Introduction
Ischemic stroke is a major type of stroke that is
characterized by high mortality and morbidity rates (1). The oxygen and glucose deprivation
(OGD) that results from complete or partial blockade of arterial
blood supply to the brain is considered as the leading cause for
the occurrence of ischemic stroke (2). Ischemia-reperfusion (I/R) injury,
which is defined as the restoration of blood supply after a given
duration of ischemia, is a common characteristic of ischemic stroke
(3). Although the incidence of
ischemic stroke is high, effective treatment strategies for this
disease are still lacking.
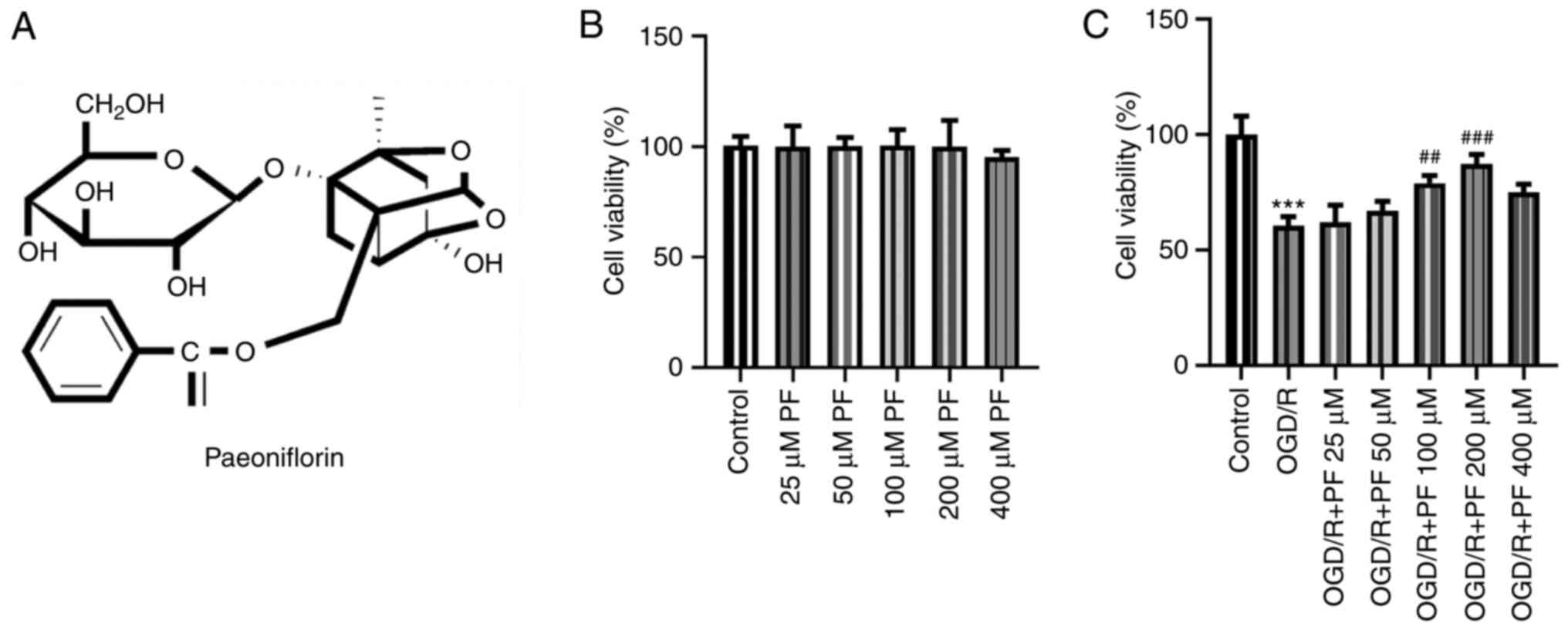
Paeoniflorin (PF; Fig.
1A), a natural compound, is the main active ingredient of
Radix Paeoniae (4). A
previous study demonstrated that PF protected HT-22 cells from
H2O2-induced oxidative injury via regulating
the expression of microRNA (miR)-135a (5). In addition, 6'-O-galloylpaeoniflorin,
the galloylated derivative of PF isolated from peony root,
attenuated neuroinflammation and oxidative stress in a cerebral I/R
injury rat model via activating the PI3K/AKT/nuclear factor
erythroid-2-like 2 signaling pathway (6). Compelling evidence has indicated that
PF combined with β-ecdysterone protected PC12 cells against
neurotoxicity triggered by rotenone (7). Additionally, 6-hydroxydopamine-induced
PC12 cell apoptosis was suppressed by PF via blocking the reactive
oxygen species-induced protein kinase C δ/NF-κB signaling pathway
(8). Another study revealed that
the function of PF in preventing mitochondrial dysfunction may
alleviate cytotoxicity in glutamate-induced PC12 cells (9). Importantly, it has also been reported
that the natural neuroprotector PF can inhibit a number of pro- and
anti-inflammatory signals in differentiated PC12 cells (10).
It has been suggested that Janus kinase 2 (JAK2), a
crucial factor involved signaling through a variety of cytokine
receptors, phosphorylates signal transducer and activator of
transcription 3 (STAT3) upon activation (11). A previous study indicated a strong
association between the phosphorylation/activation of the
JAK2/STAT3 pathway and a neuroprotection-related signaling pathway
(12). Blocking the JAK2/STAT3
signaling pathway by PF may protect the kidneys of diabetic rats
(13). Furthermore, the
melatonin-mediated regulation of the miR-26a-5p/neuron-restrictive
silencer factor and JAK2/STAT3 pathways may attenuate cerebral I/R
injury via alleviating inflammation and oxidative stress (14). In addition, the activation of the
JAK2/STAT3 pathway by Src homology 2B adaptor protein 1 protected
PC12 cells from oxygen-glucose deprivation/reoxygenation
(OGD/R)-induced apoptosis (15).
Therefore, it was hypothesized that PF may play a significant role
against OGD/R-induced PC12 cell injury via regulating the
JAK2/STAT3 pathway.
The aim of the present study was to investigate the
function of PF in OGD/R-induced PC12 cell injury and the role of
the JAK2/STAT3 signaling pathway in this process, in order to
elucidate whether PF may be considered as a promising candidate for
the treatment of ischemic stroke.
Materials and methods
PC12 cell culture and treatment
The rat pheochromocytoma cell line, PC12, was
obtained from the American Type Cell Culture Collection. The cells
were cultured in DMEM (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.),
100 U/ml penicillin and streptomycin at 37˚C in a humidified
incubator containing 5% CO2. PF (purity, >98%) was
purchased from the National Institute for the Control of
Pharmaceutical and Biological Products (Beijing, China). PF was
dissolved in DMSO (Sigma-Aldrich; Merck KGaA) to a final
concentration of 200 µM. A total of 100 µl PF (200 µM) was diluted
to 100, 50 and 25 µM PF by adding into 100, 300 and 700 µl DMEM,
respectively. The final concentration of DMSO in the cultures was
<1%. The cells were detached and re-seeded into six-well plates
(1x106 cells per well) for the subsequent experiments.
Finally, the cells were left untreated or were pre-treated with
various concentrations of PF for 24 h, followed by treatment with
FLLL32, a specific inhibitor of JAK2/STAT3 signaling, at a dose of
25 µM for an additional 24 h.
Establishment of OGD/R injury
model
PC12 cells were cultured in Earle's Balanced Salt
Solution (Sigma-Aldrich; Merck KGaA) without glucose to induce cell
ischemia. The cells were maintained in a three-gas incubator
containing 94% N2, 5% CO2 and 1%
O2 at 37˚C for 4 h. Subsequently, the medium was
discarded and replaced with normal medium supplemented with 10%
FBS, and cells were reoxygenated in a normal atmosphere for an
additional 24 h for the establishment of the reperfusion model.
Cell Counting Kit-8 (CCK-8) assay
To determine PC12 cell viability, the cells were
seeded into a 96-well plate at a density of 3x104 cells
per 100 µl medium in each well. Following incubation for 24 h, 10
µl CCK-8 reagent was added into each well, and the plate was
incubated at 37˚C for an additional 4 h. The absorbance of each
well was measured at 450 nm using a microplate reader (BioTek
Instruments, Inc.).
Determination of oxidative stress
Briefly, 2x105 PC12 cells/well were
seeded into 96-well plates. The activity of lactate dehydrogenase
(LDH; cat. no. A020-2-2), myeloperoxidase (MPO; cat. no. A044-1-1)
and superoxide dismutase (SOD; cat. no. A001-3-2) was determined
using the corresponding kits (Nanjing Jiancheng Bioengineering
Institute), according to the manufacturers' instructions.
Subsequently, the absorbance was determined at 450 nm (LDH and SOD)
or 460 nm (MPO) using a microplate reader (Bio-Rad Laboratories,
Inc.).
Determination of the levels of
inflammatory factors
ELISA kits were used to measure the levels of TNF-α
(cat. no. F16960), IL-6 (cat. no. F15870) and IL-10 (cat. no.
F15900) in the cell culture medium. The cells were first treated
with 500 µl ice-cold carbonate buffer (100 mM
Na2CO3, 50 mM NaCl, pH 11.5) supplemented
with protease inhibitors, and were then dissociated using an
ultrasonic cell disruption system (Ningbo Haishu Kesheng Ultrasonic
Equipment Co., Ltd.). The mixture was centrifuged at 12,000 x g for
45 min at 4˚C. The supernatant was then collected, and the levels
of the inflammatory factors were measured according to the
manufacturer's instructions (Shanghai Xitang Biotechnology Co.,
Ltd.).
Flow cytometric analysis
Flow cytometry using the Annexin V-FITC apoptosis
kit (Beyotime Institute of Biotechnology) was performed to
determine cell apoptosis. Following a series of treatments as
aforementioned, PC12 cells were collected by centrifugation (200 x
g; 10 min; room temperature), then washed twice by ice-cold PBS,
resuspended in 195 µl pre-chilled 1X Annexin V binding buffer.
Subsequently, cells were doubled-stained with 5 µl Annexin V-FITC
and 5 µl propidium iodide (PI) for 15 min in the dark at room
temperature according to the manufacturer's instructions.
Subsequently, cell apoptosis of each sample was assessed by flow
cytometer (BD Accuri™ C6; BD Biosciences). The data were analyzed
using FlowJo software (version 7.6.1; FlowJo LLC). Annexin V and PI
single-stained positive cells were used to regulate compensation
(as control). The sum of apoptosis rate in right upper quadrant
(Q2, late apoptotic cells) and right lower quadrant (Q3, early
apoptotic cells) were considered as the cell apoptosis rate. The
apoptotic cells were expressed as a percentage of the total number
of cells.
Western blot analysis
Total proteins were extracted from PC12 cells using
RIPA lysis buffer (Beyotime Institute of Biotechnology) and the
protein concentration was determined by a BCA Protein Assay kit
(Beyotime Institute of Biotechnology). The protein samples (40
µg/lane) were then separated by 10% SDS-PAGE and transferred onto a
PVDF membrane (EMD Millipore). Subsequently, the membrane was
blocked with 5% skimmed milk for 2 h at room temperature, washed
with Tris-buffered saline containing 0.2% Tween-20 (TBST; Boster
Biological Technology), and incubated with the primary antibodies
at 4˚C overnight. After washing with TBST, the membrane was
incubated with a goat anti-rabbit horseradish peroxidase
(HRP)-conjugated secondary antibody (1:3,000; cat. no. 7074S; Cell
Signaling Technology, Inc.) or horse anti-mouse HRP-conjugated
secondary antibody (1:3,000; cat. no. 7076S; Cell Signaling
Technology, Inc.) at room temperature for 2 h. Finally, protein
bands were visualized using an enhanced chemiluminescence substrate
(Pierce; Thermo Fisher Scientific, Inc.) on a chemiluminescence
imaging equipment (Ultra-Lum, Inc.). The proteins bands were
quantified using the ImageJ software (version 1.52r; National
Institutes of Health). The gray value of the target protein was
normalized to that of GAPDH. The following primary antibodies were
used: Anti-Bax (cat. no. 14796S; 1:1,000), anti-cleaved caspase-9
(cat. no. 20750S; 1:1,000), anti-cleaved poly(adenosine
diphosphate-ribose) polymerase (PARP) (cat. no. 9185S; 1:1,000),
anti-p-JAK2 (cat. no. 3776S; 1:1,000), anti-JAK2 (cat. no. 3230T;
1:1,000), anti-p-STAT3 (cat. no. 9145S; 1:1,000), anti-STAT3 (cat.
no. 4904T; 1:1,000) and anti-GAPDH (cat. no. 5174S; 1:1,000). All
antibodies were obtained from Cell Signaling Technology, Inc.,
apart from anti-Bcl-2 (cat. no. sc-7382; 1:1,000), which was
purchased from Santa Cruz Biotechnology, Inc.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism (version 6.0; GraphPad Software, Inc.) and data are expressed
as the mean ± SD. All experiments were performed three times.
Statistical comparisons among multiple groups were analyzed using
one-way ANOVA followed by a Tukey's post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
PF enhances the viability of PC12
cells subjected to OGD/R
The viability of PC12 cells was determined to
evaluate the effect of different concentrations of PF. As shown in
Fig. 1B, treatment of PC12 cells
with 25, 50, 100 or 200 µM PF did not affect cell viability,
whereas treatment with 400 µM PF decreased PC12 cell viability.
After the establishment of the OGD/R cell model, the viability of
PC12 cells was notably decreased compared with the control group,
and it was gradually restored following treatment with increasing
concentrations of PF (25, 50, 100 or 200 µM). This finding
suggested that PF could increase the viability of OGD/R-treated
PC12 cells (Fig. 1C). Since
treatment with 400 µM PF promoted PC12 cell injury and exerted a
weaker effect on OGD/R-treated PC12 cells compared with 200 µM PF,
doses of 25-200 µM PF were selected for the subsequent
experiments.
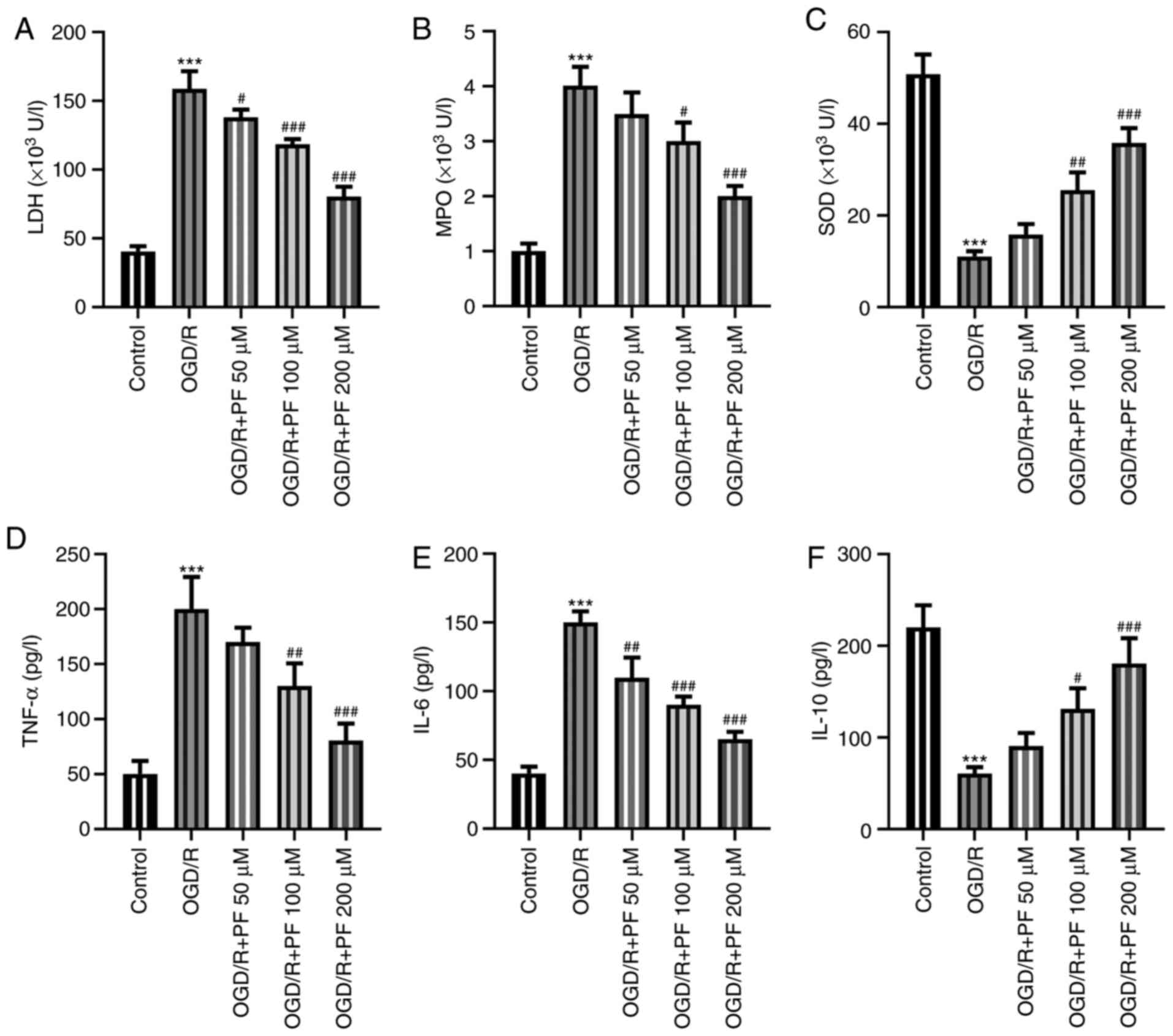
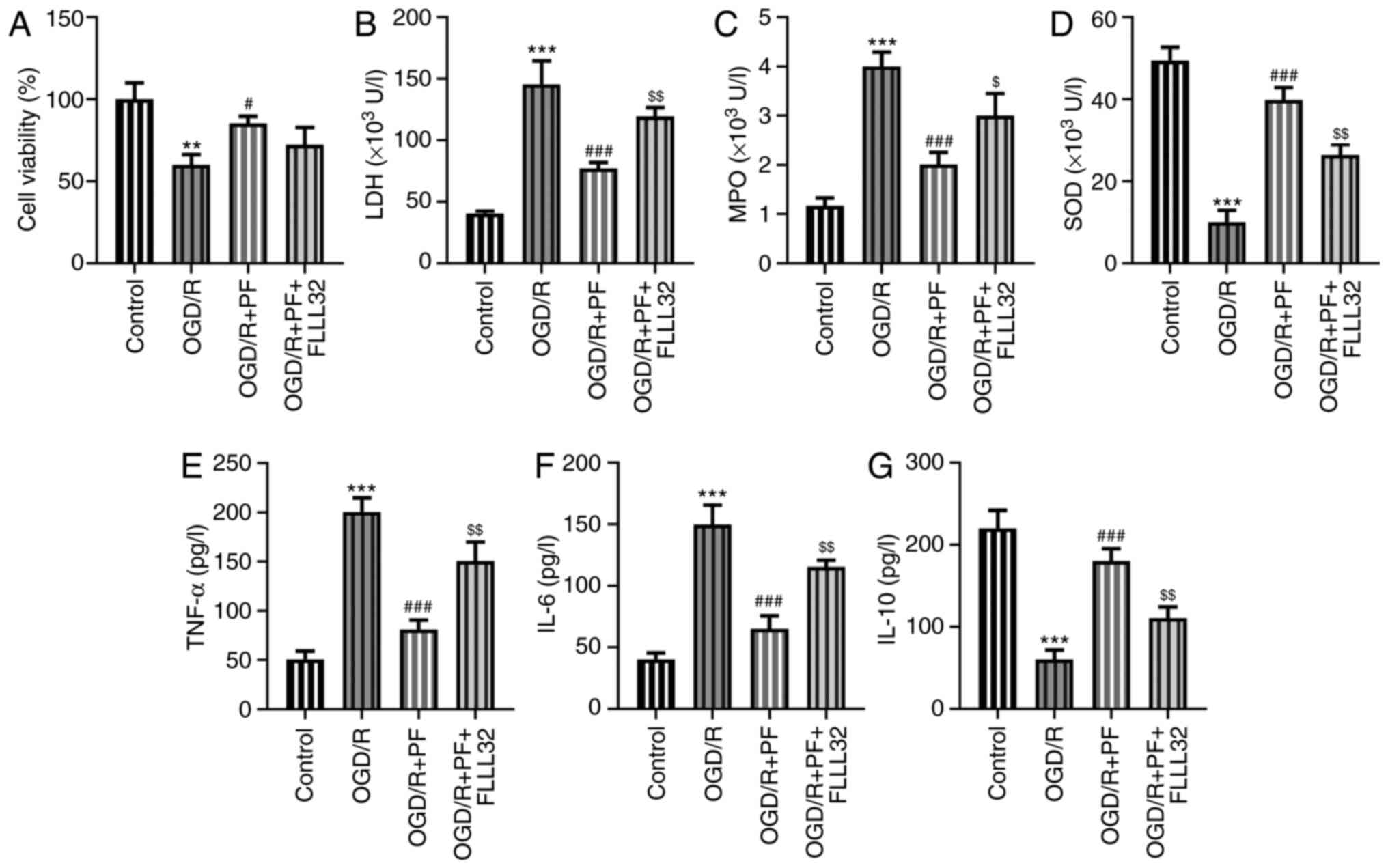
PF alleviates oxidative stress,
inflammation and apoptosis in OGD/R-treated PC12 cells
To determine whether PF could mitigate the
OGD/R-induced cell injury, the levels of oxidative stress
indicators and inflammatory factors were determined. The levels of
LDH and MPO were significantly increased, while those of SOD were
markedly reduced by OGD/R. Treatment with increasing concentrations
of PF reduced the levels of LDH and MPO, and increased those of SOD
(Fig. 2A-C). Additionally, the
ELISA results demonstrated that the levels of TNF-α and IL-6 were
increased and those of IL-10 were decreased in the OGD/R group
compared with the control group, while treatment with PF had the
opposite effect (Fig. 2D-F).
Overall, the aforementioned results suggested that PF could
attenuate oxidative stress and inflammation in OGD/R-treated PC12
cells.
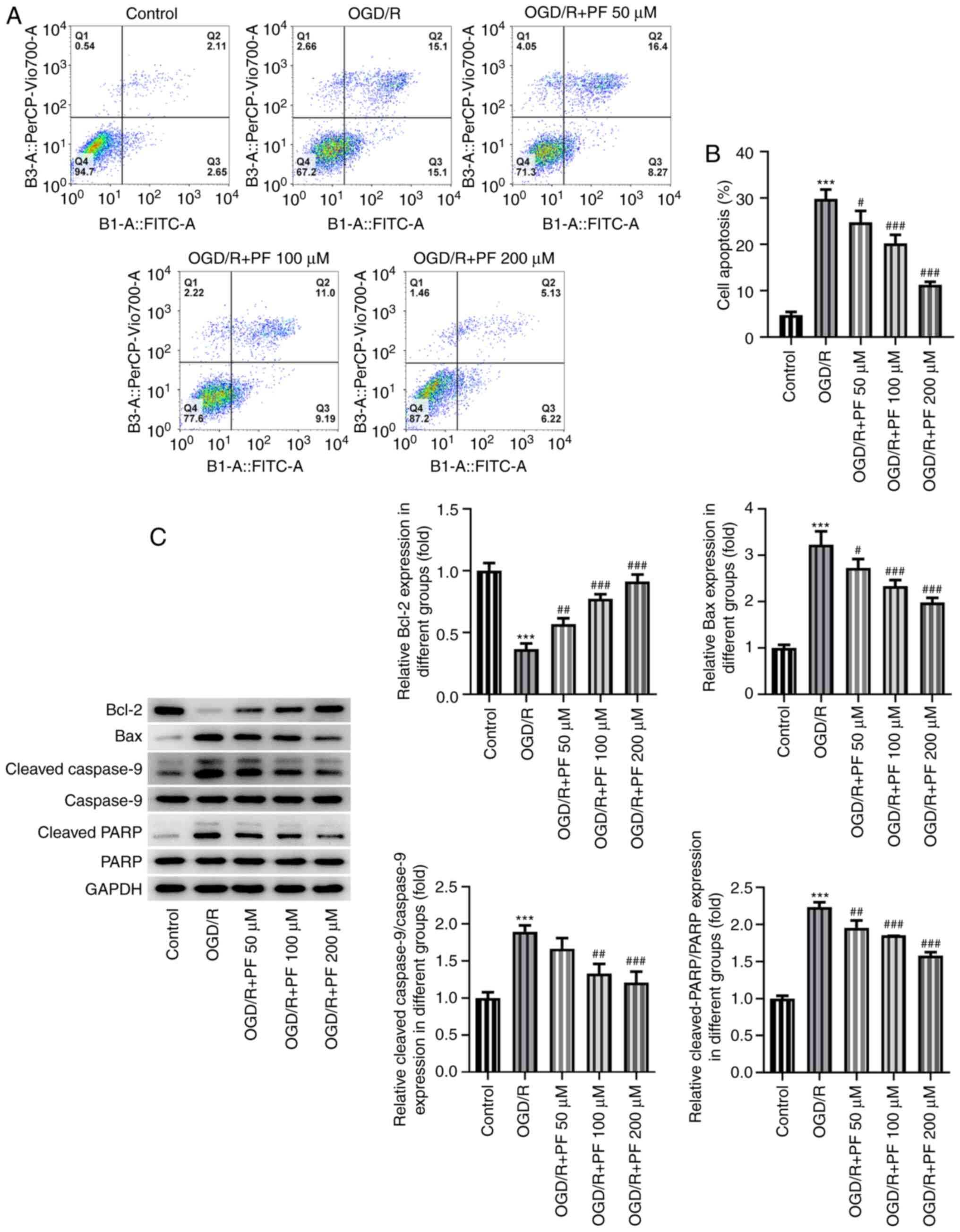
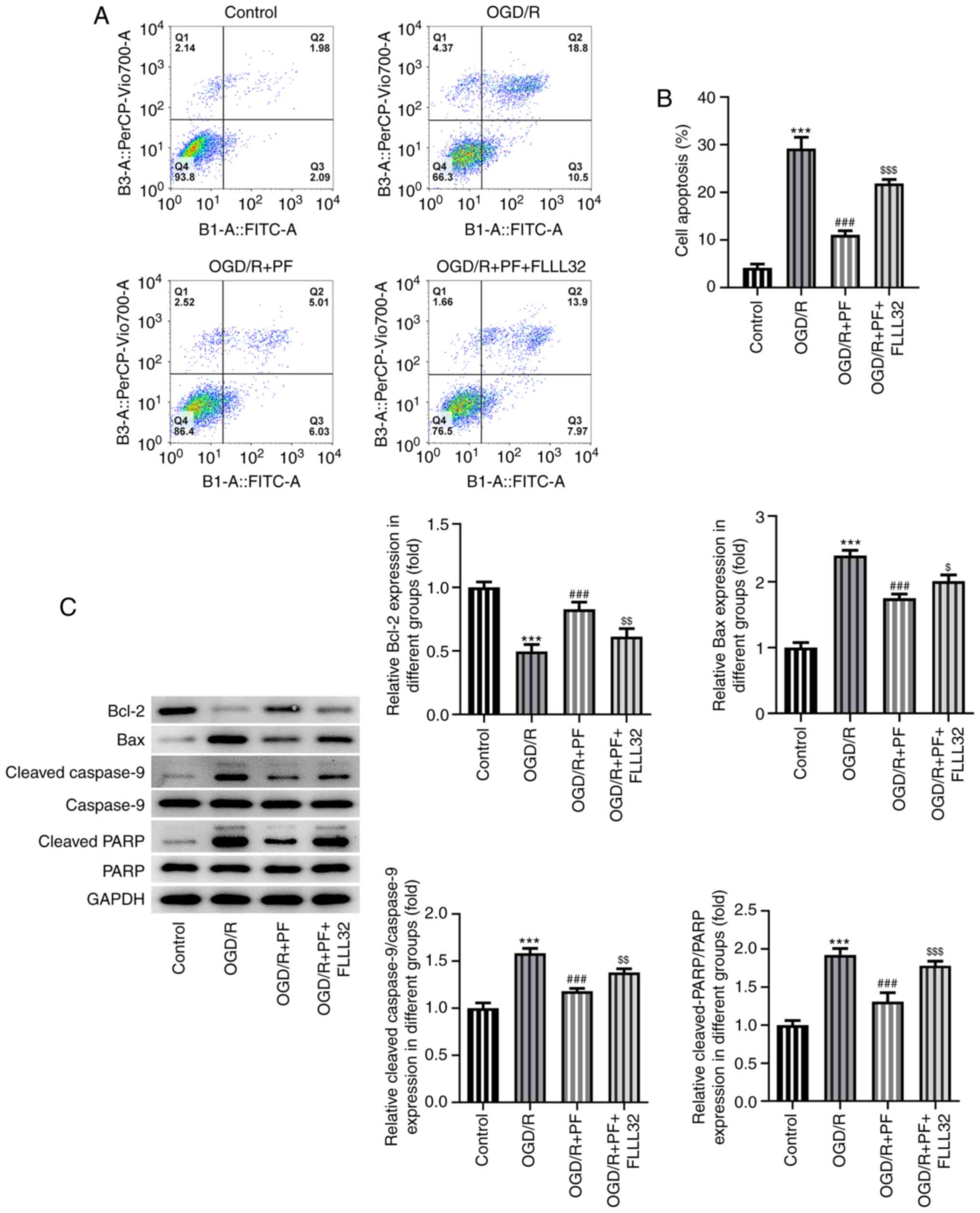
Apoptosis is considered as one of the most important
cellular processes in OGD/R-induced injury. Therefore, the
apoptosis of OGD/R-treated PC12 cells was determined. As shown in
Fig. 3A and B, cell apoptosis was enhanced among
OGD/R-treated PC12 cells; however, PF alleviated cell apoptosis in
a dose-dependent manner. Furthermore, the expression of the
pro-apoptotic proteins Bax, cleaved caspase-9 and cleaved PARP was
notably upregulated, while that of the anti-apoptotic protein Bcl-2
was downregulated by OGD/R. These effects were reversed by
increasing concentrations of PF (Fig.
3C). Collectively, these results revealed that PF attenuated
oxidative stress, inflammation and apoptosis in OGD/R-treated PC12
cells.
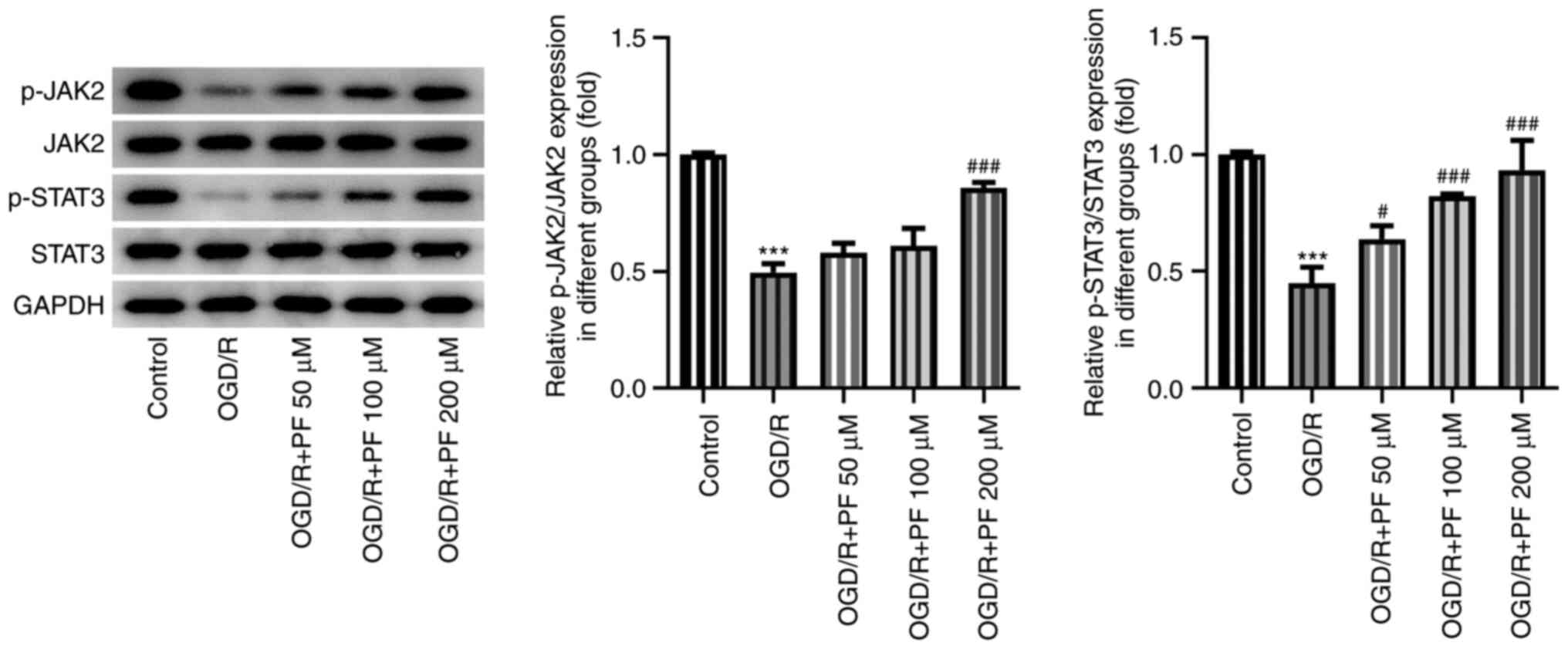
PF activates JAK2/STAT3 signaling in
OGD/R-treated PC12 cells
Subsequently, the possible association between PF
and the JAK2/STAT3 signaling pathway was evaluated by western blot
analysis. It was found that the protein expression levels of
phosphorylated (p)-JAK2 and p-STAT3 were significantly decreased in
the OGD/R-treated group compared with the untreated group (Fig. 4). In addition, treatment with
increasing doses of PF gradually enhanced the expression levels of
p-JAK2 and p-STAT3 compared with the OGD/R group. These results
suggested that the JAK2/STAT3 signaling pathway may be activated by
PF. To better evaluate the effects of PF, the concentration of 200
µM was selected for the subsequent experiments.
FLLL32 abrogates the inhibitory
effects of PF on oxidative stress, inflammation and apoptosis in
OGD/R-treated PC12 cells
Subsequently, PC12 cells subjected to OGD/R were
co-treated with the JAK2/STAT3 signaling inhibitor, FLLL32, after
PF addition, to verify whether PF exerted its protective effects on
these cells via the JAK2/STAT3 signaling pathway. As shown in
Fig. 5A, the OGD/R-mediated reduced
PC12 cell viability was restored by PF, while co-treatment with
FLLL32 decreased the cell viability. The effect of PF on
alleviating oxidative stress and inflammatory responses was
partially counteracted by FLLL32 intervention (Fig. 5B-G). As regards cell apoptosis, PF
suppressed the OGD/R-mediated PC12 cell apoptosis, which was
further promoted by FLLL32 (Fig. 6A
and B). Consistent with the
previous findings, treatment with FLLL32 markedly reduced the
expression levels of Bcl-2, which was accompanied by the
upregulated expression of Bax, cleaved caspase-9 and cleaved PARP,
compared with the OGD/R + PF group. The aforementioned findings
verified that FLLL32 could abrogate the protective effects of PF on
OGD/R-treated PC12 cells.
Discussion
In vivo and in vitro studies have
suggested that PF has potent anti-inflammatory and
immunosuppressive properties, and it can reduce pain, joint
swelling, synovial hypertrophy, bone erosion and cartilage
degradation in arthritic rats (16-18).
In addition, PF was shown to regulate the immune responses and
increase the survival rate of septic rats (19). Furthermore, PF exerted antioxidant
and anti-apoptotic effects in the treatment of cholestatic liver
injury (20). Therefore, the
present study sought to determine whether PF could also attenuate
inflammation, oxidative stress and apoptosis in the treatment of
ischemic stroke.
Oxidative stress is widely involved in several
pathophysiological processes, such as aging, inflammation and
tumorigenesis (21). A study
suggested that pre-treatment of PC12 cells with PF may enhance cell
viability and decrease the release of LDH (4). This finding was consistent with the
results of the present study. MPO is a critical inflammatory enzyme
and therapeutic target triggering both oxidative stress and
neuroinflammation during the pathological process of cerebral I/R
injury (22). MPO is often
upregulated in various inflammatory cells, while its inhibition has
been associated with the development of a relatively protective
environment from brain damage in a murine model of stroke (23). Furthermore, SOD can directly affect
the antioxidant capacity (24,25).
Increased SOD activity was shown to enhance the protective
mechanism against cerebral I/R injury in diabetic rats (26). Emerging evidence has suggested that
PF can protect HT-22 cells from H2O2-induced
oxidative injury via regulating the expression of miR-135a
(5). The present study demonstrated
that PF alleviated oxidative stress in OGD/R-treated PC12
cells.
Previous studies have reported that PF exerts
anti-inflammatory effects in order to regulate cellular functions
(27-30).
In the present study, treatment with PF also reduced the production
of inflammatory cytokines. Apoptosis is one of the most important
pathophysiological effects of ischemic stroke, and the aberrant
expression of apoptosis-related proteins has been considered to
serve as indicator or marker of cell death (11,31).
Other studies also supported the neuroprotective effects of PF,
mediated by decreased Bax and increased Bcl-2 expression (4). Consistent with previous findings, in
the present study PF promoted the down- and upregulation of pro-
and anti-apoptotic proteins, respectively, in OGD/R-treated PC12
cells. Therefore, PF attenuated oxidative stress, inflammation and
apoptosis in OGD/R-treated PC12 cells.
It has been reported that the JAK2/STAT3 signaling
pathway serves as a potent effector in attenuating cell death and
apoptosis (32). JAK2/STAT3
signaling has also been associated with the occurrence and
progression of several inflammatory diseases, including arthritis,
apical periodontitis and Alzheimer's disease (33-35).
JAK2 is essential for signaling through a variety of key class I
cytokine receptors, such as IL-3, IL-6, interferon-γ and leptin
(36). The STAT transcription
factors are crucial for cell fate. Among the members of the STAT
family, STAT3 is involved in classic inflammatory diseases
(37). A previous study
demonstrated that the activation of the JAK2/STAT3 signaling
pathway by different drugs protected mice from a series of
pathological stress stimuli and I/R injury (38). In the present study, the expression
levels of p-JAK2 and p-STAT3 were increased by PF in OGD/R-treated
PC12 cells, suggesting that this signaling pathway may be activated
by PF. Consistent with the findings of the present study, a
previous study demonstrated that abolishment of the JAK2/STAT3
signaling pathway by a pharmacological inhibitor aggravated
apoptosis and OGD/R injury (39).
Overall, the present study demonstrated that PF may
protect PC12 cells from OGD/R-induced injury partly via activating
JAK2/STAT3 signaling. Furthermore, the findings of the present
study may provide a better understating of the mechanism through
which PF alleviates the adverse effects of ischemic stroke and
indicate a promising approach to treating this disease. The lack of
studies in vivo is a limitation of the present research.
Based on the present findings, the effects of PF on the functional
recovery of animals with cerebral I/R injury will be studied in a
middle cerebral artery occlusion and reperfusion rat model.
Additionally, whether PF can inhibit oxidative stress, inflammation
and apoptosis and the regulatory effects of JAK2/STAT3 signaling
and other potential pathways will be further investigated in the
following experiments.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of materials and data
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZZ and WY searched the literature, designed the
experiments and performed the experiments. ZZ analyzed, interpreted
the data and wrote the manuscript. WY revised the manuscript. ZZ
and WY confirmed the authenticity of all the raw data. Both authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Martini ML, Neifert SN, Lara-Reyna JJ,
Shuman WH, Ladner TR, Hardigan TH, Fifi JT, Mocco J and Yaeger KA:
Trials in thrombectomy for acute ischemic stroke: Describing the
state of clinical research in the field. Clin Neurol Neurosurg.
200(106360)2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Chen H, Yoshioka H, Kim GS, Jung JE, Okami
N, Sakata H, Maier CM, Narasimhan P, Goeders CE and Chan PH:
Oxidative stress in ischemic brain damage: Mechanisms of cell death
and potential molecular targets for neuroprotection. Antioxid Redox
Signal. 14:1505–1517. 2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bavarsad K, Barreto GE, Hadjzadeh MA and
Sahebkar A: Protective effects of curcumin against
ischemia-reperfusion injury in the nervous system. Mol Neurobiol.
56:1391–1404. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Chen A, Wang H, Zhang Y, Wang X, Yu L, Xu
W, Xu W and Lin Y: Paeoniflorin exerts neuroprotective effects
against glutamate induced PC12 cellular cytotoxicity by inhibiting
apoptosis. Int J Mol Med. 40:825–833. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhai A, Zhang Z and Kong X: Paeoniflorin
alleviates H2O2-induced oxidative injury
through down-regulation of microRNA-135a in HT-22 cells. Neurochem
Res. 44:2821–2831. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wen Z, Hou W, Wu W, Zhao Y, Dong X, Bai X,
Peng L and Song L: 6'-O-Galloylpaeoniflorin attenuates cerebral
ischemia reperfusion-induced neuroinflammation and oxidative stress
via PI3K/Akt/Nrf2 activation. Oxid Med Cell Longev.
2018(8678267)2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Liu H, Yu C, Xu T, Zhang X and Dong M:
Synergistic protective effect of paeoniflorin and β-ecdysterone
against rotenone-induced neurotoxicity in PC12 cells. Apoptosis.
21:1354–1365. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Shio MT, Christian JG, Jung JY, Chang KP
and Olivier M: PKC/ROS-mediated NLRP3 inflammasome activation is
attenuated by leishmania zinc-metalloprotease during Infection.
PLoS Negl Trop Dis. 9(e0003868)2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Li J, Ji X, Zhang J, Shi G, Zhu X and Wang
K: Paeoniflorin attenuates Aβ25-35-induced neurotoxicity in PC12
cells by preventing mitochondrial dysfunction. Folia Neuropathol.
52:285–290. 2014.PubMed/NCBI
|
|
10
|
Wang D, Wong HK, Feng YB and Zhang ZJ:
Paeoniflorin, a natural neuroprotective agent, modulates multiple
anti-apoptotic and pro-apoptotic pathways in differentiated PC12
cells. Cell Mol Neurobiol. 33:521–529. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hou Y, Wang K, Wan W, Cheng Y, Pu X and Ye
X: Resveratrol provides neuroprotection by regulating the
JAK2/STAT3/PI3K/AKT/mTOR pathway after stroke in rats. Genes Dis.
5:245–255. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
de Couto G, Liu W, Tseliou E, Sun B,
Makkar N, Kanazawa H, Arditi M and Marbán E: Macrophages mediate
cardioprotective cellular postconditioning in acute myocardial
infarction. J Clin Invest. 125:3147–3162. 2015.PubMed/NCBI View
Article : Google Scholar
|
|
13
|
Li X, Wang Y, Wang K and Wu Y: Renal
protective effect of Paeoniflorin by inhibition of JAK2/STAT3
signaling pathway in diabetic mice. Biosci Trends. 12:168–176.
2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yang B, Zang LE, Cui JW, Zhang MY, Ma X
and Wei LL: Melatonin plays a protective role by regulating
miR-26a-5p-NRSF and JAK2-STAT3 pathway to improve autophagy,
inflammation and oxidative stress of cerebral ischemia-reperfusion
injury. Drug Des Devel Ther. 14:3177–3188. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yuan J, Zeng L, Sun Y, Wang N, Sun Q,
Cheng Z and Wang Y: SH2B1 protects against OGD/R induced apoptosis
in PC12 cells via activation of the JAK2/STAT3 signaling pathway.
Mol Med Rep. 18:2613–2620. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhang LL, Wei W, Wang NP, Wang QT, Chen
JY, Chen Y, Wu H and Hu XY: Paeoniflorin suppresses inflammatory
mediator production and regulates G protein-coupled signaling in
fibroblast-like synoviocytes of collagen induced arthritic rats.
Inflamm Res. 57:388–395. 2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ling L, Tong J and Zeng L: Paeoniflorin
improves acute lung injury in sepsis by activating Nrf2/Keap1
signaling pathway. Sichuan Da Xue Xue Bao Yi Xue Ban. 51:664–669.
2020.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
18
|
Zhou H, Bian D, Jiao X, Wei Z, Zhang H,
Xia Y, He Y and Dai Y: Paeoniflorin protects against
lipopolysaccharide-induced acute lung injury in mice by alleviating
inflammatory cell infiltration and microvascular permeability.
Inflamm Res. 60:981–990. 2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Jiang WL, Chen XG, Zhu HB, Gao YB, Tian JW
and Fu FH: Paeoniflorin inhibits systemic inflammation and improves
survival in experimental sepsis. Basic Clin Pharmacol Toxicol.
105:64–71. 2009.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wei S, Ma X, Niu M, Wang R, Yang T, Wang
D, Wen J, Li H and Zhao Y: Mechanism of paeoniflorin in the
treatment of bile duct ligation-induced cholestatic liver injury
using integrated metabolomics and network pharmacology. Front
Pharmacol. 11(586806)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Poprac P, Jomova K, Simunkova M, Kollar V,
Rhodes CJ and Valko M: Targeting free radicals in oxidative
stress-related human diseases. Trends Pharmacol Sci. 38:592–607.
2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Chen S, Chen H, Du Q and Shen J: Targeting
myeloperoxidase (MPO) mediated oxidative stress and inflammation
for reducing brain ischemia injury: Potential application of
natural compounds. Front Physiol. 11(433)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yu G, Liang Y, Huang Z, Jones DW,
Pritchard KA Jr and Zhang H: Erratum to: Inhibition of
myeloperoxidase oxidant production by N-acetyl lysyltyrosylcysteine
amide reduces brain damage in a murine model of stroke. J
Neuroinflammation. 13(166)2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Briyal S, Gulati K and Gulati A: Repeated
administration of exendin-4 reduces focal cerebral ischemia-induced
infarction in rats. Brain Res. 1427:23–34. 2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhao L, Xu J, Wang Q, Qian Z, Feng W, Yin
X and Fang Y: Protective effect of rhGLP-1 (7-36) on brain
ischemia/reperfusion damage in diabetic rats. Brain Res.
1602:153–159. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Fang Y, Liu X, Zhao L, Wei Z, Jiang D,
Shao H, Zang Y, Xu J, Wang Q, Liu Y, et al: RhGLP-1 (7-36) protects
diabetic rats against cerebral ischemia-reperfusion injury via
up-regulating expression of Nrf2/HO-1 and increasing the activities
of SOD. Korean J Physiol Pharmacol. 21:475–485. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Dai X, Wang LW, Jia XY, Chang Y, Wu HX,
Wang C and Wei W: Paeoniflorin regulates the function of human
peripheral blood mononuclear cells stimulated by rhIL-1β by
up-regulating Treg expression. Immunopharmacol Immunotoxicol.
37:252–257. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Jin L, Zhang LM, Xie KQ, Ye Y and Feng L:
Paeoniflorin suppresses the expression of intercellular adhesion
molecule-1 (ICAM-1) in endotoxin-treated human monocytic cells. Br
J Pharmacol. 164:694–703. 2011.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhai T, Sun Y, Li H, Zhang J, Huo R, Li H,
Shen B and Li N: Unique immunomodulatory effect of paeoniflorin on
type I and II macrophages activities. J Pharmacol Sci. 130:143–150.
2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Shi D, Wang Q, Zheng H, Li D, Shen Y, Fu
H, Li T, Mei H, Lu G, Qiu Y, et al: Paeoniflorin suppresses
IL-6/Stat3 pathway via upregulation of Socs3 in dendritic cells in
response to 1-chloro-2,4-dinitrobenze. Int Immunopharmacol.
38:45–53. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Adams JM and Cory S: The Bcl-2 protein
family: Arbiters of cell survival. Science. 281:1322–1326.
1998.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Shyu WC, Lin SZ, Chiang MF, Chen DC, Su
CY, Wang HJ, Liu RS, Tsai CH and Li H: Secretoneurin promotes
neuroprotection and neuronal plasticity via the Jak2/Stat3 pathway
in murine models of stroke. J Clin Invest. 118:133–148.
2008.PubMed/NCBI View
Article : Google Scholar
|
|
33
|
Hossain E, Li Y and Anand-Srivastava MB:
Role of JAK2/STAT3 pathway in angiotensin II-induced enhanced
expression of Gialpha proteins and hyperproliferation of aortic
vascular smooth muscle cells. Can J Physiol Pharmacol. 99:237–246.
2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
El-Ghafar OAMA, Helal GK and Abo-Youssef
AM: Apixaban exhibits anti-arthritic effects by inhibiting
activated factor X-mediated JAK2/STAT3 and MAPK phosphorylation
pathways. Inflammopharmacology. 28:1253–1267. 2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Pan S, Chen Y, Zhang X and Xie Y: The
JAK2/STAT3 pathway is involved in dexmedetomidine-induced
myocardial protection in rats undergoing cardiopulmonary bypass.
Ann Transl Med. 8(483)2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wu QY, Ma MM, Fu L, Zhu YY, Liu Y, Cao J,
Zhou P, Li ZY, Zeng LY, Li F, et al: Roles of germline JAK2
activation mutation JAK2 V625F in the pathology of
myeloproliferative neoplasms. Int J Biol Macromol. 116:1064–1073.
2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Leong PL, Andrews GA, Johnson DE, Dyer KF,
Xi S, Mai JC, Robbins PD, Gadiparthi S, Burke NA, Watkins SF, et
al: Targeted inhibition of Stat3 with a decoy oligonucleotide
abrogates head and neck cancer cell growth. Proc Natl Acad Sci USA.
100:4138–4143. 2003.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Li L, Li H and Li M: Curcumin protects
against cerebral ischemia-reperfusion injury by activating
JAK2/STAT3 signaling pathway in rats. Int J Clin Exp Med.
8:14985–14991. 2015.PubMed/NCBI
|
|
39
|
Li L, Sun L, Qiu Y, Zhu W, Hu K and Mao J:
Protective effect of stachydrine against cerebral
ischemia-reperfusion injury by reducing inflammation and apoptosis
through P65 and JAK2/STAT3 signaling pathway. Front Pharmacol.
11(64)2020.PubMed/NCBI View Article : Google Scholar
|