Introduction
Polycystic ovary syndrome (PCOS) is an endocrine
disorder that causes health problems in 6-10% of premenopausal
women (1). Hyperandrogenemia with
chronic anovulation is the main phenotype of PCOS (2). The typical clinical manifestations of
PCOS include acanthosis, alopecia, subfertility, acne vulgaris,
obesity, seborrhea (3), hirsutism
and menstrual disorders (4). PCOS
is one of the main causes of anovulatory sterility in women
(5). Furthermore, women with PCOS
are at an increased risk of dyslipidemia, insulin resistance, type
2 diabetes and hypertension (6).
However, although current research has provided more information on
genetic background and the effect of environmental factors, the
mechanism underlying the development of PCOS remains elusive.
During the development of ovarian follicles, it was
observed that all stages of follicular atresia are associated with
the apoptosis of granulosa cells (GCs). Based on this observation,
GC apoptosis is considered to be the main mechanism underlying
follicular atresia. A variety of factors have been shown to cause
GC apoptosis, including depletion of cell survival factors
(7). In addition, there is evidence
that abnormal GC function may be the cause of abnormal follicle
formation in PCOS (8). The high
rate of apoptotic GCs is associated with low pregnancy rates,
fertilization rates and embryonic dysplasia (9). However, the exact etiology of PCOS
remains to be fully elucidated.
MicroRNAs (miRs/miRNAs) are highly conserved
single-stranded non-coding RNA molecules composed of 20-24
nucleotides. miRNAs are important regulators of biological
processes, such as cell proliferation, differentiation, migration
and apoptosis (10). Dysregulation
and differential expression of miRNAs have been associated with
ovarian cancer, PCOS and uterus-related diseases. Furthermore,
miRNAs have been found to be crucial for reproductive function,
gonadal development and sex differentiation (11).
miR-451a is located on human chromosome 17q11.2. A
previous study by Ibáñez et al suggested that one of the
biomarkers for the diagnosis and treatment of PCOS during puberty
may be circulating miR-451a (12).
Low circulating levels of miR-451a in women with PCOS were
previously reported, but its specific role remained unclear
(13).
Although the pathogenic mechanism of PCOS must be
further elucidated, previous findings indicated that the
proliferation and survival of GCs may be implicated. The ovarian
granulosa cell tumour cell line KGN has been widely used to
investigate the biological function of ovarian granulosa cells in
PCOS (14-16).
Therefore, the aim of the present study was to determine whether
miR-451a could regulate the biological function of KGN cells to
verify its role in PCOS and to further explore the underlying
molecular mechanisms, in order to provide new insights into the
treatment of PCOS.
Materials and methods
Cell culture, treatment and
transfection
Human ovarian granulosa cell tumour cell line KGN
cells, normal ovarian surface epithelial IOSE80 cells and 293 cells
were all purchased from the American Type Culture Collection. All
cell lines were cultured in DMEM/F12 supplemented with 10% FBS, 100
U/ml penicillin G (Life Technologies; Thermo Fisher Scientific,
Inc.) and 0.1 mg/ml streptomycin sulfate (Gibco; Thermo Fisher
Scientific, Inc.) in a humidified incubator at 37˚C with 5%
CO2.
For the cell transfection experiments, vectors
including miR-451a mimic, miR-451a mimic control, control-plasmid,
ATF2-plasmid, miR-451a mimic + control-plasmid, or miR-451a mimic +
ATF2-plasmid were transfected into KGN cells using Lipofectamine™
2000 (Life Technologies; Thermo Fisher Scientific, Inc.) according
to the manufacturer's protocol.
Target prediction and luciferase
reporter assay
TargetScan version 7.2 (www.targetscan.org) was used to analyze and predict
potential targets for miR-451a. The 3'-untranslated region (3'-UTR)
sequence of mutated (MUT) or wild-type (WT) ATF2, containing the
putative miR-451a-binding site, was amplified by PCR and cloned
into a psiCHECK vector (Promega Corporation). Site-directed
mutagenesis using the QuikChange Lightning Site-Directed
Mutagenesis kit (Agilent Technologies, Inc.) was performed
according to the manufacturer's instructions to obtain the MUT
3'-UTR. Lipofectamine™ 2000 (Invitrogen; Thermo Fisher Scientific,
Inc.) was used to co-transfect miR-451a mimic or mimic control with
WT or MUT 3'-UTR vectors encoding Renilla luciferase into
293 cells. After 48 h, the activity of firefly luciferase was
detected using the Dual-Luciferase Report Detection kit (Promega
Corporation).
Determination of cell
proliferation
MTT analysis was used to assess the proliferation of
KGN cells. Briefly, KGN cells were seeded into 96-well plates at a
density of 5x103 cells/well. KGN cells were transfected
with the vectors (mimic control, miR-451a mimic, miR-451a mimic +
ATF2-plasmid or miR-451a mimic + control-plasmid) and incubated for
48 h. KGN cells were incubated with 20 µl MTT for 4 h at 37˚C. The
cells were then lysed with 150 µl DMSO for 10 min at room
temperature at 24, 48, 72 and 96 h after transfection. An
absorption spectrophotometer (Olympus Corporation) was used to
detect the optical density at a wavelength of 570 nm.
Reverse transcription-quantitative PCR
(RT-qPCR) analysis
The TRIzol one-step method (Invitrogen; Thermo
Fisher Scientific, Inc.) was used to extract total RNA from KGN
cells. Total RNA concentration was detected using a nucleic acid
protein analyzer (Beckman Coulter, Inc.). RT was immediately
performed using the Prime Script RT-PCR kit (Takara Biotechnology
Co., Ltd.) according to the manufacturer's instructions to avoid
RNA degradation. qPCR analysis was performed using the quantitative
SYBR-Green PCR kit (Qiagen GmbH) and the Mx4000 quantitative PCR
system (Stratagene; Agilent Technologies, Inc.). The reaction
conditions used for the qPCR were as follows: Initial denaturation
for 5 min at 95˚C; followed by 40 cycles of denaturation at 95˚C
for 10 sec, annealing at 60˚C for 30 sec and extension at 72˚C for
34 sec. The internal controls used were GAPDH or U6. Gene
expression was analyzed using the 2-ΔΔCq method
(17). The primer sequences for PCR
were listed as follows: GAPDH forward, 5'-CTTTGGTATCGTGGAAGGACTC-3'
and reverse, 5'-GTAGAGGCAGGGATGATGTTCT-3'; U6 forward,
5'-GCTTCGGCAGCACATATACTAAAAT-3' and reverse,
5'-CGCTTCACGAATTTGCGTGTCAT-3'; miR-451a forward,
5'-ACACTCCAGCTGGGAAACCGTTACCATTAC-3' and reverse,
5'-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGCTTACAG-3; ATF2 forward,
5'-TACAAGTGGTCGTCGG-3' and reverse, 5'-CGGTTACAGGGCAATC-3'; and
cyclin D1 forward, 5'-CCGTCCATGCGGAAGATC-3 and reverse,
5'-GAAGACCTCCTCCTCGCACT-3'.
Flow cytometric analysis of
apoptosis
KGN cells (5x104 cells per well)
transfected with indicated plasmids or oligonucleotides were seeded
in 6-well plates and incubated for 48 h. For cell apoptosis
analysis, the Annexin V-fluorescein isothiocyanate/propidium iodide
Apoptosis Detection kit (Abcam) was used according to the
manufacturer's instructions. Cell apoptosis was detected using the
FACScan flow cytometry system equipped with CellQuest software
version 5.1 (Becton, Dickinson and Company).
Caspase-3 activity detection
After transfection for 48 h, KGN cells were
collected through centrifugation (600 x g; 4˚C; 5 min). Then the
caspase-3 activity was immediately detected using a caspase-3
activity assay kit (cat no. C1116; Beyotime Institute of
Biotechnology), following with the manufacturer's protocol.
Western blotting
Total protein was extracted from cells using 1 ml
RIPA cell lysis buffer (Sangon Biotech, Co., Ltd.). The proteins
were quantified using a bicinchoninic acid protein assay reagent
(Thermo Fisher Scientific, Inc.). A total of 40 µg protein from
each sample was separated by 10% SDS-PAGE, and subsequently
transferred onto PVDF membranes (Bio-Rad Laboratories, Inc.). The
membranes were blocked for 1 h in TBS with 0.1% Tween-20 in 5%
skimmed milk at room temperature, and incubated overnight at 4˚C
with the following primary antibodies: Anti-ATF2 (cat no. 35031;
1:1,000; Cell Signaling Technology, Inc.), anti-cyclin D1 (cat no.
55506; 1:10,000; Cell Signaling Technology, Inc.), anti-cleaved
caspase-3 (cat no. ab32042; 1:500; Abcam), anti-pro-caspase-3 (cat
no. ab3499; 1:500; Abcam) and anti-GAPDH (cat no. 5174; 1:1,000,
Cell Signaling Technology, Inc.). This was followed by incubation
with horseradish peroxidase-conjugated anti-rabbit IgG secondary
antibody (cat no. 7074; 1:1,000; Cell Signaling Technology, Inc.)
for 1 h at room temperature. Finally, enhanced chemiluminescence
(EMD Millipore) was used to examine the immune complexes and the
protein bands were quantified using ImageJ software (version 2.0;
National Institutes of Health).
Statistical analysis
All experiments were performed three times. Data are
expressed as the mean ± SD and evaluated using Student's t-test or
one-way ANOVA followed by Tukey's post hoc test. Data were analyzed
using SPSS 22.0 software (IBM Corp.). P<0.05 was considered to
indicate a statistically significant difference.
Results
miR-451a expression in the IOSE80 and
KGN cell lines
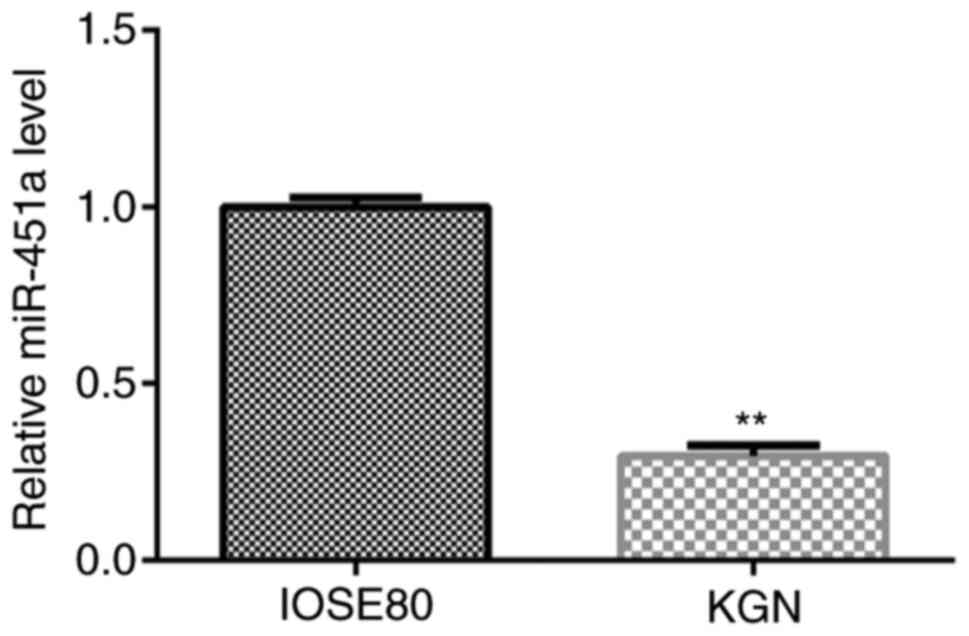
RT-qPCR analysis was performed to verify the
expression of miR-451a in IOSE80 and KGN cells. As shown in
Fig. 1, the expression of miR-451a
in KGN cells was significantly lower compared with that in IOSE80
cells.
ATF2 is a target gene of miR-451a and
is significantly overexpressed in KGN cells
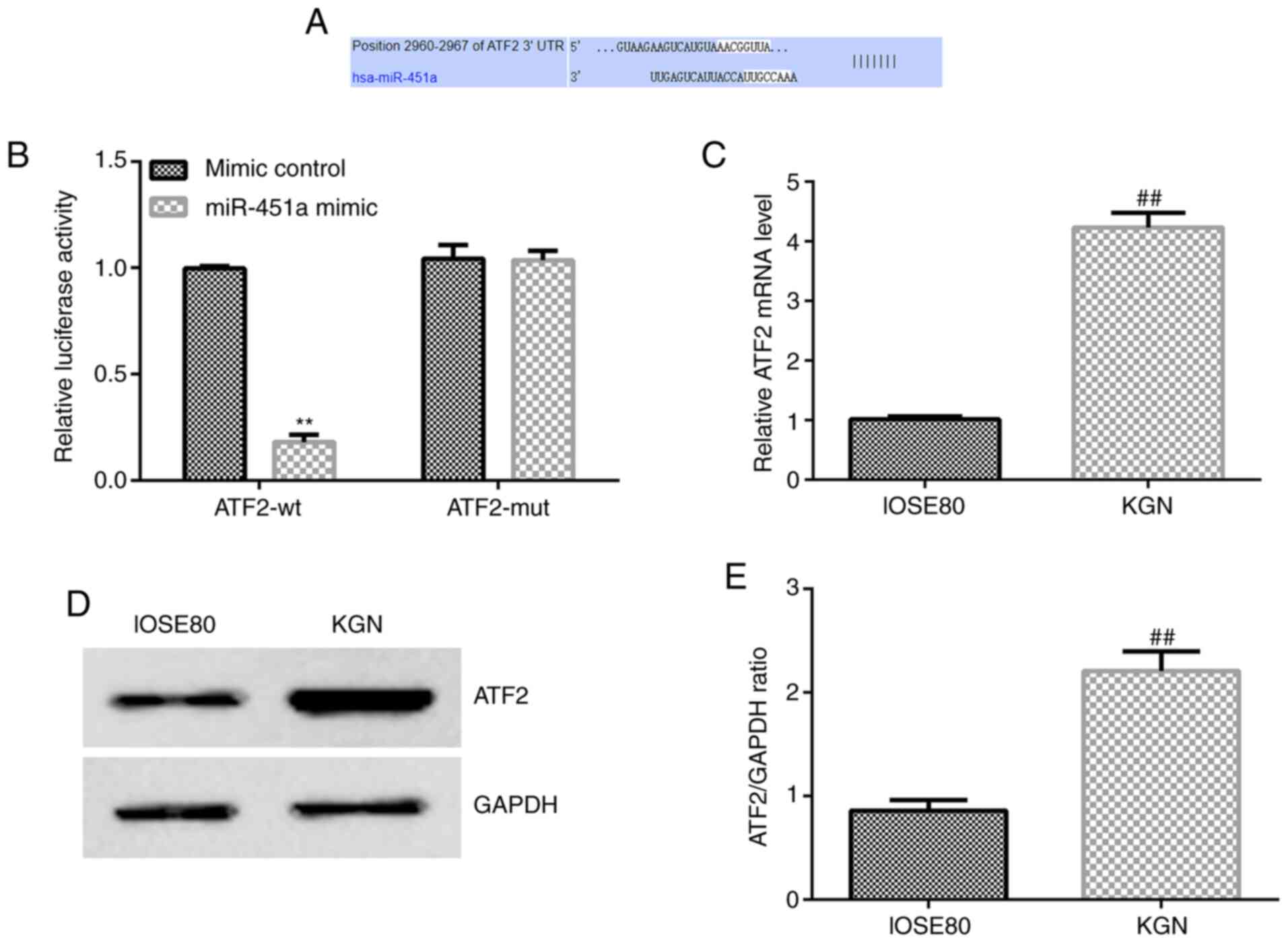
Based on the target genes predicted by
bioinformatics analysis, ATF2 was identified as a potential target
of miR-451a (Fig. 2A). To confirm
whether ATF2 is a target gene of miR-451a, a luciferase reporter
assay was performed (Fig. 2B).
Compared with the negative control, miR-451a mimic decreased WT
3'-UTR fluorescence (Fig. 2B),
while MUT 3'-UTR was not altered following miR-451a transfection.
Hence, the Dual-Luciferase reporter assay validated that ATF2 was a
target gene of miR-451a.
To further verify the potential role of miR-451a in
KGN cells, ATF2 mRNA and protein expression levels were assessed in
the KGN and IOSE80 cell lines. As shown in Fig. 2C-E, ATF2 expression was
significantly increased in KGN cells compared with that in IOSE80
cells.
miR-451a inhibits KGN cell viability
and induces apoptosis by reducing ATF2 expression
To determine the functional effects of miR-451a on
KGN cell growth in vitro, KGN cells were transfected with
mimic control, miR-451a mimic, ATF2-plasmid, control-plasmid,
miR-451a mimic + control-plasmid or miR-451a mimic + ATF2-plasmid.
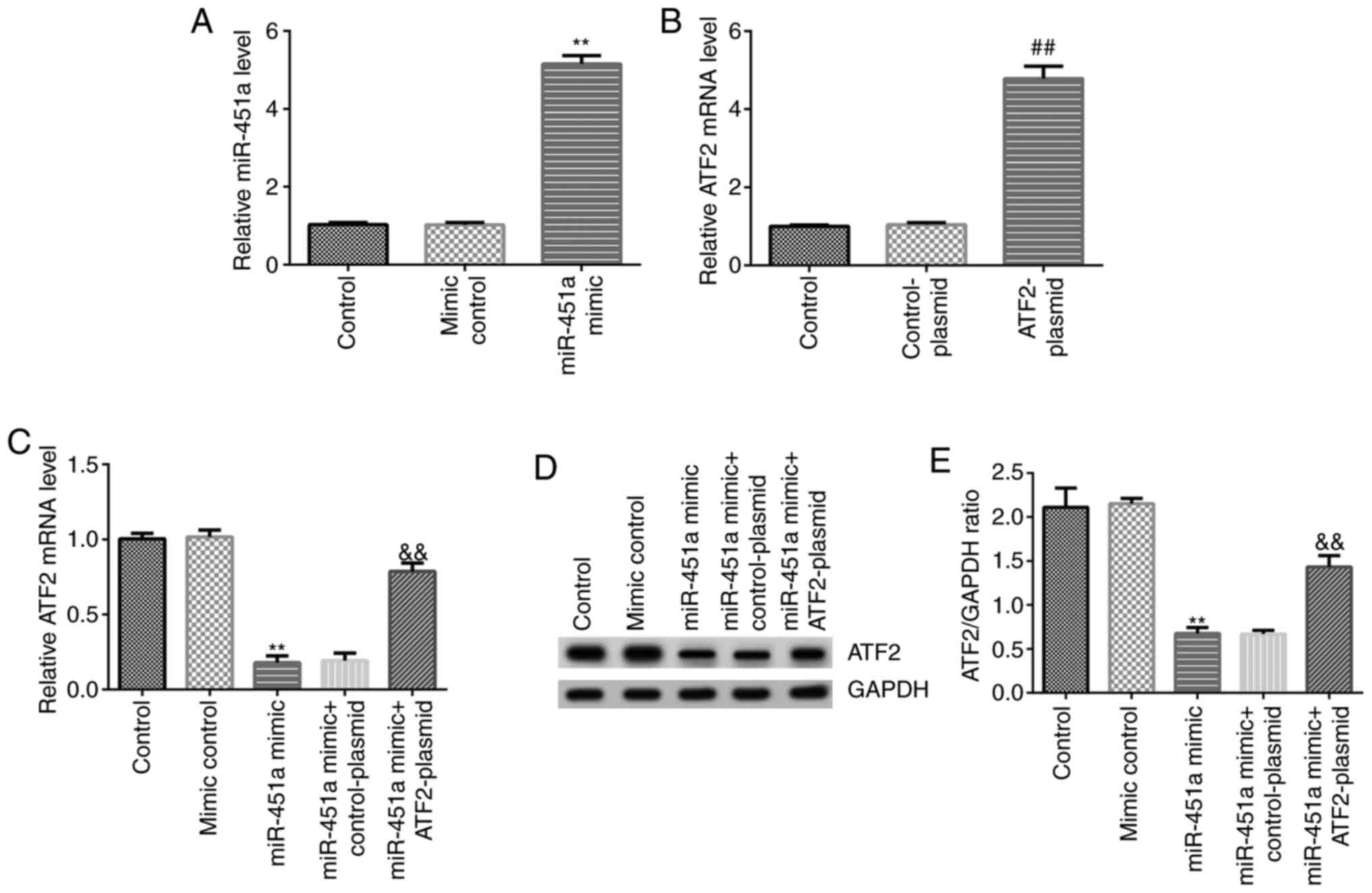
RT-qPCR analysis was performed to confirm transfection efficiency.
As shown in Fig. 3A, miR-451a mimic
significantly increased miR-451a expression levels in KGN cells
compared with the mimic control group. ATF2-plasmid significantly
enhanced ATF2 mRNA expression in KGN cells compared with the
control-plasmid group (Fig. 3B).
Simultaneously, compared with the mimic control group, miR-451a
mimic significantly decreased mRNA and protein expression of ATF2
in KGN cells, and this decrease was reversed by ATF2-plasmid
co-transfection (Fig. 3C-E).
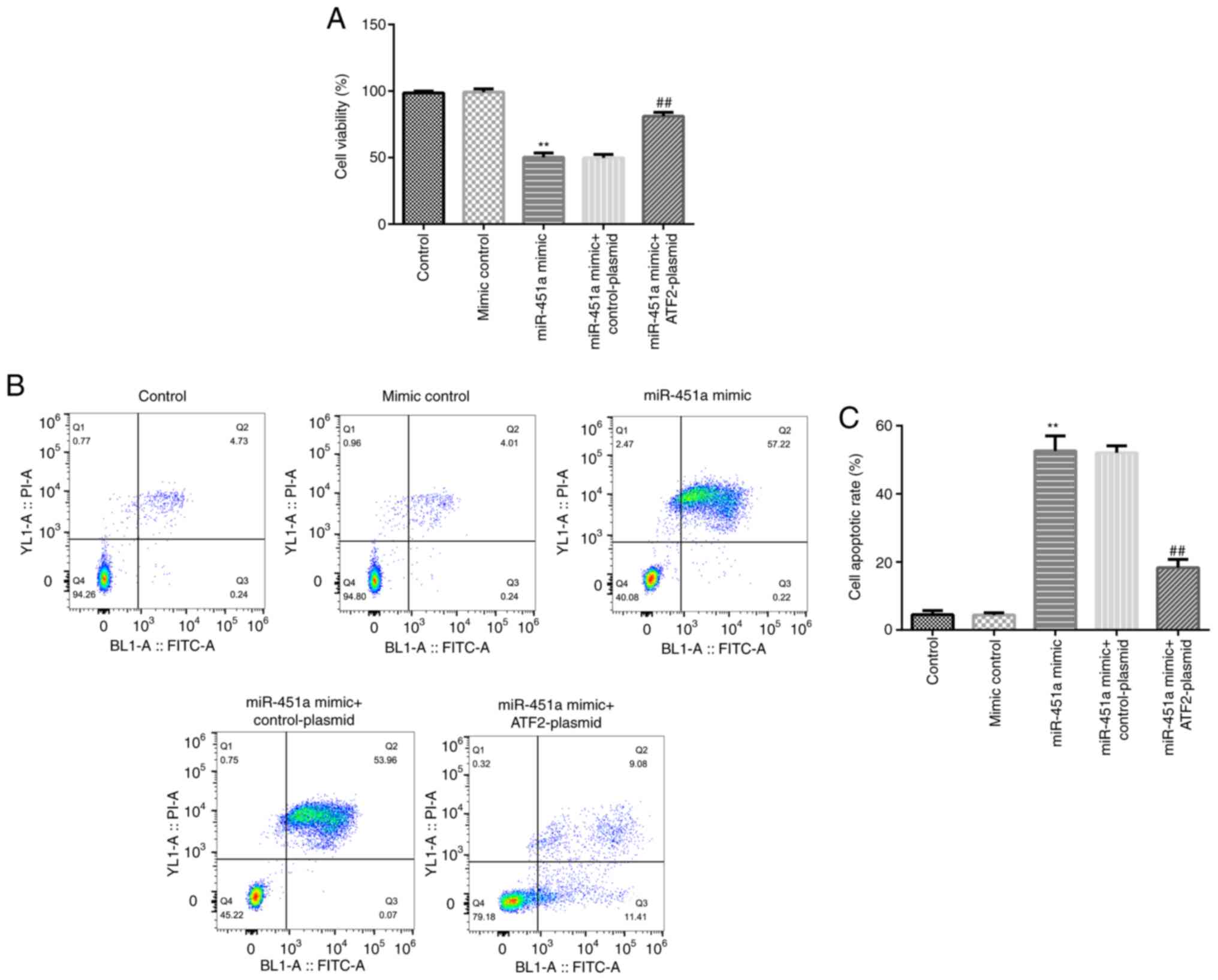
To further confirm the functional association
between ATF2 and miR-451a, KGN cells were transfected with mimic
control, miR-451a mimic, miR-451a mimic + control-plasmid or
miR-451a mimic + ATF2-plasmid. Cell viability and apoptosis was
evaluated by MTT and flow cytometry assays, respectively. As shown
in Fig. 4A-C, miR-451a mimic
significantly decreased KGN cell viability and induced apoptosis
compared with the mimic control group, and these effects were
significantly reversed by ATF2-plasmid co-transfection.
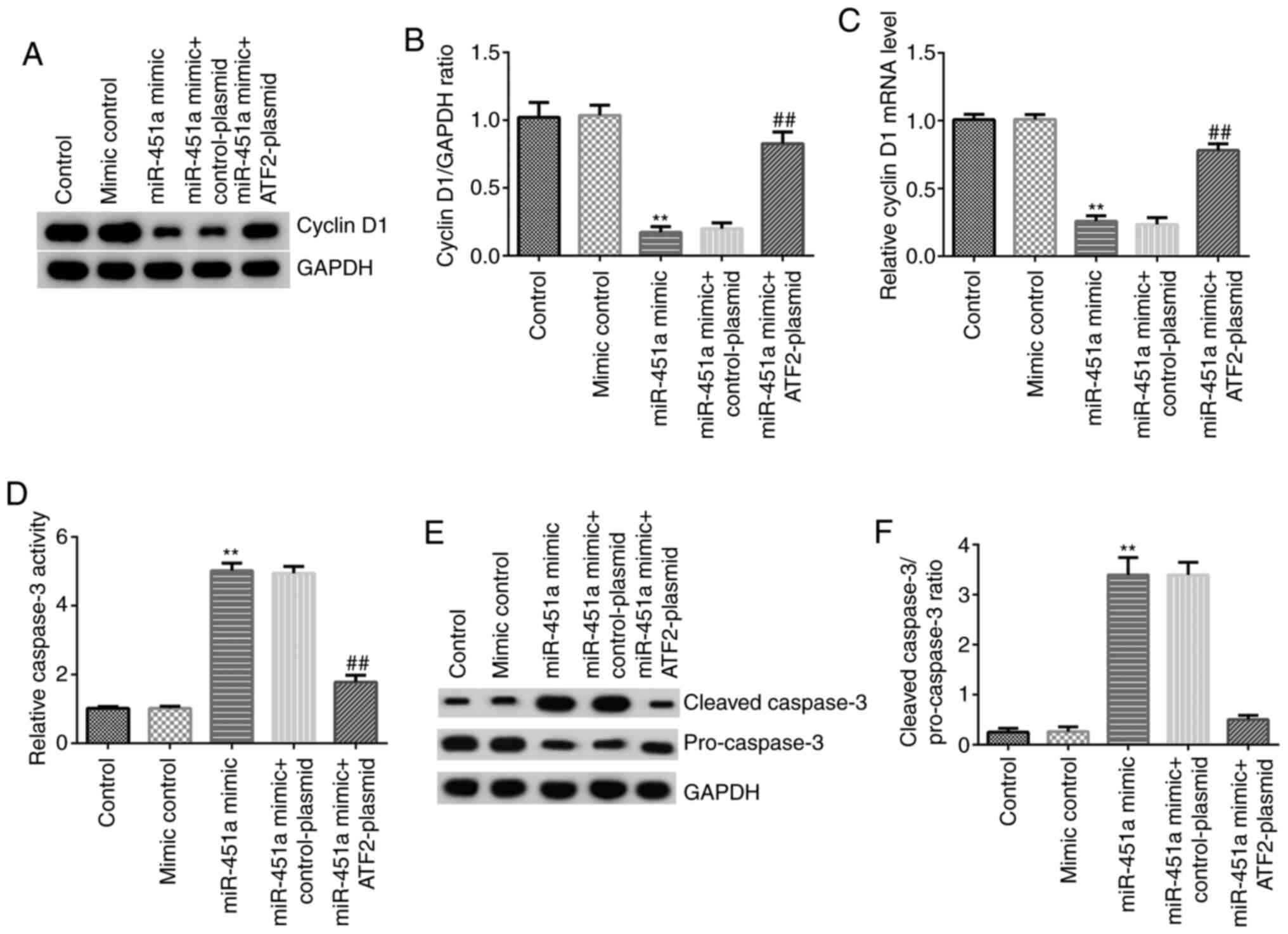
Western blotting and RT-qPCR analysis were performed
to detect cyclin D1 protein and mRNA expression, respectively. As
shown in Fig. 5A-C, compared with
mimic controls, miR-451a mimic significantly reduced the expression
of cyclin D1 at both the protein (Fig.
5A and B) and mRNA (Fig. 5C) levels in KGN cells, and these
effects were significantly reversed by ATF2-plasmid
co-transfection. Caspase-3 activity was assessed using a kit and
the protein expression of cleaved caspase-3 and pro-caspase-3 was
investigated by western blotting. The results demonstrated that
miR-451a mimic significantly increased the activity of caspase-3
(Fig. 5D), enhanced cleaved
caspase-3 protein levels, decreased the protein expression of
pro-caspase-3 (Fig. 5E) and
upregulated the cleaved caspase-3/pro-caspase-3 ratio (Fig. 5F) in KGN cells. In addition, these
effects were notably reversed by ATF2-plasmid co-transfection. The
results indicated that miR-451a inhibited KGN cell proliferation
and induced apoptosis by reducing the expression of ATF2.
Discussion
The results of the present study demonstrated that
miR-451a is downregulated in KGN cells and that miR-451a
upregulation may inhibit GC proliferation, which may be the cause
underlying the development of abnormal follicles in PCOS. The
expression of miR-451a was found to be significantly lower in KGN
cells compared with that in IOSE80 cells. Based on previous
studies, miR-451a is considered to play a role in the control of
the cell proliferation, apoptosis and metastasis, by acting as a
tumor suppressor (18-23).
However, few studies have investigated the function of miR-451a in
ovarian GCs. The present study determined that miR-451a may be a GC
proliferation inhibitor.
Consistently with the results of a previous study
(19), our further experiments
revealed that ATF2 was a target of miR-451a. Based on
Dual-Luciferase reporter gene assay results, miR-451a was shown to
directly target the 3'-UTR of ATF2. Furthermore, transfection with
miR-451a mimics reduced the protein and mRNA levels of ATF2 in KGN
cells, whereas ATF2-plasmid transfection reversed these effects.
These results indicated that miR-451a and its target ATF2 may
jointly regulate the proliferation of GCs in PCOS.
ATF2 has been investigated in the context of several
developmental and pathological conditions (24). Recent studies demonstrated that ATF2
may act as both a classic tumor suppressor (25) and as an oncogene (26). Moreover, it was reported that
miR-451a suppressed cell migration and invasion in non-small cell
lung cancer through targeting ATF2(19). However, few studies have
investigated ATF2 in ovarian GCs, and its mechanism of action
remains unclear. ATF2 expression varies among different diseases. A
previous study has shown that ATF2 is involved in the
transcriptional regulation mechanism of mouse placental trophoblast
giant cells and rat ovarian GCs in primary cultures (27). The present findings are consistent
with these previous studies, whereby inhibition of ATF2 can inhibit
GC proliferation.
The differences in miR-451a expression and cell
proliferation observed in the present study may be attributed to
differences in enzyme activity and protein and mRNA expression. The
results of the present study demonstrated that miR-451a mimic
significantly reduced the expression of cyclin D1 protein and mRNA
in KGN cells, while it concurrently significantly increased the
activity of caspase-3, increased cleaved caspase-3 and reduced the
protein expression of pro-caspase-3. These effects were
significantly reversed by ATF2-plasmid co-transfection. Cyclin D1
is a key regulatory protein that promotes the transition through
the restriction point in the G1 phase to S phase. Caspase-3 has
been reported to be an important molecule in promoting various
types of apoptosis (28). Its
activation leads to the initiation of the caspase cascade, which is
responsible for cell death (29).
Zhen et al demonstrated that the regulatory mechanism of
CCAAT/enhancer-binding protein (CEBP) in the cell cycle and steroid
synthesis was inhibited in porcine ovaries. Additionally, the
expression of cell cycle-related genes (cyclin D1, cyclin B1 and
cyclin A1) was suppressed, indicating that knocking out the CEBP
gene may inhibit apoptosis (30).
The present results were consistent with those of previous studies.
By targeting ATF2 in PCOS, miR-451a could decrease the expression
of cyclin D1 and upregulate the activity and expression of cleaved
caspase-3. However, the signaling pathway through which
miR-451a/ATF2 regulate the proliferation and apoptosis of ovarian
GCs remains largely unknown, and more in-depth investigation is
required. Future research will focus on further exploring the
molecular mechanisms underlying the regulatory effects of
miR-451a/ATF2 on ovarian GCs and elucidating the related signaling
pathways.
In summary, the results of the present study
demonstrated that miR-451a regulated the proliferation and
apoptosis of ovarian GCs by targeting ATF2, thereby providing novel
insight into GC dysfunction in PCOS, and indicating miR-451a/ATF2
as a novel potential target for PCOS treatment.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Guidance Program
of the Natural Fund of Liaoning Provincial Science and Technology
Department (grant no. 2019-ZD-0816).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
TY contributed to the conception and design of the
study and the manuscript preparation. LW, YZ and JZ contributed to
the data acquisition, analysis and interpretation. LL contributed
to the data acquisition and analysis. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Doroszewska K, Milewicz T, Mrozińska S,
Janeczko J, Rokicki R, Janeczko M, Warzecha D and Marianowski P:
Blood pressure in postmenopausal women with a history of polycystic
ovary syndrome. Prz Menopauzalny. 18:94–98. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Seyam E, Al Gelany S, Abd Al Ghaney A,
Mohamed MAA, Youseff AM, Ibrahim EM, Khalifa EM and Hefzy E:
Evaluation of prolonged use of statins on the clinical and
biochemical abnormalities and ovulation dysfunction in single young
women with polycystic ovary syndrome. Gynecolo Endocrinol.
34:589–596. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Sharma S, Mathur DK, Paliwal V and
Bhargava P: Efficacy of metformin in the treatment of acne in women
with polycystic ovarian syndrome: A newer approach to acne therapy.
J Clin Aes DermatO. 12:34–38. 2019.PubMed/NCBI
|
|
4
|
Moini Jazani A, Nasimi Doost Azgomi H,
Nasimi Doost Azgomi A and Nasimi Doost Azgomi R: A comprehensive
review of clinical studies with herbal medicine on polycystic ovary
syndrome (PCOS). Daru. 27:863–877. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kriedt KJ, Alchami A and Davies MC: PCOS:
Diagnosis and management of related infertility. Obstet Gynaecol
Reprod Med. 29:1–5. 2019.
|
|
6
|
Arentz S, Abbott JA, Smith CA and
Bensoussan A: Herbal medicine for the management of polycystic
ovary syndrome (PCOS) and associated oligo/amenorrhoea and
hyperandrogenism; a review of the laboratory evidence for effects
with corroborative clinical findings. BMC Complement Altern Med.
14(511)2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yu YS, Sui HS, Han ZB, Li W, Luo MJ and
Tan JH: Apoptosis in granulosa cells during follicular atresia:
Relationship with steroids and insulin-like growth factors. Cell
Res. 14:341–346. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Niu Z, Ye Y, Xia L, Feng Y and Zhang A:
Follicular fluid cytokine composition and oocyte quality of
polycystic ovary syndrome patients with metabolic syndrome
undergoing in vitro fertilization. Cytokine. 91:180–186.
2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Salei N, Hellberg L, Köhl J and Laskay T:
Enhanced survival of Leishmania major in neutrophil
granulocytes in the presence of apoptotic cells. PLoS One.
12(e0171850)2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Xiang Y, Song Y, Li Y, Zhao D, Ma L and
Tan L: miR-483 is down-regulated in polycystic ovarian syndrome and
inhibits KGN cell proliferation via targeting insulin-like growth
factor 1 (IGF1). Med Sci Monit. 22:3383–3393. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sun L, Zuo Z, Luo H, Chen M, Zhong Y, Chen
Y and Wang C: Chronic exposure to phenanthrene influences the
spermatogenesis of male sebastiscus marmoratus: U-shaped
effects and the reason for them. Environ Sci Technol.
45:10212–10218. 2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ibáñez L, Oberfield SE, Witchel S, Auchus
RJ, Chang RJ, Codner E, Dabadghao P, Darendeliler F, Elbarbary NS,
Gambineri A, et al: An international consortium update:
Pathophysiology, diagnosis, and treatment of polycystic ovarian
syndrome in adolescence. Horm Res Paediatr. 88:371–395.
2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Díaz M, Bassols J, López-Bermejo A, de
Zegher F and Ibáñez L: Low circulating levels of miR-451a in girls
with polycystic ovary syndrome: Different effects of randomized
treatments. J Clin Endocri Metabo. 105(dgz204)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Sun X, Su S, Zhang G, Zhang H and Yu X:
MiR-204 suppresses cell proliferation and promotes apoptosis in
ovarian granulosa cells via targeting TPT1 in polycystic ovary
syndrome. Biochem Cell Biol. 97:554–562. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
He T, Sun Y, Zhang Y, Zhao S, Zheng Y, Hao
G and Shi Y: MicroRNA-200b and microRNA-200c are up-regulated in
PCOS granulosa cell and inhibit KGN cell proliferation via
targeting PTEN. Reprod Biol Endocrinol. 17(68)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Li Y, Liu YD, Zhou XY, Chen SL, Chen X,
Zhe J, Zhang J, Zhang QY and Chen YX: MiR-29a regulates the
proliferation, aromatase expression, and estradiol biosynthesis of
human granulosa cells in polycystic ovary syndrome. Mol Cell
Endocrinol. 498(110540)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Minna E, Romeo P, Dugo M, De Cecco L,
Todoerti K, Pilotti S, Perrone F, Seregni E, Agnelli L, Neri A, et
al: miR-451a is underexpressed and targets AKT/mTOR pathway in
papillary thyroid carcinoma. Oncotarget. 7:12731–12747.
2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Shen YY, Cui JY, Yuan J and Wang X:
MiR-451a suppressed cell migration and invasion in non-small cell
lung cancer through targeting ATF2. Eur Rev Med Pharmacol Sci.
22:5554–5561. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhao S, Li J, Zhang G, Wang Q, Wu C, Zhang
Q, Wang H, Sun P, Xiang R and Yang S: Exosomal miR-451a functions
as a tumor suppressor in hepatocellular carcinoma by targeting
LPIN1. Cell Physiol Biochem. 53:19–35. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Xu K, Han B, Bai Y, Ma XY, Ji ZN, Xiong Y,
Miao SK, Zhang YY and Zhou LM: MiR-451a suppressing BAP31 can
inhibit proliferation and increase apoptosis through inducing ER
stress in colorectal cancer. Cell Death Dis. 10(152)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wei GY, Hu M, Zhao L and Guo WS: MiR-451a
suppresses cell proliferation, metastasis and EMT via targeting
YWHAZ in hepatocellular carcinoma. Eur Rev Med Pharmacol Sci.
23:5158–5167. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Li M, Song Q, Li H, Lou Y and Wang L:
Circulating miR-25-3p and miR-451a may be potential biomarkers for
the diagnosis of papillary thyroid carcinoma. PLoS One.
10(e0132403)2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Watson G, Ronai ZA and Lau E: ATF2, a
paradigm of the multifaceted regulation of transcription factors in
biology and disease. Pharmacol Res. 119:347–357. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Dai Y, Zang Y, Li J, Liu Y and Wan B:
miR-181a and miR-203 inhibit migration and invasion of laryngeal
carcinoma cells by interacting with ATF2. Int J Clin Exp Pathol.
12:133–141. 2019.PubMed/NCBI
|
|
26
|
Li Q, Gao WQ, Dai WY, Yu C, Zhu RY and Jin
J: ATF2 translation is induced under chemotherapeutic drug-mediated
cellular stress via an IRES-dependent mechanism in human hepatic
cancer Bel7402 cells. Oncol Lett. 12:4795–4802. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Yivgi-Ohana N, Sher N, Melamed-Book N,
Eimerl S, Koler M, Manna PR, Stocco DM and Orly J: Transcription of
steroidogenic acute regulatory protein in the rodent ovary and
placenta: Alternative modes of cyclic adenosine 3',
5'-monophosphate dependent and independent regulation.
Endocrinology. 150:977–989. 2009.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Riaz H, Liang A, Khan MK, Dong P, Han L,
Shahzad M, Chong Z, Ahmad S, Hua G and Yang L: Somatostatin and its
receptors: Functional regulation in the development of mice Sertoli
cells. J Steroid Biochem Mol Biol. 138:257–266. 2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Han L, Wu C, Riaz H, Bai L, Chen J, Zhen
Y, Guo A and Yang L: Characterization of the mechanism of inhibin
α-subunit gene in mouse anterior pituitary cells by RNA
interference. PLoS One. 8(e74596)2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zhen YH, Wang L, Riaz H, Wu JB, Yuan YF,
Han L, Wang YL, Zhao Y, Dan Y and Huo LJ: Knockdown of CEBPβ by
RNAi in porcine granulosa cells resulted in S phase cell cycle
arrest and decreased progesterone and estradiol synthesis. J
Steroid Biochem Mol Biol. 143:90–98. 2014.PubMed/NCBI View Article : Google Scholar
|