Introduction
Chemotherapeutic drugs, such as taxanes, vinca
alkaloids, platinum analogs, topoisomerase inhibitors and
proteasome inhibitors, can cause a series of adverse reactions. The
most common and serious side-effect is chemotherapy-induced
peripheral neuropathic pain (CIPNP) (1,2), which
negatively affects patient quality of life, rendering it a major
constraint for therapeutic drug dosage. Oxaliplatin is a platinum
derivative that is widely used in first-line colorectal cancer
therapy (3). Oxaliplatin can result
in acute and chronic distal sensory neuropathy; the former is
characterized as acral paresthesia triggered by cold temperatures
and the latter is induced by accumulative oxaliplatin (4). Oxaliplatin-induced CIPNP complicates
clinical treatment because there is a lack of effective analgesics,
therefore, the treatment process may be accompanied by unacceptable
side effects (5). There are
currently no effective ways to treat or prevent oxaliplatin-induced
CIPNP (6), necessitating the
development of novel solutions.
Nuclear factor-κB (NF-κB) has a substantial role in
regulating inflammation and immune responses (7) and is involved in the nervous system's
synaptic plasticity, memory formation, learning, neurotransmission
and neuroprotection (8,9). When the NF-κB signaling pathway is
activated, NF-κB p65 can bind to the promoters of target genes and
enhance their expression (10).
Previous research has indicated that NF-κB activation is associated
with the pathological pain caused by nerve injury or inflammation
(11,12). Recent research has also reported
NF-κB to be involved in CIPNP (13)
and the establishment and maintenance of acute and chronic pain
caused by neuroinflammation or nerve injury (14,15).
Furthermore, NF-κB activation was demonstrated to regulate the
levels of pro-inflammatory cytokines, including IL-6 and TNF-α, in
a neuropathic pain model (16).
However, it remains unclear whether oxaliplatin-induced CIPNP is
associated with NF-κB pathway activation in dorsal root ganglions
(DRGs).
Natural plant products have been shown to
effectively treat complex chronic comorbidities (17-19).
Berberine, one of the tested compounds, is an isoquinoline alkaloid
reportedly purified from herbs and featuring multiple
pharmacological effects. Its antimicrobial and antisecretory
properties have been used as a treatment to diarrhea and
gastroenteritis (20,21). Berberine and its derivatives have
also been shown to exhibit potent anti-inflammatory and anticancer
properties (22). Additionally,
berberine has alleviated allodynia and demonstrated antioxidative
effects in models of diabetic neuropathy (23) and peripheral nerve injuries
(24). However, there are no
relevant reports on berberine's analgesic function on
oxaliplatin-induced CIPNP; that is, no research has revealed
berberine having a role as an adjuvant during chemotherapy.
The present study explored the effects of single
injections and repeated doses of berberine on the induction and
prevention of oxaliplatin-induced CIPNP, respectively. To
investigate its underlying mechanism, the effects of berberine on
oxaliplatin-induced modulation of NF-κB signaling and of
pro-inflammatory cytokine secretion were also tested.
Materials and methods
Animals
Sprague Dawley rats (n=88, male, 200-220 g;
Laboratory Animal Center of Huazhong University of Science and
Technology, Wuhan, China) were housed in a room with 22-24ºC and
12-h light/dark cycle (7:00 am to 7:00 pm). Food and water were
freely available. All procedures complied strictly with the
Guidelines for the Care and Use of Laboratory Animals and were
approved by the Animal Care and Use Committee of Huazhong
University of Science and Technology (Wuhan, China).
Drug administration and experimental
design
Oxaliplatin (Dalian Meilun Biology Technology Co.,
Ltd.) was dissolved in saline. The rats were injected
intraperitoneally (i.p.) with 2.5 mg/kg oxaliplatin for 4
consecutive days, after which a final dose of 10 mg/kg was
administered to induce peripheral neuropathic pain. Berberine was
used both as a treatment in pre-established oxaliplatin-induced
CIPNP and as a prevention during the CIPNP induction process.
Berberine chloride was obtained from Shanghai
Shifeng Biotechnology, Ltd. Berberine was dissolved in dimethyl
sulfoxide (DMSO) at concentrations of 50, 100 and 200 mg/ml, and
diluted in saline (0.9%) for injection. All solutions were filtered
using a 0.22-µm membrane filter (Pall Life Sciences) prior to
injection. To evaluate the effect of berberine as a treatment in
pre-established oxaliplatin-induced CIPNP, a single dose of
berberine (5, 10 or 20 mg/kg) was administered i.p. 21 days after
the first oxaliplatin injection, according to a previous report
(23). Von Frey, acetone drop and
hot plate tests were performed 60 min after the injection of
berberine. To evaluate the effect of berberine on the prevention of
oxaliplatin-induced CIPNP, animals were injected i.p. with
berberine or saline every 24 h for 21 consecutive days after the
final injection with oxaliplatin. Animals were randomly divided
into four groups (n=8): vehicle + saline group, oxaliplatin +
saline group, oxaliplatin + berberine (10 mg/kg) group and
oxaliplatin + berberine (20 mg/kg) group. Then, tissue samples were
collected 24 h after the end of final behavioral tests. All
behavioral tests were conducted at 10:00 am of each experiment day
by researchers who were blinded to the animal groups.
Mechanical allodynia (von Frey
test)
Mechanical allodynia was evaluated by von Frey test.
The rats were placed in a plastic chamber (20x17x13 cm) without
bottom and with several compartments, and all of them were placed
on a 40-cm high wire mesh shelf. At 15 min before the test, the
rats were placed in the test box to adapt to the environment. An
electronic von Frey instrument (IITC Life Science Inc.) was used to
examine the rats' behaviors. The withdrawal threshold was reflected
by applying 0-50 g of pressure (with an accuracy of 0.2 g). Below
the wire mesh floor, a punctuate stimulus was transmitted through
the tip of the von Frey fiber to the middle part of each rats' hind
paw for 2 sec. Then the von Frey instrument automatically reads the
withdrawal threshold. The rat's sensitivity threshold is considered
as the minimum pressure required to make the hind paw produce
robust and immediate withdrawal reflex. Movement-related autonomous
motions are not considered as withdrawal responses. The stimulus
was given to the hind paw every 5 min. The measurement was repeated
three times, and the average of three measurements was recorded as
the final result.
Cold allodynia (acetone drop
test)
According to previous reports (25), cold allodynia was measured by
acetone drop tests. In brief, rats were individually placed in
plastic chambers which were put on a wire mesh shelf. Then a flat
needle of a syringe was used to aspirate some acetone and one drop
was gently dropped on the surface of the rat's hind paw. The time
of withdrawal/licking reaction was recorded within 40 sec. Acetone
was dropped every 10 min during the measurement, and the average of
the responses was calculated in each measurement.
Thermal hyperalgesia (hot-plate
test)
Thermal hyperalgesia was evaluated by a hot plate
analgesic instrument (Bioseb). Rats were placed individually on a
hot plate with a constant temperature of 53±1˚C. An electronic
timer was used to record the time required for reactions such as
jumping or hind paw-licking. To prevent scalding of rat paws, the
maximum heating time was set as 30 sec. Three repeat measurements
were obtained for each rat, with an interval between each
measurement of >10 min, then the average value of each
measurement was calculated as the final result. Normal foot-lifting
motions were not counted. The researcher performing the behavioral
testing was blind to the grouping and administration of rats.
Bodyweight and motor coordination
All rats were weighed weekly on days 0, 7, 14 and
21. A rotarod apparatus (Shanghai Mobile Data Center) was used to
evaluate the motor coordination of rats. The rats were trained for
10 min with 10, 20 and 30 rpm on the equipment for 3 consecutive
days to let them familiarize with the device, until they could
persist for 60 sec with not falling. During the test, the rats were
placed with a variable mode of 4-40 rpm and allowed to run until
they fell or reached the 5-min cut-off time. The time from the
beginning of the test to the fall was recorded.
Tissue collection
After completing the behavioral tests, rats were
anesthetized with pentobarbital sodium (50 mg/kg, i.p.;
Sigma-Aldrich; Merck KGaA) and sacrificed by cervical dislocation.
DRGs from lumbar (L) 4-L6 were dissected and quick-frozen in liquid
nitrogen, then stored in -80˚C until further experiments.
Protein preparation and western blot
analysis
Samples were homogenized in lysis buffer (50 mM
Tris-HCl, 150 mM NaCl, 1% sodium deoxycholate, 1% Triton Χ-100,
0.1% SDS, pH 7.4) to extract the protein. The protein preparations
were stored at -80˚C. The protein concentration in each sample was
determined using a BCA assay. Equal amounts of protein (25 µg) were
separated on 10% Tris-Tricine SDS-PAGE and transferred to PVDF
membranes, followed by blocking with 5% non-fat milk for 1 h at
room temperature. The membranes were then incubated with primary
antibodies targeting NF-κB p65 (1:1,000; cat. no. 4764; Cell
Signaling Technology, Inc.), phosphorylated (p-) p65 (1:1,000; cat.
no. 3033; Cell Signaling Technology, Inc.) and GAPDH (1:5,000; cat.
no. ab9485; Abcam) overnight at 4˚C. The next day, after washing,
the membranes were incubated with HRP-conjugated goat anti-rabbit
polyclonal IgG secondary antibodies (1:2,000; ab6721; Abcam) for
half hour at room temperature. Immunoblotting was detected by
chemiluminescent substrate and the experimental results were
processed with ImageJ software (version 1.51j8, National Institutes
of Health).
Enzyme-linked immunosorbent assay
(ELISA)
First, ice-cold PBS was used to homogenize DRG
samples. Then, the bicinchoninic acid assay was used to determine
the protein concentration in the samples. The levels of TNF-α and
IL-6 were measured using commercially available rat-specific ELISA
kits (IL-6 kit, cat. no. R6000B; TNF-α kit, cat. no. RTA00; R&D
Systems, Inc.), following the manufacturer's instructions.
Statistical analysis
Data were presented as the mean ± standard error of
the mean. Behavioral and western blotting results were analyzed by
single factor or mixed factor designed ANOVA, followed by Dunnett's
or simple-effects post hoc tests using SPSS software (version
21.0.0; IBM Corp.). P<0.05 was considered to indicate a
statistically significant difference.
Results
Oxaliplatin-induced mechanical
allodynia, cold allodynia and thermal hyperalgesia in rats
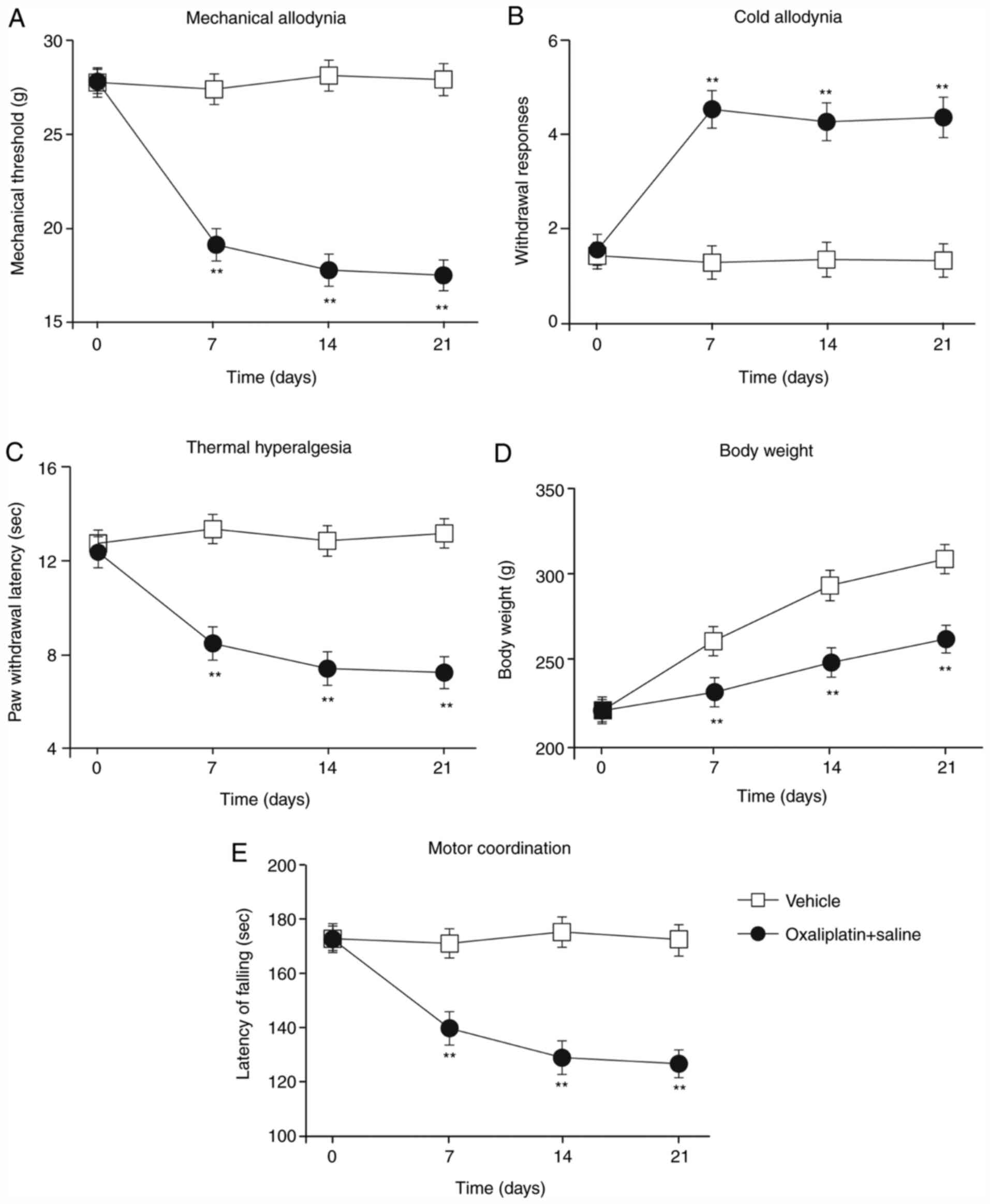
First, the rat model of oxaliplatin-induced CIPNP
was generated by injecting oxaliplatin (i.p., 2.5 mg/kg). Before
oxaliplatin injection and 1, 2 and 3 weeks post-injection, von Frey
hind-paw withdrawal thresholds, withdrawal response times for cold
plate tests and hind-paw licking latencies for hot plate tests were
assessed (Fig. 1A-C). Compared with
vehicle-treated rats, oxaliplatin-treated rats displayed
significant mechanical allodynia (Fig.
1A), cold allodynia (Fig. 1B)
and thermal hyperalgesia (Fig. 1C)
on the 7th day post-injection; these observations were sustained
throughout the experiment. Additionally, the weight of the
vehicle-treated rats increased during the experiment; although the
oxaliplatin-treated rats also gained weight, the effect was less
pronounced (Fig. 1D). Finally,
motor function was significantly impaired in the
oxaliplatin-treated rats compared with the vehicle-treated rats
(Fig. 1E). These results indicated
that treatment with oxaliplatin resulted in mechanical allodynia,
cold allodynia and thermal hyperalgesia. In addition, the impaired
weight gain and motor function suggested that the
oxaliplatin-induced CIPNP model was successfully established in the
present study.
Effects of a single injection of
berberine on oxaliplatin-induced allodynia
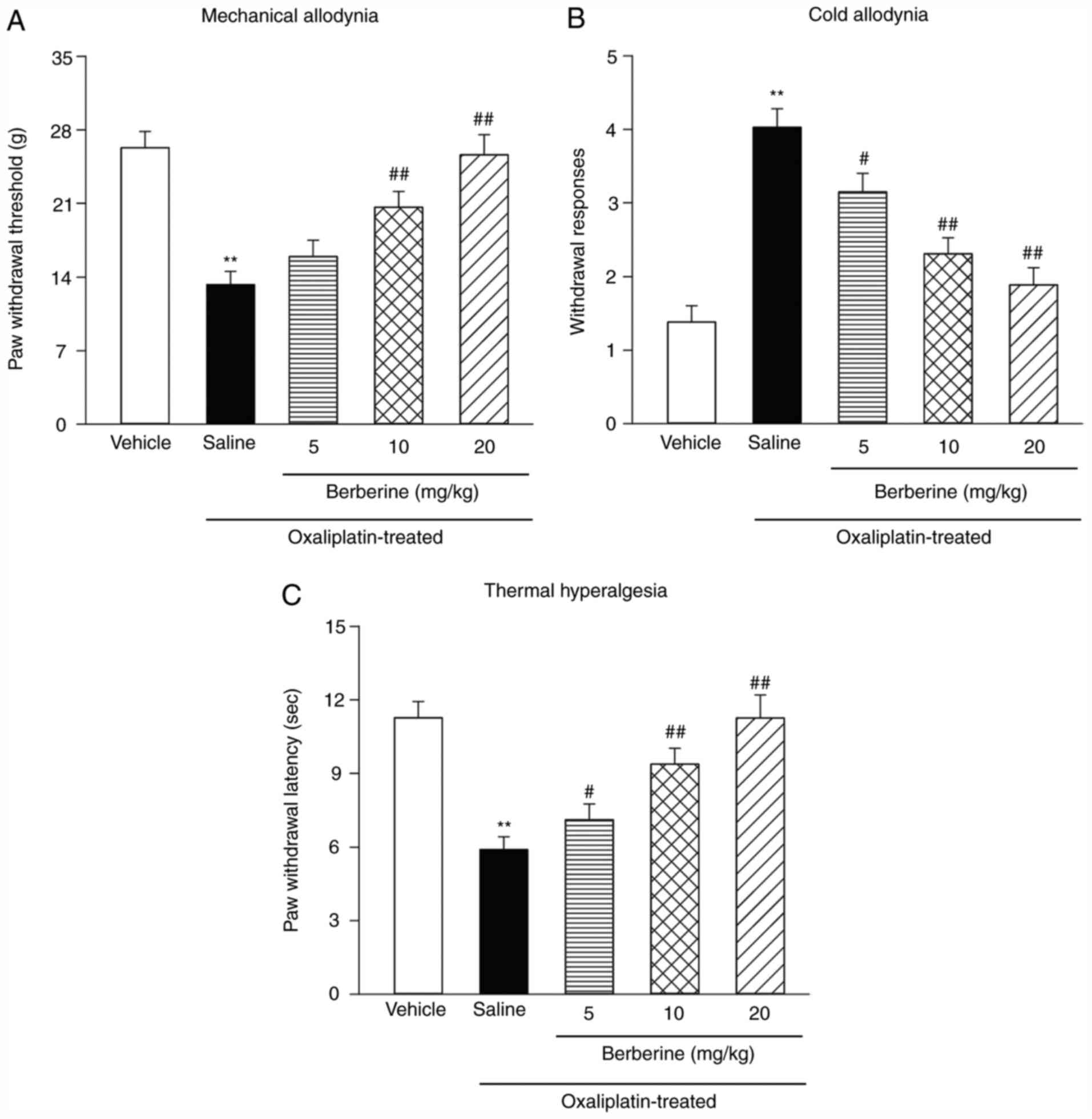
On the 21st day following the first injection of
oxaliplatin, a single injection of 5, 10 or 20 mg/kg berberine was
administered to the rats to examine the effect of berberine as a
treatment on pre-established CIPNP. A previous report (25) reported these relief effects at 60
min after the berberine injection. Fig.
2A demonstrates that, according to a von Frey test, the
hind-paw withdrawal thresholds of oxaliplatin-treated rats
decreased compared with the vehicle-treated rats. The acetone drop
tests showed increased withdrawal response times (Fig. 2B) and the hot plate tests showed
decreased hind-paw-licking latency (Fig. 2C) compared with the vehicle-treated
rats. However, a single injection of berberine significantly
reduced mechanical allodynia (Fig.
2A), cold allodynia (Fig. 2B)
and thermal hyperalgesia (Fig. 2C).
Although the dose of 5 mg/kg of berberine could effectively relieve
cold hyperalgesia and thermal hyperalgesia, it did not have a
significant effect on mechanical hyperalgesia; therefore, the doses
of 10 and 20 mg/kg of berberine were used for the following
experiments.
Effects of repeated doses of berberine
on oxaliplatin-induced mechanical allodynia, cold allodynia and
thermal hyperalgesia
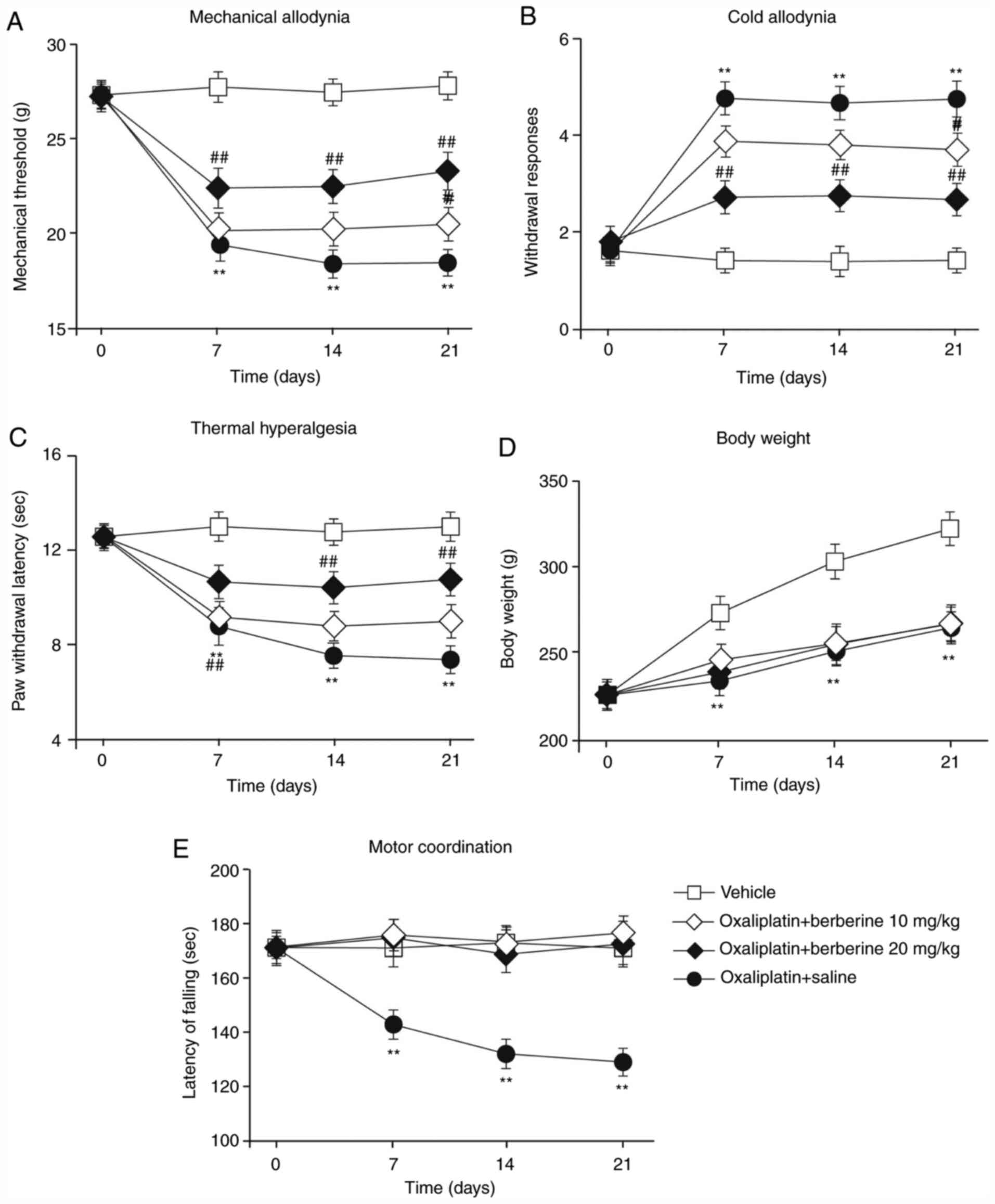
As shown in Fig. 3,
when the rats were repeatedly injected with berberine throughout
the CIPNP induction experiment, berberine reversed CIPNP
establishment, depending on the dose. The oxaliplatin and
berberine-treated rats displayed only partial development of
mechanical allodynia and cold allodynia compared with the
oxaliplatin-treated rats on all test days (Fig. 3A and B). In hot plate tests, the oxaliplatin and
berberine-treated rats displayed a distinct increase in hind paw
licking latency compared with the oxaliplatin-treated rats
(Fig. 3C). Weight gain measurements
produced results similar to those for oxaliplatin-treated rats,
with the oxaliplatin and berberine-treated rats not gaining as much
weight as the vehicle-treated rats (Fig. 3D). However, motor function in the
oxaliplatin and berberine-treated rats was significantly
ameliorated compared with the oxaliplatin-treated rats, and similar
to the levels of the control vehicle-treated rats (Fig. 3E). These results revealed that
berberine dose-dependently prevented the development of CIPNP in
rats without affecting motor function.
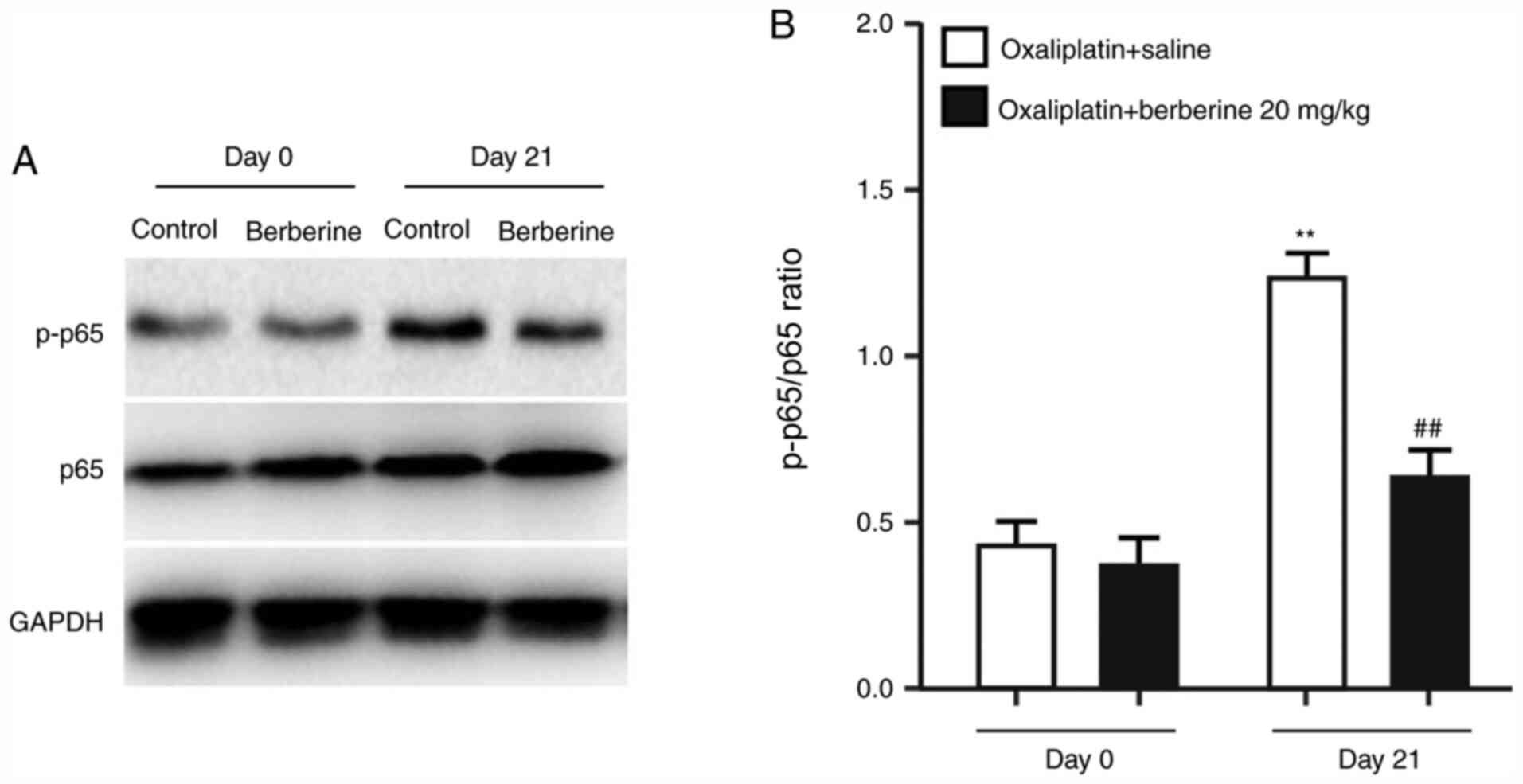
Effects of repeated injections of
berberine on oxaliplatin-induced NF-κB phosphorylation
A recent study revealed that the NF-κB signaling
pathway is associated with chemotherapy-induced chronic pain
(13). In addition, activation of
NF-κB in DRGs has been demonstrated to mediate chronic pain caused
by inflammatory reactions (14). To
understand the mechanism underlying the analgesic effect of
berberine, the protein expression levels of NF-κB p65 and the
phosphorylated NF-κB p65 were examined in DRGs from the rats
administered with oxaliplatin and either saline control or the 20
mg/kg dose of berberine. The western blotting results demonstrated
that oxaliplatin treatment significantly induced NF-κB p65
phosphorylation (Fig. 4A and
B), while repeated injections of
berberine suppressed oxaliplatin-induced NF-κB p65 phosphorylation
(Fig. 4A and B). These findings indicated that the
analgesic effect of berberine might occur through the regulation of
NF-κB phosphorylation in DRGs.
Effects of repeated injections of
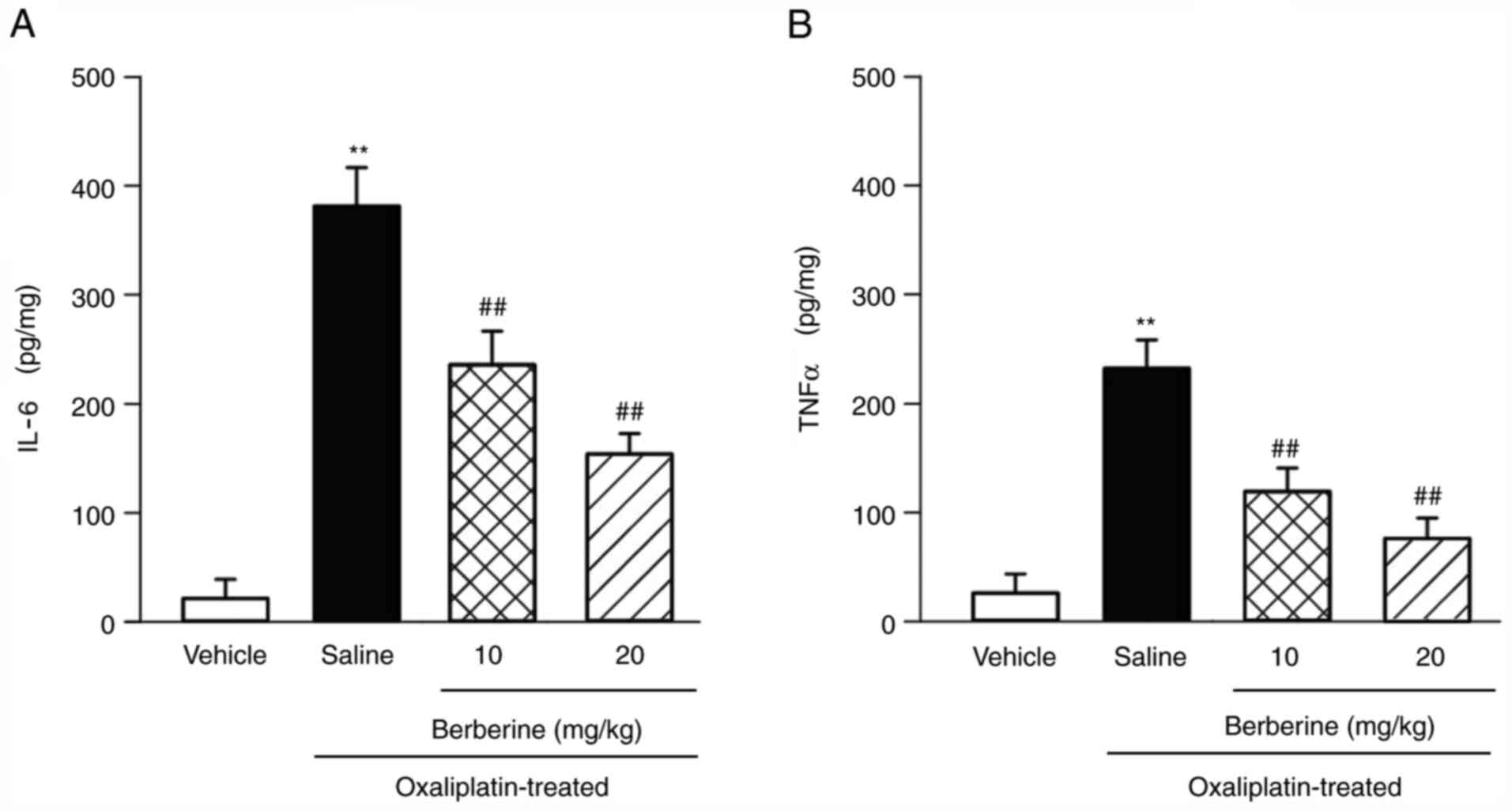
berberine on IL-6 and TNF-α levels in rat DRGs
The ELISA method was used to detect the levels of
IL-6 and TNF-α in rat DRGs. The results demonstrated that the
levels of IL-6 and TNF-α significantly increased in the
oxaliplatin-induced CIPNP model compared with the vehicle-treated
rats (Fig. 5A and B). However, berberine administration
significantly and dose-dependently suppressed these increases in
IL-6 and TNF-α levels in the oxaliplatin-induced CIPNP model
(Fig. 5A and B).
Discussion
As many as 80% of patients receiving cytostatic
drugs are treated for CIPNP (26).
As the number of cancer survivors increases, treating this
upsetting side effect has become a top priority. Although several
drugs for preventing or treating CIPNP have been considered in
experimental research, few have been applied effectively in
clinical practice (27). The
present study found that repeated doses of berberine during
oxaliplatin treatment significantly prevented the severity of CIPNP
in rats. This effect was associated with the suppressed
phosphorylation of NF-κB p65, and with decreased levels of the
cytokines IL-6 and TNF-α, in DRGs. The present results indicate
that berberine might have an analgesic role in oxaliplatin-induced
CIPNP by preventing NF-κB p65 phosphorylation and pro-inflammatory
cytokine release in DRGs.
Berberine has been reported to relieve allodynia and
to display antioxidant effects in models of diabetic neuropathy
(23) and peripheral nerve injury
(24). The present study included
behavioral observations to demonstrate that oxaliplatin injection
in rats could induce mechanical allodynia, cold allodynia and
thermal hyperalgesia. Of note, a single dose of berberine
significantly reduced oxaliplatin-induced pain behaviors in the
pre-established CIPNP process, while repeated administration acted
as a preventative treatment to the development and establishment of
oxaliplatin-induced CIPNP. However, berberine did not produce pain
behaviors in naive animals (28).
The present results suggest that berberine can potentially prevent
and treat neuropathic pain, including CIPNP. One of the limitations
of the present study was the use of only male animals in the
behavioral tests. Further analysis with both male and female
animals will be necessary to confirm these conclusions.
Berberine's analgesic mechanism in
oxaliplatin-induced CIPNP has not been extensively studied. The
main mechanisms of oxaliplatin-induced peripheral neuropathy
include ion-channel imbalance, neuronal inflammation, neuronal
damage and oxidative stress (26).
Accumulating evidence indicates that NF-κB activation and
pro-inflammatory cytokines mediate chemotherapy-induced neuropathy
and that NF-κB activation is also involved in pathological pain
caused by nerve damage or inflammation (11,12).
The most well-known functional heterodimer of NF-κB in cells is the
p50/p65 complex (29). Given NF-κB
p65 requires phosphorylation before binding to specific target
genes in the nucleus, NF-κB activation can be demonstrated by an
increase in p-p65 expression (30).
It has also been reported that berberine prevents the extracellular
matrix degradation and apoptosis of human nucleus pulposus cells by
inhibiting the NF-κB pathway (31).
The present study demonstrated that repeated administration of
berberine during the induction of CIPNP significantly inhibited the
NF-κB activation in DRGs induced by oxaliplatin. Combined with
previous studies, the present results indicate that NF-κB might
participate in the mechanism responsible for the analgesic effect
of berberine in DRGs.
Previous studies have hypothesized that the spinal
cord may be involved in the effect of berberine in CIPNP. For
example, it has been reported that berberine reduces spinal cord
neuroglia activation in streptozotocin-induced diabetic mice
(28). Protein expression levels of
inducible nitric oxide synthase and cyclooxygenase-2 in DRG and
spinal cord have also been shown to be suppressed by berberine in
the context of diabetic neuropathic pain. Levels of IL-6, IL1β and
TNF-α, in either the DRG or the spinal cord, have also been shown
to be reduced by berberine. These results indicate that the
mechanism underlying the effect of berberine might be similar in
the DRG and the spinal cord; however, this requires further
exploration.
It is known that chemotherapeutic agents, such as
paclitaxel, vincristine and oxaliplatin, can induce painful
peripheral neuropathy by inducing pro-inflammatory cytokines
(32,33). Activation of NF-κB is a critical
signaling pathway in the chronic pain context, mediating
inflammatory reactions and altering ion channel expression
(34). Additionally, NF-κB is a
pleiotropic regulator that regulates the expression of many
different genes, including genes that transcribe pro-inflammatory
cytokines such as IL-6, TNF-α and IL-1β (35). This explains the increased levels of
IL-6 and TNF-α in the DRG of rats following oxaliplatin injection
observed in the present study. The present study demonstrated that
NF-κB p65 phosphorylation and expression of the pro-inflammatory
cytokines IL-6 and TNF-α were substantially increased in the DRG of
animals with oxaliplatin-induced CIPNP. Furthermore, the present
study revealed that these increases were significantly suppressed
by berberine, suggesting that berberine might relieve CIPNP through
inhibiting NF-κB phosphorylation and the release of
pro-inflammatory cytokines. Nonetheless, other mechanisms of
berberine that might be involved with CIPNP cannot be excluded.
In conclusion, the present study demonstrated an
analgesic effect of berberine both as a treatment on
pre-established oxaliplatin-induced CIPNP and as a preventative
during the establishment of oxaliplatin-induced CIPNP, by
decreasing NF-κB p65 phosphorylation and pro-inflammatory cytokine
IL-6 and TNF-α release in DRGs. The present results indicate that
berberine may have an analgesic effect on CIPNP, thus further
research confirming its effectiveness in a clinical setting is
warranted.
Acknowledgements
Not applicable.
Funding
The present work was supported by grants from the
Natural Science Foundation of Hubei province (grant no.
2018CκB920).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
QX and WN were responsible for the concept and
design of the study. WN, XZ, LH and CK performed experiments and
data analysis. QX and WN performed data interpretation,
presentation and writing of the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
All experimental procedures were performed in
compliance with the Guide for the Care and Use of Laboratory
Animals and were approved by the Animal Care and Use Committee of
Huazhong University of Science and Technology (Wuhan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of
interest.
References
|
1
|
Park SB, Goldstein D, Krishnan AV, Lin CS,
Friedlander ML, Cassidy J, Koltzenburg M and Kiernan MC:
Chemotherapy-induced peripheral neurotoxicity: A critical analysis.
CA Cancer J Clin. 63:419–437. 2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Argyriou AA, Bruna J, Marmiroli P and
Cavaletti G: Chemotherapy-induced peripheral neurotoxicity (CIPN):
An update. Crit Rev Oncol Hematol. 82:51–77. 2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
André T, Boni C, Mounedji-Boudiaf L,
Navarro M, Tabernero J, Hickish T, Topham C, Zaninelli M, Clingan
P, Bridgewater J, et al: Multicenter International Study of
Oxaliplatin/5-Fluorouracil/Leucovorin in the Adjuvant Treatment of
Colon Cancer (MOSAIC) Investigators: Oxaliplatin, fluorouracil, and
leucovorin as adjuvant treatment for colon cancer. N Engl J Med.
350:2343–2351. 2004.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Cersosimo RJ: Oxaliplatin-associated
neuropathy: A review. Ann Pharmacother. 39:128–135. 2005.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Farquhar-Smith P: Chemotherapy-induced
neuropathic pain. Curr Opin Support Palliat Care. 5:1–7.
2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Brewer JR, Morrison G, Dolan ME and
Fleming GF: Chemotherapy-induced peripheral neuropathy: Current
status and progress. Gynecol Oncol. 140:176–183. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Vallabhapurapu S and Karin M: Regulation
and function of NF-kappaB transcription factors in the immune
system. Annu Rev Immunol. 27:693–733. 2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Meffert MK, Chang JM, Wiltgen BJ, Fanselow
MS and Baltimore D: NF-kappa B functions in synaptic signaling and
behavior. Nat Neurosci. 6:1072–1078. 2003.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Kaltschmidt B and Kaltschmidt C: NF-kappaB
in the nervous system. Cold Spring Harb Perspect Biol.
1(a001271)2009.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wan F, Anderson DE, Barnitz RA, Snow A,
Bidere N, Zheng L, Hegde V, Lam LT, Staudt LM, Levens D, et al:
Ribosomal protein S3: A KH domain subunit in NF-kappaB complexes
that mediates selective gene regulation. Cell. 131:927–939.
2007.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhao LX, Jiang BC, Wu XB, Cao DL and Gao
YJ: Ligustilide attenuates inflammatory pain via inhibition of
NFκB-mediated chemokines production in spinal astrocytes. Eur J
Neurosci. 39:1391–1402. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chu LW, Chen JY, Wu PC and Wu BN:
Atorvastatin prevents neuroinflammation in chronic constriction
injury rats through nuclear NFκB downregulation in the dorsal root
ganglion and spinal cord. ACS Chem Neurosci. 6:889–898.
2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Huang ZZ, Li D, Ou-Yang HD, Liu CC, Liu
XG, Ma C, Wei JY, Liu Y and Xin WJ: Cerebrospinal fluid oxaliplatin
contributes to the acute pain induced by systemic administration of
oxaliplatin. Anesthesiology. 124:1109–1121. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Fu ES, Zhang YP, Sagen J, Candiotti KA,
Morton PD, Liebl DJ, Bethea JR and Brambilla R: Transgenic
inhibition of glial NF-kappaB reduces pain behavior and
inflammation after peripheral nerve injury. Pain. 148:509–518.
2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Fu ES, Zhang YP, Sagen J, Yang ZQ and
Bethea JR: Transgenic glial nuclear factor-kappaB inhibition
decreases formalin pain in mice. Neuroreport. 18:713–717.
2007.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Nong X, Lan Y and Picroside II: Picroside
II Attenuates CCI-induced neuropathic pain in rats by inhibiting
spinal reactive astrocyte-mediated neuroinflammation through the
NF-κB pathway. Neurochem Res. 43:1058–1066. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Tao W, Luo X, Cui B, Liang D, Wang C, Duan
Y, Li X, Zhou S, Zhao M, Li Y, et al: Practice of traditional
Chinese medicine for psycho-behavioral intervention improves
quality of life in cancer patients: A systematic review and
meta-analysis. Oncotarget. 6:39725–39739. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yuan QL, Guo TM, Liu L, Sun F and Zhang
YG: Traditional Chinese medicine for neck pain and low back pain: A
systematic review and meta-analysis. PLoS One.
10(e0117146)2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Li Q, Yue N, Liu SB, Wang ZF, Mi WL, Jiang
JW, Wu GC, Yu J, Wang YQ, et al: Effects of chronic
electroacupuncture on depression- and anxiety-like behaviors in
rats with chronic neuropathic pain. Evid Based Complement Alternat
Med. 2014(158987)2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chen C, Lu M, Pan Q, Fichna J, Zheng L,
Wang K, Yu Z, Li Y, Li K, Song A, et al: Berberine improves
intestinal motility and visceral pain in the mouse models mimicking
diarrhea-predominant irritable bowel syndrome (IBS-D) symptoms in
an opioid-receptor dependent manner. PLoS One.
10(e0145556)2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Chen C, Tao C, Liu Z, Lu M, Pan Q, Zheng
L, Li Q, Song Z and Fichna J: A randomized clinical Trial of
berberine hydrochloride in patients with diarrhea-predominant
irritable bowel syndrome. Phytother Res. 29:1822–1827.
2015.PubMed/NCBI View
Article : Google Scholar
|
|
22
|
Tan W, Li Y, Chen M and Wang Y: Berberine
hydrochloride: Anticancer activity and nanoparticulate delivery
system. Int J Nanomedicine. 6:1773–1777. 2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kim SO and Kim HJ: Berberine ameliorates
cold and mechanical allodynia in a rat model of diabetic
neuropathy. J Med Food. 16:511–517. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Han AM, Heo H and Kwon YK: Berberine
promotes axonal regeneration in injured nerves of the peripheral
nervous system. J Med Food. 15:413–417. 2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Rezaee R, Monemi A, SadeghiBonjar MA and
Hashemzaei M: Berberine alleviates paclitaxel-induced neuropathy. J
Pharmacopuncture. 22:90–94. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Sisignano M, Baron R, Scholich K and
Geisslinger G: Mechanism-based treatment for chemotherapy-induced
peripheral neuropathic pain. Nat Rev Neurol. 10:694–707.
2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Majithia N, Loprinzi CL and Smith TJ: New
practical approaches to chemotherapy-induced neuropathic pain:
Prevention, assessment, and treatment. Oncology (Williston Park).
30:1020–1029. 2016.PubMed/NCBI
|
|
28
|
Liu M, Gao L and Zhang N: Berberine
reduces neuroglia activation and inflammation in
streptozotocin-induced diabetic mice. Int J Immunopathol Pharmacol.
33(2058738419866379)2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ghosh S and Karin M: Missing pieces in the
NF-kappaB puzzle. Cell. 109 (Suppl 1):S81–S96. 2002.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Vermeulen L, De Wilde G, Notebaert S,
Vanden Berghe W and Haegeman G: Regulation of the transcriptional
activity of the nuclear factor-kappaB p65 subunit. Biochem
Pharmacol. 64:963–970. 2002.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Lu L, Hu J, Wu Q, An Y, Cui W, Wang J and
Ye Z: Berberine prevents human nucleus pulposus cells from IL 1β
induced extracellular matrix degradation and apoptosis by
inhibiting the NF κB pathway. Int J Mol Med. 43:1679–1686.
2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Yoon SY and Oh J: Neuropathic cancer pain:
Prevalence, pathophysiology, and management. Korean J Intern Med
(Korean Assoc Intern Med). 33:1058–1069. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Carozzi VA, Canta A and Chiorazzi A:
Chemotherapy-induced peripheral neuropathy: What do we know about
mechanisms? Neurosci Lett. 596:90–107. 2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ahmed AS, Berg S, Alkass K, Druid H, Hart
DA, Svensson CI and Kosek E: NF-κB-associated pain-related
neuropeptide expression in patients with degenerative disc disease.
Int J Mol Sci. 20(20)2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Shih RH, Wang CY and Yang CM: NF-kappaB
signaling pathways in neurological inflammation: A mini review.
Front Mol Neurosci. 8(77)2015.PubMed/NCBI View Article : Google Scholar
|