Introduction
Cholangiocarcinoma (CCA) is a malignant tumor of the
bile duct epithelium. This disease has become a major public health
concern in Northeastern Thailand (1). Although the growth of CCA is quite
slow, the metastasis rate is very high (1). CCA is a rare primary liver tumor
worldwide, with an estimated 2,000-3,000 new cases occurring
annually in the United States (rate, 1-2 cases/100,000 population
between 1999-2014) (2). CCA occurs
with a high prevalence in Asia, especially in Northeastern
Thailand, where liver fluke Opisthorchis viverrini infection
is endemic (3). The 5-year survival
rates of patients with hilar, intrahepatic and distal extrahepatic
CCA receiving surgical intervention are 11-41, 22-44 and 27-37%,
respectively (4). The survival rate
of patients with CCA depends on the anatomical location and the
extent of metastasis (5). The
prognosis of patients with CCA is poor due to failure of early
diagnosis and the lack of an effective treatment for patients with
inoperable CCA (6). Therefore,
novel and effective chemotherapeutic agents for the treatment of
advanced and metastatic CCA are required.
Previous studies have suggested that cell invasion
and metastasis are associated with poor prognosis and increased
mortality rate in CCA (7,8). Mitogen-activated protein kinases
(MAPKs) influence different cellular processes, including gene
expression, mitosis, movement, metabolism and apoptosis (9). There are three major subfamilies of
MAPKs, including extracellular signal-regulated kinases 1/2 (ERK1
and ERK2), c-Jun NH2-terminal kinases (JNK1, JNK2 and JNK3) and
four p38 enzymes, namely p38α, p38β, p38δ and p38γ (9). The MAPK pathway serves an important
role in tumor development and progression via regulating several
cellular activities, such as cell apoptosis, proliferation and
differentiation (10). ERK 1/2 are
proline-directed kinases that are activated via coordinated
phosphorylation of tyrosine and threonine residues, resulting in
cell proliferation and malignant transformation (10). JNKs are activated in response to
environmental stressors, such as inflammatory cytokines, UV
irradiation and ischemia or membrane-bound receptor signaling
(11,12). Furthermore, JNKs regulate cell
viability and proliferation, and induction of apoptosis via
modulating the expression of AP-1, p53, c-Myc, nuclear factor
(NF)-κB, Sap-1 and Bcl-2 family members (11,12).
The p38 subfamily is a member of the MAPK family and is associated
with the development of CCA (13).
The activation of the p38 MAPK signaling pathway influences CCA
growth via maintaining the transformed cell phenotype in malignant
human biliary tract epithelial cells (14).
Natural products extracted from plants, have been
used for the prevention and/or treatment of cancer (15). Garcinia hanburyi is
distributed widely throughout Southeast Asia, including Cambodia
and Thailand (15). The latex of
G. hanburyi is used in Thai traditional medicine for the
treatment of potent purgative and infected wounds (15). Several caged xanthones, including
gambogic acid, isomorellin, isomorellinol, forbesione, morellic
acid, desoxygambogenin, hanburin, desoxymorellin and
dihydroisomorellin are extracted from the latex, fruits and whole
G. hanburyi plant (16-18).
Hahnvajanawong et al (19)
reported that four caged xanthones, namely isomorellin,
isomorellinol, gambogic acid and forbesione induced apoptosis in
CCA cell lines via the mitochondrial-dependent pathway. It has been
also reported that forbesione, alone or combined with 5-FU, may
strongly induce apoptosis in hamster CCA (Ham-1) cells in
vitro and in vivo (20).
Gambogic acid inhibits the invasion of highly invasive human breast
carcinoma (MDA-MB-435) cells via protein kinase C (PKC),
phosphorylation of ERK1/2 and JNK-mediated MMP-2 and -9 expression
(21). Furthermore, gambogic acid
has been demonstrated to inhibit human umbilical vascular
endothelial cell (HUVEC) proliferation, migration, invasion, tube
formation and microvessel growth, via inhibiting vascular
endothelial growth factor receptor (VEGF)-2, c-Src, focal adhesion
kinase (FAK) and AKT (22). Our
previous study revealed that four caged xanthones, namely
isomorellin, gambogic acid, forbesione and isomorellinol, exerted
no cytotoxic effect on human peripheral blood mononuclear cells
(19). Therefore, the present study
aimed to reveal the anti-invasive effect of isomorellin and its
underlying mechanism in the CCA cell line KKU-100.
Materials and methods
Materials
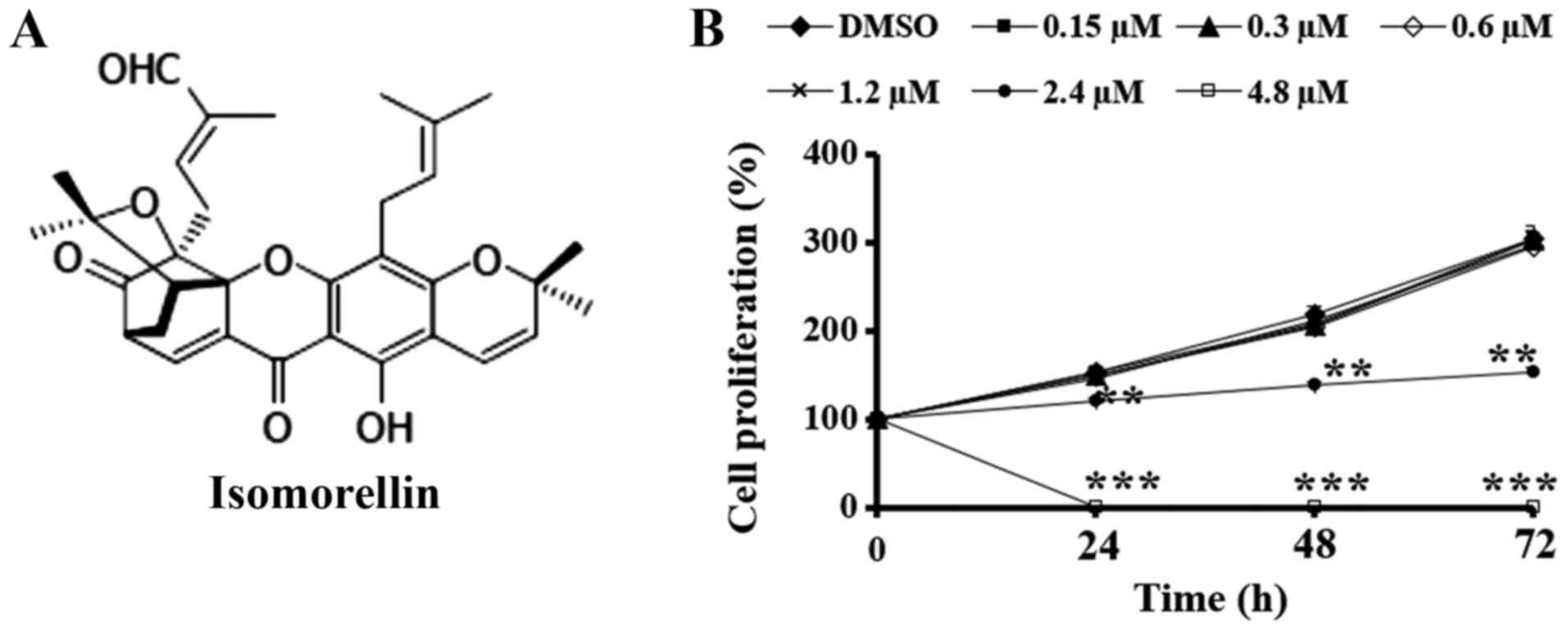
Isomorellin (Fig.
1A) was extracted from G. hanburyi Hook. f. (family
Guttiferae) using bioassay-directed fractionation and
provided by Professor Vichai Reutrakul, Department of Chemistry,
Faculty of Science, Mahidol University (Bangkok, Thailand) as
previously described (23). The
stock solution of isomorellin was prepared by dissolving in DMSO to
a concentration 1.8 mM and stored at -20˚C.
Antibodies against PKC (cat. no. sc-17804; 1:500),
FAK (cat. no. sc-558; 1:400), phosphorylated (p)-FAK (cat. no.
sc-374668; 1:200), ERK1/2 (cat. no. sc-514302; 1:400), p-ERK1/2
(cat. no. sc-7383; 1:400), JNK1/2 (cat. no. sc-7345; 1:400),
p-JNK1/2 (cat. no. sc-6254; 1:400), p38 (cat. no. sc-7149; 1:400),
p-p38 (cat. no. sc-7973; 1:400), NF-κB/p65 (cat. no. sc-109;
1:500), MMP-2 (cat. no. sc-53630; 1:400), COX-2 (cat. no.
sc-376861; 1:500) and HRP-conjugated secondary antibodies
(anti-mouse (cat. no. sc-2005; 1:10,000) or anti-rabbit (cat. no.
sc-2004; 1:10,000) and were sourced from Santa Cruz Biotechnology,
Inc. Primary antibody against β-actin (cat. no. A1978; 1:3,000) was
obtained from Sigma-Aldrich (Merck KGaA) and antibodies against
histone-H1 (cat. no. ab11079; 1:10,000) were purchased from Abcam.
The BD Biocoat Matrigel invasion chamber was purchased from Becton
Dickinson and Company, with 6.5 mm diameter polycarbonate membranes
(8-µm pore size) and coated with Matrigel (Becton Dickinson and
Company). The proteolytic enzyme standard of human gelatinases A
(MMP-2; cat. no. CC071), B (MMP-9; cat. no. CC079) and uPA (cat.
no. CC4000) was purchased from Chemicon International (Thermo
Fisher Scientific, Inc.).
Cell culture
The human CCA cell line KKU-100 was established at
the Department of Pathology, Faculty of Medicine, Khon Kaen
University, Thailand. KKU-100 cells were maintained in RPMI-1640
medium containing 10% heat-inactivated fetal bovine serum (FBS)
(Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and
100 µg/ml streptomycin and cultured at 37˚C in a humidified
incubator containing 5% CO2.
Cell viability assay
The sulforhodamine B (SRB) assay (cat. no.
3520-42-1; Sigma-Aldrich; Merck KGaA) was performed as previously
described (24). Briefly, KKU-100
cell suspension was seeded into 96-well plates at a density of
1x105 cells per well. Following 24-h incubation at 37˚C,
cells were treated with isomorellin at final concentrations of 0,
0.15, 0.3, 0.6, 1.2, 2.4 and 4.8 µM for 24, 48 and 72 h, and
subsequently the effect of isomorellin on KKU-100 cells was
determined using the SRB assay. The IC50 values were
obtained using concentration-effect curves following linear
regression analysis.
Wound healing assay
Wound healing was performed according to a procedure
described by Lu et al (25)
with certain modifications. Briefly, KKU-100 cells
(5x105 cells/ml) were seeded into 6-well plates and
cultured to 100% confluency. The denuded zone (gap) was generated
using a 1 ml plastic pipette tip. Subsequently, cells were starved
in 1% FBS medium with or without isomorellin (0, 0.3, 0.6 and 1.2
µM) for 12 h. The wound area was digitally imaged using a
microscope at x10 magnification (Eclipse Ni-U microscope; Nikon
Corporation). Finally, the area of the wound closure was calculated
using the following formula: (Area of original wound - Area of
wound during healing)/Area of original wound.
Chamber invasion assay
The Transwell invasion chamber assay was performed
as previously described by Albini et al (26). A total of 400 µl (KKU-100 cells)
cell suspension in 10% FBS (density, 1.25x105 cells/ml)
and 100 µl of isomorellin (0, 0.3, 0.6 and 1.2 µM) were added into
the upper chamber of a Matrigel invasion chamber. The medium
containing 10% FBS was added into the lower chamber as a
chemoattractant. Invasion chambers were incubated for 24 h at 37˚C,
fixed with 4% paraformaldehyde for 30 min at room temperature and
stained with 0.4% SRB for 30 min at room temperature. Cells on each
membrane were counted under a light microscope and digital images
were captured at low-power fields (magnification, x20).
Gelatin zymography and uPA assay
The activity of MMP-2, MMP-9 and uPA was determined
using gelatinase zymography assay as previously described, with
certain modifications (27). The
concentration of the conditioned medium in the homogenate was
determined using Bradford reagent (28). Briefly, cells (density,
1.5x105 cells/well) were seeded into 6-well plates and
incubated at 37˚C for 24 h. Cell monolayers were washed with PBS
and serum-free medium, and cultured in serum-free medium containing
various concentrations of isomorellin (0, 0.3, 0.6 and 1.2 µM) for
72 h at 37˚C. For the gelatin zymography assay, a total of 50 µg
protein lysate was separated in a 10% polyacrylamide gel
supplemented with 1 mg/ml gelatin, while a 10% polyacrylamide gel
supplemented with 1 mg/ml gelatin (cat. no. 9000-70-8;
Sigma-Aldrich; Merck KGaA) and 20 µg/ml plasminogen (cat. no.
528213; Sigma-Aldrich; Merck KGaA) was used for uPA assay. Gels
were washed with 2.5% Triton X-100 and then incubated at 37˚C in a
50 mM Tris-HCl buffer containing 5 mM CaCl2 and 0.02%
NaN3 for 12 h. The gel was stained overnight and
discolored using 0.5% Coomassie blue and 10% acetic acid,
respectively. The protease activity bands of MMP2, MMP-9 and uPA
were imaged using a gel documentation system (Bio-Rad Laboratories,
Inc.) and analyzed using Scion Image software (version 4.0.2; Scion
Corporation).
Total cell, cytosol, nuclear and
membrane protein extraction
Protein extraction was carried out according to the
procedure of Wattanawongdon et al (29). To prepare total cell lysates, cells
(density, 1x106 cells/dish) were seeded and then treated
with isomorellin (0, 0.3, 0.6 and 1.2 µM) at 37˚C for 24 h.
Subsequently, cells were rinsed and lysed with cold RIPA buffer (50
mM Tris-HCl, pH 7.5, 0.5% Nonidet P-40, 150 mM NaCl, 1 mM
dithiothreitol, 1 mM EDTA, 0.1% sodium dodecyl sulfate and 0.5%
deoxycholate) supplemented with protease and phosphatase inhibitor
cocktails (cat. no. 78446; Pierce Biotechnology Inc.). The
supernatants were collected following centrifugation at 13,000 x g
at 4˚C for 30 min. For the cytosolic and nuclear proteins, cells
were extracted using 500 µl buffer A (10 mM HEPES, 0.1 mM EDTA, 10
mM KC1, 0.2% NP40, 1.5 mM MgCl2, 1 mM DTT and 0.5 mM
phenylmethylsulfonyl fluoride) at 4˚C for 30 min, followed by
vortexing to shear the cytoplasmic membranes. The cell nuclei were
collected by centrifugation at 1,000 x g for 30 min at 4˚C and
nuclear proteins were extracted with 200 µl of high-salt buffer B
(20 mM HEPES, 25% glycerol, 1.5 mM MgCl2, 0.1 mM EDTA,
420 mM NaCl, 1 mM DTT and 0.5 mM phenylmethylsulfonyl fluoride) at
4˚C for 30 min. Finally, membrane proteins were extracted using
translocation buffer containing 0.1% Triton X-100, agitated at 4˚C
overnight and then centrifuged at 13,000 x g for 30 min. The
protein concentration was quantified using the Bradford method
(28).
Western blot analysis
Proteins (5 µg protein/lane) were fractionated in a
12% SDS-PAGE and electrotransferred onto a nitrocellulose membrane
(EMD Millipore). Following blocking with 5% skimmed milk dissolved
in Tris-buffered saline containing 0.1% Tween-20 at 37˚C for 1 h,
the membranes were probed with primary antibodies against PKC, FAK,
p-FAK, ERK1/2, p-ERK1/2, JNK1/2, p-JNK1/2, p38, p-p38, NF-κB-p65,
COX-2, MMP-2, β-actin or histone H1 at 4˚C overnight and then
incubated with HRP conjugated secondary antibodies (anti-mouse or
anti-rabbit) at room temperature for 1 h. The blots were visualized
using an enhanced chemiluminescence kit (Pierce Biotechnology
Inc.), quantified using densitometry (Image Quant LAS 4000; GE
Healthcare Bio-Sciences) and assessed using the Scion Image
software (version 4.0.2; Scion Corporation). The relative
intensities of total cell, cytosolic and membrane protein
expression were normalized to β-actin, while the protein expression
of the nuclear lysate was normalized to histone H1.
Statistical analysis
Data were expressed as the mean ± standard deviation
(SD) from three independent experiments. Comparisons between
untreated control cells and treated cells were made using Tukey's
post hoc test in the SPSS statistical software, version 16.0 (SPSS,
Inc.). Differences were considered significant at
*P<0.05, **P<0.01 and
***P<0.001.
Results
Isomorellin reduces the viability of
the CCA cell line KKU-100
The ability of isomorellin to inhibit KKU-100 cell
viability was assessed using an SRB assay. Following treatment with
2.4 and 4.8 µM of isomorellin for 24, 48 and 72 h, KKU-100 cell
viability was decreased in a dose-dependent manner (Fig. 1B) and the IC50 values at
24, 48 and 72 h were 3.46±0.19, 3.78±0.02 and 4.01±0.01 µM,
respectively.
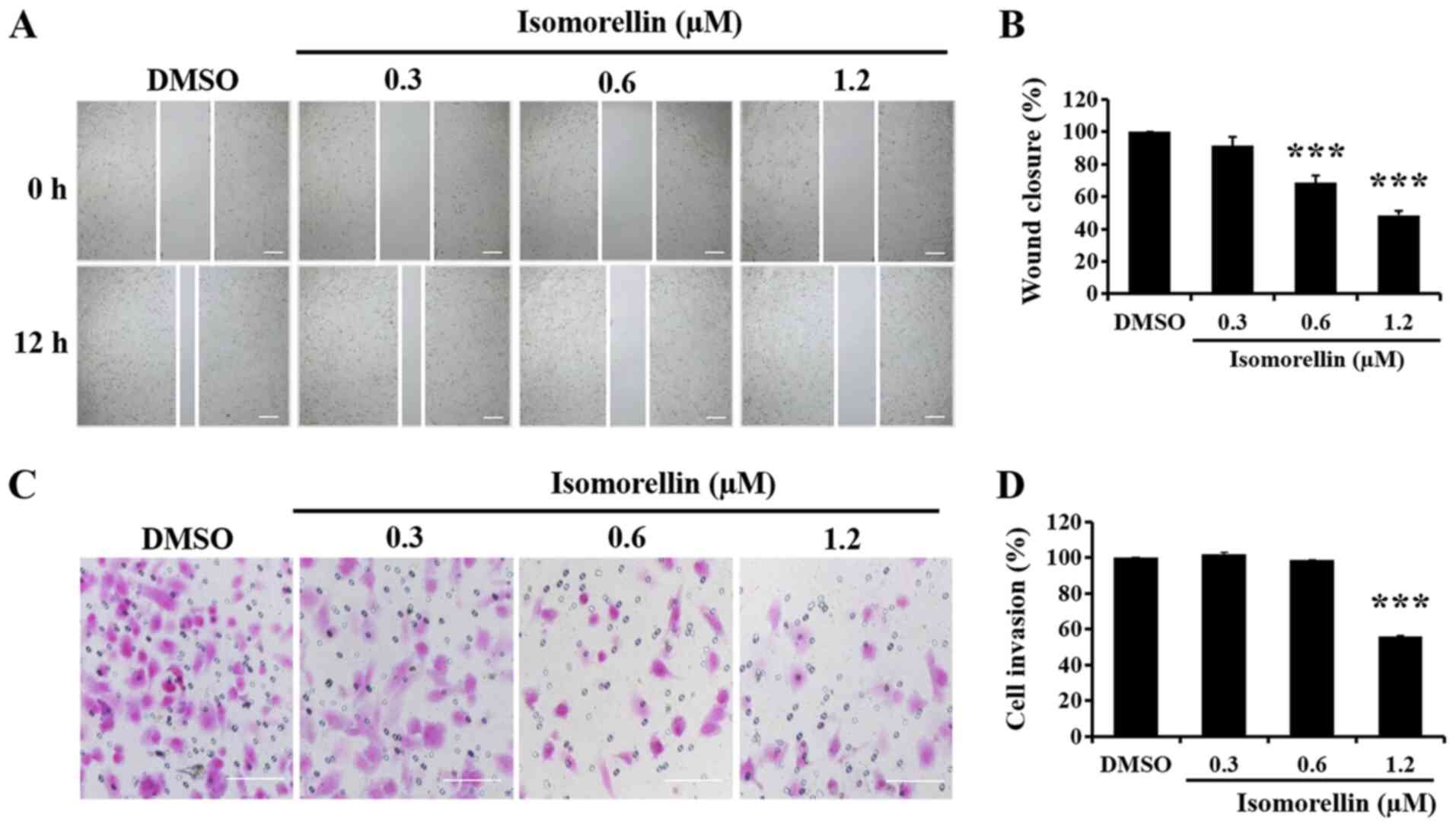
Isomorellin reduces KKU-100 cell
migration and invasion ability
The effect of isomorellin on KKU-100 cell migration
ability was evaluated using a wound healing assay. Compared with
the control group, isomorellin (0.6 and 1.2 µM) significantly
inhibited the migration of KKU-100 cells into the wound area in a
dose-dependent manner (P<0.001; Fig.
2A and B). Furthermore, an
invasion chamber assay was performed to determine the effect of
isomorellin on the invasion ability of KKU-100 cells. Isomorellin
(1.2 µM) significantly inhibited the penetration of the
Matrigel-coated filter by KKU-100 cells in a dose-dependent manner
(P<0.001; Fig. 2C and D).
Effect of isomorellin on the activity
of FAK, PKC and downstream MAPK pathway, and translocation of NF-κB
and IκB-α transcription factors
As revealed in Fig.
3, isomorellin significantly downregulated p-FAK (P<0.05),
COX-2 (P<0.05), whole (P<0.01) and membrane PKC (P<0.01)
compared with DMSO-treated control cells (Fig. 3A-D). Furthermore, FAK (P<0.001)
and cytosolic PKC (P<0.01) expression was significantly
increased in isomorellin-treated KKU-100 cells compared with the
control (Fig. 3A and B). Additionally, treatment with
isomorellin (1.2 µM) significantly decreased and increased the
protein levels of p-p38 (P<0.001) and p38 (P<0.001),
respectively, in a dose-dependent manner (Fig. 3E and F). However, isomorellin did not affect the
expression of ERK1/2, p-ERK1/2, JNK1/2 and p-JNK1/2 (Fig. 3E and F). Finally, NF-κB expression was
significantly downregulated in total cell (P<0.001; 0.6 and 1.2
µM), cytosolic (P<0.01; 1.2 µM) and nuclear fractions
(P<0.001; 1.2 µM), and IκB-α was upregulated in total cell
(P<0.001; 0.3, 0.6 and 1.2 µM) and cytosolic lysates
(P<0.001; 1.2 µM) of isomorellin-treated KKU-100 cells at 1.2 µM
(Fig. 4A-C). These findings
indicated that isomorellin inhibited the translocation of NF-κB to
the nucleus in a dose-dependent manner.
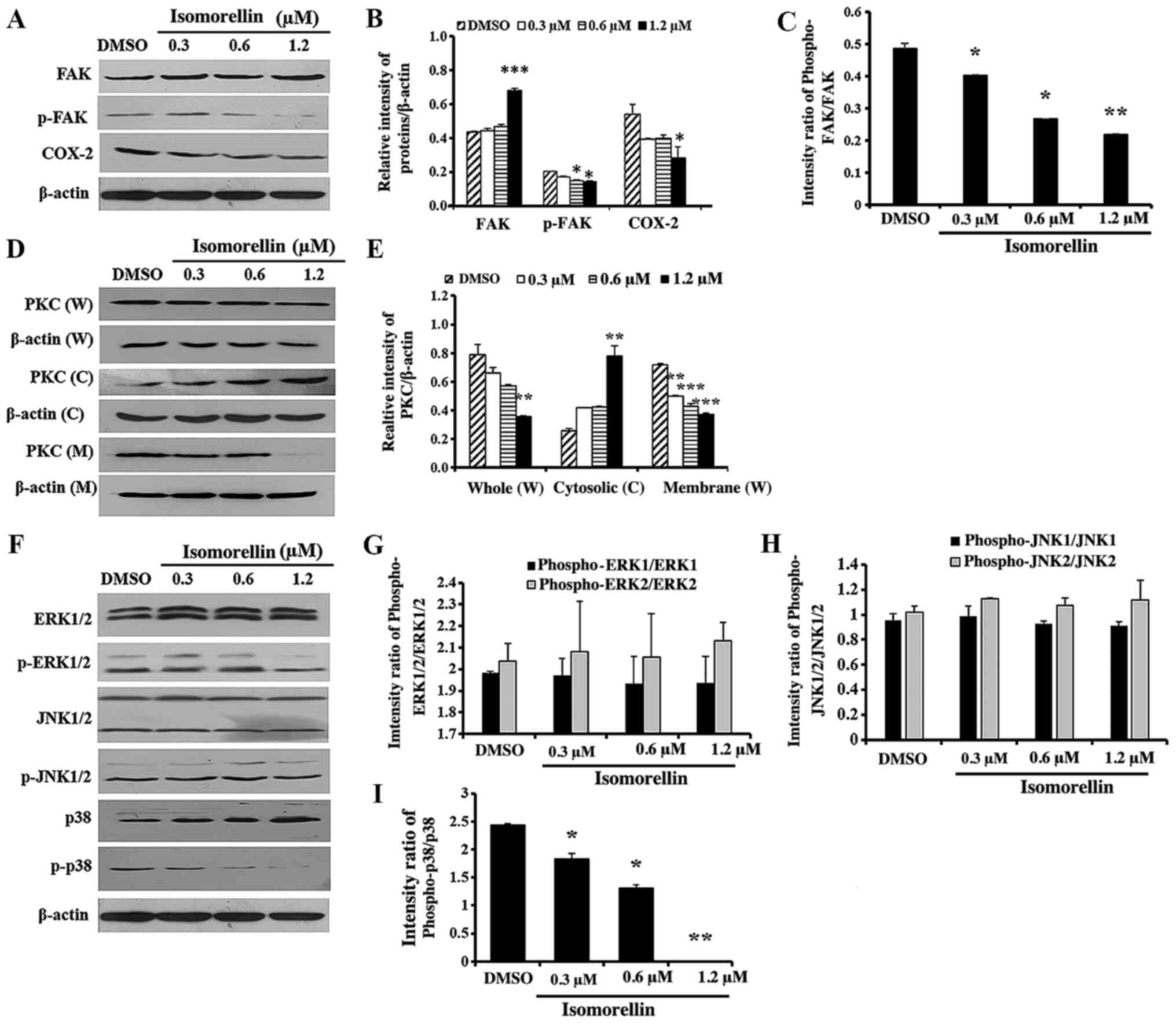 | Figure 3Effect of isomorellin on the
expression of FAK, COX-2, PKC and downstream MAPK pathway in
KKU-100 cells. (A) Western blot analysis, (B) quantification of the
relative intensity of FAK, COX-2 expression and (C) quantification
of the intensity ratio of phospho-FAK/FAK. (D) Western blot
analysis and (E) quantification of PKC in W, C and M protein
extracts. (F) Western blot analysis and (G-I) quantification of the
intensity ratio of phosho-ERK1/2-ERK1/2, the intensity ratio of
Phospho-JNK1/2/JNK1/2 and the intensity ratio of phospho-p38/p38.
The protein expression levels were normalized to β-actin and
expressed as relative intensity. Data are expressed as the mean ±
SD of three independent experiments. *P<0.05,
**P<0.01, ***P<0.001 vs. DMSO-treated
group. FAK, focal adhesion kinase; COX-2, cyclooxygenase-2; PKC,
protein kinase C; MAPK, mitogen-activated protein kinase; ERK1/2,
signal-regulated kinases 1/2; p-ERK1/2, phosphorylated ERK1/2;
JNK1/2, c-Jun NH2-terminal kinases 1/2; SD, standard deviation;
DMSO, dimethyl sulfoxide; W, whole cell; C, cytosolic; M, membrane;
phosphor, phosphorylated. |
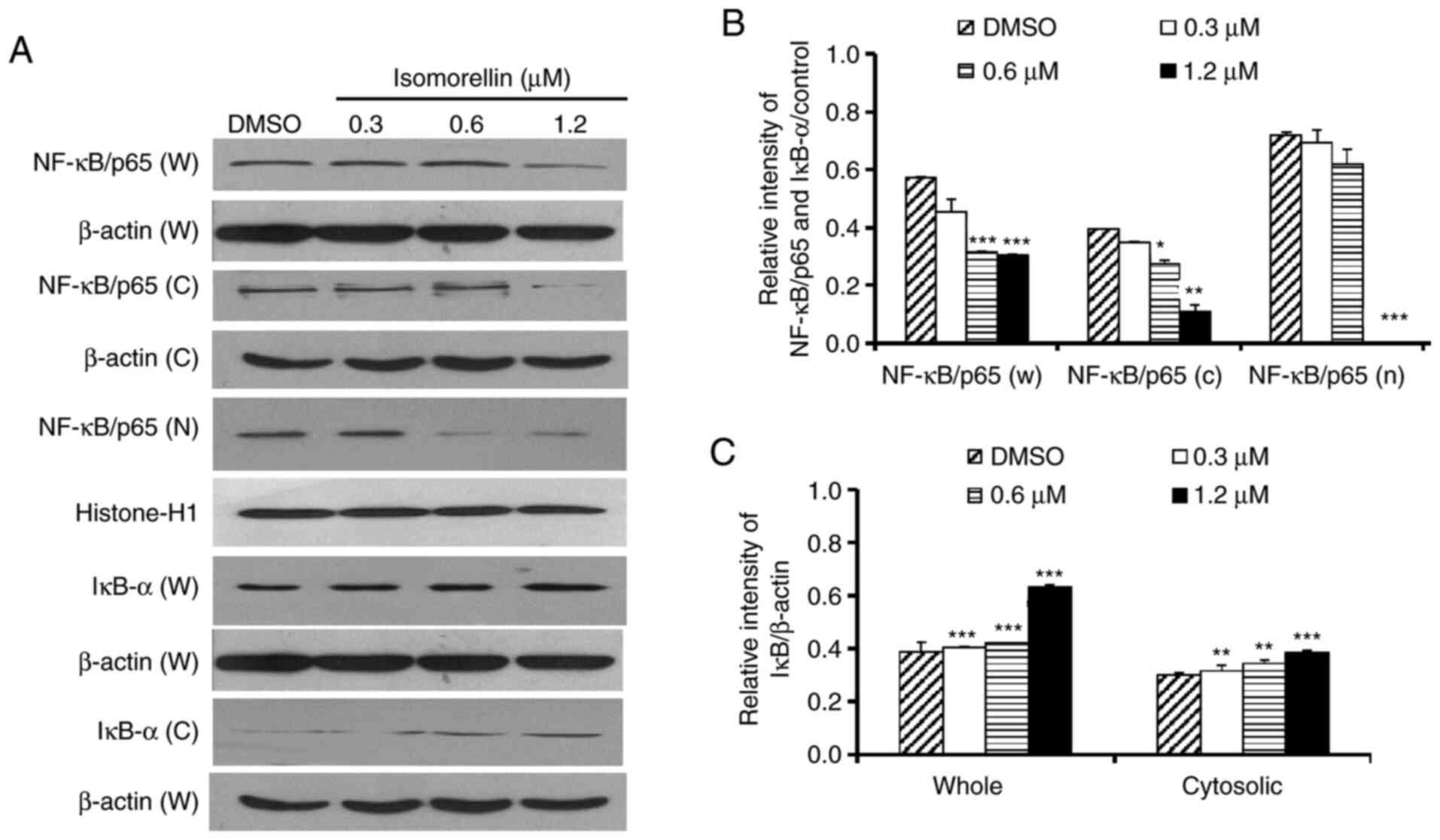 | Figure 4Isomorellin inhibits the expression
of the transcriptional factors NF-κB/p65 and IκB-α in KKU-100
cells. Cells were treated with different concentrations of
isomorellin (0, 0.3, 0.6 and 1.2 µM) for 24 h. (A) The protein
expression levels of the transcription factor NF-κB/p65 in W, C and
N and IκB-α W and C were detected via western blot analysis and
normalized to β-actin for total cell and cytosol lysates, and
histone-H1 for nuclear lysates. Quantification of (B) NF-κB/p65
blots and (C) IκB-α blots. Data are presented as the relative
intensity and expressed as the mean ± SD of three independent
experiments. *P<0.05, **P<0.01,
***P<0.001 vs. DMSO-treated group. NF-κB, nuclear
factor κB; IκB-α, NF-κB inhibitor α; SD, standard deviation; DMSO,
dimethyl sulfoxide; W, whole cell; C, cytosolic; M, membrane. |
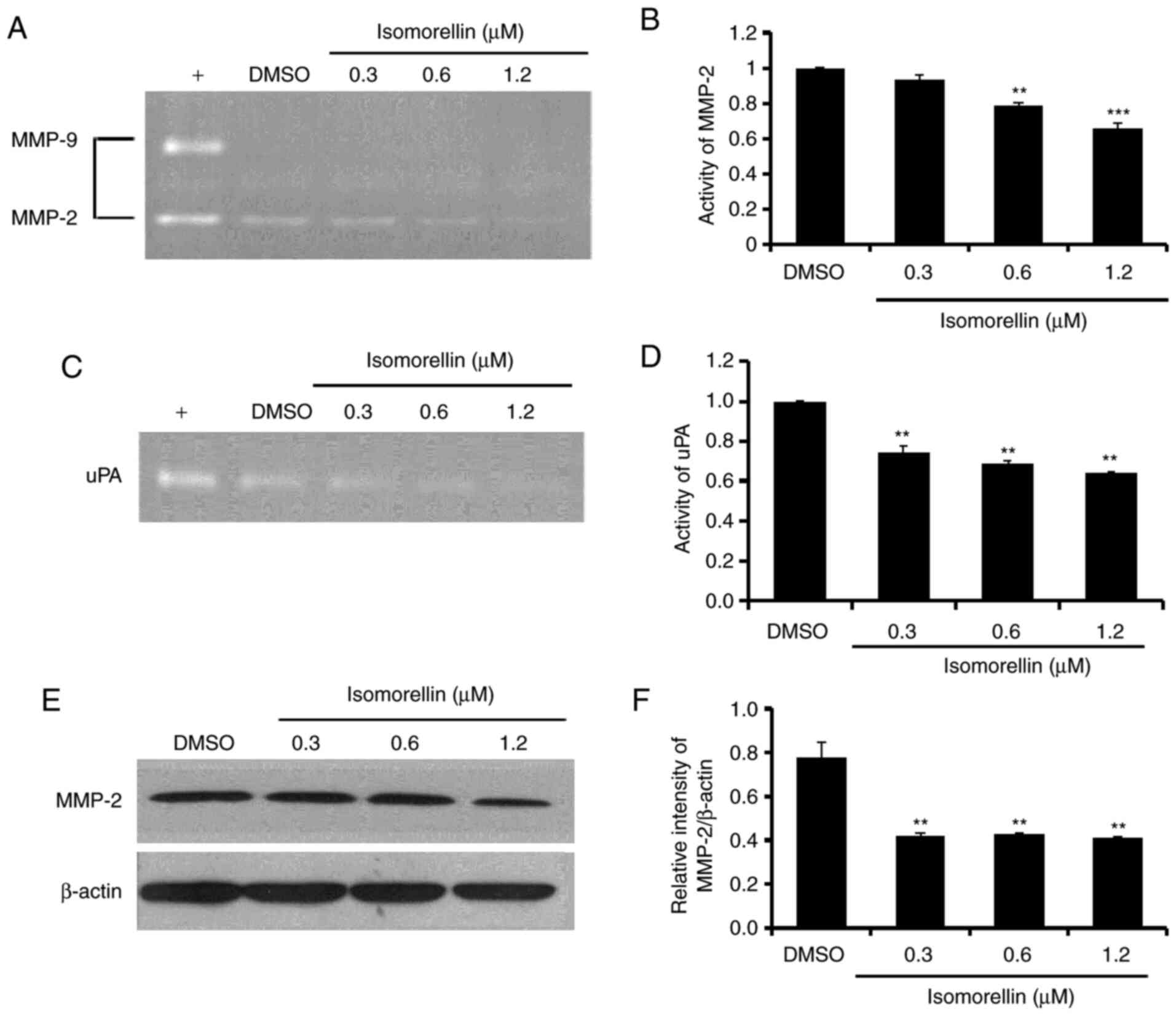
Isomorellin reduces the activity and
expression of MMP-2 and uPA in KKU-100 cells
It has been reported that the activity of the
proteolytic enzymes MMP-2 and uPA serve a critical role in cancer
cell invasion and metastasis (30).
The results indicated that isomorellin significantly reduced
KKU-100 cell migration and invasion abillities, while DMSO had no
effect on the activity of MMP-2 and uPA (Fig. 5). Therefore, the underlying
mechanism of the impaired secretion of MMP and uPA was further
investigated. Treatment with isomorellin (1.2 µM) significantly
reduced the activity of MMP-2 (P<0.001) and uPA (P<0.01)
(Fig. 5A-D). Furthermore,
isomorellin significantly downregulated MMP-2 protein expression in
KKU-100 cells (P<0.01) (Fig. 5E
and F).
Discussion
The present study provided evidence that isomorellin
reduced KKU-100 cell migration and invasion via downregulating FAK,
PKC and p-p38 MAPK pathway expression. In addition, isomorellin
inhibited the translocation of NF-κB to the nucleus, thus resulting
in decreased activity and expression of MMP-2 and uPA. The present
study suggested that the mechanism underlying the inhibitory
effects of isomorellin was associated with the inhibition of the
transcription factor NF-κB, the downstream MAPK signaling
transduction pathway, MMPs and uPA, thus leading to decreased
migration and invasion abilities of CCA cancer cells (Fig. 6).
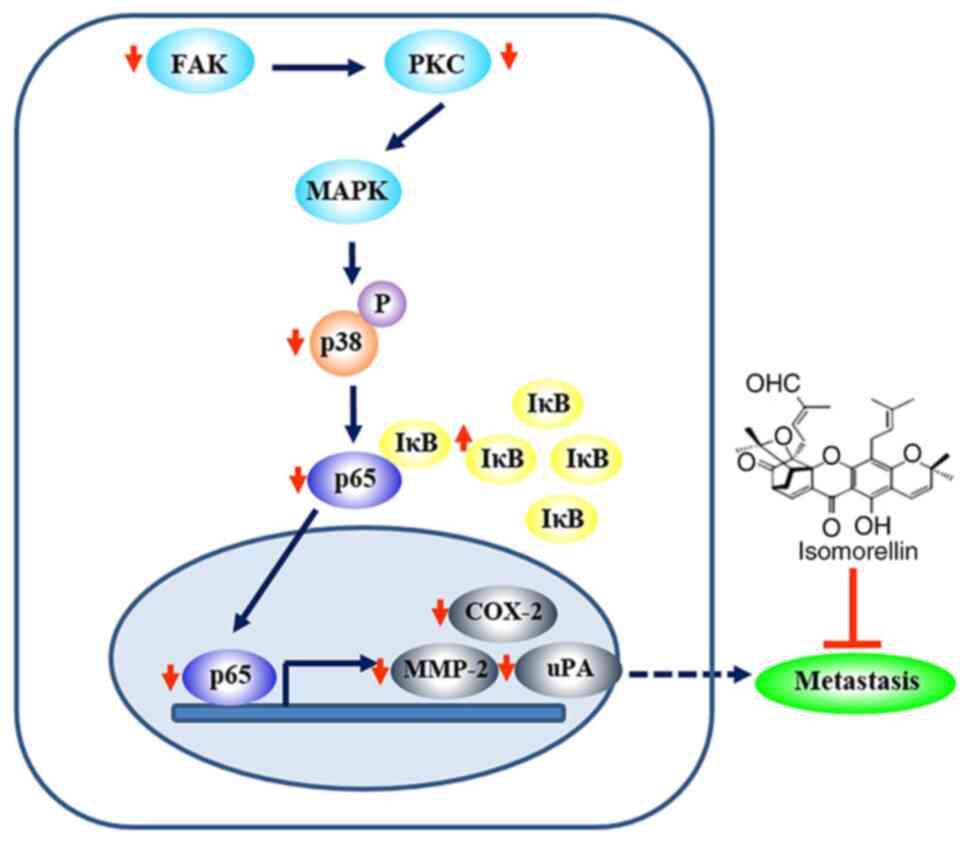 | Figure 6Possible signaling pathway involved
in the isomorellin-mediated inhibition of CCA cell migration and
invasion. Isomorellin inhibits the FAK, PKC and p38 MAPK pathway,
which in turn induces IκBα expression and inhibits the nuclear
translocation of NF-κB/p65, thus resulting in downregulation of
MMP-2, uPA and COX-2 expression in CCA. CCA, cholangiocarcinoma;
FAK, focal adhesion kinase; PKC, protein kinase C; MAPK,
mitogen-activated protein kinase; IκBα, NF-κB inhibitor α; NF-κB,
nuclear factor κB; MMP-2, metalloproteinase-2; uPA, urokinase-type
plasminogen activator; COX-2, cyclooxygenase-2. |
In recent years, there has been growing use of
natural products for medicinal purposes, including anticancer
treatment (20,31,32).
This may be due to the fact that many plants contain a variety of
active phytochemicals, including flavonoids, terpenoids, lignans,
sulfides, polyphenolics, carotenoids, coumarins, saporins, plant
sterol, curcumins and phthalides, which have been reported to
exhibit chemoprotective properties against cancer (33). These active phytochemicals serve an
important role in several processes, including antioxidation,
electrophile scavenging, immune response activation, induction of
detoxification enzymes and inhibition of hormonal function and
metabolic pathways in carcinogenesis (33). Recently, a novel approach in cancer
therapy targeting the metastatic process has been developed. For
example, the anti-metastatic properties of resveratrol
(3,4',5-trihydroxystilbene) isolated from Polygonum species
(Polygonaceae family) have been reported (34). Therefore, a previous study
demonstrated that resveratrol inhibited HUVEC growth via reducing
the gelatinolytic activitiy of MMP-2, tube formation, and
endothelial cell attachment to fibronectin and laminin (34). In addition, it has been demonstrated
that nobiletin, isolated from citrus fruits, exerts inhibitory
effects on highly metastatic human AGS gastric adenocarcinoma cell
adhesion, invasion and migration at non-cytotoxic concentrations
(35). Furthermore, curcumin,
isolated from Curcuma longa, decreases cell proliferation,
invasion, angiogenesis and metastasis in different types of cancer
via interacting with several cell signaling proteins, such as
NF-κB, AP-1 and the MAPK signaling pathway (36). Boueroy et al (37) demonstrated that rhinacanthin-C,
extracted from Rhinacanthus nasutus (L.), inhibited CCA cell
growth and metastasis via suppressing the expression of MMP-2, uPA,
FAK and the downstream MAPK pathways. Our previous study reported
that isomorellin inhibited cholangiocarcinoma cell lines by
apoptotic induction in both cell lines (KKU-100 and KKU-M156),
indicating a broad spectrum of anticancer activities (19). One limitation of current study is
that it only reported the effect of isomorellin against one CCA
cell line (KKU-100). However, the inhibition of migration and
invasion effects were first demonstrated in the present study. For
the evaluation of wound closure potential of compounds, cells were
starved for minimizing proliferation and the degree of serum
starving has to be worked out for each cell type examined (38). Many studies reported the starvation
of cells in media containing 1% FBS for depletes growth factors
that could influence the cell migration (39,40).
FAK is a signal transduction molecule associated
with the invasive and metastatic potential of different types of
cancer (41,42). FAK initiates a cascade of
intracellular signals in response to adhesion, including activation
of the MAPK pathway (43). PKC is a
family of enzymes that serves an important role in signal
transduction pathways associated with the regulation of hormone
release, mitogenesis and tumor promotion (44,45).
Furthermore, the MAPK signaling pathway is involved in the enhanced
invasive and metastatic ability of cancer (46). Additionally, it has been reported
that the expression of MMP-2, MMP-9 and uPA inhibits the p-p38 MAPK
pathway (46). MMPs serve a key
role in the degradation of the extracellular matrix (ECM) and are
involved in cell proliferation, migration, invasion and metastasis
(30). Two classes of MMPs, namely
MMP-2 and MMP-9, serve a key role in cancer cell invasion and
metastasis via degrading type IV collagen, which is a major
component of the basement membrane (30). The expression and activity of NF-κB,
a transcription factor, mediates cancer cell proliferation,
invasion, angiogenesis and metastasis (47,48).
Furthermore, the PI3K-AKT and RAS-MAPK pathways regulate the
overexpression of NF-κB (49).
Therefore, the aforementioned findings indicate that translocation
and downregulation of NF-κB, which are regulated by the inhibition
of the p-p38/MAPK pathway, may mediate the decreased expression and
activity of MMP-2 and uPA.
The expression of matrix metalloproteinases (MMP-2
and MMP-9) and COX-2 is also promoted by NF-κB (50). COX-2 serves a crucial role in cancer
metastasis and is associated with the destruction of the ECM,
initiation of epithelial-mesenchymal transition (EMT) and
angiogenesis (51). Overexpression
of COX-2 has been reported in different types of human cancer,
including breast cancer, lung cancer, colon cancer and CCA
(52,53). Herein, the inhibitory effect of
isomorellin on cancer cell migration and invasion was mediated by
inhibition of NF-κB expression and translocation, resulting in
decreased expression of MMP-2, uPA and COX-2. The present study
also demonstrated that isomorellin significantly decreased the
expression of FAK, PKC and the p-p38 MAPK pathway, which are
involved in the upstream signal transduction of NF-κB, resulting in
reduced activity and protein expression of MMP-2, uPA and COX-2 in
KKU-100 cells.
In conclusion, isomorellin significantly inhibited
cancer cell migration and invasion abilities via FAK, PKC, and the
p-p38 MAPK and NF-κB pathways, thus leading to reduced expression
levels of MMP-2, uPA and COX-2. Inhibition of MMP-2, uPA and COX-2
may result in decreased CCA cell invasion ability. Therefore,
isomorellin may represent a promising therapeutic drug for the
treatment of advanced and metastatic CCA. However, further studies
should be conducted using in vivo models and clinical
trials.
Acknowledgements
The authors would like to acknowledge Dr Andrew
Warner of the Kasetsart University Research and Development
Institute (KURDI), Bangkok, Thailand for assistance with English
editing.
Funding
The present study was supported in part by grants
from the Faculty of Medicine, Khon Kaen University (grant no.
I54230; Khon Kaen, Thailand).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CH designed the experiments. TS performed the
experiments. VR performed isomorellin separation. TB and AK
performed data analysis. PB analyzed the data and wrote and
reviewed the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Olnes MJ and Erlich R: A review and update
on cholangiocarcinoma. Oncology. 66:167–179. 2004.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Yao KJ, Jabbour S, Parekh N, Lin Y and
Moss RA: Increasing mortality in the United States from
cholangiocarcinoma: An analysis of the National Center for Health
Statistics Database. BMC Gastroenterol. 16(117)2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Landis SH, Murray T, Bolden S and Wingo
PA: Cancer statistics, 1998. CA Cancer J Clin. 48:6–29.
1998.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hasegawa S, Ikai I, Fujii H, Hatano E and
Shimahara Y: Surgical resection of hilar cholangiocarcinoma:
Analysis of survival and postoperative complications. World J Surg.
31:1256–1263. 2007.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Khan SA, Davidson BR, Goldin RD, Heaton N,
Karani J, Pereira SP, Rosenberg WM, Tait P, Taylor-Robinson SD,
Thillainayagam AV, et al: British Society of Gastroenterology:
Guidelines for the diagnosis and treatment of cholangiocarcinoma:
An update. Gut. 61:1657–1669. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hopfner M, Schuppan D and Scherubl H:
Targeted medical therapy of biliary tract cancer: Recent advances
and future perspectives. World J Gastroenterol. 14:7021–7032.
2008.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Leelawat K, Leelawat S, Tepaksorn P,
Rattanasinganchan P, Leungchaweng A, Tohtong R and Sobhon P:
Involvement of c-Met/hepatocyte growth factor pathway in
cholangiocarcinoma cell invasion and its therapeutic inhibition
with small interfering RNA specific for c-Met. J Surg Res.
136:78–84. 2006.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Leelawat K, Leelawat S, Narong S and
Hongeng S: Roles of the MEK1/2 and AKT pathways in CXCL12/CXCR4
induced cholangiocarcinoma cell invasion. World J Gastroenterol.
13:1561–1568. 2007.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Johnson GL and Lapadat R:
Mitogen-activated protein kinase pathways mediated by ERK, JNK, and
p38 protein kinases. Science. 298:1911–1912. 2002.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Li Q and Yang Z: Expression of
phospho-ERK1/2 and PI3-K in benign and malignant gallbladder
lesions and its clinical and pathological correlations. J Exp Clin
Cancer Res. 28(65)2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kaiser RA, Liang Q, Bueno O, Huang Y,
Lackey T, Klevitsky R, Hewett TE and Molkentin JD: Genetic
inhibition or activation of JNK1/2 protects the myocardium from
ischemia-reperfusion-induced cell death in vivo. J Biol Chem.
280:32602–32608. 2005.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wang XT, Pei DS, Xu J, Guan QH, Sun YF,
Liu XM and Zhang GY: Opposing effects of Bad phosphorylation at two
distinct sites by Akt1 and JNK1/2 on ischemic brain injury. Cell
Signal. 19:1844–1856. 2007.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Cuenda A and Rousseau S: p38 MAP-kinases
pathway regulation, function and role in human diseases. Biochim
Biophys Acta. 1773:1358–1375. 2007.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yamagiwa Y, Marienfeld C, Tadlock L and
Patel T: Translational regulation by p38 mitogen-activated protein
kinase signaling during human cholangiocarcinoma growth.
Hepatology. 38:158–166. 2003.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Saralamp P, Chuakul W, Temsiririrkkul R
and Clayton T: Medicinal plants in Thailand. Vol. 1. Amarin
Printing and Publishing Public Co., Ltd Bangkok, pp209-211,
1996.
|
|
16
|
Asano J, Chiba K, Tada M and Yoshii T:
Cytotoxic xanthones from Garcinia hanburyi. Phytochemistry.
41:815–820. 1996.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Sukpondma Y, Rukachaisirikul V and
Phongpaichit S: Antibacterial caged-tetraprenylated xanthones from
the fruits of Garcinia hanburyi. Chem Pharm Bull (Tokyo).
53:850–852. 2005.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Han QB, Wang YL, Yang L, Tso TF, Qiao CF,
Song JZ, Xu LJ, Chen SL, Yang DJ and Xu HX: Cytotoxic
polyprenylated xanthones from the resin of Garcinia
hanburyi. Chem Pharm Bull (Tokyo). 54:265–267. 2006.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hahnvajanawong C, Boonyanugomol W,
Nasomyon T, Loilome W, Namwat N, Anantachoke N, Tassaneeyakul W,
Sripa B, Namwat W and Reutrakul V: Apoptotic activity of caged
xanthones from Garcinia hanburyi in cholangiocarcinoma cell
lines. World J Gastroenterol. 16:2235–2243. 2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Boueroy P, Hahnvajanawong C, Boonmars T,
Saensa-ard S, Wattanawongdon W, Kongsanthia C, Salao K, Wongwajana
S, Anantachoke N and Reutrakul V: Synergistic effect of forbesione
from Garcinia hanburyi in combination with 5-fluorouracil on
cholangiocarcinoma. Asian Pac J Cancer Prev. 18:3343–3351.
2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Qi Q, Lu N, Wang XT, Gu HY, Yang Y, Liu W,
Li C, You QD and Guo QL: Anti-invasive effect of gambogic acid in
MDA-MB-231 human breast carcinoma cells. Biochem Cell Biol.
86:386–395. 2008.PubMed/NCBI View
Article : Google Scholar
|
|
22
|
Gupta SC, Kim JH, Prasad S and Aggarwal
BB: Regulation of survival, proliferation, invasion, angiogenesis,
and metastasis of tumor cells through modulation of inflammatory
pathways by nutraceuticals. Cancer Metastasis Rev. 29:405–434.
2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Reutrakul V, Anantachoke N, Pohmakotr M,
Jaipetch T, Sophasan S, Yoosook C, Kasisit J, Napaswat C, Santisuk
T and Tuchinda P: Cytotoxic and anti HIV 1 caged xanthones from the
resin and fruits of garcinia hanburyi. Planta Med. 73:33–40.
2007.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Pinmai K, Chunlaratthanabhorn S,
Ngamkitidechakul C, Soonthornchareon N and Hahnvajanawong C:
Synergistic growth inhibitory effects of Phyllanthus emblica
and Terminalia bellerica extracts with conventional
cytotoxic agents: Doxorubicin and cisplatin against human
hepatocellular carcinoma and lung cancer cells. World J
Gastroenterol. 14:1491–1497. 2008.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Lu Y, Jiang F, Jiang H, Wu K, Zheng X, Cai
Y, Katakowski M, Chopp M and To SS: Gallic acid suppresses cell
viability, proliferation, invasion and angiogenesis in human glioma
cells. Eur J Pharmacol. 641:102–107. 2010.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Albini A, Iwamoto Y, Kleinman HK, Martin
GR, Aaronson SA, Kozlowski JM and McEwan RN: A rapid in vitro assay
for quantitating the invasive potential of tumor cells. Cancer Res.
47:3239–3245. 1987.PubMed/NCBI
|
|
27
|
Heussen C and Dowdle EB: Electrophoretic
analysis of plasminogen activators in polyacrylamide gels
containing sodium dodecyl sulfate and copolymerized substrates.
Anal Biochem. 102:196–202. 1980.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wattanawongdon W, Hahnvajanawong C, Namwat
N, Kanchanawat S, Boonmars T, Jearanaikoon P, Leelayuwat C,
Techasen A and Seubwai W: Establishment and characterization of
gemcitabine-resistant human cholangiocarcinoma cell lines with
multidrug resistance and enhanced invasiveness. Int J Oncol.
47:398–410. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Librach CL, Werb Z, Fitzgerald ML, Chiu K,
Corwin NM, Esteves RA, Grobelny D, Galardy R, Damsky CH and Fisher
SJ: 92-kD type IV collagenase mediates invasion of human
cytotrophoblasts. J Cell Biol. 113:437–449. 1991.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Essafi Rhouma H, Trabelsi N, Chimento A,
Benincasa C, Tamaalli A, Perri E, Zarrouk M and Pezzi V: Olea
europaea L. Flowers as a new promising anticancer natural
product: Phenolic composition, antiproliferative activity and
apoptosis induction. Nat Prod Res. 5:1–4. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
El-Garawani IM, Elkhateeb WA, Zaghlol GM,
Almeer RS, Ahmed EF, Rateb ME and Abdel Moneim AE: Candelariella
vitellina extract triggers in vitro and in vivo cell death
through induction of apoptosis: A novel anticancer agent. Food Chem
Toxicol. 127:110–119. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Craig WJ: Health-promoting properties of
common herbs. Am J Clin Nutr. 70 (Suppl):491S–499S. 1999.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Cao Y, Fu ZD, Wang F, Liu HY and Han R:
Anti-angiogenic activity of resveratrol, a natural compound from
medicinal plants. J Asian Nat Prod Res. 7:205–213. 2005.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Lee YC, Cheng TH, Lee JS, Chen JH, Liao
YC, Fong Y, Wu CH and Shih YW: Nobiletin, a citrus flavonoid,
suppresses invasion and migration involving FAK/PI3K/Akt and small
GTPase signals in human gastric adenocarcinoma AGS cells. Mol Cell
Biochem. 347:103–115. 2011.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kunnumakkara AB, Anand P and Aggarwal BB:
Curcumin inhibits proliferation, invasion, angiogenesis and
metastasis of different cancers through interaction with multiple
cell signaling proteins. Cancer Lett. 269:199–225. 2008.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Boueroy P, Saensa-Ard S, Siripong P,
Kanthawong S and Hahnvajanawong C: Rhinacanthin-C extracted from
Rhinacanthus nasutus (L.) inhibits cholangiocarcinoma cell
migration and invasion by decreasing MMP-2, uPA, FAK and MAPK
pathways. Asian Pac J Cancer Prev. 19:3605–3613. 2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Jonkman JE, Cathcart JA, Xu F, Bartolini
ME, Amon JE, Stevens KM and Colarusso P: An introduction to the
wound healing assay using live-cell microscopy. Cell Adhes Migr.
8:440–451. 2014.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Stamm A, Strauß S, Vogt P, Scheper T and
Pepelanova I: Positive in vitro wound healing effects of functional
inclusion bodies of a lipoxygenase from the Mexican axolotl. Microb
Cell Fact. 17(57)2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Latifi-Pupovci H, Kuçi Z, Wehner S, Bönig
H, Lieberz R, Klingebiel T, Bader P and Kuçi S: In vitro migration
and proliferation (“wound healing”) potential of mesenchymal
stromal cells generated from human CD271+ bone marrow
mononuclear cells. J Transl Med. 13(315)2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Mon NN, Hasegawa H, Thant AA, Huang P,
Tanimura Y, Senga T and Hamaguchi M: A role for focal adhesion
kinase signaling in tumor necrosis factor-α-dependent matrix
metalloproteinase-9 production in a cholangiocarcinoma cell line,
CCKS1. Cancer Res. 66:6778–6784. 2006.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Weiner TM, Liu ET, Craven RJ and Cance WG:
Expression of focal adhesion kinase gene and invasive cancer.
Lancet. 342:1024–1025. 1993.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Seko Y, Takahashi N, Tobe K, Kadowaki T
and Yazaki Y and Yazaki Y: Pulsatile stretch activates
mitogen-activated protein kinase (MAPK) family members and focal
adhesion kinase (p125(FAK)) in cultured rat cardiac myocytes.
Biochem Biophys Res Commun. 259:8–14. 1999.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Blobe GC, Obeid LM and Hannun YA:
Regulation of protein kinase C and role in cancer biology. Cancer
Metastasis Rev. 13:411–431. 1994.PubMed/NCBI View Article : Google Scholar
|
|
45
|
O'Brian C, Vogel VG, Singletary SE and
Ward NE: Elevated protein kinase C expression in human breast tumor
biopsies relative to normal breast tissue. Cancer Res.
49:3215–3217. 1989.PubMed/NCBI
|
|
46
|
Shen KH, Hung SH, Yin LT, Huang CS, Chao
CH, Liu CL and Shih YW: Acacetin, a flavonoid, inhibits the
invasion and migration of human prostate cancer DU145 cells via
inactivation of the p38 MAPK signaling pathway. Mol Cell Biochem.
333:279–291. 2010.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Karin M and Greten FR: NF-kappaB: Linking
inflammation and immunity to cancer development and progression.
Nat Rev Immunol. 5:749–759. 2005.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Oya M, Takayanagi A, Horiguchi A, Mizuno
R, Ohtsubo M, Marumo K, Shimizu N and Murai M: Increased nuclear
factor-kappa B activation is related to the tumor development of
renal cell carcinoma. Carcinogenesis. 24:377–384. 2003.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Katiyar SK and Meeran SM: Obesity
increases the risk of UV radiation-induced oxidative stress and
activation of MAPK and NF-kappaB signaling. Free Radic Biol Med.
42:299–310. 2007.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Dolcet X, Llobet D, Pallares J and
Matias-Guiu X: NF-κB in development and progression of human
cancer. Virchows Arch. 446:475–482. 2005.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Neil JR, Johnson KM, Nemenoff RA and
Schiemann WP: Cox-2 inactivates Smad signaling and enhances EMT
stimulated by TGF-beta through a PGE2-dependent mechanisms.
Carcinogenesis. 29:2227–2235. 2008.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Endo K, Yoon BI, Pairojkul C, Demetris AJ
and Sirica AE: ERBB-2 overexpression and cyclooxygenase-2
up-regulation in human cholangiocarcinoma and risk conditions.
Hepatology. 36:439–450. 2002.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Stasinopoulos I, Shah T, Penet MF,
Krishnamachary B and Bhujwalla ZM: COX-2 in cancer: Gordian knot or
Achilles heel? Front Pharmacol. 4(34)2013.PubMed/NCBI View Article : Google Scholar
|