Introduction
One of the key treatments in regenerative medicine
is stem cell therapy. Thus, the maintenance of stem cells is
essential. Maintenance of the undifferentiated state and
proliferation activity of stem cells is essential to sustain their
functionality (1). It has been
hypothesized that this requirement can be met by mimicking the
in vivo extracellular matrix (ECM) configuration, thereby
modulating the activity of stem cells in vitro (2). The principle behind this hypothesis is
that the ECM not only functions as structural support for stem
cells in vivo but also provides biochemical cues for their
maintenance versus directed differentiation (3).
Basement membranes (BMs) are a subgroup of the ECM
that is necessary for cell differentiation during early
developmental processes. In addition, BMs are critical for the
formation and maintenance of mature tissues (4,5).
Laminin, one of the components of BMs, consists of three
genetically distinct subunits called α, β and γ chains, which are
assembled into cross-shaped molecules (6,7). At
present, 5α, 3β and 3γ chains, as well as at least 16 different
trimeric laminin isoforms are known in humans and mice each
(8). Laminin-mediated cell
attachment and cellular behavior are achieved by five different α
chains. Among them, laminin α5 (Lα5) plays a key role during
embryogenesis. Lα5, expressed in the inner cell mass of the
blastocyst, supports the self-renewal of embryonic stem cells
(7,9). Lα5-knockout mouse or zebrafish models
have severe developmental defects, demonstrating the effect of Lα5
on stem cell maintenance and embryonic development (10). Most of the cellular binding regions
of laminins are primarily located in the C-terminal large globular
(G) domain of the protein. The G domain consists of only the
laminin α chain and is subdivided into five homologous LG domains
(4,11). LG domains are important for
interaction with cells (12). LG1,
LG2 and LG3 are the first three of the LG domains, assembled into a
clover-leaf arrangement to form the putative binding sites of
laminin-binding integrin (11).
LG1-3 domains of the α5 chain comprise cell-binding sites, which is
mediated by integrins α3β1, α6β1 and α6β4. LG4 domain of the α5
chain binds to α-dystroglycan and heparin (12-14).
Elastin is an important ECM protein found abundantly
in blood vessels and serves as a biomechanical and physiological
signal for cells (15). The soluble
form of elastin, tropoelastin, is primarily located in elastic
tissues. It has been reported to promote the proliferation of
hematopoietic stem cells (HSCs) and mesenchymal stem cells (MSCs)
(16). Additionally, it is often
used in the form of cross-linked gel fibers or injectable scaffolds
for tissue engineering and drug delivery (17). Elastin-like polypeptides (ELPs) are
recombinant peptide polymers composed of VPGXG pentapeptides (found
in tropoelastin), where X stands for any amino acid residue except
proline (18). ELPs exhibit
biocompatibility that is similar to elastin in terms of mechanical
and viscoelastic properties. ELPs effectively modulate the
migration, proliferation, and differentiation of various type of
mammalian cells (19). These
polypeptides can be genetically engineered to modulate their
structural and biological properties and exhibit an inverse phase
transition behavior in response to the changes in their solutions
(20). Recombinant ELPs have been
expressed in E. coli and subsequently purified through a
separation process known as inverse transition cycling (ITC)
(21).
Human MSCs (hMSCs) are pluripotent stem cells that
can differentiate both in vitro and in vivo. In
particular, it is known that hMSCs can differentiate into the
mesenchymal lineage, forming bone, cartilage, adipose, and muscle
cells. These stem cells can be isolated from cord blood, placental
fluids, and multiple adult tissues, such as bone marrow, adipose
tissue, skeletal muscle, and connective tissue (22-27).
Previously, we have shown that a recombinant human laminin α2 LG1-3
promotes hMSCs physiology in vitro, including cell adhesion,
proliferation, and stemness (28).
In this study, we fused the coding sequence of ELP to lamininα5
LG1-3 domains and examined the cellular effects of this fusion
construct (Lα5LG1-3/ELP) on hMSCs.
Materials and methods
Construction and purification of
Lα5LG1-3/ELP fusion protein
Lα5LG1-3 coding sequence containing integrin-binding
modules LG1-LG3 of laminin α5 (Lα5) and ELP coding sequence were
synthesized (Genotech).
Based on the characteristics of ELP guest residues
(19-21),
ELP coding sequence was designed by inserting Val, Leu, and Gly
(ratio of 17:4:9) into ELP guest residue position. The full-length
ELP(V17L4G9-30) coding sequence containing Val, Leu, and Gly in the
fourth guest residue (Xaa) (Val-Pro-Gly-Xaa-Gly) of the
pentapeptide repeat is VPGGG VPGVG VPGGG VPGVG VPGVG VPGGG VPGVG
VPGVG VPGGG VPGLG VPGVG VPGVG VPGGG VPGVG VPGLG VPGVG VPGVG VPGGG
VPGVG VPGLG VPGVG VPGVG VPGGG VPGVG VPGLG VPGVG VPGVG VPGGG VPGVG
VPGGG.
The Lα5LG1-3 construct was double digested with
SacI and KpnI and then cloned into the expression
vector pBAD-His (Invitrogen; Thermo Fisher Scientific, Inc.) to
construct the pBAD-His-Lα5LG1-3. Next, ELP construct was double
digested with KpnI and EcoRI and then cloned into
pBAD-His-Lα5LG1-3 to construct the pBAD-His-Lα5LG1-3/ELP.
The recombinant pBAD-His-Lα5LG1-3/ELP was
transformed into E. coli TOP10 cells through heat shock at
42˚C. A single colony was selected and inoculated into 20 ml
Luria-Bertani (LB) media (LPS Solution) containing 100 µg/ml
ampicillin (LB-Amp) overnight at 37˚C. This overnight culture was
then mixed with 1 liter LB medium and incubated until the
OD600 reached 0.4. Subsequently, L-Arabinose was added
to a final concentration of 0.1% (w/v), and the culture temperature
was decreased to 20˚C. After 6 h of induction, bacterial cells were
pelleted by centrifugation at 4˚C and 6000 x g for 15 min. The
pellet was resuspended in sodium chloride-Tris-EDTA buffer and then
lysed by sonication. The lysate was obtained by centrifugation at
13,000 x g and 4˚C for 20 min. To obtain Lα5LG1-3/ELP, the
supernatant was purified through ITC. Briefly, the supernatant was
transferred into fresh tube with 3 M NaCl pre-heated to 40˚C,
followed by centrifugation at 40˚C to obtain the Lα5LG1-3/ELP
pellet. After discard of supernatant containing soluble
Lα5LG1-3/ELP, and the pellet was re-solubilized in cold
phosphate-buffered saline. The resulting solution was centrifuged
at 4˚C to remove any insoluble protein, and the supernatant,
containing pure Lα5LG1-3/ELP, was kept. The purity of this solution
was analyzed through 12% SDS-PAGE, followed by Coomassie blue
staining. Additionally, western blots analysis was performed using
a peroxidase-conjugated monoclonal anti-polyhistidine antibody (His
antibody, sc-8036 HRP; Santa Cruz Biotechnology, Inc.) diluted at
1:1,000 to assess the expression of Lα5LG1-3/ELP. The size of the
immunodetected protein was determined based on the pre-stained
protein markers (Elpis Biotech) electrophoresed in parallel
lanes.
Cell culture
Primary hMSCs were kindly provided by Dr Sung-Won
Kim (St. Mary's Hospital, the Catholic University of Korea)
(29,30). The cells were maintained in a
humidified incubator at 37 °C with 5% CO2 and cultured
in the growth medium (minimum essential medium, α-modification)
(α-MEM) (Welgene) containing 10% fetal bovine serum (Welgene), 100
µg/ml streptomycin, 100 U/ml penicillin, and 0.25 µg/ml
amphotericin B (Invitrogen; Thermo Fisher Scientific, Inc.).
Confluent cells were passaged by trypsinization (0.25% trypsin-EDTA
solution for 3 min). Cells were maintained for three passages
before use for further cell experiments.
Adhesion assay
Cell adhesion assay was carried out using crystal
violet. Culture plates were coated with 0, 0.01, 0.05, 0.1, 0.5,
1.0, or 5.0 µg/ml of purified Lα5LG1-3/ELP protein for 2 h at 37˚C.
Following three rinse with Dulbecco's phosphate-buffered saline
(DPBS) (Welgene), the plates were blocked with 0.5% (w/v) bovine
serum albumin (BSA) (Gibco; Thermo Fisher Scientific, Inc.) in DPBS
for 1 h at 37˚C. Subsequently, hMSCs were added (5x104
cells/well) and incubated at 37˚C for 30 min. Three replicates were
performed for each treatment. After incubation, non-attached cells
were removed by three rinses with DPBS, and the remaining cells
were fixed by incubation in 3.7% formalin solution for 20 min at
room temperature. The fixed cells were stained with 0.25% (w/v)
crystal violet (Sigma-Aldrich; Merck KGaA) for 30 min at room
temperature. Subsequently, they were extensively rinsed with DPBS
and then lysed using 2% SDS. The culture plates were read on a
microplate reader (BioTek) at 570 nm. Additionally, cell adhesion
was monitored with 15 min intervals to confirm the adhesion over
time. Cell adhesion activity was normalized relative to uncoated
plates.
MTT cytotoxicity assay
Twenty-four-well plates were coated with 1 µg/ml
Lα5LG1-3/ELP protein for 2 h at 37˚C and then rinsed three times
with DPBS. Subsequently, hMSCs were added (1x104
cells/well) and incubated for 7 days at 37˚C. Three replicates were
performed, and cell proliferation was assessed by the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT;
AMRESCO Inc.) assay, which measures the metabolic activity of
viable cells, according to the manufacturer's directions (Promega).
Briefly, cells were rinsed three times with DPBS and then incubated
with 0.5 ml/well of 5 µg/ml MTT in DPBS for 2 h at 37˚C. Afterward,
the MTT solution was removed, and the resulting formazan crystals
were dissolved in 150 µl/well of dimethyl sulfoxide. The plates
were read at 570 nm on a microplate reader (BioTek). Data were
normalized using the uncoated control.
Quantitative reverse
transcription-polymerase chain reaction (qRT-PCR)
The total RNA of the cell was isolated using the
Easy-Spin Total RNA Extraction Kit and then purified following the
manufacturer's directions (Intron). The RNA amount and quality were
assessed using a NanoDrop 2000 spectrophotometer (Thermo Fisher
Scientific, Inc.). Next, cDNA was synthesized from 1 µg of total
RNA by using the High Capacity cDNA Reverse Transcription Kit
according to the manufacturer's directions (Applied Biosystems;
Thermo Fishier Scientific, Inc.). qRT-PCR was performed using
SYBR-Green PCR Master Mix (Toyobo) and the ABI Step One Real-Time
PCR System (Applied Biosystems; Thermo Fisher Scientific, Inc.).
The PCR conditions were as follows: 10 min at 95˚C and 40 cycles of
95˚C for 15 sec and 60˚C for 1 min. The gene expression level was
normalized to that of β-actin and estimated with the comparative
Cq method (31). Each
experiment per sample was performed in triplicate. The primers used
are listed in Table I.
 | Table ISequences of the primers used for
reverse transcription-quantitative PCR. |
Table I
Sequences of the primers used for
reverse transcription-quantitative PCR.
| Gene | Forward primer | Reverse primer |
|---|
| β-actin |
5'-TGGCACCCAGCACAATGAAGAT-3' |
5'-TACTCCTGCTTGCTGATCCA-3' |
| CD90 |
5'-CCAAAGGCTTCTTCTTGCTG-3' |
5'-CCACCAAATGTGAAGACGTG-3' |
| CD105 |
5'-GAAAATGGAGCTCCTGGTCA-3' |
5'-ACCCTTAGCACCAACAGCAC-3' |
| CD73 |
5'-CAGTACCAGGGCACTATCTGG-3' |
5'-AGTGGCCCCTTTGCTTTAAT-3' |
Statistical analysis
ANOVA followed by a post-hoc Tukey's test was used
for comparisons between various groups. Differences between two
groups were tested via unpaired Student's t-test: P-value <0.05
was considered significant (*P<0.05,
**P<0.01 and ***P<0.001). Experiments
were repeated three times, and the results are presented as mean ±
SD.
Results
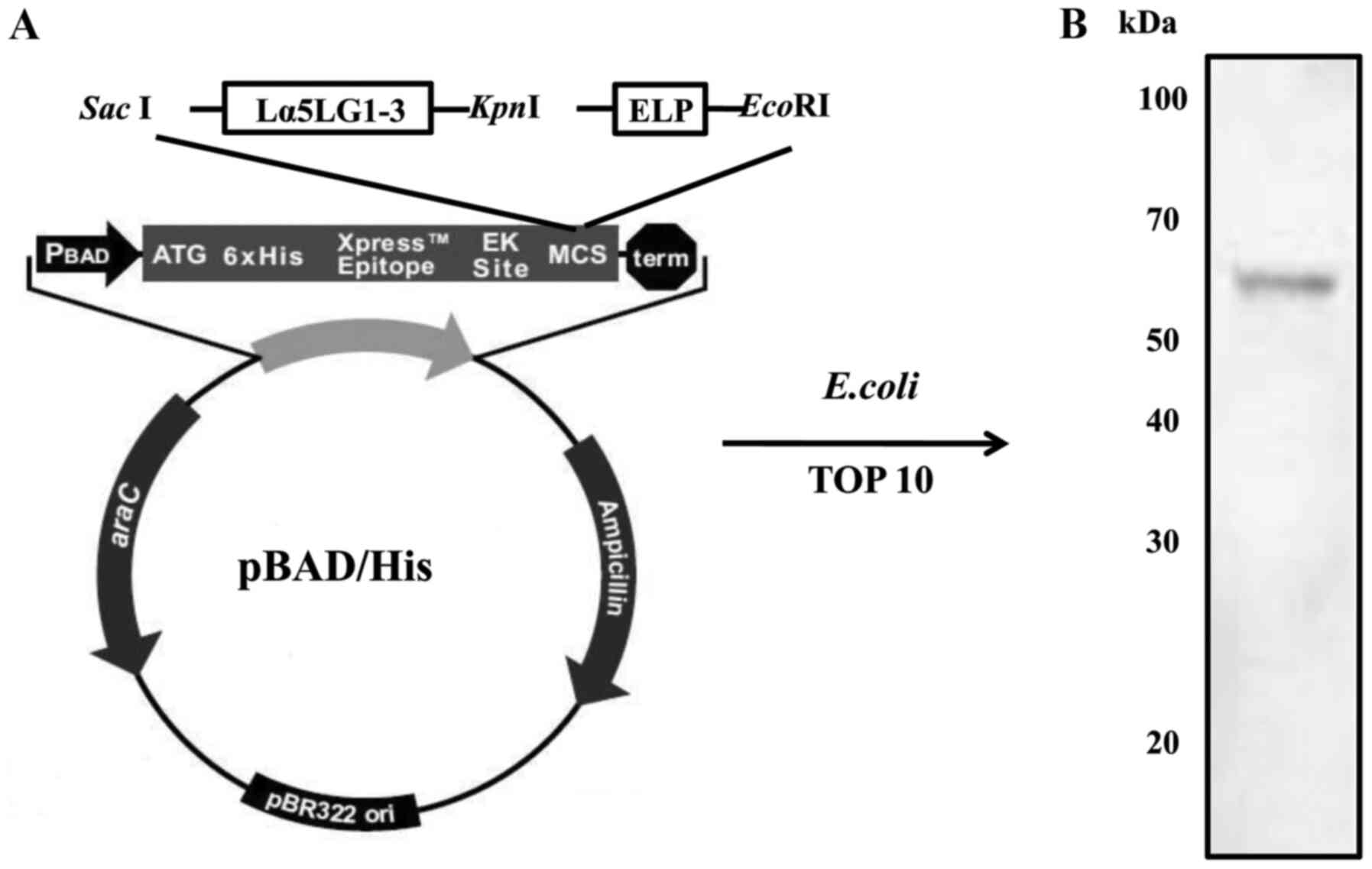
Expression and purification of
Lα5LG1-3/ELP protein
To make the recombinant Lα5LG1-3/ELP construct, the
Lα5LG1-3 sequence was inserted into the pPAD-HisA expression
vector. Then, the ELP sequence was inserted into the resulting
pBAD-His-Lα5LG1-3 expression vector. The Lα5LG1-3/ELP fusion
protein retained the phase transition property of ELP. Thus,
Lα5LG1-3/ELP could be purified through ITC. The molecular size of
Lα5LG1-3/ELP was estimated at approximately 70 kDa on 12%
SDS-polyacrylamide gels stained with Coomassie blue. The expression
of Lα5LG1-3/ELP was verified by western blotting with a
peroxidase-conjugated monoclonal anti-polyhistidine antibody
specific to the N-terminal His-tag (Fig. 1). Production of this Lα5LG1-3/ELP
protein was well-established and used in subsequent
experiments.
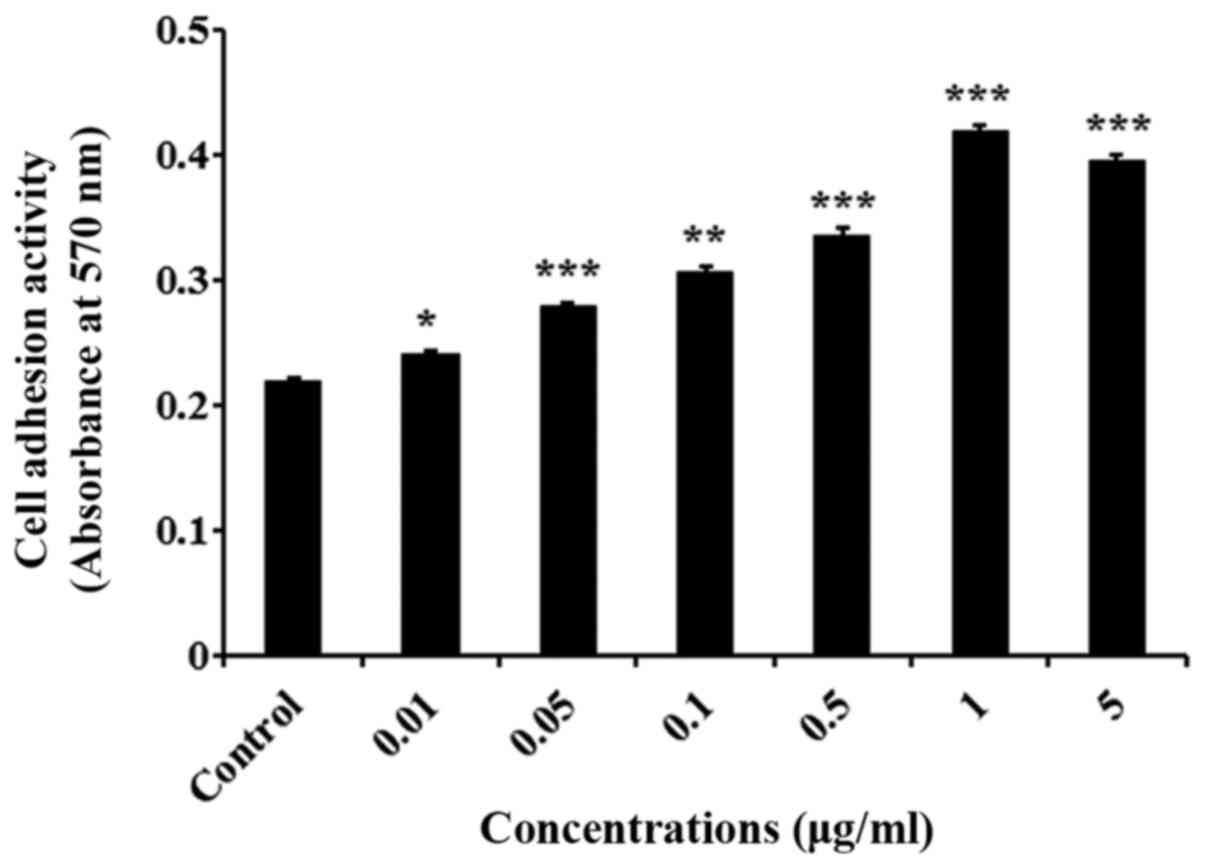
Lα5LG1-3/ELP promotes the adhesion of
hMSCs
Cell adhesion assay was carried out using crystal
violet to determine the effect of Lα5LG1-3/ELP on the adhesion of
hMSCs. As shown in Fig. 2
(P<0.001), the attachment of hMSCs was significantly better in
the wells coated with Lα5LG1-3/ELP than in the uncoated, control
wells. Moreover, Lα5LG1-3/ELP improved the cell adhesion in a
dose-dependent manner, with the cell adhesion saturated at 1 µg/ml
of Lα5LG1-3/ELP. Therefore, Lα5LG1-3/ELP was used at 1 µg/ml in the
subsequent experiments.
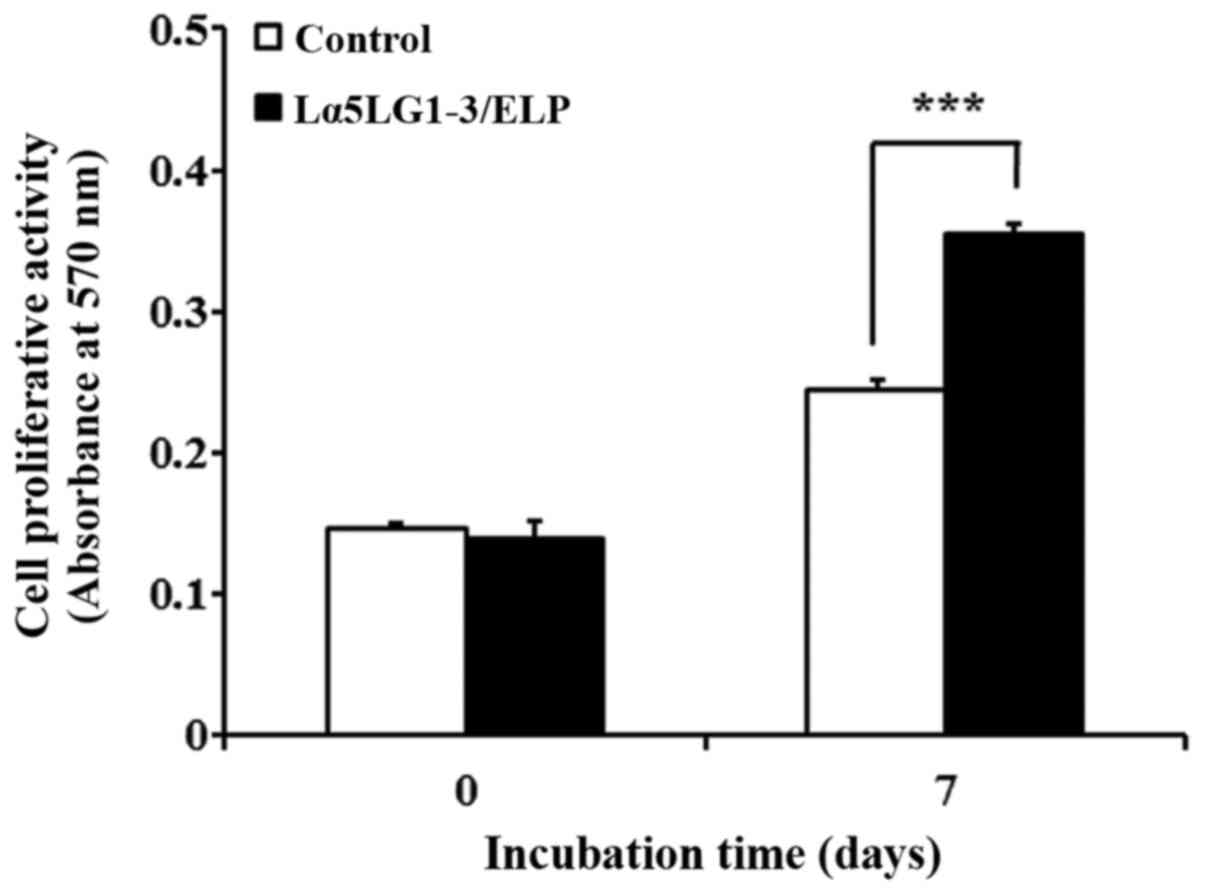
Lα5LG1-3/ELP enhances the
proliferation of hMSCs
We measured the effect of Lα5LG1-3/ELP on the
proliferative activity of hMSCs by using the MTT assay. Toward this
end, 24-well plates were coated with Lα5LG1-3/ELP, and hMSCs were
incubated in these coated wells for 7 days. Additionally,
experiments were conducted in two groups of 0 and 7 days for
comparison. After 7 days, the proliferation of hMSCs increased
1.45-fold (P<0.001; Fig. 3) in
the coated well compared with the proliferation in the controls
(uncoated wells). This result demonstrated that Lα5LG1-3/ELP
enhances the proliferation of hMSCs.
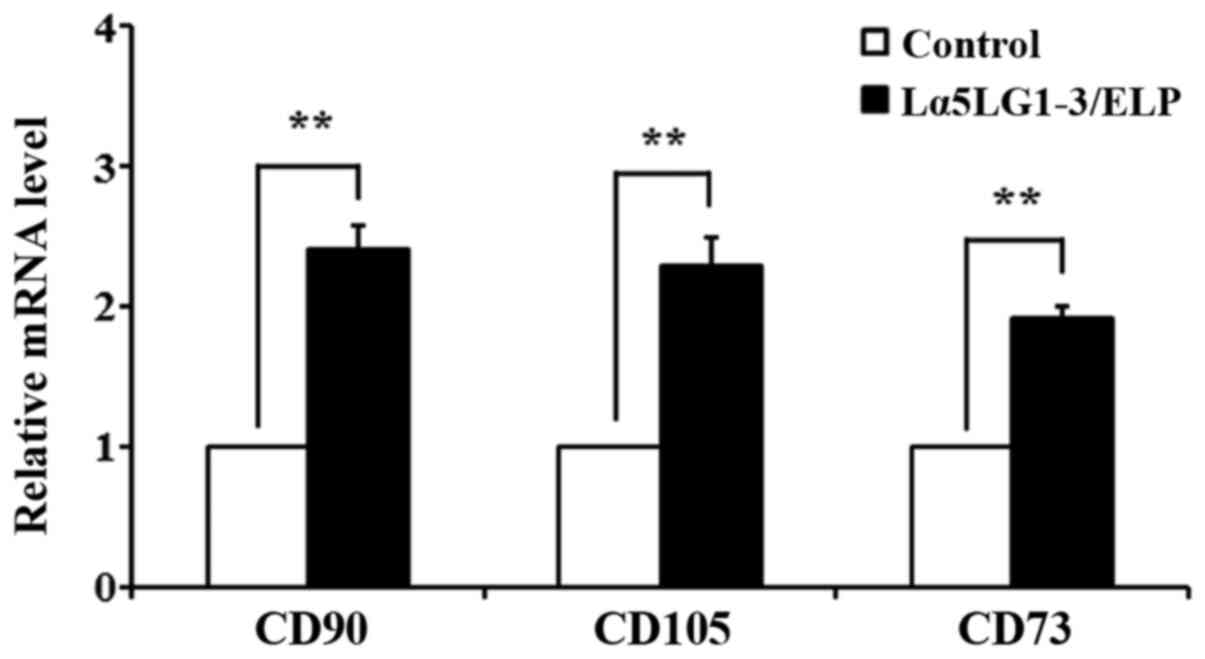
Lα5LG1-3/ELP helps maintain the
stemness of hMSCs
qRT-PCR was performed to confirm that Lα5LG1-3/ELP
maintains stemness of hMSCs at the gene expression level. The
distinctive stem cell surface marker genes cluster differentiation
90 (CD90), endoglin (CD105) and CD73 were
chosen. The mRNA levels of these markers in hMSCs grown with or
without Lα5LG1-3/ELP for 7 days are presented in Fig. 4. The gene expression levels of
CD90, CD105 and CD73 were upregulated in the
presence of Lα5LG1-3/ELP compared with the levels in the uncoated
control wells.
Discussion
Cells must interact with the ECM because it supports
many cellular activities, including adhesion, proliferation, and
differentiation (32). Laminin and
elastin are two ECM proteins predominantly present in the BMs of
most tissues in humans (33). The
major multifunctional components of the ECM include the followings:
i) Laminin is one of the glycoproteins and a major factor affecting
cell attachment, proliferation, differentiation and survival. The
five globular domains of the α-chain, LG domains, are known to have
a critical effect on cells by binding to integrins. ii) Elastin
affects the biomechanical and physiological properties of cells
(32,33). In this work, we engineered a novel
fusion protein composed of LG1-3 domains laminin α5 chain and ELPs,
recombinant forms of elastin.
The Lα5LG1-3/ELP fusion protein was purified through
ITC, based on the inverse phase transition properties of ELP. As
shown in Fig. 1, the total
molecular weight of the recombinant protein was estimated at
approximately 70 kDa by performing western blot analysis with and
antibody that detects the amino-terminal His-tag. The production of
the recombinant protein was successful, and the presence of
Lα5LG1-3/ELP was determined by protein identification. The major BM
proteins laminins, exert biological activities, such as promoting
cell attachment, differentiation, and migration, as well as
angiogenesis and formation of neurite outgrowths (5,6).
Integrins are the most characteristic laminin receptors that the
diverse biological activities of laminins. Moreover, interactions
of cells with laminins are crucial to cellular adhesion (4,33).
The hMSCs are pluripotent stem cells that can
differentiate into mesenchymal lineages (22,23).
To assess the effect of Lα5LG1-3/ELP on the adhesion of hMSCs, we
performed the cell adhesion assay by using crystal violet.
Lα5LG1-3/ELP enhanced the adhesion of hMSCs in a dose-dependent
manner, with saturation at 1 µg/ml. Therefore, Lα5LG1-3/ELP, was
used at the concentration of 1 µg/ml for the subsequent experiments
(Fig. 2). To further estimate the
effect of Lα5LG1-3/ELP on hMSC proliferation, MTT assay was carried
out. For this purpose, hMSCs were grown in Lα5LG1-3/ELP coated
24-well plates for 7 days and then used for the assay. In addition,
for comparison, experiments were conducted in two groups, 0 day and
7 day. As shown in Fig. 3, after 7
days, the cell proliferation was 1.45-fold higher in the coated
plates than in the uncoated controls. Taken together, these results
suggest that Lα5LG1-3/ELP improves the proliferation of hMSCs.
It has been reported that hMSCs express CD44, CD71,
CD73, CD90 (Thy-1), CD105 (endoglin), and CD271, but not the
co-stimulatory molecules CD80, CD86 and CD40, or the hematopoietic
markers CD14, CD34 and CD45(22).
In addition, hMSCs express various surface receptors, such as
integrin, that bind to the LG1-3 domain of the laminin α-chain.
Therefore, hMSCs are anticipated to bind to recombinant laminin α5
LG1-3 (Lα5LG1-3) (27). To
investigate the effect of Lα5LG1-3/ELP on the maintenance of the
stemness properties of hMSCs, the gene expression levels of the
stem cell surface markers CD73, CD90 and CD105 were measured
through qRT-PCR. Total RNA was isolated from cells cultured in
Lα5LG1-3/ELP coated plates for 7 days, cDNA was synthesized and
surface markers were examined, and CD90, CD105, and CD73 were stem
cell surface markers that are expressed by hMSCs at varying levels
(34). CD90 is a stem cell
surface-anchoring glycoprotein that plays a key role in cell
motility, and is essential for stem cell growth and differentiation
(35,36). CD90 expression at high levels is
associated with the undifferentiated state of MSCs (37). CD105, also called SH2, is a
component of a receptor complex that transforming growth factor-β
(TGF-β), which modulates stem cell migration, proliferation, and
differentiation. Additionally, CD105 is also used for purifying
hMSCs through an immunoisolation method. CD105-positive MSCs can
differentiate into chondrogenic, osteogenic, and adipogenic
lineages (36,38,39).
CD73, also termed as ecto-5'-nucleotidase, is a glycosyl
phosphatidylinositol-linked membrane protein found on the surface
of multiple cell types. It is found on the surfaces of
hematopoietic and mesenchymal stem cells and functions in numerous
physiological processes in various tissues (40-42).
In addition, CD73 is known to modulates epithelial-mesenchymal stem
cell transition and stemness in ovarian cancer initiating cells
(43). As shown in Fig. 4, the expression levels of the
surface markers CD90, CD105 and CD73 were 2.42-, 2.29- and
1.92-fold higher, respectively, than the levels in the control.
These results indicate that Lα5LG1-3/ELP contributes to maintaining
the undifferentiated state of hMSCs. The increase in CD90, CD105
and CD73 levels in hMSCs cultured in Lα5LG1-3/ELP-coated plates
suggest that Lα5LG1-3/ELP supports the stemness properties of
hMSCs. In order to further clarify the effect of Lα5LG1-3/ELP, and
experiment at the protein level such as western blot will be
performed later.
In conclusion, Lα5LG1-3/ELP maintains the
undifferentiated state of hMSCs and promotes their adhesion,
proliferation, and stemness. Therefore, Lα5LG1-3/ELP can
potentially be used during MSC therapy to enhance the viability of
these stem cells and their maintenance at the undifferentiated
state. Hence, in vivo experiments with recombinant laminin
are required.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Research Foundation of Korea grant funded by the Korean government
(grant no. NRF-2020R1A2C1009533) and Inha University Research
Grant.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SL, DSL and JHJ conceptualized the study. SL and JHJ
analyzed the data, performed the experiments and curated the data.
SL, DSL and JHJ prepared and wrote the original manuscript,
reviewed and edited the manuscript, including the figures. JHJ
supervised the study. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The human mesenchymal stem cells were isolated from
tissues discarded during surgery. The study was conducted with the
written informed consent of the subject who provided the tissue.
The isolation of human mesenchymal stem cells from tissues was
approved by the Institutional Review Board of the Catholic
University of Korea, St. Mary's Hospital (approval no.
KC08TISS034). All cells were provided by Dr Sung-Won Kim (St.
Mary's Hospital, the Catholic University of Korea).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Molofsky AV, Pardal R and Morrison SJ:
Diverse mechanisms regulate stem cell self-renewal. Curr Opin Cell
Biol. 16:700–707. 2004.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Rozario T and DeSimone DW: The
extracellular matrix in development and morphogenesis: A dynamic
view. Dev Biol. 341:126–140. 2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Mahapatra C, Kim JJ, Lee JH, Jin GZ,
Knowles JC and Kim HW: Differential chondro- and osteo-stimulation
in three-dimensional porous scaffolds with different topological
surfaces provides a design strategy for biphasic osteochondral
engineering. J Tissue Eng: Jan 31, 2019 (Epub ahead of print). doi:
10.1177/2041731419826433.
|
|
4
|
Suzuki N, Yokoyama F and Nomizu M:
Functional sites in the laminin alpha chains. Connect Tissue Res.
46:142–152. 2005.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Miner JH and Yurchenco PD: Laminin
functions in tissue morphogenesis. Annu Rev Cell Dev Biol.
20:255–284. 2004.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Patarroyo M, Tryggvason K and Virtanen I:
Laminin isoforms in tumor invasion, angiogenesis and metastasis.
Semin Cancer Biol. 12:197–207. 2002.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Rohn F, Kordes C, Castoldi M, Götze S,
Poschmann G, Stühler K, Herebian D, Benk AS, Geiger F, Zhang T, et
al: Laminin-521 promotes quiescence in isolated stellate cells from
rat liver. Biomaterials. 180:36–51. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Aumailley M, Bruckner-Tuderman L, Carter
WG, Deutzmann R, Edgar D, Ekblom P, Engel J, Engvall E, Hohenester
E and Jones JC: A simplified laminin nomenclature. Matrix Biol.
24:326–332. 2005.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Klaffky E, Williams R, Yao CC, Ziober B,
Kramer R and Sutherland A: Trophoblast-specific expression and
function of the integrin alpha 7 subunit in the peri-implantation
mouse embryo. Dev Biol. 239:161–175. 2001.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Miner JH, Cunningham J and Sanes JR: Roles
for laminin in embryogenesis: Exencephaly, syndactyly, and
placentopathy in mice lacking the laminin alpha5 chain. J Cell
Biol. 143:1713–1723. 1998.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Navdaev A and Eble JA: Components of
cell-matrix linkage as potential new markers for prostate cancer.
Cancers (Basel). 3:883–896. 2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yu H and Talts JF: Beta1 integrin and
alpha-dystroglycan binding sites are localized to different
laminin-G-domain-like (LG) modules within the laminin alpha5 chain
G domain. Biochem J. 371:289–299. 2003.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ido H, Harada K, Futaki S, Hayashi Y,
Nishiuchi R, Natsuka Y, Li S, Wada Y, Combs AC, Ervasti JM, et al:
Molecular dissection of the alpha-dystroglycan- and
integrin-binding sites within the globular domain of human
laminin-10. J Biol Chem. 279:10946–10954. 2004.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Nielsen PK, Gho YS, Hoffman MP, Watanabe
H, Makino M, Nomizu M and Yamada Y: Identification of a major
heparin and cell binding site in the LG4 module of the laminin
alpha 5 chain. J Biol Chem. 275:14517–14523. 2000.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Blit PH, Battiston KG, Woodhouse KA and
Santerre JP: Surface immobilization of elastin-like polypeptides
using fluorinated surface modifying additives. J Biomed Mater Res
A. 96:648–662. 2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yeo GC and Weiss AS: Soluble matrix
protein is a potent modulator of mesenchymal stem cell performance.
Proc Natl Acad Sci USA. 116:2042–2051. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Annabi N, Mithieux SM, Boughton EA, Ruys
AJ, Weiss AS and Dehghani F: Synthesis of highly porous crosslinked
elastin hydrogels and their interaction with fibroblasts in vitro.
Biomaterials. 30:4550–4557. 2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Meyer DE and Chilkoti A: Genetically
encoded synthesis of protein-based polymers with precisely
specified molecular weight and sequence by recursive directional
ligation: Examples from the elastin-like polypeptide system.
Biomacromolecules. 3:357–367. 2002.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Amruthwar SS and Janorkar AV: In vitro
evaluation of elastin-like polypeptide-collagen composite scaffold
for bone tissue engineering. Dent Mater. 29:211–220.
2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
MacEwan SR and Chilkoti A: Elastin-like
polypeptides: Biomedical applications of tunable biopolymers.
Biopolymers. 94:60–77. 2010.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Meyer DE and Chilkoti A: Purification of
recombinant proteins by fusion with thermally-responsive
polypeptides. Nat Biotechnol. 17:1112–1115. 1999.PubMed/NCBI View
Article : Google Scholar
|
|
22
|
Lotfinegad P, Shamsasenjan K,
Movassaghpour A, Majidi J and Baradaran B: Immunomodulatory nature
and site specific affinity of mesenchymal stem cells: A hope in
cell therapy. Adv Pharm Bull. 4:5–13. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lee DJ, Kwon J, Current L, Yoon K, Zalal
R, Hu X, Xue P and Ko CC: Osteogenic potential of mesenchymal stem
cells from rat mandible to regenerate critical sized calvarial
defect. J Tissue Eng. 10(2041731419830427)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Re F, Sartore L, Moulisova V, Cantini M,
Almici C, Bianchetti A, Chinello C, Dey K, Agnelli S, Manferdini C,
et al: 3D gelatin-chitosan hybrid hydrogels combined with human
platelet lysate highly support human mesenchymal stem cell
proliferation and osteogenic differentiation. J Tissue Eng.
10(2041731419845852)2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Oswald J, Boxberger S, Jørgensen B,
Feldmann S, Ehninger G, Bornhäuser M and Werner C: Mesenchymal stem
cells can be differentiated into endothelial cells in vitro. Stem
Cells. 22:377–384. 2004.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Tran TC1. Kimura K, Nagano M, Yamashita T,
Ohneda K, Sugimori H, Sato F, Sakakibara Y, Hamada H, Yoshikawa H,
et al: Identification of human placenta-derived mesenchymal stem
cells involved in re-endothelialization. J Cell Physiol.
226:224–235. 2011.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wen J, Zhao Z, Tong R, Huang L, Miao Y and
Wu J: Prussian blue nanoparticle-labeled mesenchymal stem cells:
Evaluation of cell viability, proliferation, migration,
differentiation, cytoskeleton, and protein expression in vitro.
Nanoscale Res Lett. 13(329)2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kim JE, Seo HJ, Lee S and Jang JH:
Evaluation of stemness maintenance properties of the recombinant
human laminin α2 LG1-3 domains in human mesenchymal stem cells.
Protein Pept Lett. 26:785–791. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Hwang SH, Cho HK, Park SH, Lee W, Lee HJ,
Lee DC, Park SH, Lim MH, Back SA, Yun BG, et al: Characteristics of
human turbinate-derived mesenchymal stem cells are not affected by
allergic condition of donor. PLoS One. 10(e0138041)2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hang Pham LB, Yoo YR, Park SH, Back SA,
Kim SW, Bjørge I, Mano J and Jang JH: Investigating the effect of
fibulin-1 on the differentiation of human nasal inferior
turbinate-derived mesenchymal stem cells into osteoblasts. J Biomed
Mater Res A. 105:2291–2298. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Paiva Dos Santos B, Garbay B, Pasqua M,
Chevron E, Chinoy ZS, Cullin C, Bathany K, Lecommandoux S, Amédée
J, Oliveira H, et al: Production, purification and characterization
of an elastin-like polypeptide containing the Ile-Lys-Val-Ala-Val
(IKVAV) peptide for tissue engineering applications. J Biotechnol.
298:35–44. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Tyndall A, Walker UA, Cope A, Dazzi F, De
Bari C, Fibbe W, Guiducci S, Jones S, Jorgensen C, Le Blanc K, et
al: Immunomodulatory properties of mesenchymal stem cells: A review
based on an interdisciplinary meeting held at the Kennedy Institute
of Rheumatology Division, London, UK, 31 October 2005. Arthritis
Res Ther. 9(301)2007.PubMed/NCBI View
Article : Google Scholar
|
|
34
|
Hynes RO: Integrins: Bidirectional,
allosteric signaling machines. Cell. 110:673–687. 2002.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Moraes DA, Sibov TT, Pavon LF, Alvim PQ,
Bonadio RS, Da Silva JR, Pic-Taylor A, Toledo OA, Marti LC, Azevedo
RB, et al: A reduction in CD90 (THY-1) expression results in
increased differentiation of mesenchymal stromal cells. Stem Cell
Res Ther. 7(97)2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Aslan H, Zilberman Y, Kandel L, Liebergall
M, Oskouian RJ, Gazit D and Gazit Z: Osteogenic differentiation of
noncultured immunoisolated bone marrow-derived CD105+
cells. Stem Cells. 24:1728–1737. 2006.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Sibov TT, Severino P, Marti LC, Pavon LF,
Oliveira DM, Tobo PR, Campos AH, Paes AT, Amaro E Jr, F Gamarra L,
et al: Mesenchymal stem cells from umbilical cord blood: Parameters
for isolation, characterization and adipogenic differentiation.
Cytotechnology. 64:511–521. 2012.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Maleki M, Ghanbarvand F, Reza Behvarz M,
Ejtemaei M and Ghadirkhomi E: Comparison of mesenchymal stem cell
markers in multiple human adult stem cells. Int J Stem Cells.
7:118–126. 2014.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Roura S, Farré J, Soler-Botija C, Llach A,
Hove-Madsen L, Cairó JJ, Gòdia F, Cinca J and Bayes-Genis A: Effect
of aging on the pluripotential capacity of human CD105+ mesenchymal
stem cells. Eur J Heart Fail. 8:555–563. 2006.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Yu YI, Wang W, Song L, Hu W, Dong C, Pei
H, Zhou G and Yue Z: Ecto-5'-nucleotidase expression is associated
with the progression of renal cell carcinoma. Oncol Lett.
9:2485–2494. 2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Colgan SP, Eltzschig HK, Eckle T and
Thompson LF: Physiological roles for ecto-5'-nucleotidase (CD73).
Purinergic Signal. 2:351–360. 2006.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Zimmermann H: 5'-Nucleotidase: Molecular
structure and functional aspects. Biochem J. 285:345–365.
1992.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Lupia M, Angiolini F, Bertalot G, Freddi
S, Sachsenmeier KF, Chisci E, Kutryb-Zajac B, Confalonieri S,
Smolenski RT, Giovannoni R, et al: CD73 regulates stemness and
epithelial-mesenchymal transition in ovarian cancer-initiating
cells. Stem Cell Reports. 10:1412–1425. 2018.PubMed/NCBI View Article : Google Scholar
|