Introduction
Sepsis is primarily caused by dysregulation of host
immune response as a result of infection, which can ultimately lead
to life-threatening organ dysfunction (1). Imbalances in inflammation and immune
response leads to uncontrolled microbial growth, which in turn
results in fatal inflammation, tissue damage and organ dysfunction
(1). The lung is the organ that is
the most susceptible to in sepsis-induced dysfunction, where acute
lung injury (ALI) is one of the most serious manifestations of
sepsis (2). Therefore, preventing
or alleviating ALI caused by sepsis is the key to reducing septic
mortality.
Excessive activation of inflammatory cytokines is
the main mechanism of organ damage in patients with sepsis,
especially in patients with ALI (3). It has been previously reported that
inhibition of inflammatory responses can protect lung function from
sepsis in rats (3). In particular,
pyroptosis is a form of programmed cell death that occurs when host
cells are infected by pathogenic microorganisms or are stimulated
by endogenous danger signals (4).
Pyroptosis is characterized by cell swelling and lysis, followed by
the release of a large number of proinflammatory cytokines
(4). This process can be divided
into canonical and non-canonical pathways. In the non-canonical
pathway, exogenous cytotoxins, such as lipopolysaccharide (LPS),
can directly induce the activation of caspase 11, caspase 4 and
caspase 5, which causes cleavage of the pyroptosis executor
gasdermin D (GSDMD), leading to perforation of the cell membrane
(5). In addition, the N-terminus of
GSDMD can activate nucleotide-binding oligomerization domain,
leucine rich repeat and pyrin domain containing 3 (NLRP3),
resulting in the release of interleukin (IL)-1β and IL-18(5). It has also been demonstrated that
inflammasome-mediated non-canonical cell pyroptosis can activate a
cascade of inflammatory responses, causing or aggravating
inflammation-associated diseases, such as lung injury (6).
Insulin-like growth factor 2 (IGF2) mRNA-binding
protein 2 (IGF2BP2) is a member of the conserved single-stranded
RNA-binding protein family IGF2 that is expressed in a wide range
of fetal tissues (7). IGF2BP2 can
function as a post-transcriptional regulator of mRNA localization,
stability and translation (8).
Dysregulation of IGF2BP2 has been frequently associated with human
diseases, including diabetes and cancer (8-10).
It has been reported that IGF2BP2 can serve a proinflammatory role
in non-alcoholic fatty liver disease, promoting
inflammation-induced carcinogenesis and subsequent tumor
proliferation and invasion (11).
Following ENCORI database search, IGF2BP2 was shown to bind to the
mRNA of caspase-4, which is a crucial regulator in the
non-canonical pathway of cell pyroptosis (4). Caspase-4-mediated pyroptosis can
promote a number of inflammatory signaling pathways in the airway,
serving a key role in LPS-induced tissue damage (12-15).
Therefore, the present study investigated the
potential effects of IGF2BP2 on LPS-induced lung cell inflammation
and caspase 4 activity, with emphasis on caspase 4-mediated
pyroptosis.
Materials and methods
Cell culture and treatment
Human bronchial epithelial (Beas-2B) cells (American
Type Culture Collection) were cultured in RPMI-1640 medium (Gibco;
Thermo Fisher Scientific, Inc.) with 10% FBS (Thermo Fisher
Scientific, Inc.) and 1% penicillin and streptomycin antibiotics
(Thermo Fisher Scientific, Inc.) in an atmosphere containing 5%
CO2 at 37˚C. The in vitro simulation of lung
injury was achieved by treating the cells with 0.1, 1 and 10 µg/ml
LPS (Thermo Fisher Scientific, Inc.) at 37˚C for 12 h.
Cell transfection
Short hairpin RNA (shRNA) against IGF2BP2 and its
corresponding scrambled negative control (NC) vector, shRNA-NC,
were designed and synthesized by Shanghai GenePharma Co., Ltd.
Beas-2B cells at the density of 2x106/ml were
transfected in vitro with 2 µg/ml shRNA-IGF2BP2 or shRNA-NC
using Lipofectamine 2000® (Invitrogen, Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol, which
were described in a previous study (16). At 48 h post-transfection, cells were
used for subsequent experiments.
Cell Counting Kit (CCK)-8 assay
CCK-8 assay was used for evaluating cell viability.
Briefly, cells (5x103 cells/well) were seeded into
96-well plates, treated with LPS after shRNA transfection before
being finally incubated with 10 µl CCK-8 working solution
(MedChemExpress) for 2 h under normal cell culture conditions.
Absorbance in each well was measured at 450 nm using a microplate
reader.
ELISA
The expression levels of IL-1β (cat. no. ab214025)
and IL-18 (cat. no. ab215539) in the supernatant of the cell
culture medium were detected using ELISA following the
manufacturer's protocols (Abcam). The assay was conducted as
described previously (17). The
results are expressed as optical density at 450 nm.
Western blotting
Beas-2B cells were lysed with RIPA buffer (Beyotime
Institute of Biotechnology) on ice for 30 min before the protein
concentration was determined using the bicinchoninic acid kit
(Thermo Fisher Scientific, Inc.). Equal amounts of protein (18
µg/lane) were separated by 12% SDS-PAGE and transferred onto
polyvinylidene fluoride membranes. Following blocking with 5%
non-fat milk at room temperature for 2 h, the membranes were
incubated with the designated primary antibodies overnight at 4˚C.
Goat anti rabbit IgG secondary antibodies conjugated with
horseradish peroxidase (ProteinTech Group, Inc.; cat. no.
SA00001-2; 1:10,000) were added and incubated at room temperature
for 2 h. The proteins bands were visualized using enhanced
chemiluminescence Prime Western blot detection reagent (Cytiva).
The antibodies used (ProteinTech Group, Inc.) included the
following: Rabbit polyclonal IGF2BP2 (cat. no. 11601-1-AP;
1:5,000), rabbit polyclonal caspase 4 (cat. no. 11856-1-AP;
1:1,000), rabbit polyclonal caspase 1 (cat. no. 22915-1-AP;
1:2,000), rabbit polyclonal cleaved-caspase 1 (cat. no. 22915-1-AP;
1:1,000) and rabbit polyclonal GAPDH (cat. no. 10494-1-AP;
1:10,000). Densitometry analysis was performed using Image Lab
system software (Bio-Rad Laboratories, Inc. Version 1.52)
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from Beas-2B cells using the
RNA extraction kit (RNAiso Plus; cat. no. 9108; Takara Bio, Inc.).
A total of 5 µg RNA was reverse-transcribed to cDNA (High-Capacity
cDNA Reverse Transcription kit; cat. no. 4368813; Applied
Biosystems; Thermo Fisher Scientific, Inc.). Next, a SYBR Green PCR
kit (Takara Bio, Inc.) was used to determine gene expression on an
ABI 7500 Real-Time PCR system (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The PCR reaction procedure was as follows: 5 min
at 95˚C, followed by 40 cycles of 30 sec at 95˚C and 45 sec at
65˚C. The specific primer sequences used were as follows: IGF2BP2
forward, 5'-CGGGGAAGAGACGGATGATG-3' and reverse,
5'-CGCAGCGGGAAATCAATCT-G-3'; caspase 4 forward,
5'-CCTATGGCAGAAGGCAACCA-3' and reverse, 5'-GGCAGTTGCGGTTGTTGAAT-3';
caspase 1 forward, 5'-ACAAGACCTCTGACAGCACG-3' and reverse,
5'-TTCACTTCCTGCCCACAGAC-3' and β-actin forward,
5'-AAATCGTGCGTGACATCAAAGA-3' and reverse, 5'-GGCCATCTCCTGCTCGAA-3'.
The results were normalized to those of β-actin expression and the
2-ΔΔCq method (18) was utilized to calculate the relative
changes in gene expression.
Immunofluorescence staining
Beas-2B cells were fixed with 4% paraformaldehyde at
4˚C for 15 min and permeabilized with 0.2% Triton X-100 at 37˚C for
20 min. After blocking with 10% Bovine Serum Albumin (Thermo Fisher
Scientific, Inc.) for 30 min at room temperature. The cells were
then incubated with primary antibodies against cleaved N-terminal
GSDMD (cat. no. ab215203; Abcam; 1:100) at 4˚C overnight. The
following day, the cells were incubated with 100 µl/well working
solution containing Alexa Fluor 488-conjugated goat anti-rabbit
secondary antibodies (Abcam; cat. no. ab150081; 1:1,000) at room
temperature for 1 h. DAPI was used for nuclear counterstaining. The
stained slides were imaged using an inverted fluorescence
microscope (magnification, x200; Olympus Corporation).
RNA pull-down assay
The interaction of caspase 4 mRNA and IGF2BP2
protein was examined following lysis of Beas-2B cells using RIPA
buffer (Beyotime Institute of Biotechnology) and subsequent
incubation with biotin-labeled caspase 4, caspase 1 and IgG
(Shanghai GenePharma Co., Ltd.) at 4˚C for 2 h. A total of 50 µl
streptavidin magnetic beads (Invitrogen; Thermo Fisher Scientific,
Inc.) were added to each sample and the mixtures were incubated at
4˚C for 2 h. The beads were washed with 1x wash buffer included in
the kit and elution buffer (Pierce™ Magnetic RNA-Protein Pull-Down
kit; Thermo Fisher Scientific, Inc.), centrifuged at 8,000 x g at
4˚C for 15 min and analyzed via western blotting analysis to assess
the expression levels of the IGF2BP2 protein in the pull-down
products (19). The antibodies used
for IGF2BP2 and GAPDH were same as those listed in the western
blotting section.
Statistical analysis
The data are presented as mean ± SD from ≥ three
independent experiments. Statistical analysis was performed using
one-way ANOVA followed by Tukey's test and Student's t-test using
GraphPad Prism 8 (GraphPad Software, Inc.). P<0.05 was
considered to indicate a statistically significant difference.
Results
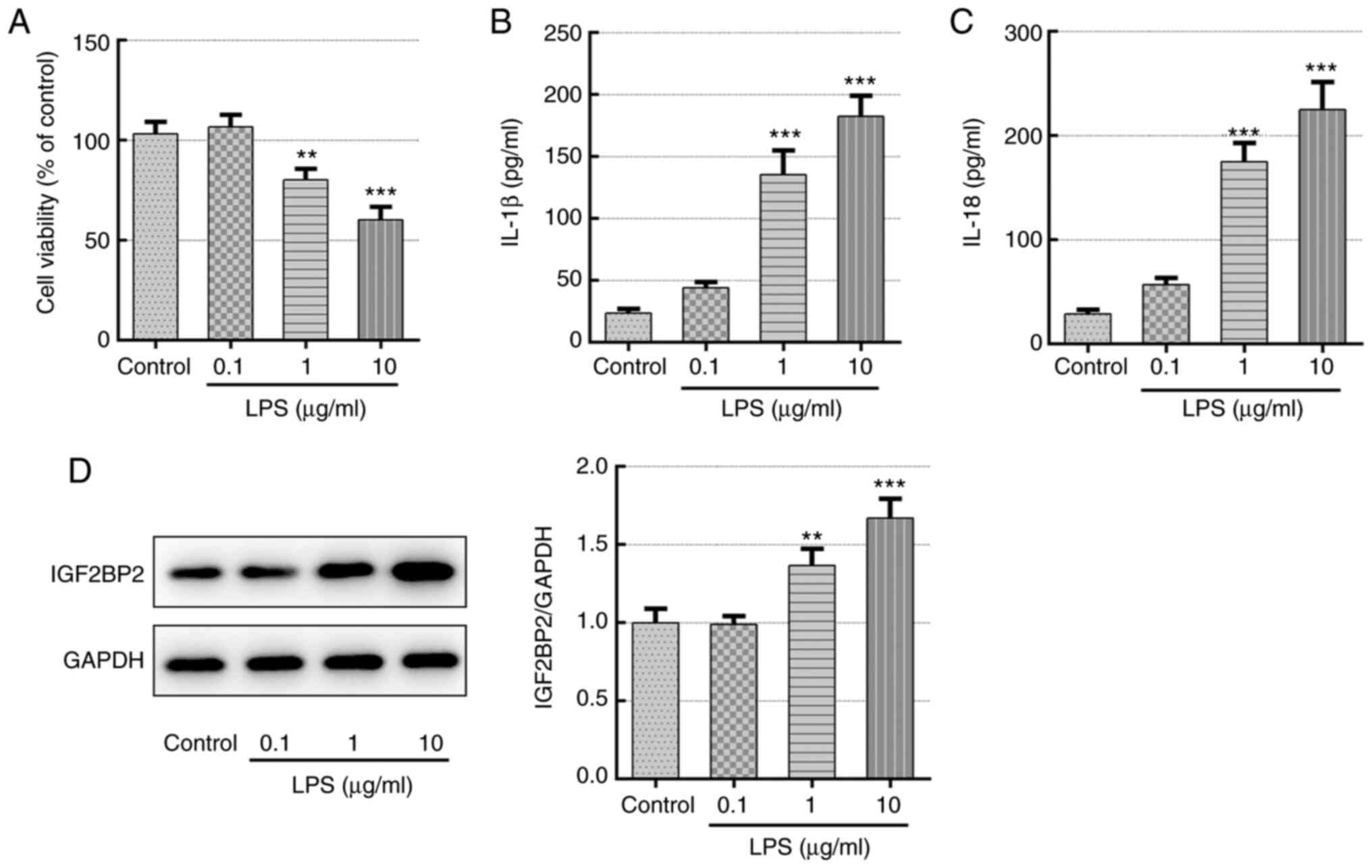
LPS stimulation increases IGF2BP2
expression of Beas-2B cells in a concentration-dependent
manner
Different concentrations of LPS (0.1, 1 and 10
µg/ml) were first used to treat Beas-2B cells for 12 h before cell
viability, inflammatory cytokine production and IGF2BP2 expression
were quantified. Compared with those in the control group, LPS was
found to significantly reduce cell viability and trigger the
generation of inflammatory cytokines IL-1β and IL-18 in a
concentration-dependent manner (Fig.
1A-C), demonstrating the successful induction of lung cell
inflammation. In addition, IGF2BP2 protein expression was also
significantly enhanced by LPS in a concentration-dependent manner
compare with that in control (Fig.
1D), suggesting a potential role of IGF2BP2 in LPS-induced lung
cell inflammation.
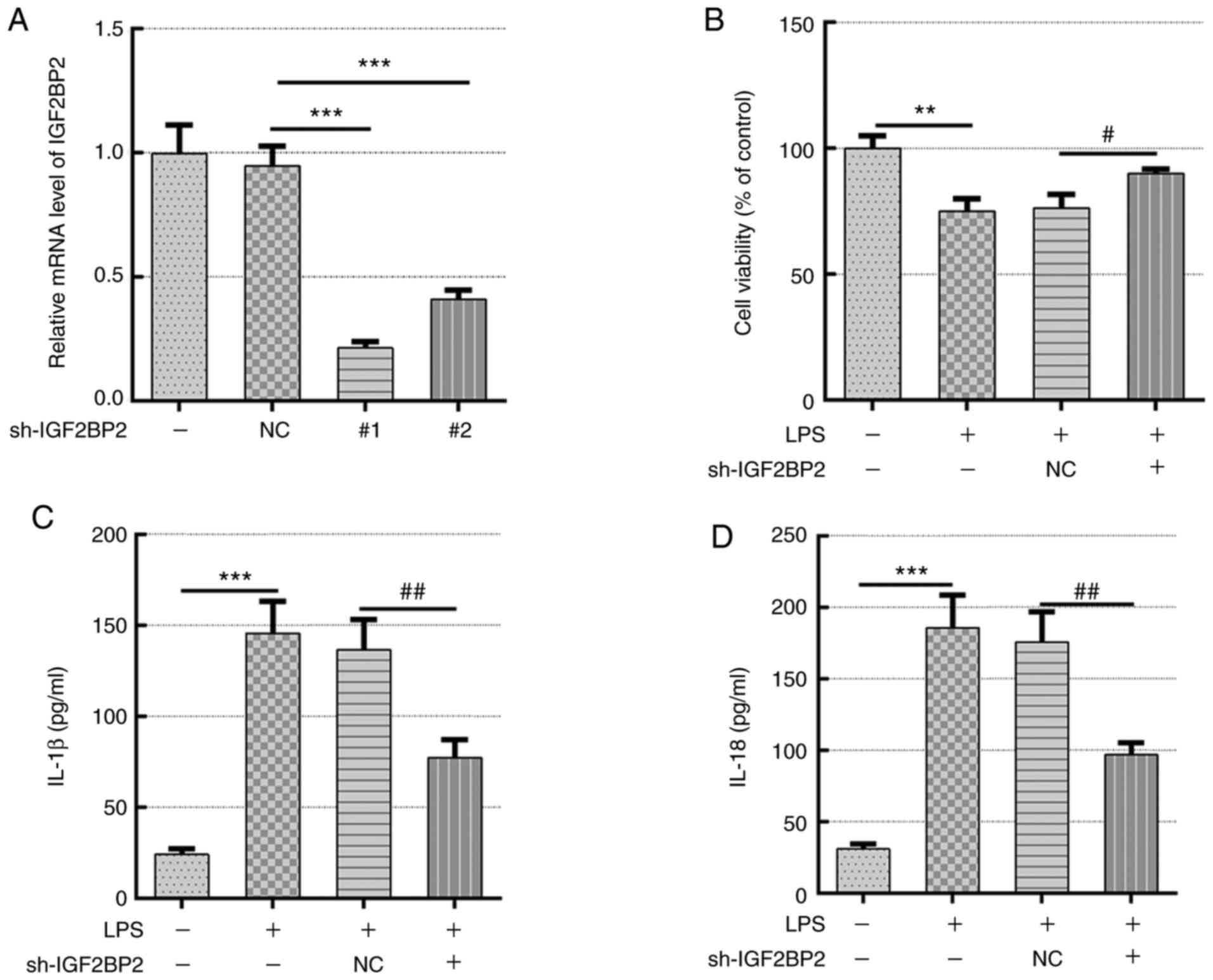
Knockdown of IGF2BP2 enhances cell
viability and inhibits the activity levels of pyroptosis-associated
inflammatory cytokines, including IL-1β and IL-18, in LPS-treated
Beas-2B cells
To explore the specific role of IGF2BP2 and its
association with caspase 4-mediated pyroptosis, two shRNA sequences
were constructed that targeted IGF2BP2 to knockdown its expression.
Scrambled shRNA was used as negative control and shRNA-IGF2BP2-1
was selected for subsequent experiments based on its superior
knockdown efficacy (Fig. 2A).
Untreated Beas-2B cells and Beas-2B cells transfected with
shRNA-IGF2BP2-1 or shRNA-NC were subsequently exposed to 1 µg/ml
LPS, which was added to the culture medium for 12 h (20). Beas-2B cells incubated in normal
culture medium were used as the control group and Beas-2B cells
stimulated with 1 µg/ml LPS (in the absence of transfection) were
considered as the model group. Cells in the model group, which was
stimulated with LPS, exhibited a significant decrease in cell
viability compared with that in cells in the control group
(Fig. 2B). However, compared with
cells transfected with shRNA-NC, IGF2BP2 knockdown resulted in
significantly increased cell viability following stimulation with
LPS (Fig. 2B). This suggested that
the knockdown of IGF2BP2 can recover the cell viability previously
reduced by LPS. In addition, the levels of the inflammatory
cytokines IL-1β and IL-18, which are typically released during cell
pyroptosis (5), were also measured.
Although LPS induced the generation of IL-1β and IL-18, IGF2BP2
knockdown significantly reduced the concentration of these two
cytokines compared with those in cells transfected with shRNA-NC in
the presence of LPS (Fig. 2C and
D). This observation indicated that
the knocking down IGF2BP2 expression rescued cell viability and
inhibited inflammation in response to LPS stimulation.
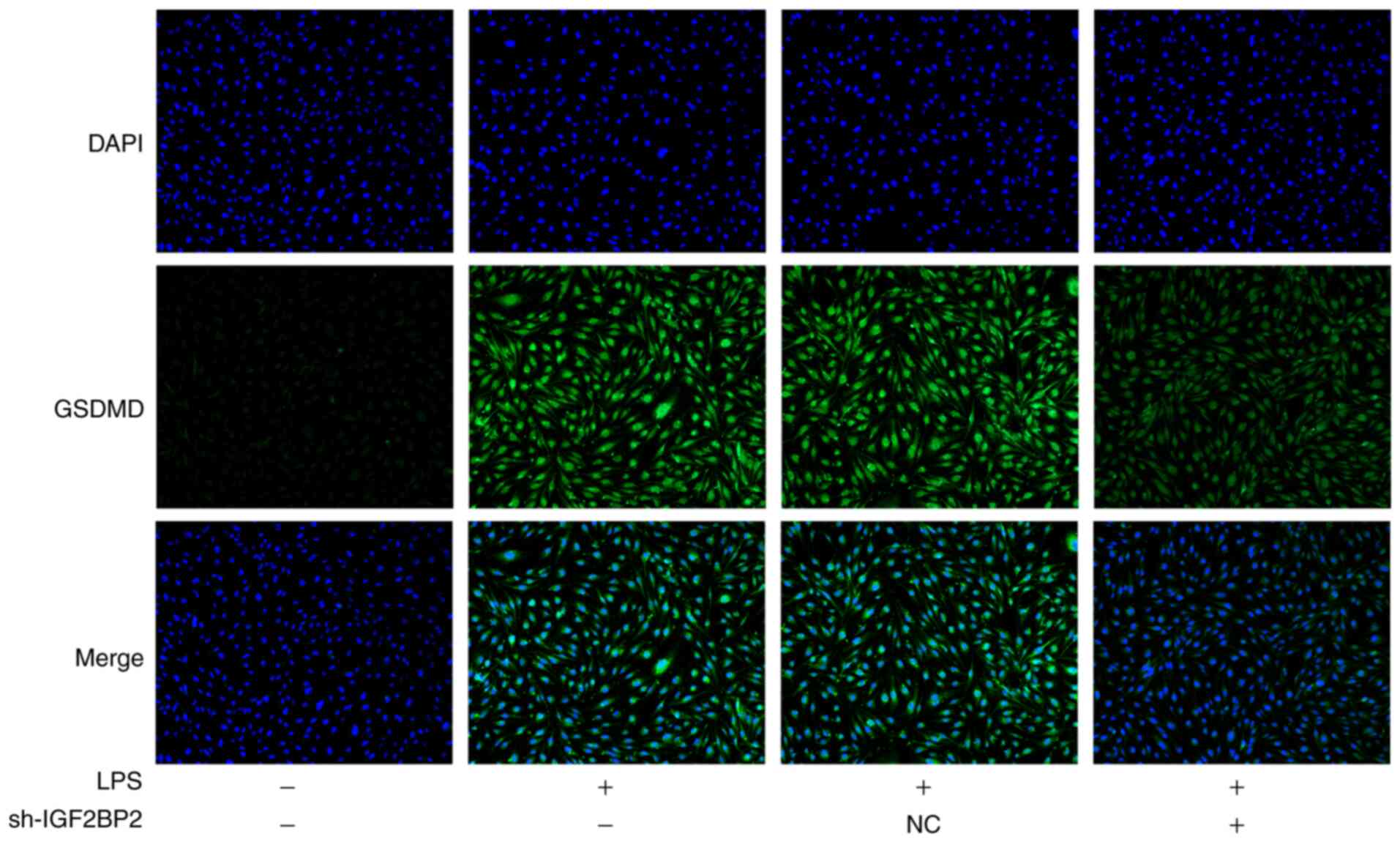
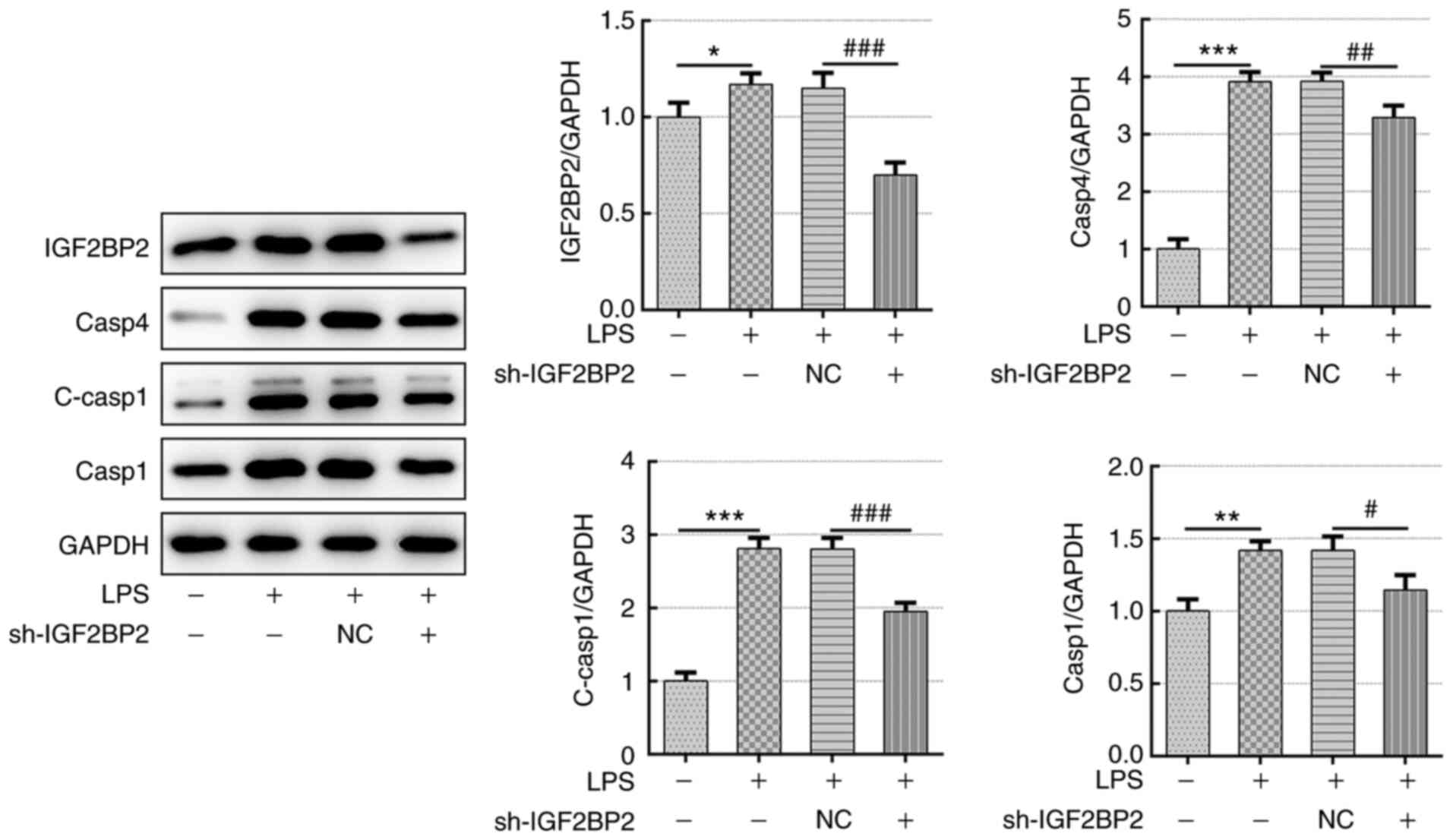
Knockdown of IGF2BP2 inhibits GSDMD
activation and caspase 4/1 expression in LPS-treated Beas-2B
cells
Subsequently, the expression level of GSDMD, caspase
4 and caspase 1, specific markers involved in cell pyroptosis
(5), were investigated. LPS
treatment led to the activation of GSDMD (Fig. 3). However, knockdown of IGF2BP2
prevented the expression of activated GSDMD. Furthermore, in cells
transfected with shRNA-IGF2BP2 that were treated with LPS, the
significant reductions in IGF2BP2 protein expression levels
compared with those in cells transfected with shRNA-NC was
observed. This appeared to be concomitant with the finding that
shRNA-IGF2BP2 transfection resulted in significant reductions in
caspase 4 and cleaved-caspase 1 levels compared with those in cells
transfected with shRNA-NC (Fig. 4).
These results implicated the inhibitory effects of IGF2BP2
knockdown on GSDMD/caspase-4- or caspase 1-mediated pyroptosis.
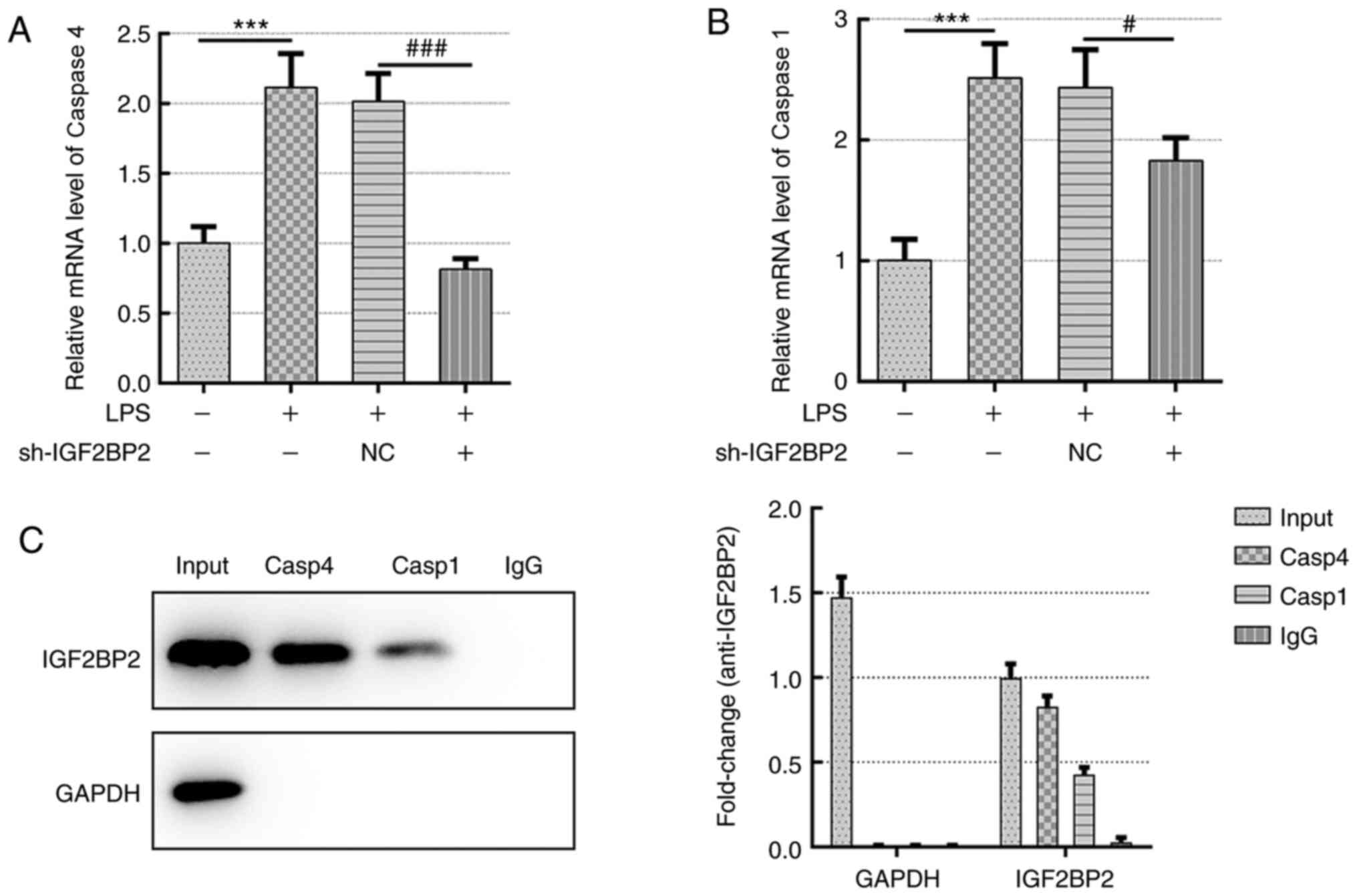
IGF2BP2 functions as an mRNA binding
protein and binds to the RNA of caspase 4
The present study next examined whether the effects
of IGF2BP2 on LPS-induced lung cell inflammation and pyroptosis
were dependent on binding of this protein to caspase 4. The mRNA
levels of both caspases 4 and 1 were assessed following IGF2BP2
knockdown. The results indicated that both caspase 4 and 1 mRNA
levels were significantly reduced following IGF2BP2 knockdown
compared with those in cells transfected with shRNA-NC (Fig. 5A and B). However, knocking down IGF2BP2
expression produced a larger reduction on caspase 4 expression
compared with that noted for caspase 1 (Fig. 5A and B). To determine the direct interaction
between IGF2BP2 and caspase 4, RNA pull-down assay was performed
and the results confirmed the direct interaction between these two
proteins (Fig. 5C).
Discussion
Aberrant activation of the inflammatory response is
one of the major mechanisms underlying lung and airway damage
(1). As a novel form of programmed
cell death that was discovered relatively recently, cell pyroptosis
has been reported to be involved in the generation of inflammatory
cytokines and amplification of the inflammatory response (6). In the present study, IGF2BP2 was
identified as a novel regulator in LPS-induced inflammation in
Beas-2B cells. Subsequently, it was demonstrated that the effects
mediated by IGF2BP2 were possibly through caspase 4, which is a key
protein involved in pyroptosis.
It has been previously reported that LPS from
gram-negative bacteria can activate the immune response and
inflammation by activating pyroptosis (6). During non-canonical pyroptosis,
intracellular LPS can activate caspase 4/5 in humans, leading to
the cleavage of GSDMD and the activation of caspase 1, which causes
the release of the inflammatory factors, including IL-1β and
IL-18(5). During canonical
pyroptosis, LPS can directly activate caspase 1 by binding to
NLRP3, thereby leading to generation of inflammatory cytokines such
as IL-1β, IL-18 and IL-6(5).
Results from the present study demonstrated that LPS treatment
inhibited cell viability in addition to triggering IL-1β and IL-18
production in a concentration-dependent manner in Beas-2B cells,
which was consistent with previous findings. In addition, LPS was
also found to activate GSDMD, caspase 4 and caspase 1, all of which
can mediate the non-canonical pyroptosis pathway (21,22).
These data suggested that cell pyroptosis is at least part
responsible for in LPS-induced Beas-2B cell inflammation,
indicating a modulatory role of pyroptosis in lung injury.
The mRNA-binding protein IGF2BP2 has been shown to
function as an oncogene by targeting long non-coding (lnc)RNAs and
microRNAs (miRs) upstream of promoting cancer cell proliferation,
migration and invasion (9,23,24).
For example, IGF2BP2 can be stabilized by the lncRNA long
intergenic non-coding RNA for IGF2BP2 stability to promote the
development of colorectal cancer (8). In addition, IGF2BP2 is downregulated
by the cellular communication network factor 6 protein in
metaplastic breast carcinomas, causing the inhibition of this tumor
(25). However, in the present
study, the data demonstrated that IGF2BP2 may be involved in
LPS-induced inflammation in Beas-2B cells based on its upregulated
expression following LPS stimulation. Knocking down IGF2BP2
expression reversed the LPS-induced reduction in cell viability, in
addition to reversing the LPS-induced production of IL-1β and
IL-18. Furthermore, IGF2BP2 knockdown also reversed the activation
of GSDMD, caspase 4 and caspase 11 in the presence of LPS. All
these data suggested the participation of IGF2BP2 in LPS-induced
pyroptosis in Beas-2B cells. Subsequently, the role of IGF2BP2 in
inflammation was also assessed. A previous study reported that
increases in the expression levels of IGFBP2 in the sputum may
contribute to the development of idiopathic pulmonary fibrosis
(26), suggesting a potential role
of the IGF family of proteins in lung-associated diseases. In
addition, IGF2 has also reported to be involved in
inflammation-associated diseases, such as acute pneumonia (27). For example, lncRNA small nucleolar
RNA host gene 16 was found to promote LPS-induced acute pneumonia
in A549 cells by targeting the miR-370-3p/IGF2 axis (27). In another study, miR-3941 targeted
IGF2 to control LPS-induced acute pneumonia in A549 cells (28).
IGF2BP2 is a secreted protein that can bind to IGF2
and regulate its localization. Therefore, it was predicted that
IGF2BP2 could also regulate LPS-induced acute pneumonia or lung
cell injury.
To identify the potential targets of IGF2BP2 in
LPS-induced Beas-2B cell inflammation, the ENCORI database
(http://starbase.sysu.edu.cn/rbpClipRNA.php?source=mRNA&flag=RBP&clade=mammal&genome=human&assembly=hg19&RBP=IGF2BP2&clipNum=&panNum=&target=)
was searched, where the RNA of caspase-4 was found to be a
potential target that could bind to IGF2BP2. In the present study,
expression levels of caspase 4 were increased following LPS
stimulation, which was reversed by IGF2BP2 knockdown. In accordance
with these findings, the direct interaction between IGFBP2 and
caspase 4 mRNA was confirmed by performing a RNA pull down assay.
In addition, the expression levels of caspase 1 were also reduced
by IGF2BP2 knockdown in the presence of LPS. During non-conical
pyroptosis, caspase 1 is one of the downstream proteins of caspase
4(5). Therefore, knocking down
IGF2BP2 in LPS-stimulated Beas-2B cells targeted caspase 4, thereby
promoting the cleavage of GSDMD and cell membrane rupture in
addition to activating of caspase 1. These events ultimately led to
the release of inflammatory cytokines, including IL-1β and IL-18.
In addition, during pyroptosis cells typically undergo phenotypic
changes, including cell swelling and lysis (5). However, immunofluorescence staining
against cleaved N-terminal GSDMD did not reflect this phenotypic
change in the cells. Therefore, other techniques, such as
transmission electron microscopy, should be utilized to further
validate these findings from the present study.
Taken together, the present study demonstrated that
in LPS-stimulated Beas-2B cells, IGF2BP2 could activate the
non-conical pyroptosis pathway by targeting caspase 4. This
promoted the release of inflammatory cytokines to aggravate the
inflammatory response. Therefore, inhibition of IGF2BP2 expression
may provide a therapeutic approach for alleviating LPS-induced lung
cell injury and ALI.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ND and JW contributed to study conception and
design. JW and XY contributed to acquisition, analysis and
interpretation of data. ND drafted the work and revised it
critically for important intellectual content. ND and JW have seen
and can confirm the authenticity of the raw data. All authors read
and approved the final version of the manuscript
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Perner A, Gordon AC, De Backer D,
Dimopoulos G, Russell JA, Lipman J, Jensen JU, Myburgh J, Singer M,
Bellomo R and Walsh T: Sepsis: Frontiers in diagnosis,
resuscitation and antibiotic therapy. Intensive Care Med.
42:1958–1969. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Mehaffey JH, Charles EJ, Schubert S,
Salmon M, Sharma AK, Money D, Stoler MH, Laubach VE, Tribble CG,
Roeser ME and Kron IL: In vivo lung perfusion rehabilitates
sepsis-induced lung injury. J Thorac Cardiovasc Surg. 155:440–448
e442. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Aji G, Li F, Chen J, Leng F, Hu K, Cheng
Z, Luo Y, Xu X, Zhang J and Lu Z: Upregulation of PCP4 in human
aldosterone-producing adenomas fosters human adrenocortical tumor
cell growth via AKT and AMPK pathway. Int J Clin Exp Pathol.
11:1197–1207. 2018.PubMed/NCBI
|
|
4
|
Van Opdenbosch N and Lamkanfi M: Caspases
in cell death, inflammation, and disease. Immunity. 50:1352–1364.
2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Robinson N, Ganesan R, Hegedűs C, Kovács
K, Kufer TA and Virág L: Programmed necrotic cell death of
macrophages: Focus on pyroptosis, necroptosis, and parthanatos.
Redox Biol. 26(101239)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hersoug LG, Møller P and Loft S: Role of
microbiota-derived lipopolysaccharide in adipose tissue
inflammation, adipocyte size and pyroptosis during obesity. Nutr
Res Rev. 31:153–163. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Huang X, Zhang H, Guo X, Zhu Z, Cai H and
Kong X: Insulin-like growth factor 2 mRNA-binding protein 1
(IGF2BP1) in cancer. J Hematol Oncol. 11(88)2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wang Y, Lu JH, Wu QN, Jin Y, Wang DS, Chen
YX, Liu J, Luo XJ, Meng Q, Pu HY, et al: lncRNA LINRIS stabilizes
IGF2BP2 and promotes the aerobic glycolysis in colorectal cancer.
Mol Cancer. 18(174)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Li T, Hu PS, Zuo Z, Lin JF, Li X, Wu QN,
Chen ZH, Zeng ZL, Wang F, Zheng J, et al: METTL3 facilitates tumor
progression via an m(6)A-IGF2BP2-dependent mechanism in colorectal
carcinoma. Mol Cancer. 18(112)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Dai N: The diverse functions of
IMP2/IGF2BP2 in metabolism. Trends Endocrinol Metab. 31:670–679.
2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Simon Y, Kessler SM, Bohle RM, Haybaeck J
and Kiemer AK: The insulin-like growth factor 2 (IGF2) mRNA-binding
protein p62/IGF2BP2-2 as a promoter of NAFLD and HCC? Gut.
63:861–863. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Baker PJ, Boucher D, Bierschenk D, Tebartz
C, Whitney PG, D'Silva DB, Tanzer MC, Monteleone M, Robertson AAB,
Cooper MA, et al: NLRP3 inflammasome activation downstream of
cytoplasmic LPS recognition by both caspase-4 and caspase-5. Eur J
Immunol. 45:2918–2926. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Gonzalez-Juarbe N, Bradley KM, Riegler AN,
Reyes LF, Brissac T, Park SS, Restrepo MI and Orihuela CJ:
Bacterial pore-forming toxins promote the activation of caspases in
parallel to necroptosis to enhance alarmin release and inflammation
during pneumonia. Sci Rep. 8(5846)2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zaslona Z, Flis E, Wilk MM, Carroll RG,
Palsson-McDermott EM, Hughes MM, Diskin C, Banahan K, Ryan DG,
Hooftman A, et al: Caspase-11 promotes allergic airway
inflammation. Nat Commun. 11(1055)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Srisaowakarn C, Pudla M, Ponpuak M and
Utaisincharoen P: Caspase-4 mediates restriction of burkholderia
pseudomallei in human alveolar epithelial cells. Infect Immun.
88(e00868)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Bao Z, Chen L and Guo S: Knockdown of
SLC34A2 inhibits cell proliferation, metastasis, and elevates
chemosensitivity in glioma. J Cell Biochem. 120:10205–10214.
2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ko IG, Hwang JJ, Chang BS, Kim HS, Jin JJ,
Hwang L, Kim CJ and Choi CW: Polydeoxyribonucleotide ameliorates
lipopolysaccharide-induced acute lung injury via modulation of the
MAPK/NF-kB signaling pathway in rats. Int Immunopharmacol.
83(106444)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Dou YQ, Kong P, Li CL, Sun HX, Li WW, Yu
Y, Nie L, Zhao LL, Miao SB, Li XK, et al: Smooth muscle SIRT1
reprograms endothelial cells to suppress angiogenesis after
ischemia. Theranostics. 10:1197–1212. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lv X, Zhou X, Yan J, Jiang J and Jiang H:
Propofol inhibits LPS-induced apoptosis in lung epithelial cell
line, BEAS-2B. Biomed Pharmacother. 87:180–187. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Aaltomaa S, Kärjä V, Lipponen P, Isotalo
T, Kankkunen JP, Talja M and Mokka R: Reduced alpha- and
beta-catenin expression predicts shortened survival in local
prostate cancer. Anticancer Res. 25:4707–4712. 2005.PubMed/NCBI
|
|
22
|
He C, Zhao Y, Jiang X, Liang X, Yin L, Yin
Z, Geng Y, Zhong Z, Song X, Zou Y, et al: Protective effect of
Ketone musk on LPS/ATP-induced pyroptosis in J774A.1 cells through
suppressing NLRP3/GSDMD pathway. Int Immunopharmacol. 71:328–335.
2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wan BS, Cheng M and Zhang L: Insulin-like
growth factor 2 mRNA-binding protein 1 promotes cell proliferation
activation of AKT and is directly targeted by microRNA-494 in
pancreatic cancer. World J Gastroenterol. 25:6063–6076.
2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Abrenica B, AlShaaban M and Czubryt MP:
The A-kinase anchor protein AKAP121 is a negative regulator of
cardiomyocyte hypertrophy. J Mol Cell Cardiol. 46:674–681.
2009.PubMed/NCBI View Article : Google Scholar
|
|
25
|
McMullen ER, Gonzalez ME, Skala SL, Tran
M, Thomas D, Djomehri SI, Burman B, Kidwell KM and Kleer CG: CCN6
regulates IGF2BP2 and HMGA2 signaling in metaplastic carcinomas of
the breast. Breast Cancer Res Treat. 172:577–586. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Guiot J, Henket M, Corhay JL, Moermans C
and Louis R: Sputum biomarkers in IPF: Evidence for raised gene
expression and protein level of IGFBP-2, IL-8 and MMP-7. PLoS One.
12(e0171344)2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Liu Y, Zhang B, Cao WB, Wang HY, Niu L and
Zhang GZ: Study on clinical significance of lncRNA EGOT expression
in colon cancer and its effect on autophagy of colon cancer cells.
Cancer Manag Res. 12:13501–13512. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Fei S, Cao L and Pan L: microRNA-3941
targets IGF2 to control LPS-induced acute pneumonia in A549 cells.
Mol Med Rep. 17:4019–4026. 2018.PubMed/NCBI View Article : Google Scholar
|