Introduction
Kawasaki disease (KD) is an acute febrile rash
disease in children, which is a type of non-specific vasculitis.
The main cause of the disease is systemic inflammatory response
(1). Arteries, veins and
capillaries may be involved. Arteries are the most vulnerable,
which is currently the leading cause of acquired heart disease in
children (2,3). KD can be divided into complete KD and
incomplete KD. Incomplete KD has fewer types of symptoms than
complete KD. However, the diagnosis and treatment of patients with
incomplete symptoms are often delayed, and patients with incomplete
KD have a similar risk of coronary artery abnormalities as those
with complete KD (4,5). Therefore, improving the rate of a
correct diagnosis and timely treatment is valuable to reduce the
incidence of KD and its risk of coronary artery damage. KD in
children under 1 year of age is difficult to be diagnosed. Infants
show less classic clinical features; thus it is difficult to meet
the typical KD diagnostic criteria (6), which increases the possibility of
misdiagnosis. At present, apart from clinical manifestations, the
diagnosis of KD also depends on the support of laboratory tests. KD
biomarkers may facilitate the early diagnosis of KD (7). Typical KD biomarkers include
N-terminal brain natriuretic peptide, neutrophil to lymphocyte
ratio (NLR), C-reactive protein (CRP), D-dimer (DD), tumor necrosis
factor-α (TNF-α) and interleukin-6 (IL-6) (8-11).
However, it is still unknown whether the above-mentioned biomarkers
can be used as judgment criteria for gamma globulin
(IVIG)-sensitive and -insensitive incomplete KD. miRNAs are a type
of non-coding single-strand RNA molecules approximately 22
nucleotides long. They can bind to target mRNAs and participate in
post-transcriptional gene expression regulation. A variety of
miRNAs are related to inflammatory cell differentiation and
development, regulation of inflammatory factors, and vascular
inflammatory damage (12). Studies
have shown that miRNA-21, miRNA-145, miRNA-155 and miRNA-199b-5p
are closely related to the occurrence and development of KD
(13-15).
However, there are few studies concerning the expression of the
above-mentioned miRNAs in patients with IVIG-sensitive and
-insensitive incomplete KD. Therefore, the present study provides a
basis for the clinical diagnosis of incomplete KD, and for drug
selection and prognostic evaluation of these patients.
Patients and methods
Patient information
One hundred and eighty-five patients with incomplete
KD were collected as the KD group. They were admitted to Xuzhou
Children's Hospital Affiliated to Xuzhou Medical University from
March 2016 to December 2018. There were 92 males and 93 females,
with a mean age of 4.3±3.1 years. According to the American Heart
Association (AHA), inclusion criteria were as follows: Patients
aged 2 months to 12 years, with fever that lasts for at least 5
days without any other explanation (>38˚C). Patients had at
least 2 symptoms, including: polymorphous rash (rash in any form),
bilateral bulbar conjunctival injection without exudate, erythema
of the oral mucosa (including lips, pharynx or tongue), changes in
peripheral limbs (including erythema on the palms or soles and/or
swelling of the hands or feet) and cervical lymphadenopathy (at
least the diameter of 1 lymph node ≤1.5 cm) (4,16).
Exclusion criteria: Patients with severe hematological diseases;
patients with combined liver and kidney dysfunction; patients
allergic to treatment drugs. One hundred and eighty-two patients
with respiratory infections were taken as the control group. They
had a mean age of 4.2±3.0) years, and included 90 males and 92
females. Inclusion criteria consisted of patients with respiratory
infections aged 2 months to 11 years. Exclusion criteria included
patients with severe blood system diseases; patients with combined
liver and kidney dysfunction; patients with previous history of KD.
The study was approved by the Ethics Committee of Xuzhou Children's
Hospital. Signed written informed consents were obtained from the
patients and/or guardians.
All patients in the KD group received aspirin (30
mg/kg orally daily) and gamma globulin (IVIG, 1 g/kg intravenously
daily). The patients were divided into the IVIG-sensitive KD group
(n=104) and IVIG-insensitive KD group (n=81). The criteria for the
IVIG-insensitive KD group included patients for whom the first
treatment of IVIG was ineffective, the body temperature remained
over 38˚C after 48 h, or the body temperature rose again within 2-7
days after administration, and who had at least 1 symptom in the
inclusion criteria of incomplete KD as mentioned above.
Methods
Neutrophil to lymphocyte ratio (NLR)
and C-reactive protein (CRP) testing
Five milliliters of fasting venous blood was
collected from the control group, IVIG-sensitive KD group and
IVIG-insensitive KD group in the morning. The Siemens BN-II
automatic protein analyzer was used to determine CRP before
treatment and 2 days after IVIG was administered. The blood cell
meter was used to determine the neutrophil to lymphocyte ratio
(NLR), C-reactive protein (CRP) levels, white blood cell count
(WBC), hemoglobin level (Hb), platelet count (PLT) levels before
treatment and 2 days after IVIG was used. Au5400 automatic
biochemical analyzer (Beckman Coulter, Inc.) was used to test the
indicators of liver function [alanine aminotransferase (ALT) and
aspartate aminotransferase (AST)] in patients before treatment.
Beckman Coulter FC500 flow cytometer (Beckman Coulter, Inc.) was
used to test treatment pre-T cell subset index (CD3+,
CD3+CD4+).
miRNA relative expression levels
Peripheral blood mononuclear cells were obtained
from the control group, IVIG-sensitive KD group and
IVIG-insensitive KD group, and a certain amount of cell lysate was
added. Total RNA extraction kit was used to extract total RNA
according to the TRIzol method. RevertAid™ H Minus First Strand
cDNA Synthesis Kit (Fermentas) was used to prepare the mRNA reverse
transcription system to synthesize cDNA by reverse transcription.
The primer sequences of miRNA-21, miRNA-145, miRNA-155, and
miRNA-199b-5p are as previously documented (17-20).
The reaction conditions of PCR consisted of
pre-denaturation at 94˚C for 3 min, for a total of 40 cycles (at
94˚C for 30 sec at 60˚C for 30 sec, and at 72˚C for 45 sec). Each
samples was set up with 3 parallel duplicate wells. U6 was taken as
an internal reference, and 2-ΔΔCq (21) was used to calculate the relative
expression of miRNA-21, miRNA-145, miRNA-155, and miRNA-199b-5p in
peripheral blood. The primers are documented in Table I.
 | Table IPrimer sequences of miRNA-21,
miRNA-145, miRNA-155 and miRNA-199b-5p. |
Table I
Primer sequences of miRNA-21,
miRNA-145, miRNA-155 and miRNA-199b-5p.
| Name | Upstream | Downstream | (Refs.) |
|---|
| miRNA-21 |
5'-ACACTCCAGCTGGGTAGCTTATCAGACTGATG-3' |
5'-CTCAACTGGTGTCGTGGA-3' | (17) |
| miRNA-145 |
5'-GTCCAGTTTTCCCAGGAATCCCT-3' |
5'-TCCAGTCCTATTGAATGTGGGA-3' | (18) |
| miRNA-155 |
5'-TTAATGCTAATCGTGACT-3' |
5'-ACCTGAGAGTAGACCAGA-3' | (19) |
| miRNA-199b-5p |
5'-CAGCCCAGTGTTTAGACTATC-3' |
5'-CAGTGCAGGGTCCGAGGT-3' | (20) |
| U6 |
5'-CTCGCTTCGGCAGCACATATACT-3' |
5'-ACGCTTCACGAATTTGCGTGTC-3' | (20) |
Inflammatory factor expression
The fasting venous blood of the study subjects in
the above three groups was collected from 9:00-10:00 Beijing time
in the morning. After centrifugation, the samples were marked with
the date, group and name. They were tested in strict accordance
with the instructions contained in the TNF-α, IL-6 and IL-1β kits
(Shanghai Yubo Biological Technology Co., Ltd., product nos.
YBC102g, YBA079Ov01 and YBA056Bo01). The blank holes were adjusted
to zero, the absorbance (OD value) of each well samples was tested
sequentially at 450 nm wavelength. The concentration of the
standard was taken as the abscissa, and the OD value as the
ordinate to draw the standard curve. According to the OD value of
samples, the corresponding concentration was determined from the
standard curve, and then timed by the dilution factor to obtain the
values of TNF-α, IL-6 and IL-1β.
Statistical processing
All data were analyzed using SPSS 20.0 software (IBM
Corp.). Measurement data are expressed as mean ± standard deviation
(SD). The data of experimental indicators in each group conformed
to the normal distribution and the variances are equal. The
comparison between multiple groups was conducted using one-way
analysis of variance (ANOVA), followed by the Student-Newmnan-Keuls
test. The sample comparison between two groups was conducted using
the independent sample t-test (unpaired). Receiver operating
characteristic (ROC) curve was used to determine the best
predictive value. P<0.05 was considered as indicative of a
statistically significant result.
Results
General results
There was no significant difference in age and sex
between the two groups (P>0.05; P>0.05). Before treatment,
the levels of NLR, C-reactive protein (CRP), and white blood cells
(WBC) in the KD group were significantly higher compared with the
control group, and the differences were statistically significant
(P<0.05, P<0.05, P<0.05). There were no changes in alanine
aminotransferase (ALT), aspartate aminotransferase (AST),
hemoglobin (Hb), platelets (PLT), CD3+ and
CD3+CD4+ in patients of the KD group; the
differences were not statistically significant (P>0.05,
P>0.05, P>0.05, P>0.05, P>0.05, P> 0.05). The
results are shown in Table II.
 | Table IIComparison of the related indicators
before treatment in patients in the control and KD groups. |
Table II
Comparison of the related indicators
before treatment in patients in the control and KD groups.
| Basic indicators | KD group (N=185) | Control group
(N=182) | t | P-value |
|---|
| NLR | 5.91±1.24 | 1.01±0.81 | 44.888 | <0.001 |
| CRP (mg/l) | 78.07±59.03 | 19.22±28.83 | 12.165 | <0.001 |
| ALT (U/l) | 41.21±70.04 | 39.71±14.44 | 0.285 | 0.776 |
| AST (U/l) | 39.08±67.54 | 38.61±16.64 | 0.092 | 0.927 |
| WBC
(x109/l) | 17.61±3.85 | 10.21±2.64 | 21.504 | <0.001 |
| Hb (g/l) | 102.26±10.85 | 104.08±10.65 | 1.621 | 0.106 |
| PLT
(x109/l) | 397.61±47.85 | 405.01±87.65 | 1.002 | 0.317 |
| CD3+
(%) | 64.51±9.25 | 65.31±7.45 | 0.913 | 0.362 |
|
CD3+CD4+ (%) | 42.71±8.95 | 41.69±8.79 | 1.101 | 0.271 |
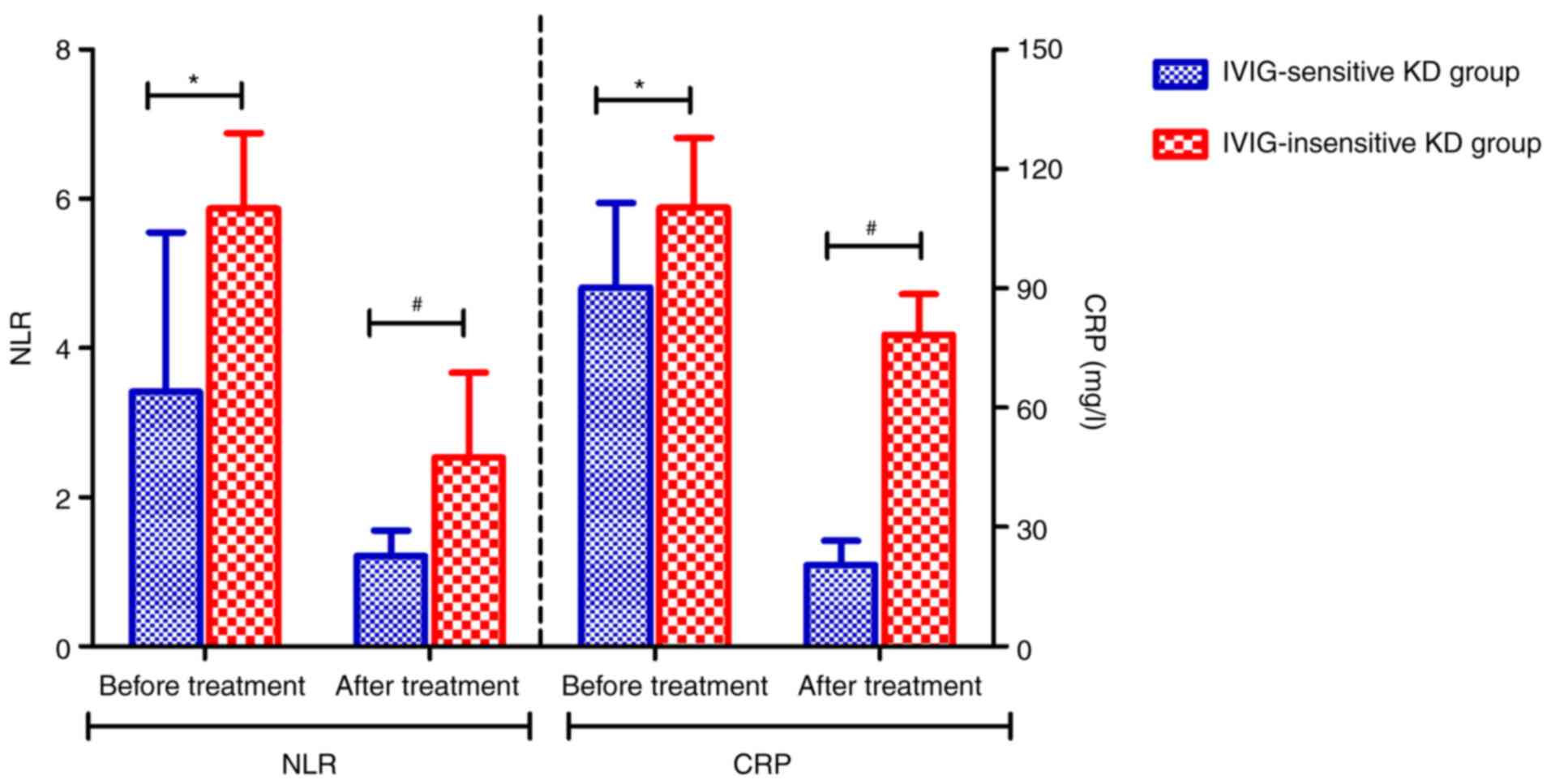
Comparison of the levels of NLR and
CRP in IVIG-sensitive and IVIG-insensitive KC groups
The results showed that the levels of NLR and CRP of
patients in the IVIG-insensitive KD group were higher than those in
the IVIG-sensitive KD group before IVIG treatment, and the
differences were statistically significant (P<0.05, P<0.05).
Compared with the IVIG-sensitive KD group, the levels of NLR and
CRP in the IVIG-insensitive KD group were increased after IVIG
treatment, and the difference was statistically significant
(P<0.05, P<0.05) (Fig.
1).
Predictive value of NLR and CRP to the
sensitivity of IVIG of incomplete KD
Before IVIG treatment, the AUC indicated that the
predictive value of NLR for IVIG-insensitive patients was 0.72 (95%
CI, 0.64-0.80), the sensitivity was 0.74, and the specificity was
0.67. AUC indicated that the predictive value of CRP for
IVIG-insensitive patients was 0.72 (95% CI, 0.64-0.79), the
sensitivity was 0.77, and the specificity was 0.61. After IVIG
treatment, AUC indicated that the predictive value of NLR for
IVIG-insensitive patients was 0.74 (95% CI, 0.68-0.81), the
sensitivity was 0.64, and the specificity was 0.73. AUC indicated
that the predictive value of CRP for IVIG-insensitive patients was
0.82 (95% CI, 0.76-0.88), the sensitivity was 0.68, and the
specificity was 0.82 (Fig. 2).
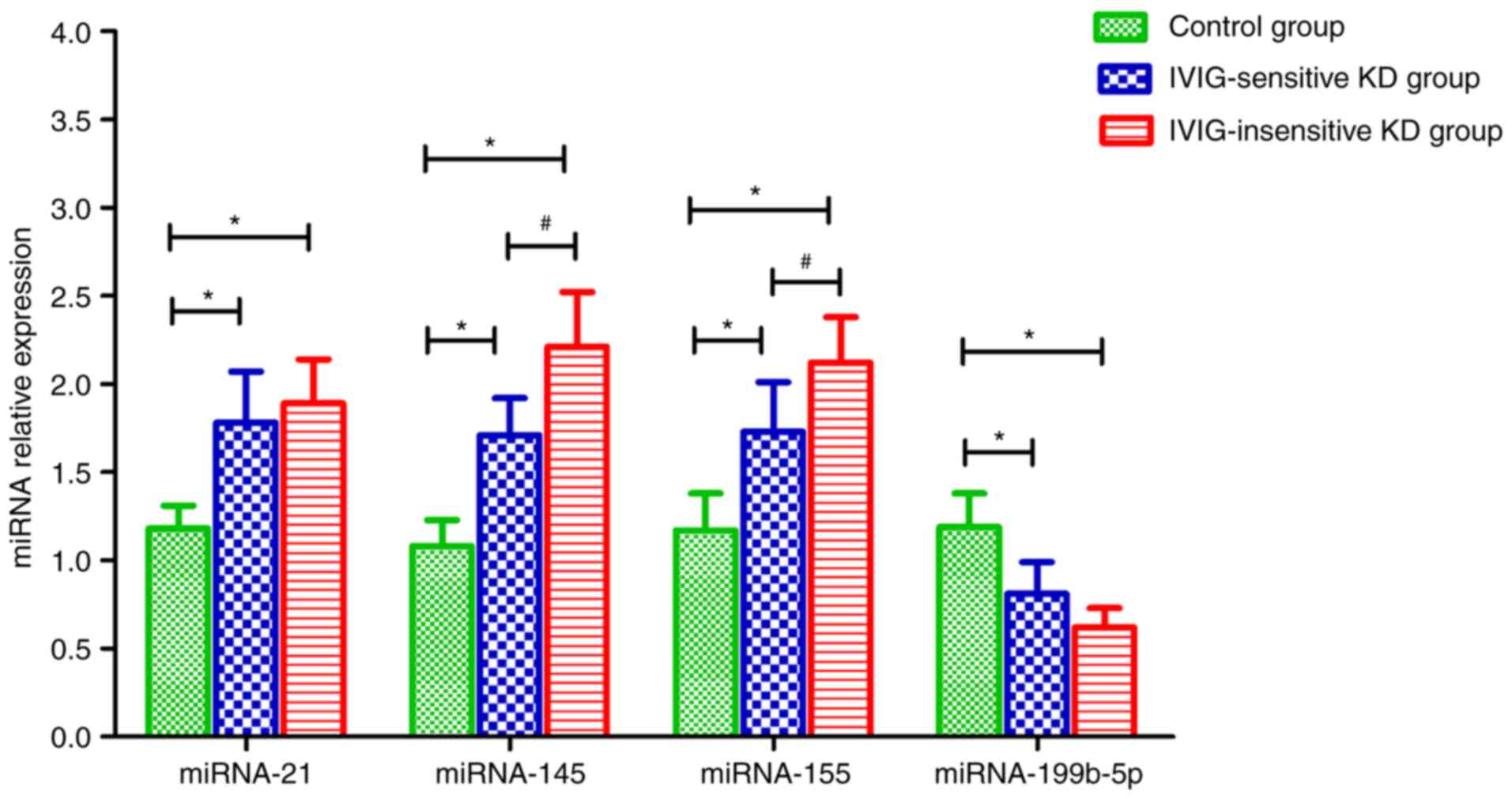
miRNA relative expression
The results showed that compared with the control
group, the relative expression of miRNA-21, miRNA-145 and miRNA-155
in the serum of patients in the IVIG-sensitive and IVIG-insensitive
KD groups was increased, and the difference was statistically
significant (P<0.05, P<0.05, P<0.05, P<0.05).
Meanwhile, the relative expression of miRNA-199b-5p was decreased
compared to the control group, and the difference was statistically
significant (P<0.05). Compared with the IVIG-sensitive KD group,
the relative expression of miRNA-145 and miRNA-155 was increased,
and the difference was statistically significant (P<0.05,
P<0.05) (Fig. 3).
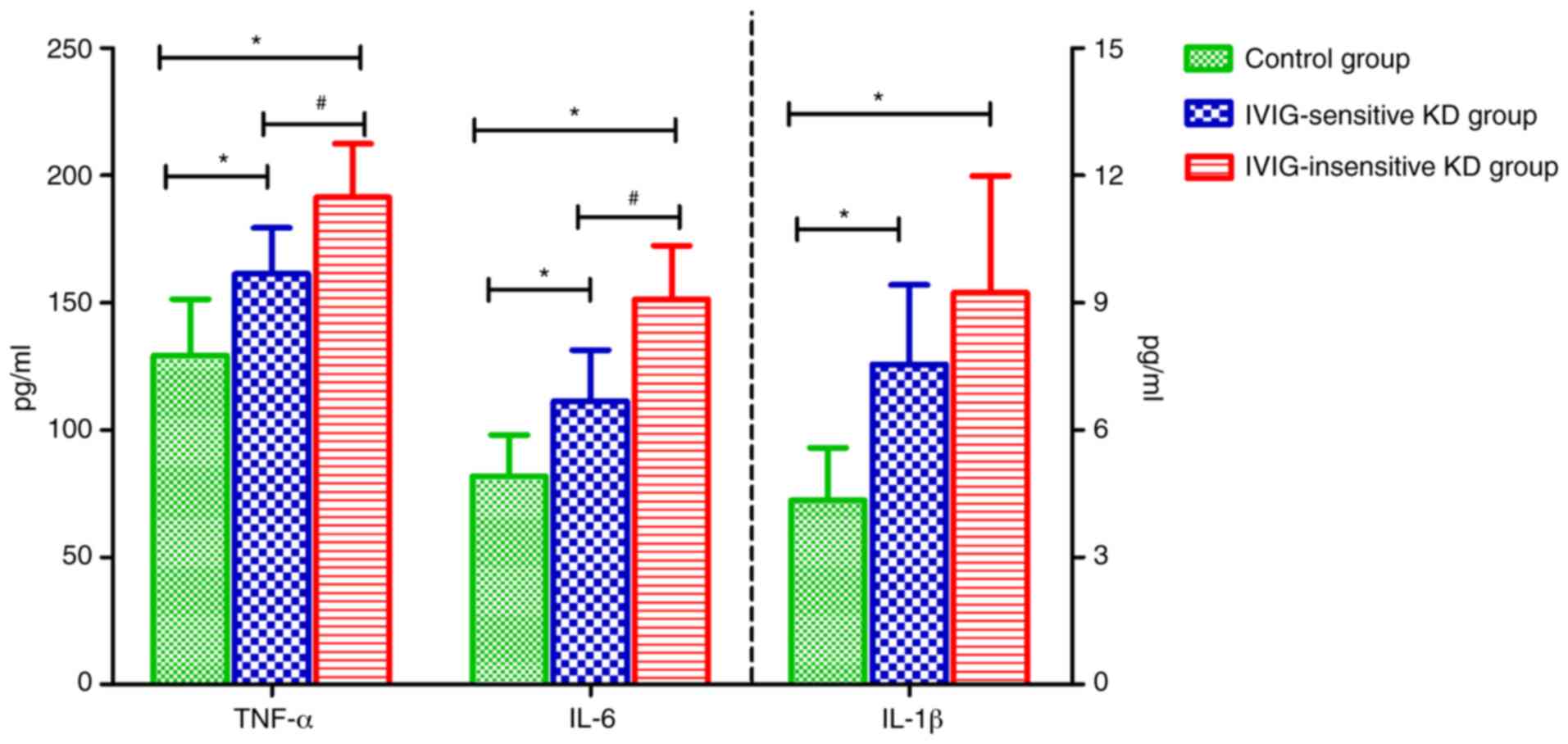
Inflammatory factor expression
The results showed that compared with the control
group, the levels of TNF-α, IL-6 and IL-1β in the serum of patients
in the IVIG-sensitive and IVIG-insensitive KD group were increased,
and the differences were statistically significant (P<0.05,
P<0.05, P<0.05). Compared with the IVIG-sensitive KD group,
the levels of TNF-α and IL-6 in the serum of the patients in the
IVIG-insensitive KD group were increased, and the differences were
statistically significant (P<0.05, P<0.05) (Fig. 4).
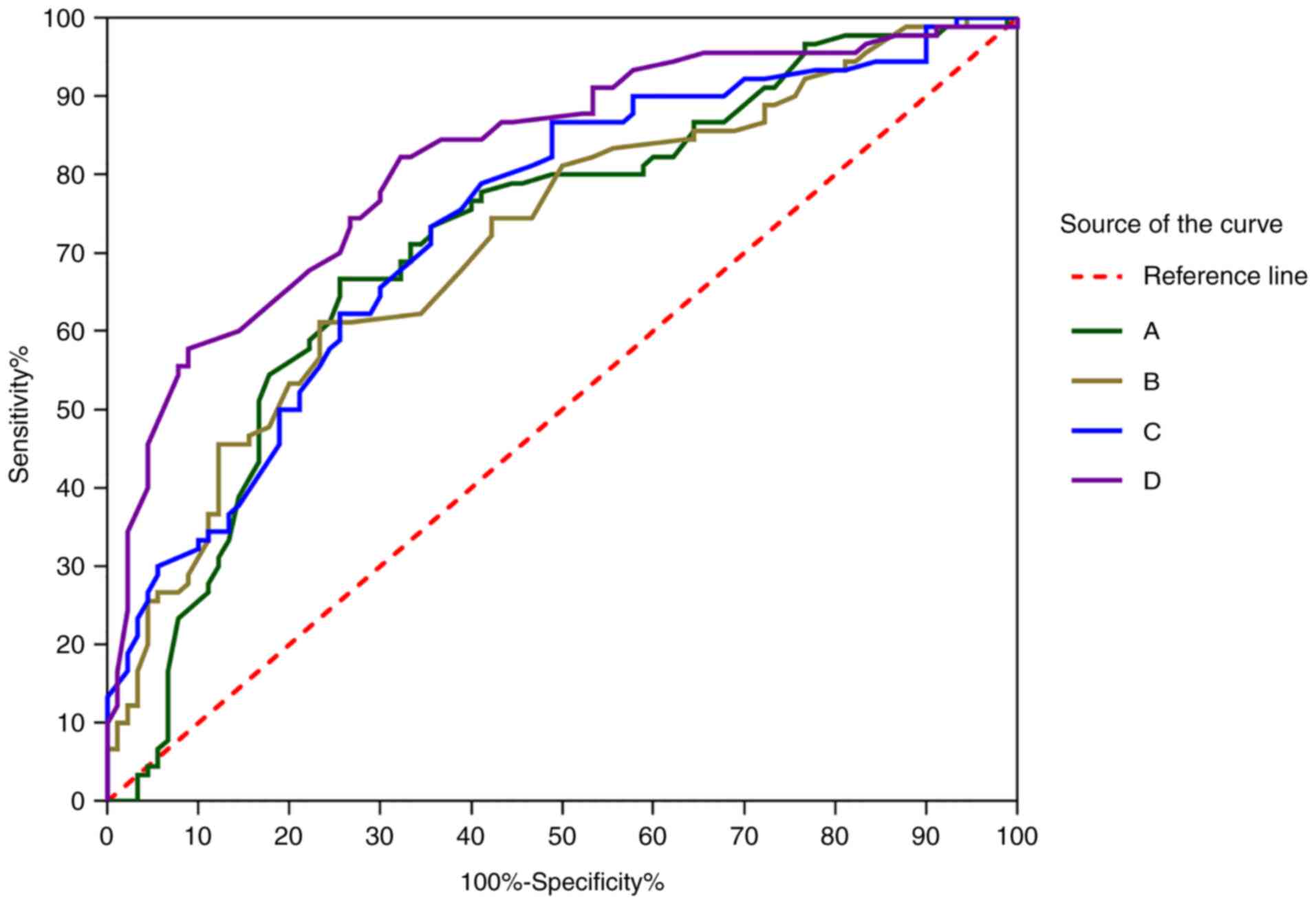
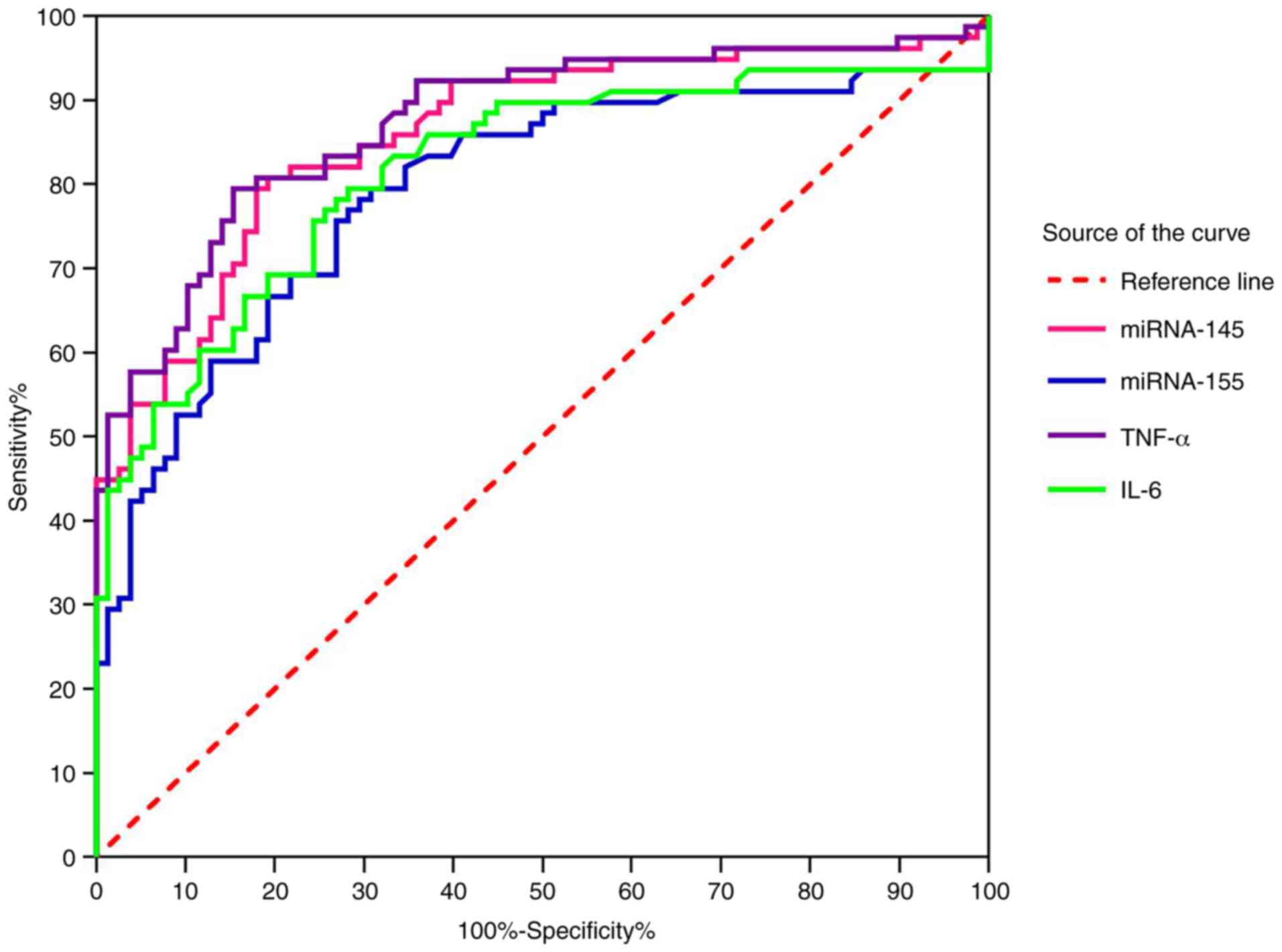
Predictive value of miRNA and
inflammatory factors to IVIG sensitivity
We predicted whether patients with incomplete KD are
sensitive to IVIG. The area under the ROC curve (AUC), the
sensitivity and the specificity of miRNA-145 were 0.86 (95% CI,
0.80-0.92), 0.81 and 0.81, respectively. The AUC, the sensitivity
and the specificity of miRNA-155 were 0.79 (95% CI, 0.72-0.87),
0.80 and 0.69, respectively. The AUC, the sensitivity and the
specificity of TNF-α were 0.87 (95% CI, 0.82-0.93), 0.80 and 0.85,
respectively. The AUC, the sensitivity and the specificity of IL-6
were 0.82 (95% CI, 0.75-0.89), 0.80 and 0.72, respectively.
(Fig. 5).
Discussion
In most children with Kawasaki disease (KD),
intravenous IVIG and oral aspirin therapy can quickly reduce
inflammatory factor levels, fever, and other clinical symptoms. But
approximately 15-20% of children receiving the initial gamma
globulin (IVIG) infusion show persistent or recurrent fever, which
is classified as IVIG-insensitive KD (11). Neutrophil to lymphocyte ratio (NLR)
and platelet to lymphocyte ratio (PLR) are related to the onset of
KD, which can be used as a basis for early diagnosis of KD. NLR is
a predictive factor of IVIG-insensitive KD (22). The present study demonstrated that
NLR and CRP were significantly increased in the KD group compared
with those in the control group, which was statistically
significant. The results were consistent with the reports in the
literature, indicating that NLR and CRP can be used as reference
indicators for the diagnosis of incomplete KD. In addition, this
study also found that before and after IVIG treatment, the levels
of NLR and CRP in patients in the IVIG-insensitive KD group were
significantly higher than those in the IVIG-sensitive KD group. The
results suggested that NLR and CRP can also be used as indicators
to predict whether patients with incomplete KD are sensitive to
IVIG, to provide a basis for drug selection and prognosis
evaluation.
IL-6 is an inflammatory cytokine produced in
response to the activation of monocytes and macrophages during the
acute phase of KD. It can stimulate other inflammatory markers, for
example, CRP. Higher levels of serum IL-6 and CRP are related to
coronary artery damage and insensitivity to IVIG. Therefore, serum
IL-6 can be used as a novel marker (23) to predict coronary artery involvement
and resistance to IVIG. TNF-α is an inflammatory cytokine, which
plays an important role in the defense against infection and immune
response. Compared with other cases without treatment or without
IVIG, TNF-α blockers have a beneficial effect on treatment
resistance after the start of KD treatment (11). The present study found that compared
with the control group, the levels of TNF-α and IL-6 in the serum
of patients with IVIG-sensitive and IVIG-insensitive KD group were
increased, which were basically consistent with those reported in
the literature (22,23). In addition, compared with the
IVIG-sensitive KD group, the levels of TNF-α and IL-6 in the serum
of patients in the IVIG-insensitive KD group were higher. In this
study, the levels of TNF-α and IL-6 in the three groups were
consistent with the trends of NLR and CRP levels. The results
confirmed that there may be a close positive correlation between
incomplete KD and inflammation, but its mechanism needs to be
further studied.
Inflammatory response is a key mechanism of KD
pathogenesis, and miRNAs may be the main regulators of this
inflammatory response (24).
Multiple studies (18-21)
have shown that compared with the control group, the expression
levels of serum miRNA-21, miRNA-145 and miRNA-155 in KD are
relatively increased. In comparison, the relative expression of
miRNA-199b-5p is reduced. miRNA-199b-5p can be used as an auxiliary
indicator for KD diagnosis. The present study found that compared
with the control group, the relative expression of serum miRNA-21,
miRNA-145 and miRNA-155 of patients in the IVIG-sensitive and
IVIG-insensitive KD groups were all increased, while the relative
expression of miRNA-199b-5p was decreased. The results were
basically consistent with the reports in the literature. The
difference is that there was no reclassification of patient
coronary artery lesions, which is a deficiency of this study. In
addition, the present study also found that the relative expression
levels of miRNA-145 and miRNA-155 in the serum of patients with
IVIG-insensitive KD group were higher than those in the
IVIG-sensitive KD group; the difference was statistically
significant. The results suggest that the relative expression
levels of serum miRNA-145 and miRNA-155 can also be used as
indicators to predict whether patients with incomplete KD are
sensitive to IVIG. No similar report has been reported at present.
The sample size of the IVIG-sensitive KD group (N=104) and the
IVIG-insensitive KD group (N=81) in this study was small. To
further confirm whether miRNA-145 and miRNA-155 can be sensitive
indicators of IVIG, it is necessary to further expand the sample
size.
Studies have reported that plasma miRNA-155 can show
the calculation ability of atrial fibrillation recurrence after
cardioversion. It is positively correlated with serum B-type
natriuretic peptide (BNP), TNF-α, CRP and IL-6(25). IL-6 can stimulate inflammation
marker CRP. In addition, there have been studies that have reported
that miRNA-217 can regulate the Toll-like receptor 4/nuclear
factor-κB (TLR4/NF-κB) signaling transduction pathway, block
inflammatory response, and improve lung injury caused by lung
tissue protection (26).
Lipopolysaccharide (LPS) of TLR4/NF-κB signaling pathway can
increase the expression of TNF-α and IL-6 in hippocampus tissue of
rats, induce brain neuroinflammation, and ultimately lead to
cognitive memory impairment (27).
The above studies have shown that miRNAs can mediate the TLR4/NF-κB
signaling pathway and affect the expression of inflammatory
factors. In this study, it was found that the relative expression
of miRNAs and the levels of inflammatory factors are consistent.
Therefore, we speculated that changes in serum-related miRNAs in
patients with incomplete KD may activate the TLR4/NF-κB signal
transduction pathway, promote inflammatory responses, leading to
changes in the inflammatory factors of IL-6 and TNF-α, and
stimulating the increase in NLR and CRP levels. Eventually they
lead to systemic inflammation, KD and related complications, which
needs to be further studied.
To summarize, the expression of miRNAs and
inflammatory factors in serum of patients with IVIG-sensitive and
-insensitive incomplete KD differ. It is necessary to further
verify whether the levels of NLR, CRP, TNF-α and IL-6 and the
expression of miRNA-145 and miRNA-155 in serum can be used as
potential predictors of the sensitivity of patients with incomplete
KD to IVIG.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YW, CL, LN and XA conceived and designed this study.
YW, CL, MF, JT and XA helped with data collection and summary. CL,
LN, MF and JT were responsible for data analysis and
interpretation. XA made contributions to manuscript writing. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Xuzhou Children's Hospital. Signed written informed consents were
obtained from the patients and/or guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lazea C, Man O, Sur LM, Serban R and Lazar
C: Unusual presentation of kawasaki disease with gastrointestinal
and renal manifestations. Ther Clin Risk Manag. 49:1411–1416.
2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Eleftheriou D, Levin M, Shingadia D,
Tulloh R, Klein NJ and Brogan PA: Management of Kawasaki disease.
Arch Dis Child. 99:74–83. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kowalczyk M, Turska-Kmieć A, Ziółkowska L,
Raszek M and Kawalec W: Symptoms, diagnosis and characteristic
abnormalities in the coronary arteries in Kawasaki disease in
children. Med Wieku Rozwoj. 14:344–349. 2010.PubMed/NCBI(In Polish).
|
|
4
|
Pacheco DA, Miller CR, Boor PJ and Mambo
NC: Incomplete Kawasaki disease with development of fatal coronary
artery thrombosis in a 13-year-old male. Cardiovasc Pathol.
42:54–58. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Shi H, Qiu H, Jin Z, Li C, Yang X, Huang
C, Wu R, Zhuang G and Chu M: Coronary artery lesion risk and
mediating mechanism in children with complete and incomplete
Kawasaki disease. J Investig Med. 67:950–956. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ellepola C, Borsheim K, James J, Mitchell
M, Gudausky T and Frommelt PC: Giant thrombotic right coronary
aneurysm in an infant with undiagnosed incomplete Kawasaki disease
and rapidly progressive cardiovascular collapse. CASE (Phila).
3:250–254. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ha KS, Lee J, Jang GY, Lee JB, Lee KC, Son
CS and Lee JW: Value of neutrophil-lymphocyteratio in predicting
outcomes in Kawasaki disease. Am J Cardiol. 116:301–306.
2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Tazón-Varela MA, Alonso-Valle H and
Muñoz-Cacho P: On the usefulness of N-terminal prohormone of brain
natriuretic peptide for the diagnosis of incomplete Kawasaki
disease. Emergencias. 31:366–367. 2019.PubMed/NCBI
|
|
9
|
Hwang SY, Shin TG, Jo IJ, Jeon K, Suh GY,
Lee TR, Yoon H, Cha WC and Sim MS: Neutrophil-to-lymphocyte ratio
as a prognostic marker in critically-ill septic patients. Am J
Emerg Med. 35:234–239. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Azab B, Zaher M, Weiserbs KF, Torbey E,
Lacossiere K, Gaddam S, Gobunsuy R, Jadonath S, Baldari D, McCord D
and Lafferty J: Usefulness of neutrophilto lymphocyte ratio in
predicting short- and long-term mortalityafter non-ST-elevation
myocardial infarction. Am J Cardiol. 106:470–476. 2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yamaji N, da Silva Lopes K, Shoda T,
Ishitsuka K, Kobayashi T, Ota E and Mori R: TNF-α blockers for the
treatment of Kawasaki disease in children. Cochrane Database Syst
Rev. 8(CD012448)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kyuno D, Zhao K, Bauer N, Ryschich E and
Zöller M: Therapeutic targeting cancer-initiating cell markers by
exosome mirna: Efficacy and functional consequences exemplified for
claudin7 and EpCAM. Transl Oncol. 12:191–199. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sheng W, Gao C and Guan H: Expression and
its significance of MicroRNA-1 4 5 and MicroRNA-1 4 3 in plasma in
children with Kawasaki disease. J Mod Lab Med. 31:34–37. 2016.
|
|
14
|
Xie Y, Li W, Feng J, Wu T and Li J:
MicroRNA-363 and GATA-1 are regulated by HIF-1α in K562 cells under
hypoxia. Mol Med Rep. 14:2503–2510. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Busch A, Eken SM and Maegdefessel L:
Prospective and therapeutic screening value of non-coding RNA as
biomarkers in cardiovascular disease. Ann Transl Med.
4(236)2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yim D, Curtis N, Cheung M and Burgner D:
An update on Kawasaki disease II: Clinical features, diagnosis,
treatment andoutcomes. J Paediatr Child Health. 49:614–623.
2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zou YC, Yan LM, Gao YP, Wang ZY and Liu G:
miR-21 may act as a potential mediator between inflammation and
abnormal bone formation in ankylosing spondylitis based on TNF-α
concentration-dependent manner through the JAK2/STAT3 pathway. Dose
Response. 18(1559325819901239)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yong Q, Nan Y, Lei Z, Li P, Liu K, Wu HW,
Shen Y and Li ZD: Expression of serum microRNA-145 in patients with
non-small cell lung cancer and its clinical significance. J Med
Postgra. 29:62–65. 2016.
|
|
19
|
Zhang X, Wang PH and Liao L: The test of
miRNA-155 and miRNA-26a for the children with bronchial asthma in
airway remodeling in the clinic application. J Chin Phys.
18:1506–1510. 2016.
|
|
20
|
LI WH, LI YX, Guo JK, Pan HG, Zhang YL and
Wang X: Overexpression of miR-199b-5p inhibits Ewing's sarcoma cell
lines by targeting CCNL1. Mol Med Rep. 12:3359–3364.
2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Chen Y, Hua Y, Zhang C, Chen S, Zhang Q,
Liao Y, Yan H, Wang Y, Liu P, Qi J, et al: Neutrophil-to-lymphocyte
ratio predicts intravenous immunoglobulin-resistance in infants
under 12-months old with Kawasaki disease. Front Pediatr.
7(81)2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Nandi A, Pal P and Basu S: A comparison of
serum IL6 and CRP levels with respect to coronary changes and
treatment response in Kawasaki disease patients: A prospective
study. Rheumatol Int. 39:1797–1801. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yun KW, Lee JY, Yun SW, Lim IS and Choi
ES: Elevated serum level of MicroRNA (miRNA)-200c and miRNA-371-5p
in children with Kawasaki disease. Pediatr Cardiol. 35:745–752.
2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhang X, Xiao XP, Ren XA and Cui T: Plasma
miRNA-155 levels predict atrial fibrillation recurrence after
cardioversion. Heart Surg Forum. 22:E140–E148. 2019.PubMed/NCBI View
Article : Google Scholar
|
|
26
|
Li J, Feng Shao F, Xu J, et al: Inhibition
effects of miR-217 on sepsis induced lung injury in rats based on
TLR4 signaling pathway. J Hainan Med University. 26:6–11. 2020.
|
|
27
|
Tang S, Yan LR, Ma ZG and Ji C: Influences
of the TLR4/NF-κB pathway on memory function and inflammatory
factors in rats with cerebral small vessel disease. Eur Rev Med
Pharmacol Sci. 23:6264–6271. 2019.PubMed/NCBI View Article : Google Scholar
|