBI
|
|
3
|
Arcaroli JJ, Liu N, Yi N and Abraham E:
Association between IL-32 genotypes and outcome in
infection-associated acute lung injury. Crit Care.
15(R138)2011.PubMed/NCBI View
Article : Google Scholar
|
|
4
|
El-Far M, Kouassi P, Sylla M, Zhang Y,
Fouda A, Fabre T, Goulet JP, van Grevenynghe J, Lee T, Singer J, et
al: Proinflammatory isoforms of IL-32 as novel and robust
biomarkers for control failure in HIV-infected slow progressors.
Sci Rep. 6(22902)2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bao F, Wen X, Liu A, Dai X, Zhao Q, Wang Y
and Lu S: Elevated levels of serum IL-32 in patients with active
pulmonary tuberculosis. Afr J Microbiol Res. 6:7292–7294. 2012.
|
|
6
|
Plantinga TS, Costantini I, Heinhuis B,
Huijbers A, Semango G, Kusters B, Netea MG, Hermus AR, Smit JW,
Dinarello CA, et al: A promoter polymorphism in human
interleukin-32 modulates its expression and influences the risk and
the outcome of epithelial cell-derived thyroid carcinoma.
Carcinogenesis. 34:1529–1535. 2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Gonzalez-Hormazabal P, Musleh M,
Bustamante M, Stambuk J, Escandar S, Valladares H, Lanzarini E,
Chiong H, Rojas J, Castro VG, et al: Role of cytokine gene
polymorphisms in gastric cancer risk in Chile. Anticancer Res.
34:3523–3530. 2014.PubMed/NCBI
|
|
8
|
Yu X, Zhou B, Zhang Z, Gao Q, Wang Y, Song
Y, Pu Y, Chen Y, Duan R, Zhang L and Xi M: Significant association
between IL-32 gene polymorphisms and susceptibility to endometrial
cancer in Chinese Han women. Tumour Biol. 36:5265–5272.
2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wang Y, Zhou B, Zhao Y, Yu X, Liu Y and
Zhang L: Association of plasma IL-32 levels and gene polymorphisms
with systemic lupus erythematosus in Chinese Han population. Dis
Markers. 2016(2460206)2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wang Y, Yang Y, Zhu Y, Li L, Chen F and
Zhang L: Polymorphisms and expression of IL-32: Impact on genetic
susceptibility and clinical outcome of lung cancer. Biomarkers.
22:165–170. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Damen MS, Agca R, Holewijn S, de Graaf J,
Dos Santos JC, van Riel PL, Fransen J, Coenen MJ, Nurmohamed MT,
Netea MG, et al: IL-32 promoter SNP rs4786370 predisposes to
modified lipoprotein profiles in patients with rheumatoid
arthritis. Sci Rep. 7(41629)2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Seo EH, Kang J, Kim KH, Cho MC, Lee S, Kim
HJ, Kim JH, Kim EJ, Park DK, Kim SH, et al: Detection of expressed
IL-32 in human stomach cancer using ELISA and immunostaining. J
Microbiol Biotechnol. 18:1606–1612. 2008.PubMed/NCBI
|
|
13
|
Xuan W, Huang W, Wang R, Chen C, Chen Y,
Wang Y and Tan X: Elevated circulating IL-32 presents a poor
prognostic outcome in patients with heart failure after myocardial
infarction. Int J Cardiol. 243:367–373. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Alehagen U, Ericsson A and Dahlström U:
Are there any significant differences between females and males in
the management of heart failure? Gender aspects of an elderly
population with symptoms associated with heart failure. J Card
Fail. 15:501–507. 2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yancy CW, Jessup M, Bozkurt B, Butler J,
Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi
JL, et al: 2013 ACCF/AHA guideline for the management of heart
failure: Executive summary: A report of the American college of
cardiology foundation/american heart association task force on
practice guidelines. Circulation. 128:1810–1852. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Qi H, Shen J and Zhou W: Up-regulation of
long non-coding RNA THRIL in coronary heart disease: Prediction for
disease risk, correlation with inflammation, coronary artery
stenosis, and major adverse cardiovascular events. J Clin Lab Anal.
34(e23196)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ebadi N, Ghafouri-Fard S, Taheri M,
Arsang-Jang S and Omrani MD: Expression analysis of inflammatory
response-associated genes in coronary artery disease. Arch Physiol
Biochem: Jan. 8:1–7. 2020.PubMed/NCBIdoi: 10.1080/13813455.2019.1708953.
|
|
18
|
Das AA, Chakravarty D, Bhunia D, Ghosh S,
Mandal PC, Siddiqui KN and Bandyopadhyay A: Elevated level of
circulatory sTLT1 induces inflammation through SYK/MEK/ERK
signalling in coronary artery disease. Clin Sci (Lond).
133:2283–2299. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kura B, Szeiffova Bacova B, Kalocayova B,
Sykora M and Slezak J: Oxidative stress-responsive MicroRNAs in
heart injury. Int J Mol Sci. 21(358)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Tzoulaki I, Castagné R, Boulangé CL,
Karaman I, Chekmeneva E, Evangelou E, Ebbels TMD, Kaluarachchi MR,
Chadeau-Hyam M, Mosen D, et al: Serum metabolic signatures of
coronary and carotid atherosclerosis and subsequent cardiovascular
disease. Eur Heart J. 40:2883–2896. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Matsuzawa Y and Lerman A: Endothelial
dysfunction and coronary artery disease: Assessment, prognosis, and
treatment. Coron Artery Dis. 25:713–724. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Anderson RD, Petersen JW, Mehta PK, Wei J,
Johnson BD, Handberg EM, Kar S, Samuels B, Azarbal B, Kothawade K,
et al: Prevalence of coronary endothelial and microvascular
dysfunction in women with symptoms of ischemia and no obstructive
coronary artery disease is confirmed by a new cohort: The
NHLBI-sponsored women's ischemia syndrome evaluation-coronary
vascular dysfunction (WISE-CVD). J Interv Cardiol.
2019(7169275)2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Damen MSMA, Popa CD, Netea MG, Dinarello
CA and Joosten LAB: Interleukin-32 in chronic inflammatory
conditions is associated with a higher risk of cardiovascular
diseases. Atherosclerosis. 264:83–91. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Nold-Petry CA, Rudloff I, Baumer Y, Ruvo
M, Marasco D, Botti P, Farkas L, Cho SX, Zepp JA, Azam T, et al:
IL-32 promotes angiogenesis. J Immunol. 192:589–602.
2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Shamoun L, Kolodziej B, Andersson RE and
Dimberg J: Protein expression and genetic variation of IL32 and
association with colorectal cancer in Swedish patients. Anticancer
Res. 38:321–328. 2018.PubMed/NCBI View Article : Google Scholar
|