Introduction
Acute respiratory distress syndrome (ARDS) is one
leading cause of neonatal death, which is characterized by
excessive inflammation in lung tissues and results in loss of
alveolar-capillary membrane integrity and lung dysfunctions
(1,2). Previous studies have confirmed that
inhibition of inflammatory mediators and oxidative stress
alleviates acute lung injury (ALI) (3). Unfortunately, despite significant
advances in ALI treatment, the mortality among patients with ARDS
reached 20.0% according to a retrospective cohort study by Killien
et al (4) (data from the
National Trauma Data Bank). Consequently, it is imperative to study
the molecular mechanism of ALI/ARDS and explore new treatment
methods.
microRNAs (miRNAs; miRs), typical endogenous short
noncoding RNAs, have been revealed to greatly contribute to the
regulation of cell proliferation, apoptosis and inflammation
(5). Furthermore, miRNAs are
considered to be diagnostic biomarkers of certain human
inflammations, either promoting or inhibiting inflammatory
molecules (6). For example,
miR-215-5p has been revealed to be downregulated in sepsis-induced
inflammatory damage in H9c2 cells (7). In addition, miR-214-3p has been
revealed to be upregulated in the serum of patients with high-fat
diet-induced hyperlipidemic pancreatitis, aggravating tissue damage
and inflammation of the accompanied acute kidney injury (8). At present, an increasing number of
miRNAs have been found to have a function in ALI. For instance,
miR-150 has been revealed to be downregulated in LPS-induced ALI,
while overexpressed miR-150 reduced the lung injury (9). In addition, miRNA-1246 has been
indicated to repress ALI-induced lung inflammation and apoptosis
via NF-κB activation and Wnt/β-catenin suppression (10). As a novel miRNA, miR-490-3p has been
found to regulate the development of various tumors. For example,
miR-490-3p suppressed gliomas by inhibiting the expression of the
high-mobility group AT-hook 2(11).
However, the function and regulatory principal of miR-490-3p in ALI
is elusive.
Interleukin 1 receptor associated kinase 1 (IRAK1)
and TNF receptor associated factor 6 (TRAF6), as multifunctional
signal transduction molecules in cells, have been revealed to have
a crucial part in the regulation of inflammation by promoting the
synthesis and secretion of molecules such as IL-1β, IL-6 and TNFα
(12,13). Furthermore, accumulating studies
have verified that IRAK1 is regulated by multiple miRNAs. For
example, miR-146a has been revealed to regulate gouty arthritis
progression by targeting TRAF-6(14). Overexpressed miR-223 downregulated
IRAK-1 in H. pylori-infected macrophages, thereby inhibiting
the pro-inflammatory response (15). In the present study, bioinformatics
analysis (http://starbase.sysu.edu.cn) revealed
a potential binding between miR-490-3p and IRAK1. However, whether
miR-490-3p regulates the inflammatory response of LPS-induced ALI
by targeting IRAK1 remains unknown.
Initially, the present study determined that
miR-490-3p was expressed at a low level in LPS-induced ALI, while
IRAK1 and TRAF6 were upregulated and their expression was
negatively correlated with miR-490-3p. miR-490-3p overexpression
has been revealed to markedly inhibit LPS-induced cell injury and
inflammation. Furthermore, the present mechanistic experiments
demonstrated that miR-490-3p targeted IRAK1 and negatively
regulated its expression. Collectively, the present study explored
the molecular mechanism of neonatal ALI, with the hope of providing
a new theoretical basis for the treatment of this disease.
Materials and methods
Animal experiments and ARDS
induction
Newborn SD rats, (male; age, 3-8 days; weight, 10-15
g; Experimental Animal Center of Shandong University, Jinan, China)
were injected with LPS intraperitoneally at 3 mg/kg to induce ALI
48 h after LPS administration. The rats were housed at room
temperature (18-23˚C) with a 12 h-light/dark cycle. Rats were
randomly fed with standard chow and water and adapted to
experimental conditions at least 3 days before the experiment. All
experiments were carried out following the ‘Guidelines for the Use
of Laboratory Animal Care’ and approved by the Animal Care Use
Committee of Shanxi Medical University (Taiyuan, China). All the
rats (n=24) were randomly divided into a control and LPS group
(n=12 each). The rats were anesthetized by administering
intraperitoneal ketamine hydrochloride (60 mg/kg body weight) and
xylazine hydrochloride (5 mg-kg body weight) (16-18).
LPS (at 10 mg/kg body weight, E. coli O111:B4;
Sigma-Aldrich; Merck KGaA) was dissolved in 20 µl sterile
phosphate-buffered saline (PBS) for 24 h and then injected
intratracheally. The control group received the same volume of PBS.
The health and behaviors of rats were monitored every 12 h. Before
the rats were sacrificed to collect the lung tissues, the rats were
placed in a closed container with a low concentration of
CO2 (10-30%). Once the rats lost consciousness under the
low concentration of CO2 environment, they were
euthanized with 100% CO2 (the experiment was carried out
in September 2019). The euthanasia was confirmed when lack of
respiration and color fading of eyes were observed in the rats for
a minimum of 1 min. All efforts were made to minimize their
suffering. Preemptive euthanasia was performed for humane reasons
if rats exhibited any of the following signs: Emaciated, gasping,
no response to touch or an anal temperature <25˚C.
Immunohistochemistry
Clinical specimens were fixed with 10% formaldehyde
at room temperature for 24 h and embedded in paraffin, then the
sections (4 µm) were dewaxed and hydrated. Firstly, dewaxed
sections were dried at 37˚C for 2 h, and its endogenous peroxide
was blocked with 1% H2O2 for 5 min.
Subsequently, the sections were washed 3 times with PBS, blocked
with 5% BSA (cat. no. ST025; Beyotime Institute of Biotechnology)
for 1 h at room temperature, and then incubated with anti-IRAK1
antibody (1:200; cat. no. ab238; Abcam) at 4˚C overnight.
Subsequently, they were washed with PBS and incubated with goat
anti-rabbit IgG H&L (HRP) secondary antibody (1:500; cat. no.
ab205718; Abcam) for 1 h at room temperature. The sections were
rinsed again and stained with 3,3-diaminobenzidine hydrochloride
for 1 min. Finally, they were rinsed with double-distilled water
and stained with hematoxylin and eosin (H&E) for 1 min at room
temperature, and observed under a light microscope (magnification,
x200).
Cell culture and treatments
The A549 cell line (cat. no. CCL-185™) was obtained
from American Type Culture Collection (ATCC) and HPAEpiC (cat. no.
3200) (human type II alveolar epithelial cell) was purchased from
ScienCell Research Laboratories, Inc. These two cell lines were
incubated in Roswell Park Memorial Institute (RPMI-1640) medium
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% fetal
bovine serum and 1% antibiotic antifungal solution (both from
Sigma-Aldrich; Merck KGaA). In addition, the cells were cultured in
an incubator with humidified CO2 at 37˚C. miR-490-3p
mimics (5'-CAACCUGGAGGACUCCAUGCUG-3') and its negative control
(miR-NC, 5'-ACCGCUAAUCAUACGAAUACAC-3') were obtained from Shanghai
GenePharma Co., Ltd. The cells (1x105) were seeded in
12-well plates and transfected with miR-490-3p or miR-NC (50
pmoles) using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) at 37˚C for 24 h according to the
manufacturer's instructions. Cells were used for subsequent
experiments after 24 h. Four groups were designed, namely the
control group, the LPS group, the LPS + NC group and the LPS +
miR-490-3p. LPS (100 ng/ml) was used to treat the cells for 4
h.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from the cells using
TRIzol® reagent (Thermo Fisher Scientific, Inc.) and
reverse-transcribed into cDNA with a cDNA Synthesis Kit (Sangon
Biotech Co., Ltd.) according to the manufacturer's protocol.
RT-qPCR was conducted with a SYBR Prime Script RT-PCR kit
(Invitrogen; Thermo Fisher Scientific, Inc.). The thermocycling
conditions were as follows: 10 min at 95˚C; 40 cycles of 1 min at
95˚C, 2 min at 63˚C and 1 min at 72˚C. The primers were synthesized
by Sangon Biotech Co., Ltd. The miRNA relative expression was
calculated by normalizing to U6 small nuclear RNA. The primer
sequences were as follows: miR-490-3p forward, 5'-CGTGGATCCTTCTTCA
ACCAACGGTGGTG-3' and reverse, 5'-CCAGAATT
CAAAGCAGGAAGAGTAAGACTTCC-3'; IRAK1 forward,
5'-CCTCCAGGTTCCACTCTCTG-3' and reverse, 5'-AACCACCCTCTCCAATCCTG-3';
TRAF6 forward, 5'-TTGCACATGAGACTGTTGGC-3' and reverse,
5'-CTTCGAATGGTCCGCTTGAG-3'; GAPDH forward, 5'-AACGGATTT
GGTCGTATTG-3' and reverse, 5'-GGAA GATGGTGATGGGATT-3'; U6 forward,
5'-GGAGCGAG ATCCCTCCAAAAT-3' and reverse, 5'-GGCTGTTGTCAT
ACTTCTCATGG-3'. Relative expression was calculated using the
2-ΔΔCq method (19),
using U6 or GAPDH as normalization controls.
Western blot analysis
RIPA lysate (Roche Diagnostics) was used to isolate
the total proteins from both lung tissues and cells. Protein
concentration was determined using a BCA kit (Beyotime Institute of
Biotechnology). A total of 50 µg of protein was loaded on a 12%
polyacrylamide gel and electrophoresed at 100 V for 2 h and
electrically transferred to polyvinylidene fluoride (PVDF)
membranes. After being blocked with 5% skimmed milk (room
temperature; 1 h), the membranes were washed 3 times with TBS-0.1%
Tween for 10 min each time, then incubated with antibodies of
anti-IRAK1 (1:1,000; cat. no. ab238), anti-TRAF6 (dilution 1:1,000;
cat. no. ab227560), anti-phosphorylated (p-)NF-κB (dilution
1:1,000; cat. no. ab76302) and anti-NF-κB (dilution 1:1,000; cat.
no. ab32536; all from Abcam) at 4˚C overnight. Then, the membranes
were washed with TBST again and incubated with goat anti-rabbit IgG
H&L (HRP) (dilution 1:3,000; cat. no. ab205718; Abcam) at room
temperature for 1 h. Subsequently, the membranes were washed 3
times, 10 min each with TBST. Finally, Pierce™ ECL Western Blotting
Substrate (Invitrogen; Thermo Fisher Scientific, Inc.) was used for
imaging, and ImageJ (v1.48; National Institutes of Health) was
adopted to semi-quantify the gray value of each protein.
Cell Counting Kit-8 (CCK-8) assay
Cells of each group in the logarithmic growth phase
were trypsinized, centrifuged (500 x g) for 5 min at room
temperature and counted, and then inoculated in 96-well plates at
2x103 cells/well. After 24 h of incubation (at 37˚C with
5% CO2), the culture solution was removed. After
treatment according to the experimental group, 10 µl of CCK-8
solution (Beyotime Institute of Biotechnology) was added to each
well and incubated at 37˚C for 1 h. A microplate reader was used to
detect the absorbance value of each well at 450 nm. Four parallel
wells were set in each group, and each experiment was repeated 3
times.
BrdU assay
The cells were seeded in a 12-well plate at
1x105 cells/well and incubated at 37˚C with 5%
CO2 in a humidified environment for 12 h until fully
adherent. After treating the cells of each group, BrdU at a final
concentration of 30 μmol/l was added and incubated at 37˚C for 4 h.
Subsequently, the cell slides were washed with PBS and fixed with
4% formaldehyde at room temperature for 10 min, and then acidified
and denatured in PBST (PBS with 0.1% Tween-20) containing 2 mol/l
of hydrochloric acid for 30 min. After being blocked with PBST
containing 5% bovine serum albumin (Beyotime Institute of
Biotechnology) for 1 h, the cells were incubated with BrdU antibody
(1:1,000 in PBS; cat. no. ab8152; Abcam) for 2 h at room
temperature. Then the cells were washed 3 times with PBS, and
incubated with goat anti-mouse IgG H&L (Alexa Fluor®
647; cat. no. ab150115; Abcam) (1:1,000 in PBS) for 1 h. Finally,
DAPI (Beyotime Institute of Biotechnology) was used for labeling
the nuclei, and BrdU was observed with a fluorescence microscope
(magnification, x200). The cell proliferation rate was calculated
as follows: Cell proliferation rate = Number of positive
cells/number of total cells x100%. The experiment was repeated
three times.
Enzyme-linked immunosorbent assay
(ELISA)
The supernatant of cell culture media was collected
and centrifuged on a low-speed centrifuge (500 x g) for 5 min at
room temperature. Then the expression of IL-6, TNFα and IL-1β in
the supernatant was detected using ELISA kits for IL-6 (cat. no.
70-EK206/3-96), TNF-α (cat. no. 70-EK282/3-96) and IL-1β (cat. no.
70-EK201B/3-96) according to the manufacturer's instructions. All
of the detection kits were obtained from Hangzhou Multi Sciences
(Lianke) Biotech, Co., Ltd.
Luciferase reporter assay
Bioinformatic analysis was conducted to predict the
downstream target of miR-490-3p using StarBase v3.0 (http://starbase.sysu.edu.cn) (20). The amplified DNA sequence was cloned
into pmirGLO Dual-luciferase vectors (Promega Corporation) to
construct wild-type (WT) IRAK1 3'-untranslated region (UTR) and
mutant (MUT) IRAK1 3'-UTR reporter vectors. A549 cells
(1x105) were seeded in 24-well plates overnight and then
transfected with IRAK1-WT or IRAK1-MUT reporter vector and
transfected with miR-490-3p mimics or negative controls using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). A Dual-luciferase reporter gene assay system
(Promega Corporation) was adopted to determine luciferase viability
48 h after transfection. Renilla luciferase activity was
used as the internal control.
Statistical analysis
SPSS (version 20.0; IBM Corp.) was used for data
analysis. All data were expressed as the mean ± SD. Statistical
analysis was performed using an unpaired Student's t-test.
Differences between the two groups were analyzed using
χ2. The correlation of the molecules was analyzed using
Pearson's correlation test. P<0.05 was considered to indicate a
statistically significant difference.
Results
miR-490-3p is decreased in LPS-induced
ALI
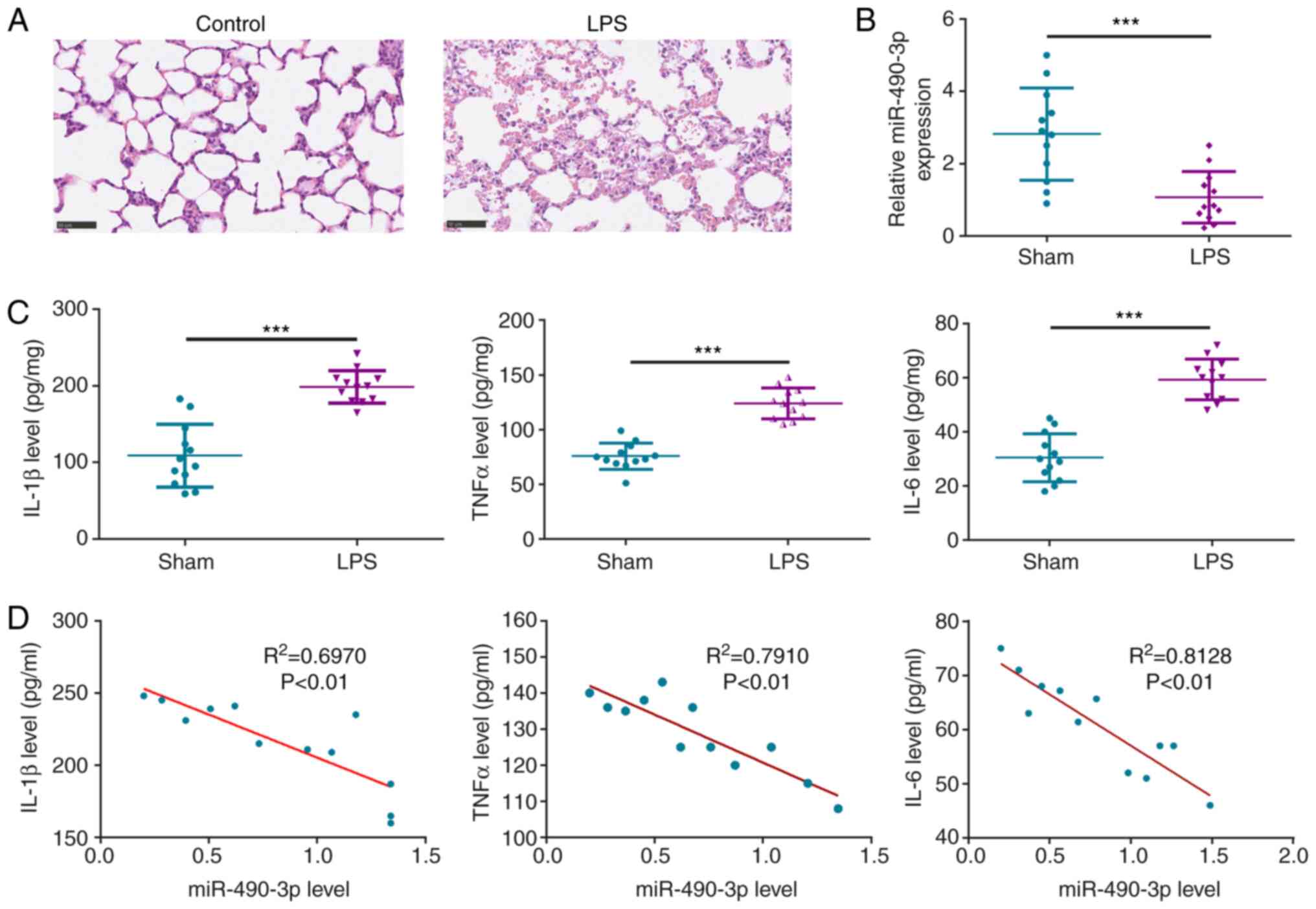
To probe miR-490-3p function in ALI, an LPS-induced
ALI rat neuronal model was established and the pathological
alteration in lung tissue of LPS rats was examined using H&E
staining (Fig. 1A). As a result, it
was revealed that there was edema, lung tissue alteration,
incomplete nuclear staining, unclear cell boundaries and
accumulating inflammatory cell and neutrophil infiltration in the
lungs of the LPS-treated rats (Fig.
1A). In addition, RT-qPCR revealed that miR-490-3p was
downregulated in LPS-induced ALI (Fig.
1B). Furthermore, it was also revealed that the inflammatory
factors IL-1β, IL-6 and TNFα were significantly upregulated in the
lung tissues of LPS-induced ALI compared with the control group
(Fig. 1C). Furthermore, Pearson
correlation analysis revealed that miR-490-3p was negatively
correlated to IL-1β, IL-6 and TNFα expression in LPS-induced ALI
(Fig. 1D). The aforementioned
findings indicated that miR-490-3p was downregulated in LPS-induced
ALI, and inversely related to the inflammatory response.
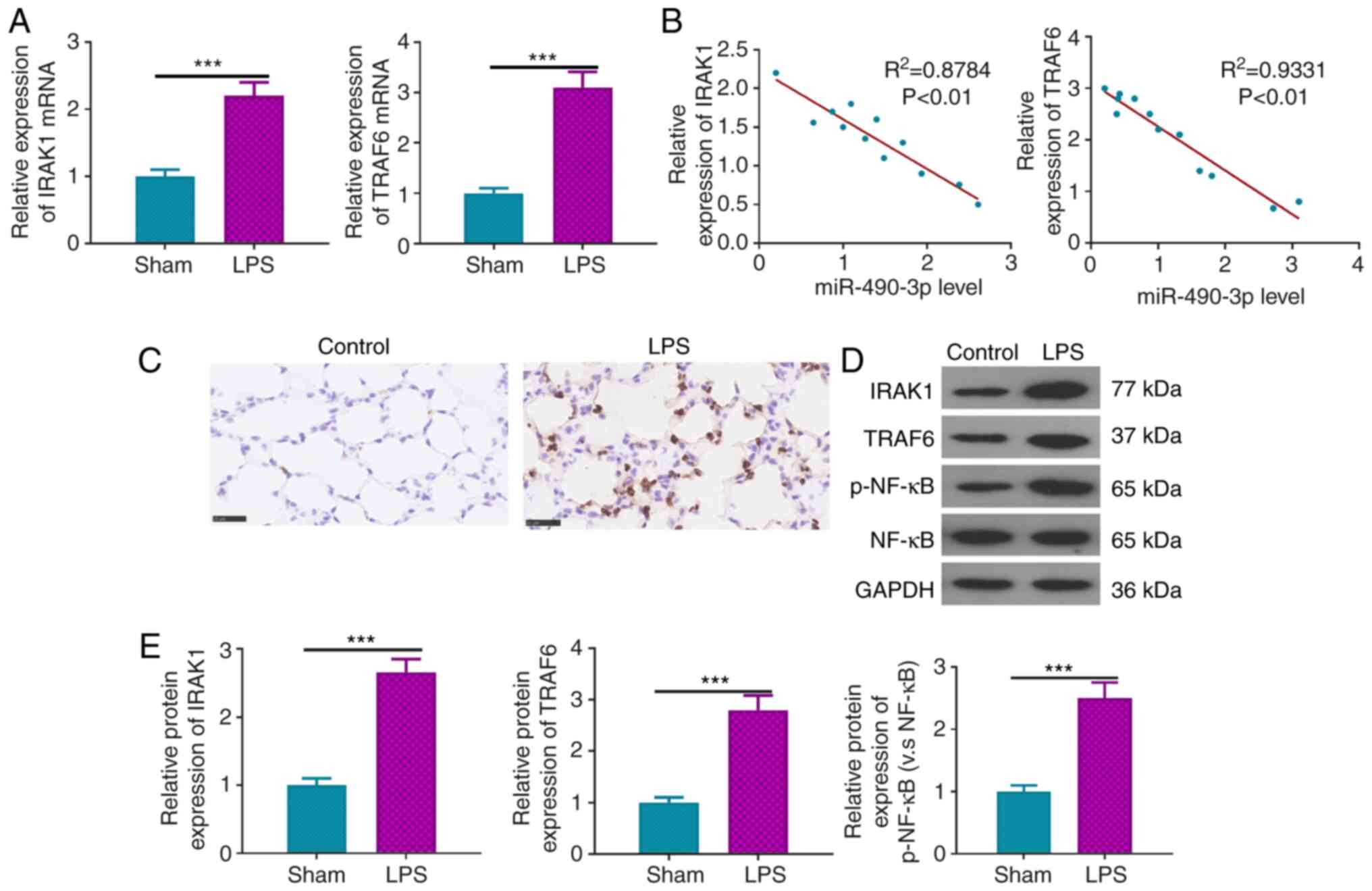
IRAK1 and TRAF6 are upregulated in ALI
and negatively correlated with miR-490-3p
The relative expression of IRAK1 and TRAF6 in
various groups was examined by RT-qPCR. The results indicated that
both IRAK1 and TRAF6 mRNAs were significantly upregulated in
LPS-induced ALI (Fig. 2A). In
addition, it was confirmed that miR-490-3p was negatively
correlated with IRAK1 and TRAF6 expression (Fig. 2B). Moreover, immunohistochemistry
indicated that the number of IRAK1-positive cells were increased in
LPS-induced ALI (Fig. 2C).
Furthermore, western blot analysis demonstrated that the expression
of IRAK1, TRAF6 and p-NF-κB proteins in LPS-induced ALI was
significantly enhanced compared with that of the control group
(Fig. 2D and E). These studies indicated that IRAK1 and
TRAF6 were upregulated in ALI and were inversely related to
miR-490-3p.
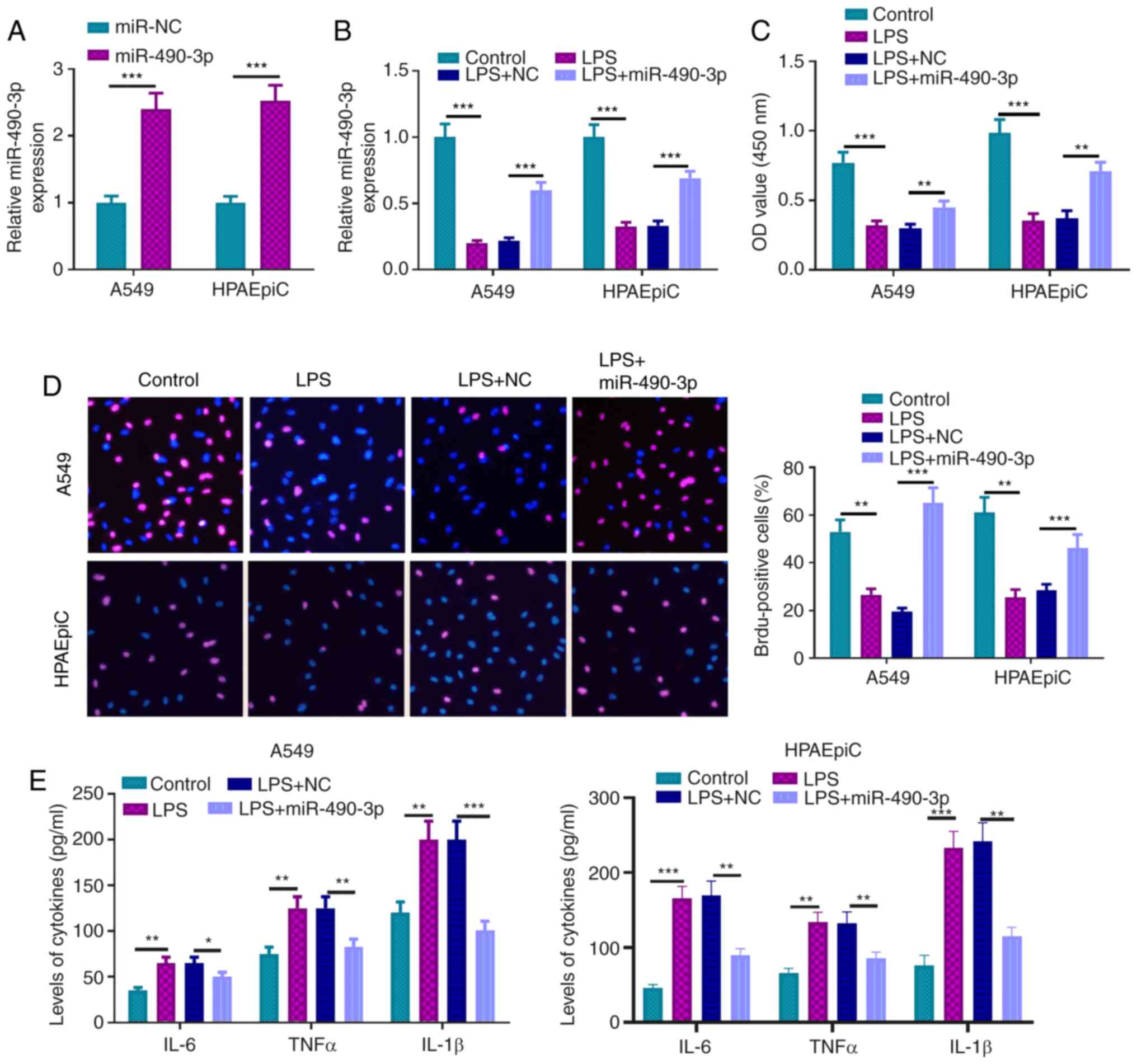
miR-490-3p overexpression distinctly
inhibits LPS-induced cell injury and inflammatory response
miR-490-3p-overexpressed A549 cells were constructed
to further investigate its mechanism in LPS-induced ALI (Fig. 3A). RT-qPCR revealed that miR-490-3p
was increased in the LPS + miR-490-3p-treated group compared with
the LPS + NC-treated group (Fig.
3B). Moreover, cell proliferation of LPS + miR-490-3p was
enhanced compared with the LPS + NC-treated group (Fig. 3C). Furthermore, BrdU assays affirmed
that the cell viability of the LPS + miR-490-3p group was
significantly increased compared with the LPS + NC group (Fig. 3D). ELISA assays also indicated that
the inflammatory factors IL-1β, IL-6 and TNFα were decreased in the
LPS + miR-490-3p-treated group compared with the LPS + NC group
(Fig. 3E). These studies indicated
that miR-490-3p overexpression significantly inhibited LPS-induced
cell injury and inflammatory response.
miR-490-3p overexpression suppresses
IRAK1 and TRAF6 expression
To further study the miR-490-3p mechanism in
LPS-induced ALI, RT-qPCR and western blot analysis was used to
examine the relative expression of IRAK1, TRAF6 and p-NF-κB. It was
revealed that the mRNA and protein expression of IRAK1, TRAF6 and
p-NF-κB were significantly downregulated in the LPS + miR-490-3p
treatment group when compared with the LPS + NC treatment group
(Fig. 4A-D). As a result,
miR-490-3p appeared to inhibit the LPS-activated IRAK1/TRAF6/NF-κB
pathway.
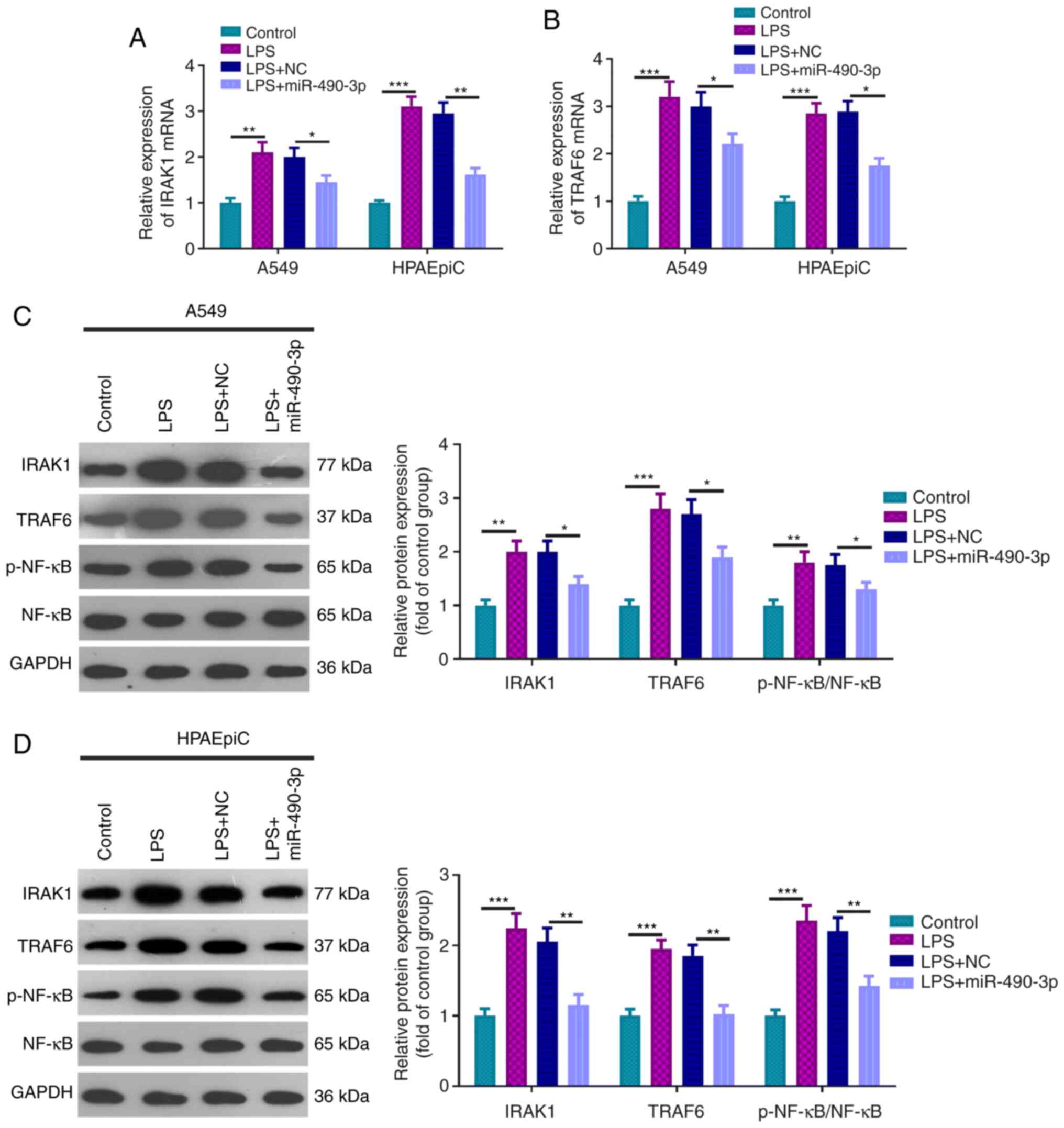 | Figure 4miR-490-3p overexpression restrains
IRAK1 and TRAF6 expression. The relative expression levels of (A)
IRAK1 and (B) TRAF6 mRNA in the control group, LPS group, LPS + NC
group, LPS + miR-490-3p group were verified by reverse
transcription-quantitative PCR. Western blot assays were conducted
to examine the relative expression levels of IRAK1, TRAF6 and
p-NF-κB in the aforementioned treatment groups in (C) A549 and (D)
HPAEpiC cells. *P<0.05, **P<0.01 and
***P<0.001. miR, microRNA; IRAK1, interleukin 1
receptor associated kinase 1; TRAF6, TNF receptor associated factor
6; LPS, lipopolysaccharide; p-, phosphorylated; NC, negative
control. |
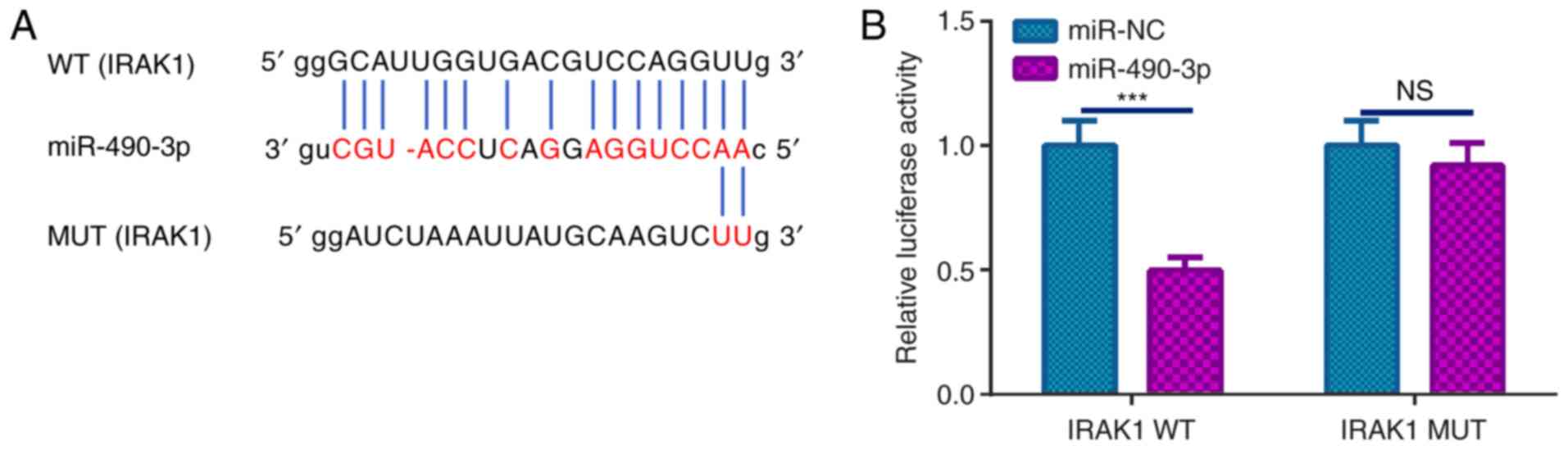
miR-490-3p targets IRAK1
To identify the downstream molecular mechanism of
miR-490-3p, its candidate targets were analyzed via StarBase
(http://starbase.sysu.edu.cn). The
results indicated that miR-490-3p could bind to IRAK1 (Fig. 5A). Similarly, the targeted binding
relationship between miR-490-3p and IRAK1 was verified using a dual
luciferase reporter assay. miR-490-3p mimics were revealed to
attenuate the luciferase viability of IRAK1-WT but had no
significant effects on that of the IRAK1-MUT (Fig. 5B). Thus, these results verified that
miR-490-3p decreased LPS-induced inflammation by directly targeting
IRAK1.
Discussion
Neonates are particularly susceptible to ALI, which
is one of the most frequent causes of mortality in newborns
(21). As the main pathogenic
component of Gram-negative bacteria, LPS has been indicated to be
an important cause of ALI (22).
LPS causes the activation of related inflammatory cells, thus
releasing IL-1β, IL-6, TNFα and other inflammatory mediators, which
results in diffuse injuries of alveolar epithelial cells, alveolar
capillary endothelial cells and pulmonary interstitium (1,23).
Consequently, studying the pathogenesis of ALI has a direct guiding
significance for its treatment. Initially, miR-490-3p was revealed
to be significantly downregulated in LPS-induced ALI, while IL-1β,
IL-6, TNFα, IRAK1 and TRAF6 were all upregulated. Subsequent,
correlation analysis revealed that the miR-490-3p expression level
was negatively correlated to IL-1β, IL-6, TNFα, IRAK1 and TRAF6.
Additionally, miR-490-3p overexpression significantly relieved
LPS-induced lung epithelial cell injury and the inflammatory
response. Mechanistically, miR-490-3p appeared to regulate IRAK1
expression. Overall, the present study revealed that overexpressed
miR-490-3p inhibited the IRAK1/TRAF6 pathway, thereby reducing the
inflammatory responses in LPS-induced ALI.
miRNAs regulate gene expression by binding to the
3'-UTR of target mRNAs at the post-transcriptional level, thereby
modulating its translation (24-26).
Previously, miRNAs have been revealed to regulate a variety of
inflammatory processes by regulating target genes. For example,
miR-29c was revealed to inhibit the production of inflammatory
cytokines and reduce the apoptosis rate in Parkinson's disease by
targeting specificity protein 1(27). miR-485 has been revealed to inhibit
the inflammation and multiplication of mesangial cells in
vitro by acting on NAPDH oxidase 5(28). Similarly, multiple miRNAs have been
revealed to regulate inflammation in ALI. Meng et al (29)
revealed that miR-539-5p relieved sepsis-induced ALI by targeting
the protein Rho associated coiled-coil containing protein kinase 1.
Yang et al (30) revealed
that miR-142a-3p alleviated ALI induced by E. coli
lipopolysaccharides by acting on TGF-β-activated kinase 1 and
MAP3K7-binding protein 2. Xie et al (31) claimed that miR-34b-5p aggravated ALI
in LPS-induced rat models by targeting progranulin (PGRN), and its
knockdown reduced lung inflammation and cell apoptosis. The
aforementioned studies have revealed that miRNAs exert protective
effects against lung injury via repressing inflammatory responses.
Moreover, a number of studies have indicated that attenuating
pulmonary inflammation ameliorated acute lung injury in neonatal
animal models (32-34).
The present research confirmed that miR-490-3p was significantly
downregulated in the LPS-induced neonatal ALI rat model. Via in
vitro experiments, it was revealed that upregulating miR-490-3p
attenuated LPS-induced pulmonary epithelial cell damage, as well as
induced inflammatory cytokine expression. Therefore, these data
demonstrated that miR-490-3p presents a potential value in treating
neonatal ALI.
When further exploring the specific mechanism by
which miR-490-3p alleviated LPS-induced ALI, IRAK was hypothesized
to be the downstream targeting molecule. Currently, IRAK and TRAF6
are downstream signaling molecules of toll-like receptors (TLR).
After LPS infects the body, it first binds to the serum binding
protein LPS binding protein (LBP), and transmits to CD14 molecules
to produce a LPS-LBP-CD14 complex, which reciprocates TLR4 and its
accessory protein myeloid differentiation protein 2, thereby
activating IRAK and TRAF6 in succession (35-37).
As an important signaling molecule, IRAK1 exerts a vital part in
TLR/IL-1R-mediated innate immune and inflammation response
(38).
Notably, it has been demonstrated that various
miRNAs target TLR4 and its downstream signaling molecules IRAK1 and
TRAF6 to regulate inflammatory responses. Considering miR-382-3p as
an example, it was revealed to inhibit IL-1β-induced chondrocyte
inflammatory response by directly targeting connexin 43 of the
TLR4/MyD88/NF-κB signaling pathway (39). miR-451 attenuated the inflammation
caused by microglial activation and alleviated inflammatory pain by
targeting TLR4(40). Similarly,
miRNAs have similar pathway regulation in ALI. For instance,
miR-34b-5p targeted PGRN, and its knockdown reduced lung
inflammation and apoptosis in an LPS-induced ALI rat model
(31). miR-16 impeded ALI by
regulating the TLR4/NF-κB pathway and restricting the inflammatory
response (41). It can be observed
that miRNAs have been revealed to regulate TLR4 signaling pathways
through their target genes in diversified inflammations (42). IRAK1, as a key molecule in the TLR4
signaling pathway, is also regulated by miRNAs. For example,
miR-146a was revealed to bind to the 3'-UTR of IRAK1 and TRAF6,
which attenuated their expression, thereby restricting activation
of the inflammatory proteins including NF-κB, p38 and
ERK1/2(43). Overexpressing miR-146
reduced IRAK-1 and TRAF6 expression levels, thereby attenuating the
inflammatory factors released, and attenuating LPS-induced ALI
(44). A more general view is that
IRAK-1 exacerbates injury by aggravating the inflammatory response,
and it is also targeted by multiple miRNAs. The present study also
revealed that miR-490-3p targeted IRAK1 and attenuated its
expression.
Overall, the present study indicated that miR-490-3p
overexpression distinctly inhibited LPS-induced ALI and
inflammatory responses by suppressing the IRAK1/TRAF6 pathway.
However, future studies are required to further explore the
therapeutic effects of miR-490-3p in ALI in vivo and
validate the expression pattern of miR-490-3p in neonates with
ALI.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The data sets used and analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contribution
GY conceived, designed and performed the experiments
and subsequently wrote the manuscript. YZ conducted the statistical
analysis. Both authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics Review
Board of Shanxi Medical University (Taiyuan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen H, Bai C and Wang X: The value of the
lipopolysaccharide-induced acute lung injury model in respiratory
medicine. Expert Rev Respir Med. 4:773–783. 2010.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Huang X, Xiu H, Zhang S and Zhang G: The
role of macrophages in the pathogenesis of ALI/ARDS. Mediators
Inflamm. 2018(1264913)2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Liu H, Yu X, Yu S and Kou J: Molecular
mechanisms in lipopolysaccharide-induced pulmonary endothelial
barrier dysfunction. Int Immunopharmacol. 29:937–946.
2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Killien EY, Mills B, Watson RS, Vavilala
MS and Rivara FP: Morbidity and mortality among critically injured
children with acute respiratory distress syndrome. Crit Care Med.
47:e112–e119. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Saliminejad K, Khorram Khorshid HR,
Soleymani Fard S and Ghaffari SH: An overview of microRNAs:
Biology, functions, therapeutics, and analysis methods. J Cell
Physiol. 234:5451–5465. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Essandoh K, Li Y, Huo J and Fan GC:
MiRNA-mediated macrophage polarization and its potential role in
the regulation of inflammatory response. Shock. 46:122–131.
2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yao Y, Xu K, Sun Y, Tian T, Shen W, Sun F,
Yuan W, Wu H, Chen G, Yuan L, et al: MiR-215-5p inhibits the
inflammation injury in septic H9c2 by regulating ILF3 and LRRFIP1.
Int Immunopharmacol. 78(106000)2020.PubMed/NCBIdoi: 10.1016/j.intimp.2019.106000.
|
|
8
|
Yan Z, Zang B, Gong X, Ren J and Wang R:
MiR-214-3p exacerbates kidney damages and inflammation induced by
hyperlipidemic pancreatitis complicated with acute renal injury.
Life Sci. 241(117118)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Li P, Yao Y, Ma Y and Chen Y: MiR-150
attenuates LPS-induced acute lung injury via targeting AKT3. Int
Immunopharmacol. 75(105794)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Suo T, Chen GZ, Huang Y, Zhao KC, Wang T
and Hu K: miRNA-1246 suppresses acute lung injury-induced
inflammation and apoptosis via the NF-κB and Wnt/β-catenin signal
pathways. Biomed Pharmacother. 108:783–791. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhang F, Wu A, Wang Y and Liu J:
miR-490-3p functions as a tumor suppressor in glioma by inhibiting
high-mobility group AT-hook 2 expression. Exp Ther Med. 18:664–670.
2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Liu Y, Zhang M, Lou L, Li L, Zhang Y, Chen
W, Zhou W, Bai Y and Gao J: IRAK-M Associates with susceptibility
to adult-onset asthma and promotes chronic airway inflammation. J
Immunol. 202:899–911. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sharma A, Maurya CK, Arha D, Rai AK, Singh
S, Varshney S, Schertzer JD and Tamrakar AK: Nod1-mediated
lipolysis promotes diacylglycerol accumulation and successive
inflammation via PKCδ-IRAK axis in adipocytes. Biochim Biophys Acta
Mol Basis Dis. 1865:136–146. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhang QB, Qing YF, Yin CC, Zhou L, Liu XS,
Mi QS and Zhou JG: Mice with miR-146a deficiency develop severe
gouty arthritis via dysregulation of TRAF 6, IRAK 1 and NALP3
inflammasome. Arthritis Res Ther. 20(45)2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wang J, Wu J, Cheng Y, Jiang Y and Li G:
Over-expression of microRNA-223 inhibited the proinflammatory
responses in Helicobacter pylori-infection macrophages by
down-regulating IRAK-1. Am J Transl Res. 8:615–622. 2016.PubMed/NCBI
|
|
16
|
Shekarforoush S, Fatahi Z and Safari F:
The effects of pentobarbital, ketamine-pentobarbital and
ketamine-xylazine anesthesia in a rat myocardial ischemic
reperfusion injury model. Lab Anim. 50:179–184. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Francischi JN, Frade TIC, Almeida MPA,
Queiroz BFG and Bakhle YS: Ketamine-xylazine anaesthesia and
orofacial administration of substance P: A lethal combination in
rats. Neuropeptides. 62:21–26. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kocaturk H, Bedir F, Altay MS, Bakan E,
Suleyman B, Yazici GN, Sunar M, Suleyman Z and Suleyman H: The
effect of desloratadine on ischemia reperfusion induced oxidative
and inflammatory renal injury in rats. Ren Fail. 42:531–538.
2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Δ Δ C(T)) Method. Methods. 25:402–408. 2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Li JH, Liu S, Zhou H, Qu LH and Yang JH:
starBase v2.0: Decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA
interaction networks from large-scale CLIP-Seq data. Nucleic Acids
Res. 42 (D1):D92–D97. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Chakraborty M, McGreal EP and Kotecha S:
Acute lung injury in preterm newborn infants: Mechanisms and
management. Paediatr Respir Rev. 11:162–170; quiz 170.
2010.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Cheng N, Liang Y, Du X and Ye RD: Serum
amyloid A promotes LPS clearance and suppresses LPS-induced
inflammation and tissue injury. EMBO Rep. 19(e45517)2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wang Q and Xiao L: Isochlorogenic acid A
attenuates acute lung injury induced by LPS via Nf-κB/NLRP3
signaling pathway. Am J Transl Res. 11:7018–7026. 2019.PubMed/NCBI
|
|
24
|
Yang H, Song Z and Hong D: CRBN knockdown
mitigates lipopolysaccharide-induced acute lung injury by
suppression of oxidative stress and endoplasmic reticulum (ER)
stress associated NF-κB signaling. Biomed Pharmacother.
123(109761)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Lee HM, Kim TS and Jo EK: MiR-146 and
miR-125 in the regulation of innate immunity and inflammation. BMB
Rep. 49:311–318. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Haneklaus M, Gerlic M, O'Neill LA and
Masters SL: miR-223: Infection, inflammation and cancer. J Intern
Med. 274:215–226. 2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wang R, Yang Y, Wang H, He Y and Li C:
MiR-29c protects against inflammation and apoptosis in Parkinson's
disease model in vivo and in vitro by targeting SP1. Clin Exp
Pharmacol Physiol. 47:372–382. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wu J, Lu K, Zhu M, Xie X, Ding Y, Shao X,
Chen Y, Liu J, Xu M, Xu Y, et al: miR-485 suppresses inflammation
and proliferation of mesangial cells in an in vitro model of
diabetic nephropathy by targeting NOX5. Biochem Biophys Res Commun.
521:984–990. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Meng L, Cao H, Wan C and Jiang L:
MiR-539-5p alleviates sepsis-induced acute lung injury by targeting
ROCK1. Folia Histochem Cytobiol. 57:68–178. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yang Y, Yang C, Guo Y-F, Liu P, Guo S,
Yang J, Zahoor A, Shaukat A and Deng G: MiR-142a-3p alleviates
Escherichia coli derived lipopolysaccharide-induced acute
lung injury by targeting TAB2. Microb Pathog.
136(103721)2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Xie W, Lu Q, Wang K, Lu J, Gu X, Zhu D,
Liu F and Guo Z: miR-34b-5p inhibition attenuates lung inflammation
and apoptosis in an LPS-induced acute lung injury mouse model by
targeting progranulin. J Cell Physiol. 233:6615–6631.
2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Cheng K, Yang A, Hu X, Zhu D and Liu K:
Curcumin attenuates pulmonary inflammation in lipopolysaccharide
induced acute lung injury in neonatal rat model by activating
peroxisome proliferator-activated receptor γ (PPARγ) pathway. Med
Sci Monit. 24:1178–1184. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Duan Q, Jia Y, Qin Y, Jin Y, Hu H and Chen
J: Narciclasine attenuates LPS-induced acute lung injury in
neonatal rats through suppressing inflammation and oxidative
stress. Bioengineered. 11:801–810. 2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wang X, Zhang C, Chen C, Guo Y, Meng X and
Kan C: Allicin attenuates lipopolysaccharide-induced acute lung
injury in neonatal rats via the PI3K/Akt pathway. Mol Med Rep.
17:6777–6783. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Yang D, Li S, Duan X, Ren J, Liang S,
Yakoumatos L, Kang Y, Uriarte SM, Shang J, Li W, et al: TLR4
induced Wnt3a-Dvl3 restrains the intensity of inflammation and
protects against endotoxin-driven organ failure through
GSK3β/β-catenin signaling. Mol Immunol. 118:153–164.
2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhang B, Zeng M, Li M, Kan Y, Li B, Xu R,
Wu Y, Wang S, Zheng X and Feng W: Protopine protects mice against
LPS-induced acute kidney injury by inhibiting apoptosis and
inflammation via the TLR4 signaling pathway. Molecules.
25(25)2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Yan S, Wang P, Wang J, Yang J, Lu H, Jin
C, Cheng M and Xu D: Long non-coding RNA HIX003209 promotes
inflammation by sponging miR-6089 via TLR4/NF-κB signaling pathway
in rheumatoid arthritis. Front Immunol. 10(2218)2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Giriwono PE, Shirakawa H, Ohsaki Y, Sato
S, Aoyama Y, Ho HJ, Goto T and Komai M: Geranylgeraniol suppresses
the expression of IRAK1 and TRAF6 to inhibit NF-κB activation in
lipopolysaccharide-induced inflammatory responses in human
macrophage-like cells. Int J Mol Sci. 20(20)2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Lei J, Fu Y, Zhuang Y, Zhang K and Lu D:
miR-382-3p suppressed IL-1β induced inflammatory response of
chondrocytes via the TLR4/MyD88/NF-κB signaling pathway by directly
targeting CX43. J Cell Physiol. 234:23160–23168. 2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Sun X and Zhang H: miR-451 elevation
relieves inflammatory pain by suppressing microglial
activation-evoked inflammatory response via targeting TLR4. Cell
Tissue Res. 374:487–495. 2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Yang Y, Yang F, Yu X, Wang B, Yang Y and
Zhou X, Cheng R, Xia S and Zhou X: miR-16 inhibits NLRP3
inflammasome activation by directly targeting TLR4 in acute lung
injury. Biomed Pharmacother. 112(108664)2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Xie MY, Hou LJ, Sun JJ, Zeng B, Xi QY, Luo
JY, Chen T and Zhang YL: Porcine milk exosome MiRNAs attenuate
LPS-induced apoptosis through inhibiting TLR4/NF-κB and p53
pathways in intestinal epithelial cells. J Agric Food Chem.
67:9477–9491. 2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Zeng R, Xu H, Liu Y, Du L, Duan Z, Tong J,
He Y, Chen Q, Chen X and Li M: miR-146a inhibits biofilm-derived
cutibacterium acnes-induced inflammatory reactions in human
keratinocytes. J Invest Dermatol. 139:2488–2496.e4. 2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Zeng Z, Gong H, Li Y, Jie K, Ding C, Shao
Q, Liu F, Zhan Y, Nie C, Zhu W, et al: Upregulation of miR-146a
contributes to the suppression of inflammatory responses in
LPS-induced acute lung injury. Exp Lung Res. 39:275–282.
2013.PubMed/NCBI View Article : Google Scholar
|