Introduction
Oxidative stress, defined as the imbalance between
pro-oxidants and antioxidant capacity, plays an important role in
the course of inflammatory, metabolic and proliferative chronic
liver disease (CLD). Chronic liver injury can be manifested as
fibrosis, cholestasis, necrosis and cirrhosis (1). Liver cirrhosis is the final stage of
various types of CLD and fibrosis is the precursor of cirrhosis.
The burden of liver disease is underestimated but continues to grow
worldwide (2). Ethanol consumption
and chronic infections due to hepatitis B virus (HBV) and/or
hepatitis C virus (HCV) constitute the main causes of liver
cirrhosis which was reported to represent the 11th most common
cause of mortality worldwide in 2018(3), with first-year mortality ranging from
1 to 57% depending on the stage (1,4).
Many types of cells, cytokines and microRNAs are
involved in the initiation and progression of liver fibrosis and
cirrhosis. Pathological features are common to all cases of liver
cirrhosis, including hepatocyte degeneration and necrosis,
replacement of liver parenchyma by fibrotic tissues and
regenerative nodules, and loss of liver function. The liver that is
exposed to high amounts of ethanol undergoes structural and
functional alterations as a consequence of two linked phenomena:
Oxidative stress and inflammation (5). Ethanol may increase the production of
reactive oxygen and nitrogen species (ROS, RNS), and these reactive
intermediates are able to induce pro-fibrogenic cytokines and the
release of several inflammatory markers and collagen synthesis
during the progression of liver fibrosis (1,6). ROS
are oxygen-containing molecules that are produced during normal
metabolism. The organism has two types of systems able to
neutralize the harmful effects of endogenous ROS, enzymatic and
non-enzymatic antioxidants (7).
Under normal circumstances, the liver maintains a balance between
internal antioxidants and ROS in order to be able to neutralize the
free radicals generated by viruses and various endogenous and
exogenous compounds processed by the liver. Under certain
conditions, the oxidative to antioxidative balance shifts towards
the oxidative status as a result of an increase in ROS production
or antioxidant depletion. However, when the liver is overwhelmed by
continuous oxidative insults (e.g., long-lasting ethanol abuse,
infection with HBV or HCV), the damage from free radicals
increases, resulting in inflammation and fibrosis (8).
Oxidative stress causes liver injury by the
alteration of main biological molecules (DNA, proteins, and lipids)
(9). We know from previous studies
that DNA and protein oxidation as well as lipid peroxidation
products are involved in the modulation of signaling pathways
associated with gene transcription, protein expression, apoptosis,
and hepatic stellate cell activation, contributing to both the
onset and progression of liver fibrosis (10,11).
Regarding inflammation, it is an essential event in the immune
response manifested as infiltration of inflammatory cells to fight
against various aggressive stimuli.
The close interplay between oxidative stress and
inflammation in the development of liver disease has stimulated the
interest of researchers for a long time. Excessive inflammatory
cells may produce more ROS and RNS and further these are able to
increase the expression of genes coding proinflammatory cytokines.
The general consensus is that oxidative stress and inflammation are
tightly correlated and create a vicious cycle which is involved in
the progression to cirrhosis and ultimately hepatocellular
carcinoma of liver diseases (12).
Recently, the trend of research has been focused on
the role of hematological markers of inflammation from complete
blood count (CBC) panel [ratios including neutrophil/lymphocyte
(NLR), monocyte/lymphocyte (MLR) and platelet/lymphocyte (PLR)] in
assessing the prognosis of various disorders (13-17).
Thus, NLR and PLR have been validated as prognostic markers in
cancer, sepsis, cardiac conditions, pneumonia and acute respiratory
distress syndrome (18-20).
Few studies have evaluated the role of these ratios as prognostic
indexes of disease outcome in patients with liver cirrhosis.
According to our knowledge, none of these reported the use of these
indexes to assess the association between oxidative stress,
inflammation and the severity of liver disease.
Therefore, the aim of the present study was to
determine the usefulness of such hematological indicators to assess
the relationship between inflammation and oxidative stress in order
to provide new predictive tools for a non-invasive paraclinical
investigation of disease outcome in liver cirrhosis patients.
Patients and methods
Statement of ethics
According to the European Union Guidelines
(Declaration of Helsinki), the study received the approval of the
Institutional Ethics Committee of the University of Medicine and
Pharmacy of Craiova (registration no. 116/11.11.2019) and the
registered participants gave their written informed consent to be
included.
Patients
A total of 35 subjects, hospitalized at the First
Clinic of Internal Medicine, Clinical City Hospital ‘Filantropia’
and Second Clinic of Internal Medicine, County Hospital of Craiova,
Romania from November 2019 to February 2020, with compensated or
decompensated liver cirrhosis aged between 38-75 years and 10
age-matched healthy volunteers were enrolled in this study. The
diagnosis was established based on medical history, clinical
examination, laboratory tests, ultrasonography and endoscopy.
Decompensated liver cirrhosis is associated with ascites,
esophageal varices or hepatic encephalopathy. Exclusion criteria
were the following: Pregnancy, drug abuse, comorbidities that could
increase the systemic inflammation (e.g., diabetes, metabolic
syndrome, inflammatory and autoimmune diseases), corticoids or
non-steroidal anti-inflammatory drug use (17). The patients were divided into two
groups: Group 1, patients (n=25) with toxic metabolic cirrhosis due
to ethanol consumption (all of these patients had consumed at least
70 g of pure alcohol per day for more than 5 years); group 2,
patients (n=10) with liver cirrhosis following HBV and HCV
infection. The control group, included 10 age-matched healthy
subjects without any clinical or paraclinical sign of disease.
Sample collection and handling
In the morning, after a minimum of 12 h of fasting,
blood samples were collected in commercially available covered test
tubes without any anticoagulant and, in order to prevent blood
clotting, in lavender topped K2EDTA BD vacutainers
(Becton-Dickinson). Blood samples collected in K2EDTA
tubes were used to perform a complete blood count (CBC).
For each patient, a sample of blood was also
collected in black capped BD ESR (Becton-Dickinson) tubes. Plasma
and blood cell fractions were separated by centrifugation of blood
also collected in vacutainers containing K2EDTA at 2,000
x g, for 10 min, at 4˚C (5417R Eppendorf centrifuge;
Eppendorf AG). Immediately after separation, the plasma was
aliquoted in Eppendorf tubes and stored under proper conditions (at
-80˚C, avoiding repeated freezing/refreezing cycles)
until determination of several oxidative stress markers. The
sediment was processed to obtain a hemolysate that was preserved
for further analyses.
Serum was separated by centrifugation of blood
collected in red topped BD vacutainers (Becton-Dickinson) at 1,000
x g for 10 min, after which it was allowed to clot for 20 min at
room temperature, and used for the measurement of several
inflammatory markers and biochemical parameters.
Laboratory and clinical
assessments
We recorded the following general information for
each subject: Age, sex, time of disease progression. Counts of
white blood cells (WBC), red blood cells (RBC), neutrophils,
monocytes, lymphocytes and platelets were performed in samples of
peripheral blood obtained by standard venipuncture in
K2EDTA BD vacutainers using an automatic flow cytometry
analyzer (CELL-DYN Ruby System; Abbott Diagnostics). Using the
hematological data, we calculated the monocyte/lymphocyte ratio
(MLR), neutrophil/lymphocyte ratio (NLR) and platelet/lymphocyte
ratio (PLR) by dividing the number of respective subtypes of blood
cells (monocytes, neutrophils and platelets) by lymphocyte number
(21) and also a systemic
immune-inflammation index (SII) according to the formula:
SII=platelet count x neutrophil count/lymphocyte count (22). The erythrocyte sedimentation ratio
(ESR) was assessed according to the Westergren method. C-reactive
protein (CRP) analysis was performed using an automated immunoassay
analyzer (Cobase411; Roche Diagnostics GmbH). Fibrinogen was
measured using an ACL Top 500 coagulometer (Instrumentation
Laboratory, USA). Total proteins, albumin, alanine-aminotransferase
(ALT), aspartate aminotransferase (AST), alkaline phosphatase
(Palk) and γ-glutamyl transpeptidase (GGT) were assessed using an
automated analyzer Architect c8000 (Abbott Diagnostics).
Lipid peroxidation analysis as
thiobarbituric acid reactive substances assay
The thiobarbituric acid reactive substances (TBARS)
assay in plasma was performed using a spectrophotometric method in
order to evaluate the lipid peroxidation level as previously
described (23,24). The lipid peroxidation level was
evaluated by quantifying malondialdehyde (MDA) concentration, a
major product of fatty acid peroxidation, from deproteinized
plasma. Human plasma (0.1 ml) was treated with 5% trichloroacetic
acid (TCA) and 0.2 M Tris-HCl pH=4.7 (v/v). After 10 min of
incubation at room temperature, the sample was mixed with 1 ml of
0.55 M thiobarbituric acid (TBA) in 2 M sodium sulphate, heated at
90˚C for 45 min and cooled in ice (25). After cooling, the mixture was
centrifuged at 15,000 x g for 3 min in a refrigerated centrifuge
(Eppendorf 5417R; Eppendorf AG). MDA reacts with TBA and forms a
pink color product which has a specific absorption at 532 nm. The
optical density (OD) was measured using an UV-VIS spectrophotometer
(Kruss). The TBARS concentration was calculated using the molar
extinction coefficient of MDA (1.55x105
M-1cm-1). The results are expressed as µmol/l
TBARS. Except TBA produced by Fluka, all other reagents used were
provided by Sigma-Aldrich; Merck KGaA.
Protein carbonyl content assay
A spectrophotometric assay using
2,4-dinitrophenylhydrazine (DNPH) was performed to assess
carbonylated protein (PCARB) content as a marker of protein
oxidation (23,25,26).
The plasma samples were mixed with 20% TCA (v/v), incubated for 15
min on ice and separated by centrifugation at 15,000 x g, for 5 min
at 4˚C. After centrifugation, the supernatant was
discarded and the pellet was treated with 0.5 ml 10 mM DNPH in 2.5
M HCl. The samples were incubated in the dark for 1 h with
intermittent shaking every 15 min. After incubation, the upper
phase was removed and two washing steps were performed with
ethanol:ethyl acetate (1:1, v/v) to remove the excess DNPH. The
protein pellet was solved in 1 ml of 5 M urea (pH=2.3) at
37˚C for 10 min and separated by centrifugation at
15,000 x g, for 5 min at 4˚C. Finally, the OD of the
samples was measured at 375 nm using a UV-VIS spectrophotometer
(Kruss). The PCARB content was calculated based on the molar
extinction factor of DNFH (22,000 M-1cm-1).
PCARB concentration is expressed as nmol/mg of protein. Total
protein concentration in the samples was assessed using Bradford
method (27). All reagents used
were provided by Sigma-Aldrich; Merck KGaA.
Total antioxidant capacity (TAC)
assay
TAC assay is one of the analyses usually performed
to assess the antioxidant status in human blood samples related to
various diseases. Evaluation of TAC characterizes the general
ability of the body to fight oxidative stress by making antioxidant
compounds. TAC can be easily assessed in human plasma using a
spectrophotometric method (24,28).
Plasma samples diluted at 1:25 in phosphate-buffered saline (PBS,
pH=7.4) were mixed with 0.1 mM 2,2 diphenyl-1-picrylhydrazyl
radical reagent (DPPH, v/v) and incubated in a dark room for 30
min. After incubation, the samples were separated by centrifugation
for 3 min at 20,000 x g and OD was read at 520 nm using a UV-VIS
spectrophotometer. TAC was expressed as mmol DPPH/l. All reagents
used were provided by Sigma-Aldrich; Merck KGaA.
Statistical analysis
Data were analyzed using GraphPad Prism 5.0 software
(GraphPad Software, Inc.). Data are expressed as mean ± standard
deviation (SD). The comparison of oxidative stress markers between
groups was performed using several statistical tests: Unpaired
non-parametric Mann-Whitney t-test, one-way ANOVA with Tukey's and
Bonferroni's multiple comparison tests. A P-value <0.05 was
considered to indicate a statistically significant difference.
Results
Demographic data, biochemical and
hematological markers of inflammation
We included in this study 35 patients with liver
cirrhosis divided into two groups according to the etiological
factor: Group 1, patients with toxic metabolic cirrhosis due to
ethanol consumption and group 2, patients with liver cirrhosis
following HBV and HCV infection.
Demographic data and various biochemical findings
for the patients in the liver cirrhosis subgroups are presented in
Table I.
 | Table IDemographic and biochemical findings
of the patients in the liver cirrhosis subgroups. |
Table I
Demographic and biochemical findings
of the patients in the liver cirrhosis subgroups.
| Characteristic | Group 1 (alcoholic
cirrhosis) | Group 2 (cirrhosis
due to viral infection) |
|---|
| Mean age
(years) | 63.17±10.4 | 59.14±10.52 |
| Sex ratio
(M/F) | 19:6 | 7:3 |
| ALT (UI) | 31.63±20.96 | 31.28±10.07 |
| AST (UI) | 62.04±58.75 | 49.85±24.43 |
| GGT (UI) | 134.58±143.16 | 77.83±74.69 |
| Palk (UI) | 226.52±184.26 | 291.66±149.52 |
| Total protein
(g/dl) | 7.35±0.83 | 7±0.28 |
| Albumin (g/dl) | 3.35±0.89 | 2.92±0.66 |
| Fibrinogen
(mg/dl) | 246.66±76.78 | 406.25±17.42 |
Table II contains a
parallel between the hematological markers of inflammation found in
the patients from the healthy control group and the liver cirrhosis
subgroups.
 | Table IIHematological markers of inflammation
in the subjects from the liver cirrhosis subgroups and healthy
control group. |
Table II
Hematological markers of inflammation
in the subjects from the liver cirrhosis subgroups and healthy
control group.
| Characteristic | Group 1 (alcoholic
cirrhosis) | Group 2 (cirrhosis
due to viral infection) | Control group |
|---|
| Mean age
(years) | 63.17±10.4 | 59.14±10.52 | 56.4±6.73 |
| Sex ratio
(M/F) | 19:6 | 7:3 | 7:3 |
| ESR (mm/h) | 55 (12-120) | 43.42 (18-90) | 8.4 (7-8) |
| CRP | Negative
(n=22) | Negative(n=9) | Negative |
| | Positive (n=3) | Positive (n=1) | |
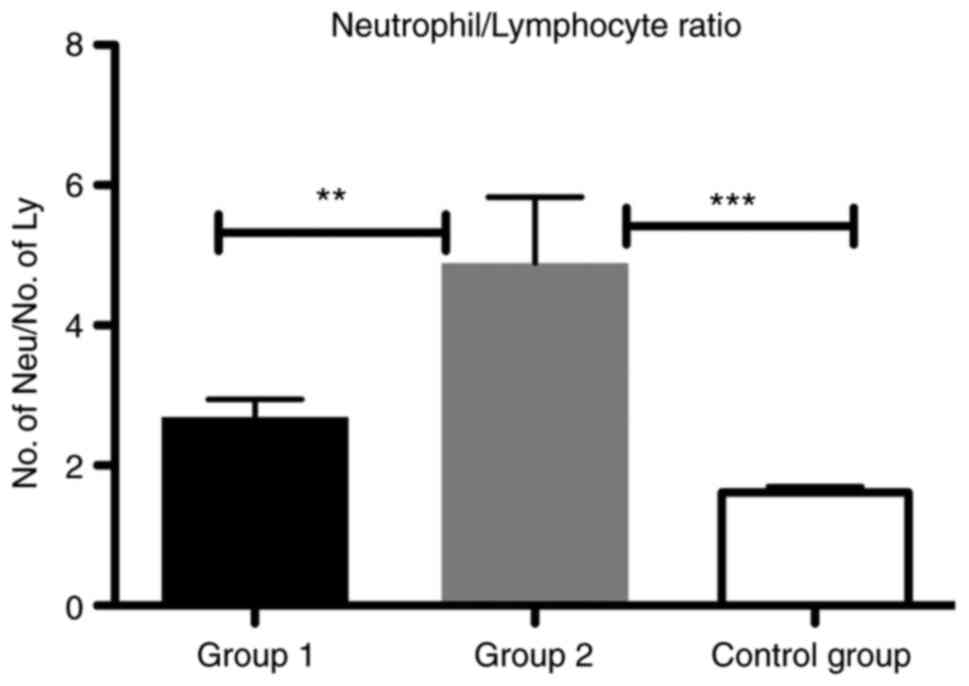
We showed that NLR was significantly increased in
group 2 compared with group 1 (P<0.01) and with the control
group (P<0.001) (Fig. 1).
Receiver operator characteristic (ROC) analysis,
area under the curve (AUC) and 95% confidence interval (CI) were
performed in order to establish hematological markers of
inflammation performance.
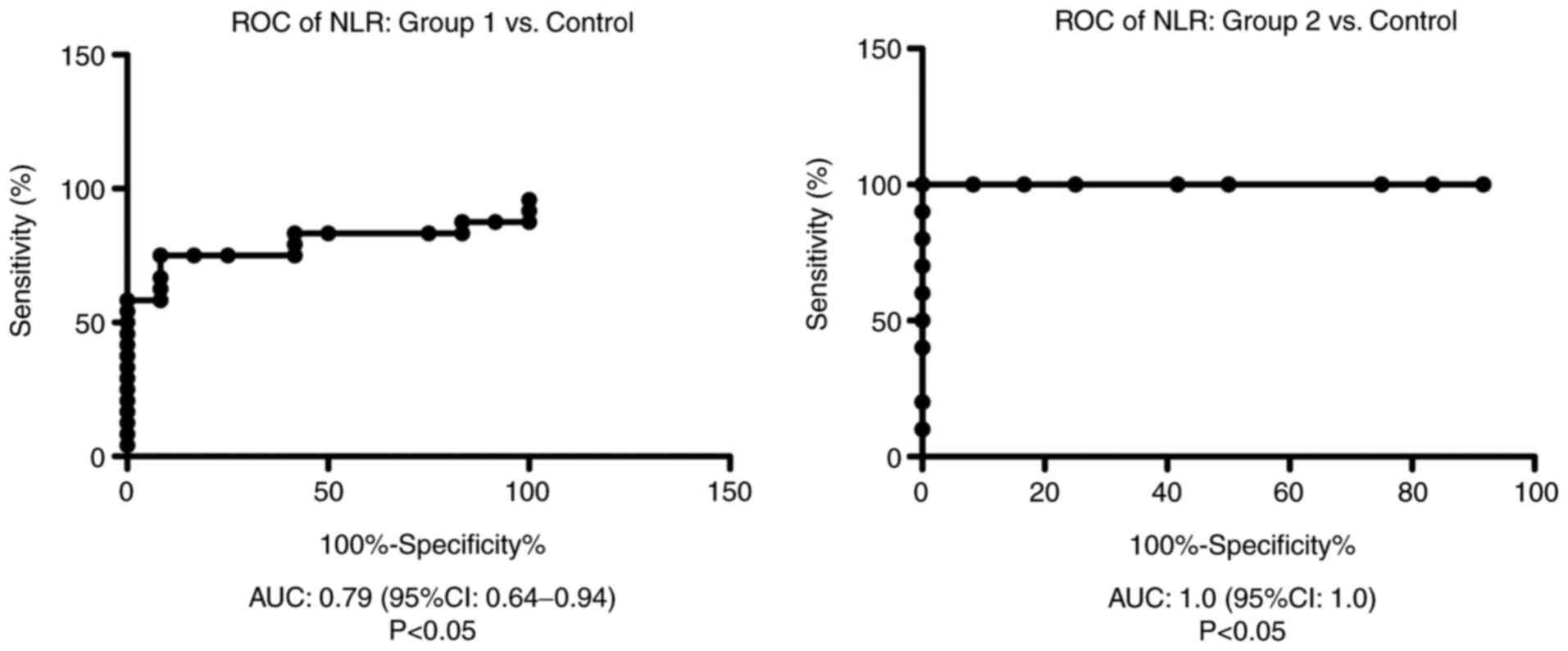
ROC analysis of NLR was performed for group 1 and
group 2 vs. the control group. We found AUC of 0.79 (95% CI:
0.64-0.94) for NLR in group 1 vs. the control group and AUC of 1.00
(95% CI: 1.00-1.00) for NLR in group 2 compared with the control
group (Fig. 2).
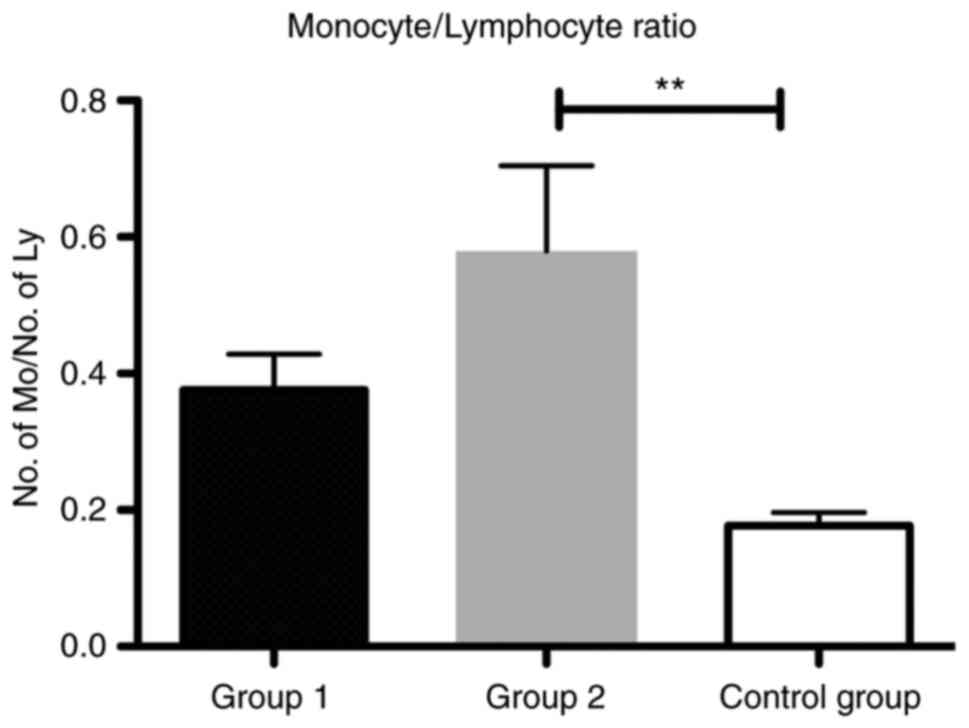
As a biomarker of the immune system, we found that
the MLR was significantly increased in group 2 compared with that
in the control group (P<0.01) (Fig.
3). The ratio was also increased in patients from group 1
compared with the control, yet this difference was not
significant.
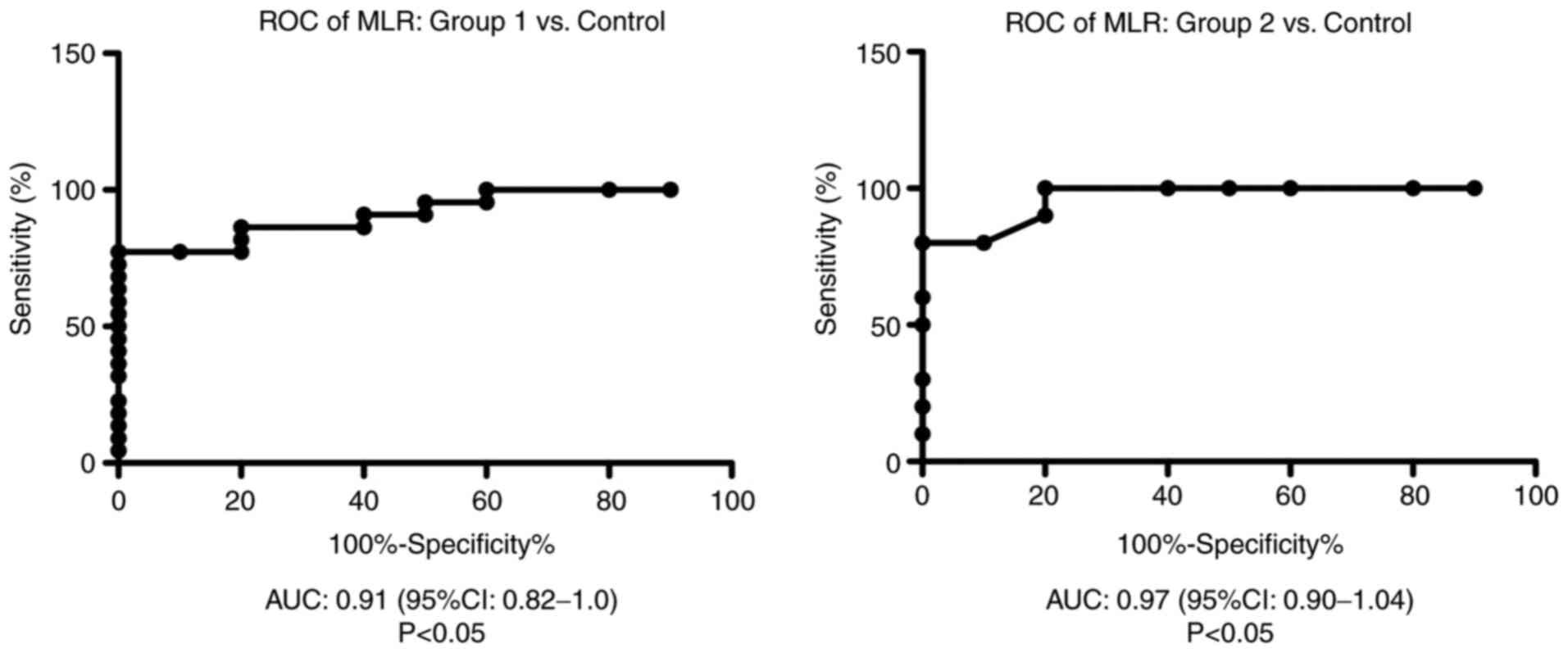
ROC analysis of MLR was performed for group 1 and
group 2 vs. the control group. We found an AUC of 0.9136 (95% CI:
0.82-1) for MLR in group 1 vs. the control group and AUC of 0.97
(95% CI: 0.9-1.04) for MLR in group 2 compared with the control
group (Fig. 4).
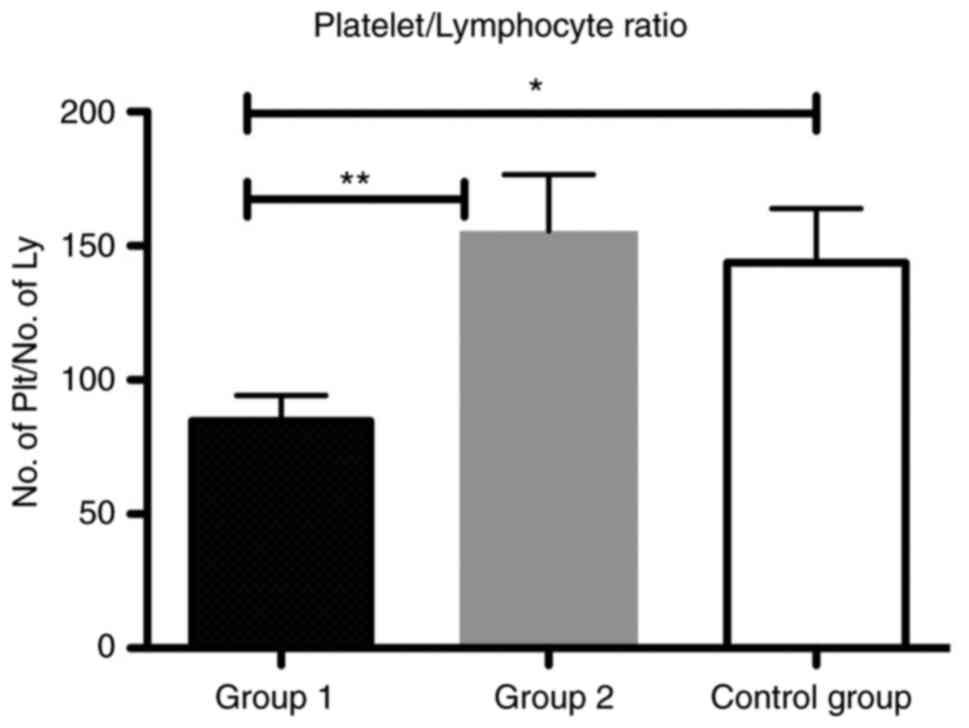
In addition, we showed that the PLR was
significantly increased in group 2 compared with group 1
(P<0.01) and in the group 1 compared to control group
(P<0.05) (Fig. 5).
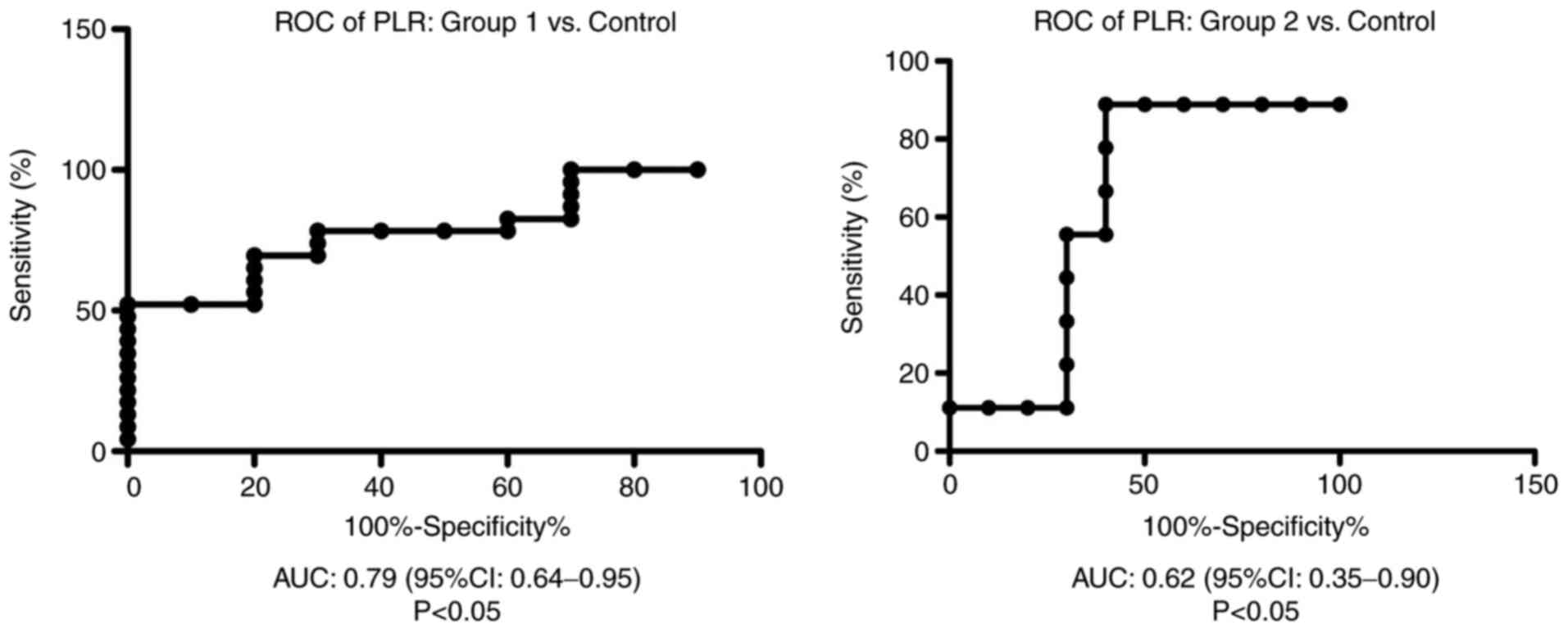
ROC analysis of PLR was performed for group 1 and 2
vs. the control group. We found AUC of 0.79 (95% CI: 0.64-0.95) for
PLR in group 1 vs. the control group and AUC of 0.62 (95% CI:
0.35-0.9) for PLR in group 2 compared with the control group
(Fig. 6).
Variation in the ratios for various blood cells
between the subgroups with liver cirrhosis was sustained by the
difference between the SII values
(505.55x109±106.16x109 cells/l for the
patients with viral cirrhosis from group 2, compared to
410.56x109±280.91x109 cells/l for those from
group 1, with alcoholic liver disease).
Markers of oxidative stress and total
antioxidant capacity
The effects of ROS damage against biomolecules, such
as quantification of plasma TBARS for lipid peroxidation and plasma
PCARB for protein oxidation, were assessed in the controls and
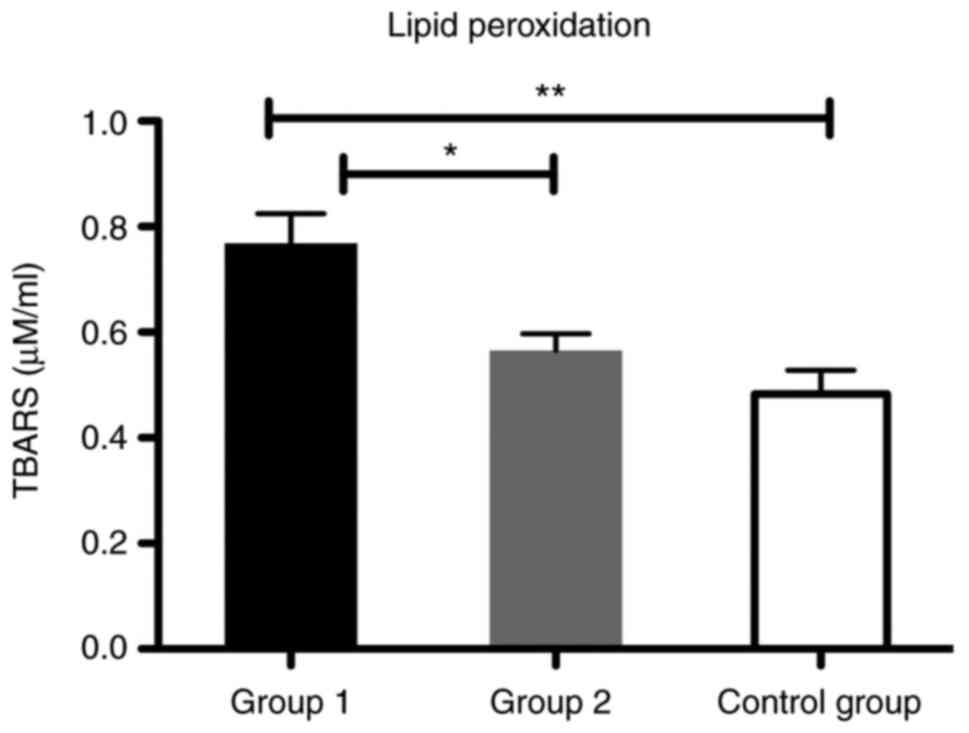
patients from the liver cirrhosis subgroups. We found a
significantly increased level of TBARS in group 1 compared with
group 2 (P<0.05) and also for group 1 compared with the control
group (P<0.01) (Fig. 7).
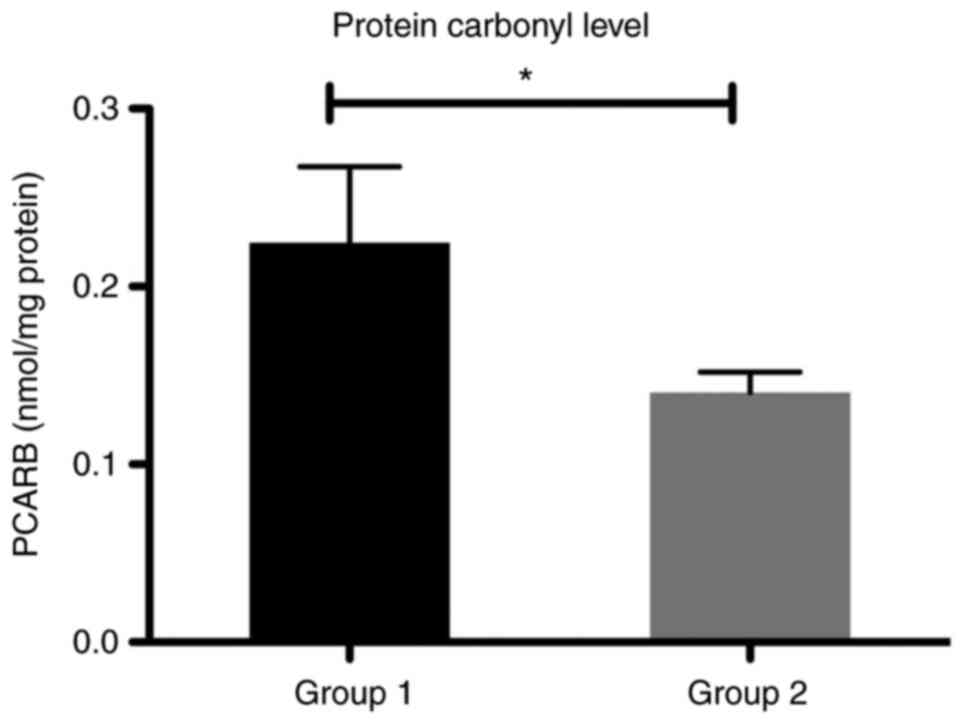
In our study, the level of protein damage by
carbonylation of the lateral chain of amino acids from protein
structure (PCARB) was significantly increased in group 1 compared
with group 2 in the patients with liver cirrhosis (P<0.05)
(Fig. 8).
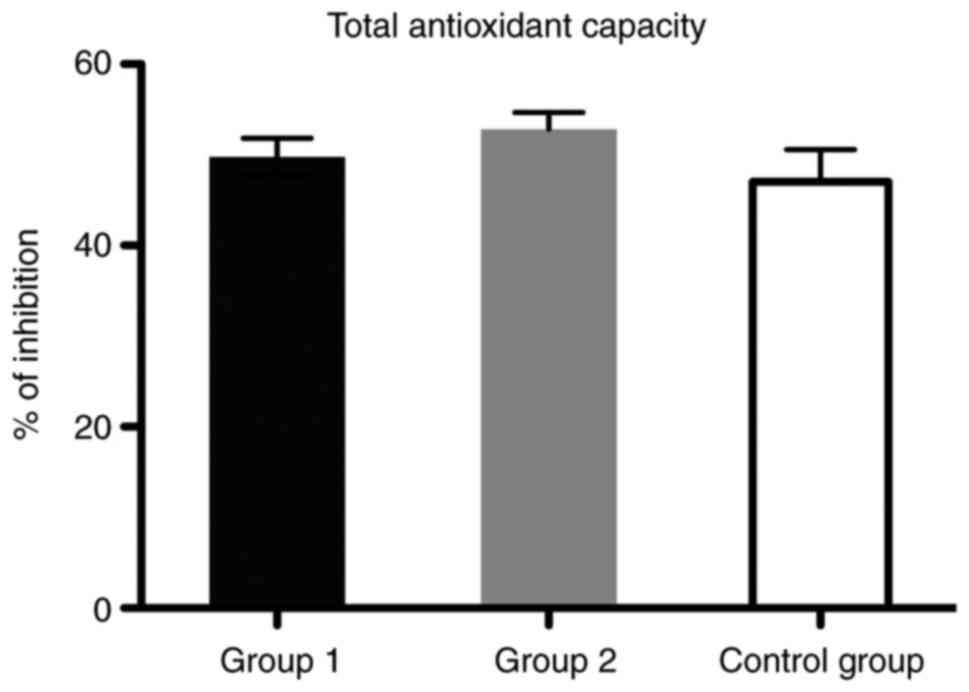
Regarding the total antioxidant defense capacity
(TAC), we found that the values did not differ significantly
between the control age-matched group and patients from the two
groups with liver cirrhosis (Fig.
9).
Unfortunately, oxidative stress markers (TBARS and
PCARB) did not correlate significantly with any of the ratios
between blood cells investigated as predictive markers for the
unfavorable progression of liver cirrhosis.
Discussion
To the best of our knowledge, this is the first
study that aimed to evaluate the association of hematological
markers of inflammation and oxidative stress in liver cirrhosis
patients. In the present study, we found that the patients included
showed a significant increase in plasma oxidative stress markers,
evaluated as thiobarbituric acid reactive substances (TBARS) and
carbonylated protein (PCARB), confirming that oxidative stress acts
as a continuous pathogenic mechanism in all stages of liver disease
irrespective of the etiological factor. The increased synthesis of
these products is due to an imbalance between different agents
(ethanol, HCV or HBV) that trigger oxidative changes and the body's
ability to scavenge reactive oxygen and nitrogen species (ROS and
RNS) through its antioxidants, evaluated in the present study as
total antioxidant capacity (TAC).
Involvement of oxidative stress in liver diseases
has been extensively investigated and the impact of ROS and RNS in
the pathogenesis of various liver diseases such as alcoholic liver
disease (ALD), non-alcoholic fatty liver disease (NAFLD), viral
hepatitis and hepatocellular cancer (HCC) has been reported
(5,9).
The liver is the main site of ethanol metabolism and
also one of the first targets for alcohol-induced injuries. In
alcoholic liver disease (ALD), metabolic processing of ethanol
requires the activation of cytochrome P450 2E1 (CYP2E1) isoform
that is able to commit formation of ROS. ROS can react with fatty
acids from lipids to produce various peroxides which may undergo
fragmentation to generate multiple reactive intermediates, mainly
malondialdehyde (MDA) and 4-hydroxynonenal (4-HNE) (12). These are able to interact further
with proteins and DNA forming adducts responsible for structural
and functional alterations of liver cells and finally for cell
death signaling. Alternative liver processing of ethanol through
alcohol dehydrogenase reaction generates acetaldehyde, another
reactive intermediate able to interact with proteins and DNA as
well to form adducts that augment hepatocellular damage (29).
Through complex signaling pathways, alcohol
consumption alters antioxidant systems involved in ROS removal.
Finally, ROS may lead to excessive liver fibrosis and cirrhosis via
activation of hepatic stellate cells that contribute to
accumulation of the extracellular matrix within the liver (12).
It has been clearly established that HCV is
associated with strong oxidative stress. HCV triggers oxidative
stress by induction of several ROS-producing pathways:
Ca2+-mediated mitochondrial dysfunction, NADPH oxidases
(NOX), CYP2E1 and ER oxidoreductin-1α (Ero1α) (30). HCV core proteins are believed to
possess the highest pro-oxidant potential to trigger mitochondrial
dysfunction. However, another important factor responsible for
HCV-induced ROS production is the activation of several NOX
isoforms (31).
Regarding chronic viral hepatitis B, many studies
have shown that continuous HBV infection can promote the oxidative
response, with patients exhibiting signs of pronounced oxidative
stress in liver and blood (32,33).
Plasma of these patients is also characterized by elevated levels
of ROS and oxidation products of lipids and proteins with a
concomitant reduction in total antioxidant status (33).
Alteration of the pro-oxidant/antioxidant balance
was revealed in liver and blood samples of patients using various
techniques, either direct ROS/RNS quantification, identification of
tissue storage of oxidative/nitrosative stress markers, measurement
of lipid, protein and DNA oxidation products, or assessments of the
individual antioxidants and total antioxidant capacity.
Quantification of MDA and 4-HNE (thiobarbituric acid
reactive substances, TBARS) and of protein carbonylation (PCARB)
performed in our study as indexes of lipid peroxidation and protein
oxidation respectively, are useful laboratory tools to explore
redox status imbalance in liver diseases. Our results are congruent
with those from other studies, with serum/plasma of the patients
included in our study characterized by increased levels of lipid
peroxides and protein carbonyl content.
As well as in ALD, another feature of oxidative
stress in chronic hepatitis B and C is an impaired antioxidant
capacity in liver and blood. These patients often exhibit reduced
total blood glutathione levels and total antioxidant status, as
well as an imbalance between oxidized and reduced glutathione in
plasma and blood cells (12).
In our study, the values of the total antioxidant
capacity did not differ significantly between the patients from the
group with liver cirrhosis due to viral hepatitis and the control
group. This finding could be explained by the fact that oxidative
stress is biphasic: Low or moderate ROS concentrations trigger
signaling cascades to switch on the antioxidant protection;
instead, higher amounts of ROS inhibit the expression of gene
coding antioxidant enzymes leading to cell damage. Moreover, the
effects of viral proteins on antioxidants is different; some are
induced (catalase, glutathione peroxidase) while others (SOD
isoenzymes) are downregulated (34,35).
Thus, evaluation of a cumulative marker as TAC must be accompanied
by an individual assessment of the most enzymatic and non-enzymatic
antioxidants.
Individually, the roles of oxidative stress and
inflammation in the pathophysiological events of gastrointestinal
diseases, including liver disorders, have been extensively
investigated for some time now (36,37),
yet, currently studies are exploring the interrelationship between
oxidative stress and inflammation (38,39).
When the liver is attacked by exogenous or endogenous stimuli such
as viruses and toxins, neutrophils, monocytes and lymphocytes
infiltrate the liver and inflammation occurs to protect it from
injury. Since many studies have shown that alterations in the
amount of peripheral blood cells can demonstrate body inflammatory
response, hematological indicators such as the
neutrophil/lymphocyte (NLR), monocyte/lymphocyte (MLR) and
platelet/lymphocyte (PLR) ratios have emerged as accepted
biomarkers for the assessment of overall inflammatory status as
well as significant prognostic factors for various inflammatory and
ischemic conditions including cardiovascular diseases, different
types of malignancies, and inflammatory bowel disease. NLR, PLR,
and also MLR or LMR are simple and cost-effective biomarkers that
can be easily derived from a cell blood counting diagram during
routine examinations (40,41).
As far as we know, there has been no investigation
regarding the normal range of NLR, MLR and PLR in individuals from
our country. In this study, we compared these hematological
indicators in patients with toxic metabolic alcoholic cirrhosis and
liver cirrhosis due to HCV and HBV infection and their association
with oxidative stress markers in order to assess their usefulness
to predict disease outcome in terms of the relationship between
oxidative stress and inflammation. Our results are inconsistent
with the intended purpose for some of the markers.
ROC analysis of the MLR showed that this index is a
very good biomarker for increased inflammatory status estimation in
both groups (toxic-metabolic liver cirrhosis and viral liver
cirrhosis), with an AUC of 0.9136 (95% CI: 0.82-1) in group 1 and
0.97 (95% CI: 0.9-1.04) in group 2. Interestingly, we founded that
the NLR has a fair power for increased inflammatory status
estimation in group 1 with an AUC of 0.79 (95% CI: 0.64-0.94). In
contrast, we found it to be a good tool for inflammatory status in
group 2, with an AUC of 1.00 (95% CI: 1.00-1.00). On the other
hand, PLR was demonstrated to be a poor biomarker for both groups,
with an AUC of 0.79 (95% CI: 0.64-0.95) for group 1 and AUC of 0.62
(95% CI: 0.35-0.9) in group 2.
These indicators have been previously described as
predictive markers of disease progression, and they provide
supplementary means for an effective management of chronic HBV, HCV
infection and also ALD (42,43).
In addition, lymphocyte and platelet-related parameters have
recently been investigated in alcohol consumption disorders
(44). A retrospective analysis of
patients with cirrhosis revealed that NLR is a biomarker of immune
dysregulation in patients with cirrhosis; the associated risk of
death persisting long after their initial hospitalization (45). Another study revealed that
HBV-related-compensated cirrhosis patients had a significantly
lower PLR, and HBV-related-decompensated cirrhosis patients had a
significantly higher NLR than did any other patients (46).
Unfortunately, due to the limitation of our study,
for none of these ratios between blood cells we did not find any
significant correlation with oxidative stress markers (TBARS and
PCARB) assessed as predictive markers for the unfavorable
progression of liver cirrhosis.
In conclusion, increased levels of oxidative stress
markers and an insignificant alteration of total antioxidant
capacity was found in our cirrhosis patients. In addition, we
showed that NLR, MLR and PLR are easy-to-perform and accurate
biomarkers associated with liver inflammatory status, even if they
did not shown a significant correlation with all oxidative stress
markers assessed.
Taken together, the data demonstrated that early
detection of increased oxidative insult associated with a
proinflammatory status can be a preclinical sign applied for proper
intervention to support antioxidant homeostasis in order to limit
an unfavorable disease progression.
However, a limitation of our study was the small
number of patients included. Shortly after the beginning of our
research, patient access to hospitals was restricted due to the
pandemic period of SARS-Cov-2 infection. Thus, further exploration
of these topics in a large cohort must be conducted.
Acknowledgements
This study is part of the PhD thesis of Mihnea
Marian Pomacu from the University of Medicine and Pharmacy of
Craiova, Romania. We are thankful to Mrs. Loredana Colhon from the
Department of Biochemistry for the technical support.
Funding
Funding: This research received no external funding.
Availability of data and materials
The data analyzed during the current study are
available from the authors on reasonable request.
Authors' contributions
Conceptualization of the study was achieved by MMP,
VP, MDT, CGP and AMB. Methodology was designed by MMP, VP, AMA,
ECS, DR and CGP. Formal analysis of the results were conducted by
AMB, CGP and IMB. Data curation was conducted by MMP, VP, MDT, CGP
and AMB. Writing of the manuscript was performed by MMP, CGP and
AMB. Supervision was headed by AMB. All authors have read and
agreed to the published version of the manuscript.
Ethics approval and consent to
participate
The study was approved by The Ethics Committees of
The University of Medicine and Pharmacy of Craiova, Romania (Nr.
116/11.11.2019). Patients included in this study provided informed
consent for data publication.
Patients consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ezhilarasan D: Oxidative stress is bane in
chronic liver diseases: Clinical and experimental perspective. Arab
J Gastroenterol. 19:56–64. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Romanelli RG and Stasi C: Recent
advancements in diagnosis and therapy of liver cirrhosis. Curr Drug
Targets. 17:1804–1817. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Asrani SK, Devarbhavi H, Eaton J and
Kamath PS: Burden of liver diseases in the world. J Hepatol.
70:151–171. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
D'Amico G, Garcio-Tsao G and Pagliaro L:
Natural history and prognostic indicators of survival in cirrhosis:
A systematic review of 118 studies. J Hepatol. 44:217–231.
2006.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Cederbaum AI, Lu Y and Wu D: Role of
oxidative stress in alcohol-induced liver injury. Arch Toxicol.
83:519–548. 2009.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhou WC, Zhang QB and Qiao L: Pathogenesis
of liver cirrhosis. World J Gastroenterol. 20:7312–7324.
2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Aruoma OI: Characterization of drugs as
antioxidant prophylactics. Free Radic Biol Med. 20:675–705.
1996.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Valko M, Leibfritz D, Moncol J, Cronin MT,
Mazur M and Telser J: Free radicals and antioxidants in normal
physiological functions and human disease. Int J Biochem Cell Biol.
39:44–84. 2007.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Li S, Tan HY, Wang N, Zhang ZJ, Lao L,
Wong CW and Feng Y: The role of oxidative stress and antioxidants
in liver diseases. Int J Mol Sci. 16:26087–26124. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Poli G: Pathogenesis of liver fibrosis:
Role of oxidative stress. Mol Aspects Med. 21:49–98.
2000.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Irshad M, Chaudhuri PS and Joshi YK:
Superoxide dismutase and total anti-oxidant levels in various forms
of liver diseases. Hepatol Res. 23:178–184. 2002.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Li S, Hong M, Tan HY, Wang N and Feng Y:
Insights into the role and interdependence of oxidative stress and
inflammation in liver diseases. Oxid Med Cell Longev.
2016(4234061)2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hao X, Li D, Wu D and Zhang N: The
relationship between hematological indices and autoimmune rheumatic
diseases (ARDs), a meta-analysis. Sci Rep. 7(10833)2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Mititelu RR, Padureanu R, Băcănoiu M,
Pădureanu V, Docea AO, Calina D, Barbulescu AL and Buga AM:
Inflammatory and oxidative stress markers-mirror tools in
rheumatoid arthritis. Biomedicines. 8(125)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Prabawa IPY, Bhargah A, Liwang F, Tandio
DA, Tandio AL, Lestari AAW, Budiana ING and Manuaba IBAP:
Pretreatment neutrophil-to-lymphocyte ratio (NLR) and
platelet-to-lymphocyte ratio (PLR) as a predictive value of
hematological markers in cervical cancer. Asian Pac J Cancer Prev.
20:863–868. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Fest J, Ruiter R, Ikram MA, Voortman T,
van Eijck CHJ and Stricker BH: Reference values for white
blood-cell-based inflammatory markers in the Rotterdam Study: A
population-based prospective cohort study. Sci Rep.
8(10566)2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Padureanu R, Albu CV, Mititelu RR,
Bacanoiu MV, Docea AO, Calina D, Padureanu V, Olaru G, Sandu RE,
Malin RD and Buga AM: Oxidative stress and inflammation
interdependence in multiple sclerosis. J Clin Med.
8(1815)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kartal O and Kartal AT: Value of
neutrophil to lymphocyte and platelet to lymphocyte ratios in
pneumonia. Bratisl Lek Listy. 118:513–516. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Angkananard T, Anothaisintawee T, McEvoy
M, Attia J and Thakkinstian A: Neutrophil lymphocyte ratio and
cardiovascular disease risk: A systematic review and meta-analysis.
BioMed Res Int. 2018(2703518)2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Liberski PS, Szewczyk M and Krzych LJ:
Haemogram-derived indices for screening and prognostication in
critically Ill septic shock patients: A case-control study.
Diagnostics (Basel). 10(638)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Gao K, Zhu W, Liu W, Ma D, Li H, Yu W,
Wang L, Cao Y and Jiang Y: Diagnostic value of the blood
monocyte-lymphocyte ratio in knee osteoarthritis. J Int Med Res.
47:4413–4421. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Liu J, Li S, Zhang S, Liu Y, Ma L, Zhu J,
Xin Y, Wang Y, Yang C and Cheng Y: Systemic immune-inflammation
index, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio
can predict clinical outcomes in patients with metastatic
non-small-cell lung cancer treated with nivolumab. J Clin Lab Anal.
33(e22964)2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Liu J, Yeo HC, Doniger SJ and Ames BN:
Assay of aldehydes from lipid peroxidation: Gas chromatography-mass
spectrometry compared to thiobarbituric acid. Anal Biochem.
245:161–166. 1997.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Spanidis Y, Goutzourelas N, Stagos D,
Mpesios A, Priftis A, Bar-Or D, Spandidos DA, Tsatsakis AM, Leon G
and Kouretas D: Variations in oxidative stress markers in elite
basketball players at the beginning and end of a season. Exp Ther
Med. 11:147–153. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Keles MS, Taysi S, Sen N, Aksoy H and
Akçay F: Effect of corticosteroid therapy on serum and CSF
malondialdehyde and antioxidant proteins in multiple sclerosis. Can
J Neurol Sci. 28:141–143. 2001.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Colombo G, Clerici M, Garavaglia ME,
Giustarini D, Rossi R, Milzani A and Dalle-Donne I: A step-by-step
protocol for assaying protein carbonylation in biological samples.
J Chromatogr B Analyt Technol Biomed Life Sci. 1019:178–190.
2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Janaszewska A and Bartosz G: Assay of
total antioxidant capacity: Comparison of four methods as applied
to human blood plasma. Scand J Clin Lab Invest. 62:231–236.
2002.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhu H, Jia Z, Misra H and Li YR: Oxidative
stress and redox signaling mechanisms of alcoholic liver disease:
Updated experimental and clinical evidence. J Dig Dis. 13:133–142.
2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ivanov AV, Valuev-Elliston VT, Tyurina DA,
Ivanova ON, Kochetkov SN, Bartosch B and Isaguliants MG: Oxidative
stress, a trigger of hepatitis C and B virus-induced liver
carcinogenesis. Oncotarget. 8:3895–3932. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ivanov AV, Smirnova OA, Ivanova ON,
Masalova OV, Kochetkov SN and Isaguliants MG: Hepatitis C virus
proteins activate NRF2/ARE pathway by distinct ROS-dependent and
independent mechanisms in HUH7 cells. PLoS One.
6(e24957)2011.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Alavian SM and Showraki A: Hepatitis B and
its relationship with oxidative stress. Hepat Mon.
16(e37973)2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Xianyu J, Feng J, Yang Y, Tang J, Xie G
and Fan L: Correlation of oxidative stress in patients with
HBV-induced liver disease with HBV genotypes and drug resistance
mutations. Clin Biochem. 55:21–27. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Abdalla MY, Ahmad IM, Spitz DR, Schmidt WN
and Britigan BE: Hepatitis C virus-core and non structural proteins
lead to different effects on cellular antioxidant defenses. J Med
Virol. 76:489–497. 2005.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Brault C, Lévy P, Duponchel S, Michelet M,
Sallé A, Pécheur EI, Plissonnier ML, Parent R, Véricel E, Ivanov
AV, et al: Glutathione peroxidase 4 is reversibly induced by HCV to
control lipid peroxidation and to increase virion infectivity. Gut.
65:144–154. 2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Robles L, Vaziri ND and Ichii H: Role of
oxidative stress in the pathogenesis of pancreatitis: Effect of
antioxidant therapy. Pancreat Disord Ther. 3(112)2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Tache DE, Stănciulescu CE, Baniţă IM,
Purcaru ŞO, Andrei AM, Comănescu V and Pisoschi CG: Inducible
nitric oxide synthase expression (iNOS) in chronic viral hepatitis
and its correlation with liver fibrosis. Rom J Morphol Embryol. 55
(Suppl 2):S539–S543. 2014.PubMed/NCBI
|
|
38
|
Choghakhori R, Abbasnezhad A, Hasanvand A
and Amani R: Inflammatory cytokines and oxidative stress biomarkers
in irritable bowel syndrome: Association with digestive symptoms
and quality of life. Cytokine. 93:34–43. 2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Popa SL, Leucuta DC and Dumitrascu DL:
Pressure management as an occupational stress risk factor in
irritable bowel syndrome: A cross-sectional study. Medicine
(Baltimore). 97(e13562)2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Avci BŞ, Avci A, Dönmez Y, Kaya A, Gülen
M, Özer Aİ, Bulut A, Koç M, Nazik H and Satar S: The effectiveness
of neutrophil-lymphocyte ratio in predicting In-hospital mortality
in Non-ST-elevation myocardial infarction. Emerg Med Int.
2020(8718304)2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Hu J, Zhou W, Zhou Z, Han J and Dong W:
Elevated neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios
predict post-stroke depression with acute ischemic stroke. Exp Ther
Med. 19:2497–2504. 2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Zhao Z, Liu J, Wang J, Xie T, Zhang Q,
Feng S, Deng H and Zhong B: Platelet-to-lymphocyte ratio (PLR) and
neutrophil-to-lymphocyte ratio (NLR) are associated with chronic
hepatitis B virus (HBV) infection. Int Immunopharmacol. 51:1–8.
2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Liu H, Zhang H, Wan G, Sang Y, Chang Y,
Wang X and Zeng H: Neutrophil-lymphocyte ratio: A novel predictor
for short-term prognosis in acute-on-chronic hepatitis B liver
failure. J Viral Hepat. 21:499–507. 2014.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Orum MH and Kara MZ: Platelet to
lymphocyte ratio (PLR) in alcohol use disorder. J Immunoassay
Immunochem. 41:184–194. 2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Rice J, Dodge JL, Bambha KM, Bajaj JS,
Reddy KR, Gralla J, Ganapathy D, Mitrani R, Reuter B, Palecki J, et
al: Neutrophil-to-lymphocyte ratio associates independently with
mortality in hospitalized patients with cirrhosis. Clin
Gastroenterol Hepatol. 16:1786–1791.e1. 2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Mao W and Wu J: Haematologic indices in
hepatitis B virus-related liver disease. Clin Chim Acta.
500:135–142. 2020.PubMed/NCBI View Article : Google Scholar
|