Introduction
Large variations in CF phenotypes observed among the
patients cannot be simply explained by the CFTR mutations since,
frequently, patients with identical genotype show varying severity
of clinical presentations even within the same family. Indeed,
modifier genes and environmental factors are likely to modulate the
disease severity.
Over the past few years, microRNAs (miRNAs) have
been suggested to act as modulators of CF disease severity.
Aberrant levels and functions of these molecules have been observed
in CF tissues (1). miRNAs are small
non-coding RNAs that negatively regulate gene expression at the
post-transcriptional level by repressing translation or decreasing
mRNA stability (2). Mature miRNAs
are able to bind target transcripts through base pairing with their
3'-untranslated regions (3'UTRs). Recently, some studies have
investigated differentially expressed miRNAs in CF cells: miR-126
that regulates the expression of Target Of Myb1 protein 1(3), which in turn is a negative regulator
of the Interleukin 1 beta (IL1β) and tumor necrosis factor alpha
(TNFα) signal pathways (3);
miR-155, implicated in the phosphoInositide 3-kinase/protein kinase
B pathway, which leads to the expression of the proinflammatory
cytokine IL8, thus contributing to exaggerated inflammation
observed in CF patients (4);
miR-145, which is able to regulate the expression of Smad family
member 3 (SMAD 3), a component of the Transforming Growth Factor
beta (TGFβ) inflammatory pathway (5). Altogether, these results support the
notion that changes in miRNAs may contribute to CF clinical
phenotype, also considering the important regulatory role of these
molecules in the expression of a wide range of genes (1,6), whose
deregulation has been implicated in different disease (7-9).
Oxidative stress is a biological response with key
role in a variety of inflammatory diseases. It is caused by an
imbalance between antioxidants and radical oxygen species. When
oxidative stress occurs, cells try to counteract its effects and to
restore the redox balance by modulating genes encoding defensive
enzymes, transcription factors, and structural proteins. The master
gene of this cellular defense response is nuclear factor erythroid
derived-2 like2 (NRF2), a transcription factor that positively
regulates genes encoding proteins implicated in detoxification.
Inactive NRF2 is restrained in the cytoplasm associated with
Kelch-like Ech-associated protein 1 (KEAP1). Upon exposure of cells
to oxidants, it is phosphorylated and translocated to the nucleus
where it binds the antioxidant response elements within the
promoter of target genes and transactivates them.
Oxidative stress resulting in ROS increase is often
observed in several human diseases. CF is characterized by the
disruption of many processes as aberrant ion transport,
inflammation responses, metabolism of lipids and proteins that are
modulated by oxidants and antioxidants. In CF context, defective
function of CFTR prompts to produce a redox imbalance in epithelial
cells and extracellular fluids and causes an abnormal flux of ROS.
The occurrence of ROS increase is dependent on a diminished
availability of important dietary antioxidants, such as vitamin E
and carotenoids, as a result of maldigestion, malabsorption,
increased turnover (10) and
sustained activation of neutrophils (11). In this disease, a suboptimal
antioxidant protection is a main contributor to oxidative stress
together with a poor control of immuno-inflammatory pathway.
As previously reported, cellular response to ROS is
carried out by transcriptional activity of NRF2 which induces some
deoxidant ARE (antioxidant responsive elements) genes, which in
turn help to recover cellular homeostasis. In different cell
context, the well-known and studied targets are: NAD(P)H:Quinone
Oxidoreductase (NQO-1), glutathione S-transferase 1 (GST-T1) and
heme oxygenase (HO-1).
Specifically, HO-1 is strongly upregulated during
stress and exerts its function by catabolizing heme to biliverdin,
free iron and carbon monoxide to counter sequential imbalance. Its
expression was demonstrated cytoprotective against lung injury of
oxidative stress and inflammation not only in CF patients (12) but also in subjects with other lung
diseases (11). HO-1 prompts to a
better prognosis by protecting the airway epithelial and
endothelial cells and pancreatic beta cells from injury and
apoptosis (13,14).
Recently, some studies have shown that NRF2 directly
regulates the expression of different miRNAs at transcriptional
level, including miR-125b (15,16).
miR-125b is a highly conserved homolog of lin-4 and,
recently, it has been found to be modulated by CFTR-mediated
HCO3- influx through the sAC-PKA-NFKB cascade
in mice embryo development (17),
this suggesting a role of epigenetic regulator of CFTR in addiction
to the anion channel. Moreover, in a genetic condition affecting
keratinocytes, our previous studies showed that miR-125b expression
is regulated by oxidative stress-dependent mechanisms (18). As miRNAs are thought to function
both as drivers and modifiers of CF phenotype and several studies
highlighted that these small molecules can regulate and be
regulated by oxidative stress (19), we hypothesized that miR-125b is a
good candidate to play an important role in the CF
pathophysiology.
All these remarks prompted us to investigate whether
oxidative stress may represent a mechanism involved in the CF
pathogenesis, given its role in the CF lung disease. In this pilot
study we analyzed a possible correlation among miR-125b, NRF2
together with its targets and CFTR expressions in 13 CF patients.
Indeed, the validation of the miR-125b/NRF2/CFTR circuitry role in
CF disease may lead to the identification of new therapeutic
strategies against oxidative stress along with airway inflammation
in CF patients.
Materials and methods
Sample collection
We analyzed 13 unrelated CF individuals (mean age,
30.77 years, 8:5 male:female patients) who received a diagnosis of
Cystic Fibrosis at the Regional center of Cystic
Fibrosis-Policlinico Umberto I of Rome, Italy. Patients were
F508del/F508del homozygotes and were eligible if they were 18 years
old or older. The segregation of CFTR mutated alleles was verified
in parents. CF subjects are stratified into two groups: Group 1
(P1-P4) shared mild lung function impairment with a FEV1 mean value
79.5% without P. aeruginosa (PA) chronic infection (PA[-]);
group 2 (P5-P13) shared recurrent or chronic pathogen infections
(PA[+]) with FEV1 mean value 53.78%. Furthermore, group 2 PA[+]
were stratify in two subgroups based on the best or worse FEV1:
FEV1>60% or FEV1<60%, respectively. The values of sweat test,
a measure of CFTR-associated functionality, resulted higher than 30
mmol/l. Three non-CF unrelated individuals, without known airway
diseases, were used as healthy control group.
RNA extraction and reverse
transcription (RT)
By cytobrushing, primary nasal epithelial cells were
isolated from nasal cavity of patients and controls. Total RNA was
extracted using TRIzol Reagent (Thermo Fisher Scientific) according
to the manufacturer's instructions. RT for miR-125b was carried out
with TaqMan MicroRNA Assay kit (Thermo Fisher Scientific) using 20
ng of RNA sample; High capacity cDNA Reverse Transcription kit
(Thermo Fisher Scientific) was used for RT of total RNA for CFTR
and NRF2 expression analysis using 500 µg-1 mg of RNA sample
(20).
Quantitative Real Time (qPCR)
CFTR, NRF2, HO-1 and NQO1 mRNAs and miR-125b levels
were analyzed by StepOnePlus Real-Time PCR System machine using
Taqman Gene expression Assay (CFTR Hs00357011; NRF2 Hs 00975961_g1;
HO1, Hs01110250_m1; NQO1, Hs02512143_s1; miR-125b, hsa-miR125b
000449) with TaqMan Universal PCR Master Mix II (Thermo Fisher
Scientific). GAPDH (Hs02758991_g1) mRNA and U6 small nuclear RNA
(Hs001973) were used as endogenous controls to normalize sample
data. SYBR green fluorescence chemistry for GST-T1 and GAPDH
expression analysis were carried out using specific primers (GST-T1
Fw: AAGGTCCCTGACTACTGGTA; GST-T1 Rev: ATACTGGCTCACCCAGGAAA; GAPDH
Fw: TGCACCACCAACTGCTTAG; GAPDH Rev: GAGGCAGGGATGATGTTC) with
SensiFAST SyBr Hi-ROX kit (Bioline, UK). The fold change of the
mRNA gene and miRNA in the CF samples relative to the mean values
of healthy control samples was calculated using ΔΔCt method
(21,22).
Statistical analysis
Results of real time PCR are expressed as mean
standard deviation (SD) from at least three separate experiments
and P-value was used for significance using Student's t-test.
Correlation coefficients were calculated by Pearson's correlation
test. All statistical analyses were performed using Excel
software.
Results
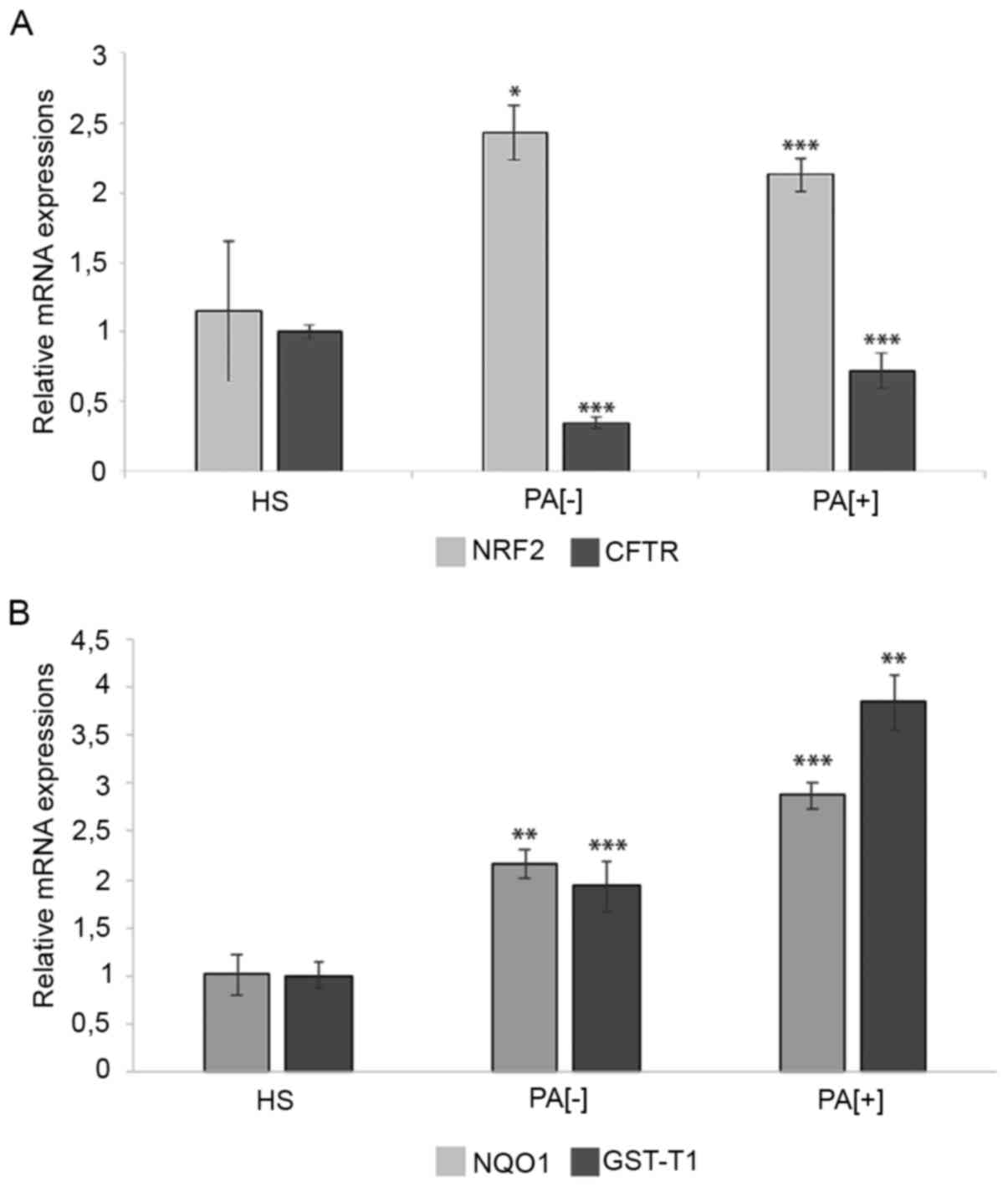
We analyzed CFTR and NRF2 mRNA levels together with
its target expressions in CF patients compared to non-CF healthy
individuals (n.3), by qPCR using TaqMan chemistry. The lost
function of CFTR generally correlates with increased oxidative
stress caused by impaired antioxidant response (23). Interestingly, qPCR analysis showed
doubled mRNA levels of the master detoxification gene NRF2
(Fig. 1A) in both patient groups
PA[-] and PA[+] (P-value=0.018 and 0.00013, respectively) and so
might be used as marker of oxidative stress onset. Moreover, in
line with these data, the expression levels of NRF2 targets, NQO1
(P-value=0.0079 and 0.00084 respectively) and GST-T1
(P-value=0.00029 and 0.0016, respectively) were also upregulated
(Fig. 1B). These results support
the hypothesis that oxidative stress induces the transcriptional
activity of NRF2, which in turn activates NQO1 and GST-T1 though
this protective cascade failed to rescue anyhow the unbalanced
redox microenvironment, possibly because ROS levels are too high to
be counteracted in CF patients.
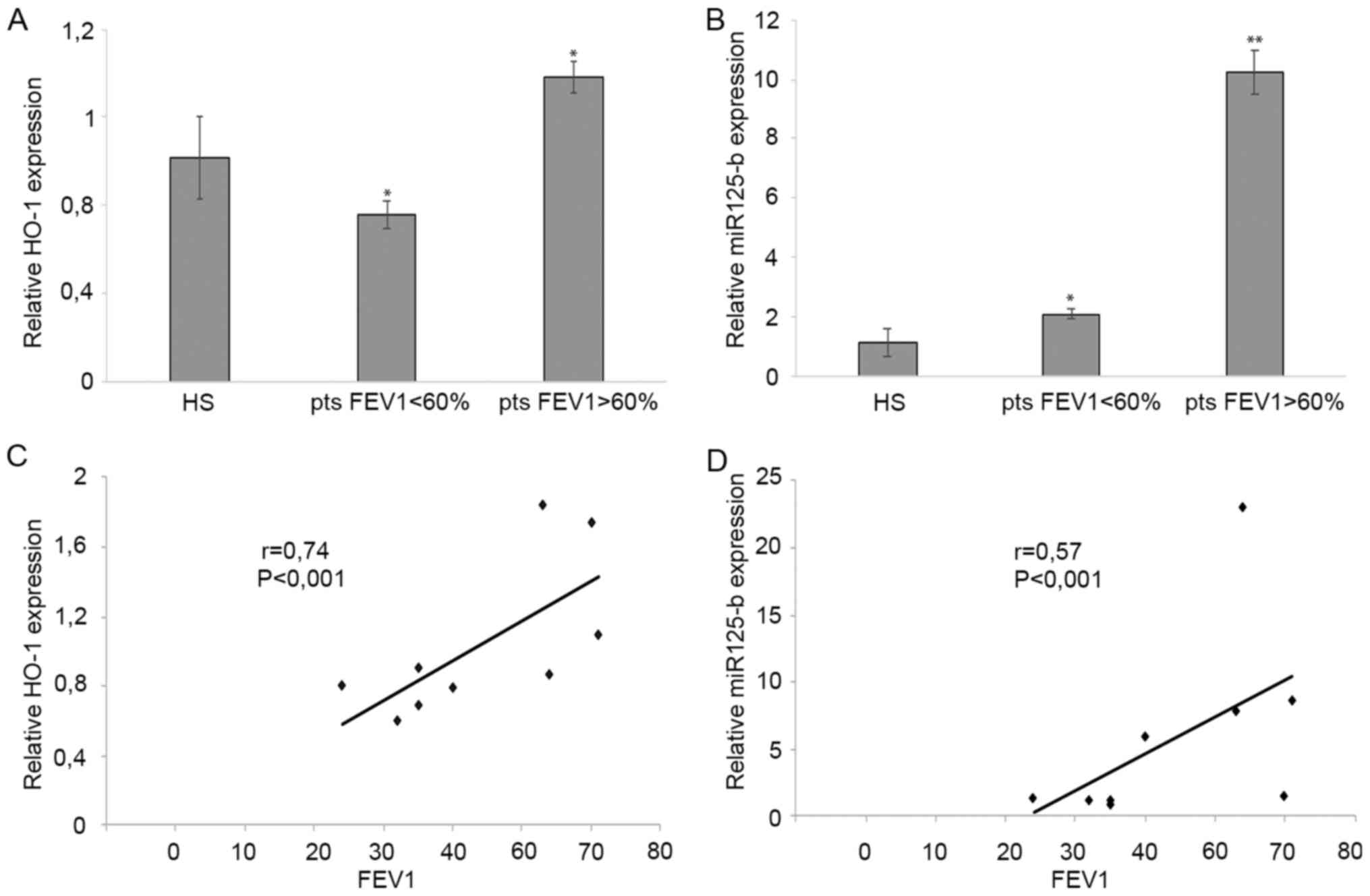
Furthermore, we evaluated expression profiles of
HO-1 and miR-125b. Interestingly, we observed not significantly
changed expression neither in HO-1 deoxidant enzyme nor in miR-125b
in PA[-] patients (data not shown). Instead, after further
stratification of PA[+] patients in mild or severe phenotype group,
based on FEV1>60% and FEV1<60% respectively, the analysis
displayed induced expressions of HO-1 and miR-125b mainly in the
first group than in the second (P-value=0.015 and 0.0023,
respectively) (Fig. 2A and B).
To further confirm these results, using the
Pearson's correlation test, we showed that the abundance of HO-1
and miR-125b transcripts was directly and significantly associated
with FEV1: r=0.74 P<0.001 and r=0.57 P<0.001, respectively
(Fig. 2C and D).
We speculated that the concomitant upregulation of
HO-1 and miR-125b might supply a synergistic protective effect,
which might promote a better prognosis in CF patients by
attenuating ROS-dependent damage and reducing apoptotic
signals.
Discussion
Oxidative stress in Cystic Fibrosis has been widely
demonstrated. Because of a defective CFTR channel, redox imbalance
develops and in turn induces the increase of ROS with all
well-known effects.
In particular, CF lung displays a chronic
inflammation that sustains the recruitment of neutrophils to
destroy pathogenic bacteria through phagocytosis and release of
cytokines, resulting in the release of large amount of ROS and
other immunological mediators. This increased oxidant load produces
a redox imbalance which affects antioxidant capacity of the cells,
thus resulting in oxidative insults. Moreover, Pseudomonas
aeruginosa infection produces pyocyanin
(N-methyl-1-hydroxiphenazine), a redox-active virulence factor that
further increases ROS occurrence (24). Lastly, high ROS amount causes cell
death through apoptosis or necrosis.
For basic research studies the in vitro cell
models play a key role. The best physiological model of airway
epithelium are primary bronchial epithelia, but this kind of cells
are limited due to human lung tissue availability. Fortunately, it
has been shown primary nasal cells represent a valid alternative
model for CF pathophysiology studies summarizing the properties of
bronchial cultures and have been used in different airway
functional and inflammation studies (25-27).
We extracted mRNA from nasal epithelial cells of 13
ΔF508 CF patients stratified in low risk (P1-P4 PA[-]) or high risk
(P5-P13 PA[+]) (Table 1).
 | Table IClinical characteristics of patients
with cystic fibrosis (n=13). |
Table I
Clinical characteristics of patients
with cystic fibrosis (n=13).
| Characteristic | Value |
|---|
| Sex, male:female
ratio | 8:5 |
| Mean age ± SD,
years | 30.77±6.70 |
| Genotype | F508del CFTR |
| Positive for
Pseudomonas aeruginosa chronic colonization, n | 9 |
| Negative for
Pseudomonas aeruginosa chronic colonization, n | 4 |
We found that NRF2 mRNA expression was doubled,
together with CFTR downmodulation, in nasal epithelia from ΔF508 CF
patients compared to healthy subjects. NQO-1 and GST-T1, two direct
targets of NRF2, resulted overexpressed also.
NRF2 mRNA is expressed broadly and independently of
inducers sustaining a post-transcriptional mechanism for its
activation, so it might be important show the correlation between
the protein and the RNA expressions of CFTR and NRF2.
Unfortunately, we are not able to perform protein extraction or
FACS analysis because of too low amount of the obtained nasal cells
with cytobrush.
However, our results imply the onset of oxidative
stress that cells are unable to neutralize due to the too high
levels of ROS. Moreover, our results suggest that a protective
circuitry might be functional, but not sufficient to maintain
physiological levels of ROS, which would allow to avoid oxidative
injury in CF context.
Then we analyzed the expression values of two
additional NRF2 targets: HO-1 and miR-125b. HO-1 gene encodes an
antioxidant enzyme that is inducible in response to ROS,
proinflammatory cytokines or endotoxins (13). Its action is carried out through
degradation of heme into bilirubin, Fe2+ and CO, so
modulating the oxidative damage. Moreover, Heme-oxygenase was
demonstrated to be involved in cytotoxic protection against
cellular lesion induced by P. Aeruginosa in vitro in CF
patients (12) as well as active
against oxidative injury not only in lung-affected diseases [CF,
chronic Obstructive Pulmonary Disease (COPD), idiopathic pulmonary
fibrosis (IPF)] but also in other diseases, as neurodegenerative or
cardiovascular, and in hematological malignancies (28-30).
Furthermore, invasive P. aeruginosa strains have been
demonstrated to induce mitochondrial apoptosis in target host cells
through some virulence factors (31) and HO-1 resulted defensive against
apoptosis because oxidative insults prompt a broad range of effects
like growth arrest, senescence and cell death.
The present results displayed that the high
expression of HO-1 was positively correlated with FEV1>60% in
airway epithelial cells of PA[+] patients, this indicating that the
stress response player might protect cells from oxidative stress
injury. Accordingly, in lung aberrant inflammation HO-1 may act as
a modifier gene able to affect CF clinical manifestations.
Recently, multiple studies are exploring the
mechanisms of miRNAs as pivotal modulators of cellular activities
mostly in association with many diseases. In particular, some
studies have shown that miR-125b was regulated by NRF2 through
binding on ARE within its promoter region (15,16);
then, specifically, other studies showed that CFTR is able to
modulate miR-125b expression through HCO3 influx
(17). In addition, our previous
results demonstrated the involvement of oxidative stress in
miR-125b modulation in skin disease (18) and not least, in apoptotic signaling
repression targeting p53 in rat models (32).
Furthermore, we have found that miR-125b was
significantly overexpressed in PA [+] patients with better lung
function. The basic defect caused by CFTR mutations, recurrent
bacterial infections, impaired antioxidant protection system and
all hallmarks of CF disease bring excessive ROS production. This
ROS overload induces DNA, protein and lipids damage, inflammation
and apoptosis (23) so we
speculated that miR-125b might be protective of airway cells
against apoptosis, but future investigations need to demonstrate
this miRNA function in CF disease.
In summary, by qPCR analysis on airway epithelial
cells of CF patients we have demonstrated that oxidative stress
response mechanisms are activated but fail to counteract it;
nevertheless, synergistic effects of HO-1 and miR-125b might have
protective effect, which is involved in phenotypic variability of
CF disease and oxidative stress-related genes might be potential
biomarkers for better prognosis. Until now there are still few
biomarkers demonstrated to be predictive of CF clinical outcome.
Thus, it's very important the discovery of clinically relevant
factors able to support the diagnosis, to forecast the progress of
disease and to understand the impact of therapies. The
identification of valid biomarkers might help clinicals to treat
early patients to avoid recurrent lung exacerbations generally
involved in decline of medical case.
Future studies will be addressed to further confirm
these results in a wide cohort of CF individuals as well as in CF
in vitro models in order to examine the molecular pathways
of the potential cross-talk between of HO-1 and miR-125b in
protecting airway cells from oxidative stress and apoptosis,
finally sustaining their contribution in CF clinical
manifestations.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Italian Ministry
of Education, University and Research-Dipartimenti di Eccellenza
(law no. 232/2016).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SC conceived and designed the experiments, and wrote
the manuscript. MP and SC performed the experiments. SC, MP and DS
analyzed and interpreted the data. CT and IS provided facilities,
performed the data analysis and critically revised the manuscript.
SQ, GC and AP acquired and managed patients, provided clinical
information in interpreting experimental data and critically
revised the manuscript. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethical Committee of
Sapienza University, Policlinico Umberto I (Rome, Italy). Patients
with cystic fibrosis and healthy controls were fully informed of
the aims of the present study and freely agreed to take part to the
research by signing an institutional written informed consent
form.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
McKiernan PJ and Greene CM: MicroRNA
dysregulation in cystic fibrosis. Mediators Inflamm.
2015(529642)2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Valencia-Sanchez MA, Liu J, Hannon GJ and
Parker R: Control of translation and mRNA degradation by miRNAs and
siRNAs. Genes Dev. 20:515–524. 2006.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Oglesby IK, Bray IM, Chotirmall SH,
Stallings RL, O'Neill SJ, McElvaney NG and Greene CM: miR-126 is
downregulated in cystic fibrosis airway epithelial cells and
regulates TOM1 expression. J Immunol. 184:1702–1709.
2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Bhattacharyya S, Balakathiresan NS,
Dalgard C, Gutti U, Armistead D, Jozwik C, Srivastava M, Pollard HB
and Biswas R: Elevated miR-155 promotes inflammation in cystic
fibrosis by driving hyperexpression of interleukin-8. J Biol Chem.
286:11604–11615. 2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Megiorni F, Cialfi S, Cimino G, De Biase
RV, Dominici C, Quattrucci S and Pizzuti A: Elevated levels of
miR-145 correlate with SMAD3 down-regulation in cystic fibrosis
patients. J Cyst Fibros. 12:797–802. 2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Xu W, Hui C, Yu SS, Jing C and Chan HC:
MicroRNAs and cystic fibrosis-an epigenetic perspective. Cell Biol
Int. 35:463–466. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Sato F, Tsuchiya S, Meltzer SJ and Shimizu
K: MicroRNAs and epigenetics. FEBS J. 278:1598–1609.
2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Bandiera S, Hatem E, Lyonnet S and
Henrion-Caude A: microRNAs in diseases: From candidate to modifier
genes. Clin Genet. 77:306–313. 2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Vargas Romero P, Cialfi S, Palermo R, De
Blasio C, Checquolo S, Bellavia D, Chiaretti S, Foà R, Amadori A,
Gulino A, et al: The deregulated expression of miR-125b in acute
myeloid leukemia is dependent on the transcription factor C/EBPα.
Leukemia. 29:2442–2445. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Iuliano L, Monticolo R, Straface G, Zullo
S, Galli F, Boaz M and Quattrucci S: Association of cholesterol
oxidation and abnormalities in fatty acid metabolism in cystic
fibrosis. Am J Clin Nutr. 90:477–484. 2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Galli F, Battistoni A, Gambari R, Pompella
A, Bragonzi A, Pilolli F, Iuliano L, Piroddi M, Dechecchi MC and
Cabrini G: Working Group on Inflammation in Cystic Fibrosis.
Oxidative stress and antioxidant therapy in cystic fibrosis.
Biochim Biophys Acta. 1822:690–713. 2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhou H, Lu F, Latham C, Zander DS and
Visner GA: Heme oxygenase-1 expression in human lungs with cystic
fibrosis and cytoprotective effects against Pseudomonas
aeruginosa in vitro. Am J Respir Crit Care Med. 170:633–640.
2004.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yamada N, Yamaya M, Okinaga S, Lie R,
Suzuki T, Nakayama K, Takeda A, Yamaguchi T, Itoyama Y, Sekizawa K
and Sasaki H: Protective effects of heme oxygenase-1 against
oxidant-induced injury in the cultured human tracheal epithelium.
Am J Respir Cell Mol Biol. 21:428–435. 1999.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yachie A, Niida Y, Wada T, Igarashi N,
Kaneda H, Toma T, Ohta K, Kasahara Y and Koizumi S: Oxidative
stress causes enhanced endothelial cell injury in human heme
oxygenase-1 deficiency. J Clin Invest. 103:129–135. 1999.PubMed/NCBI View
Article : Google Scholar
|
|
15
|
Joo MS, Lee CG, Koo JH and Kim SG:
miR-125b transcriptionally increased by Nrf2 inhibits AhR
repressor, which protects kidney from cisplatin-induced injury.
Cell Death Dis. 4(e899)2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Shah NM, Zaitseva L, Bowles KM, MacEwan DJ
and Rushworth SA: NRF2-driven miR-125B1 and miR-29B1
transcriptional regulation controls a novel anti-apoptotic miRNA
regulatory network for AML survival. Cell Death Differ. 22:654–664.
2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lu YC, Chen H, Fok KL, Tsang LL, Yu MK,
Zhang XH, Chen J, Jiang X, Chung YW, Ma AC, et al: CFTR mediates
bicarbonate-dependent activation of miR-125b in preimplantation
embryo development. Cell Res. 22:1453–1466. 2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Manca S, Magrelli A, Cialfi S, Lefort K,
Ambra R, Alimandi M, Biolcati G, Uccelletti D, Palleschi C,
Screpanti I, et al: Oxidative stress activation of miR-125b is part
of the molecular switch for Hailey-Hailey disease manifestation.
Exp Dermatol. 20:932–937. 2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Banerjee J, Khanna S and Bhattacharya A:
MicroRNA regulation of oxidative stress. Oxid Med Cell Longev.
2017(2872156)2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Quaranta R, Pelullo M, Zema S, Nardozza F,
Checquolo S, Lauer DM, Bufalieri F, Palermo R, Felli MP, Vacca A,
et al: Maml1 acts cooperatively with Gli proteins to regulate sonic
hedgehog signaling pathway. Cell Death Dis. 8(e2942)2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Cialfi S, Palermo R, Manca S, De Blasio C,
Vargas Romero P, Checquolo S, Bellavia D, Uccelletti D, Saliola M,
D'Alessandro A, et al: Loss of Notch1-dependent p21(Waf1/Cip1)
expression influences the Notch1 outcome in tumorigenesis. Cell
Cycle. 13:2046–2055. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
22
|
Ficociello G, Zanni E, Cialfi S, Aurizi C,
Biolcati G, Palleschi C, Talora C and Uccelletti D: Glutathione
S-transferase ϴ-subunit as a phenotypic suppressor of pmr1Δ strain,
the Kluyveromyces lactis model for Hailey-Hailey disease. Biochim
Biophys Acta. 1863:2650–2657. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Valdivieso AG and Santa-Coloma TA: CFTR
activity and mitochondrial function. Redox Biol. 1:190–202.
2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ziady AG and Hansen J: Redox balance in
cystic fibrosis. Int J Biochem Cell Biol. 52:113–123.
2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
McDougall CM, Blaylock MG, Douglas JG,
Brooker RJ, Helms PJ and Walsh GM: Nasal epithelial cells as
surrogates for bronchial epithelial cells in airway inflammation
studies. Am J Respir Cell Mol Biol. 39:560–568. 2008.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Mosler K, Coraux C, Fragaki K, Zahm JM,
Bajolet O, Bessaci-Kabouya K, Puchelle E, Abély M and Mauran P:
Feasibility of nasal epithelial brushing for the study of airway
epithelial functions in CF infants. J Cyst Fibros. 7:44–53.
2008.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Gianotti A, Delpiano L and Caci E: In
vitro methods for the development and analysis of human primary
airway epithelia. Front Pharmacol. 9(1176)2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Neis VB, Rosa PB, Moretti M and Rodrigues
ALS: Involvement of heme oxygenase-1 in neuropsychiatric and
neurodegenerative diseases. Curr Pharm Des. 24:2283–2302.
2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ndisang JF: Synergistic interaction
between heme oxygenase (HO) and nuclear-factor E2-related factor-2
(Nrf2) against oxidative stress in cardiovascular related diseases.
Curr Pharm Des. 23:1465–1470. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Li Volti G, Tibullo D, Vanella L,
Giallongo C, Di Raimondo F, Forte S, Di Rosa M, Signorelli SS and
Barbagallo I: The heme oxygenase system in hematological
malignancies. Antioxid Redox Signal. 27:363–377. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kaminski A, Gupta KH, Goldufsky JW, Lee
HW, Gupta V and Shafikhani SH: Pseudomonas aeruginosa ExoS
induces intrinsic apoptosis in target host cells in a manner that
is dependent on its GAP domain activity. Sci Rep.
8(14047)2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Xie YL, Zhang B and Jing L: miR-125b
blocks bax/cytochrome C/caspase-3 apoptotic signaling pathway in
rat models of cerebral ischemia-reperfusion injury by targeting
p53. Neurol Res. 40:828–837. 2018.PubMed/NCBI View Article : Google Scholar
|