Introduction
Hyperthyroidism represents a type of autoimmune
disease (1-3).
Previous studies have revealed that bone mineral density (BMD) is
frequently decreased in patients, which potentially leads to
osteoporosis (4,5). In general, BMD is associated with the
metabolism of vitamin D in the body. The reduction in vitamin D may
result in the decrease in bone mineral (6). A previous study suggested the
involvement of vitamin D status, parathyroid hormone and BMD in the
pathogenesis of osteoporosis in inflammatory bowel disease (IBD)
and chronic inflammation was found to cause a reduction in BMD,
leading to osteopenia and osteoporosis (7). However, the relationship of vitamin D,
BMD and inflammatory factors with hyperthyroidism remains poorly
understood. The aim of the present study was thus to investigate
the clinical change in BMD, vitamin D and inflammatory factors in
patients with hyperthyroidism, and their correlations with the
pathogenesis of hyperthyroidism.
Patients and methods
General data
A total of 55 patients with hyperthyroidism
(observation group) (male/female: 12/43) and 53 healthy patients
(control group) (male/female: 13/40) at Weifang People's Hospital
from March 2017 to February 2018 were enrolled. General data such
as age, sex, weight and body mass index (BMI) showed no significant
differences between the observation group and the control group.
All subjects provided informed consent before enrollment into the
study, and the study protocol was approved by the Ethics Committee
of Weifang People's Hospital (Approval no. SPH20170206E) (Weifang,
Shandong, China).
Inclusion criteria were: i) patients who met the
diagnostic criteria of hyperthyroidism (8), ii) patients with good compliance to
health-care workers during examination and treatment, iii) patients
who had not previously received treatment with antithyroid
medicines or drugs that affect bone metabolism, and iv) patients
who did not suffer from major injury to organs, including the
heart, liver and kidney as kidney or heart disease may affect
inflammatory factor levels (9,10).
Exclusion criteria were: i) patients in pregnancy,
ii) patients with other immunologic diseases, such as autoimmune
hepatitis or primary sclerosing cholangitis, and iii) patients with
bone development disorders, such as rickets, osteomalacia and
osteogenesis imperfecta.
Methods
All patients did not receive antithyroid therapy at
the time of sample collection. Dual energy X-ray absorptiometry was
applied to measure the BMD at lumbar vertebrae L1-L4, the femoral
neck, the total hip and the Wards triangle of the patients with
hyperthyroidism (11).
The fasting venous blood of the patients was
collected to detect inflammatory factors, interleukin-2 (IL-2),
IL-6 and transforming growth factor-β (TGF-β) via immunoassay (cat.
no. 03-0051-00; SMC™ Human Interleukin 2 (IL-2) Immunoassay kit;
cat. no. K-03-0089-01; SMC™ Human Interleukin 6 (IL-6) Immunoassay
kit; cat. no. RAB0460; Human TGF-β 1 ELISA kit; Merck KGaA), All
the operations were conducted in strict accordance with the
instructions in the kits. Enzyme-linked immunoassay was performed
to determine the content of serum 25-hydroxyvitamin D3
[25-(OH)D3] according to the manufacturer's instructions
(cat. no. ab213966; 25(OH) Vitamin D ELISA kit; Abcam).
The thyroid function indices in the serum, including
total triiodothyronine (TT3), total thyroxine (TT4), free T3 (FT3),
free T4 (FT4) and high-sensitive thyroid stimulating hormone (TSH)
were measured using a chemiluminescent analyzer as previously
described (12).
Statistical analysis
Statistical Product and Service Solutions (SPSS)
18.0 software (SPSS, Inc.) was adopted for data analysis. The
measurement data are presented as mean ± standard deviation (SD),
and the Student t-test was performed for the comparison between two
groups. Chi-square test was used for enumeration data. Pearson
correlation analysis was performed for two-variable analysis. A
level of statistical significance was defined at P<0.05.
Results
No difference in sex, age and BMI
between the observation group and control group
There were no statistical differences in general
clinical data between the observation group and control group, such
as sex, age and body mass index (BMI) (P>0.05; Table I).
 | Table IComparisons of the general clinical
data between the two groups. |
Table I
Comparisons of the general clinical
data between the two groups.
| Group | n | Sex
(male/female) | Age (years) | BMI
(kg/m2) |
|---|
| Control group | 53 | 27/26 | 56±6 | 21.21±2.54 |
| Observation
group | 55 | 28/27a | 59±7a |
20.98±2.32a |
Thyroid function is enhanced in
patients with hyperthyroidism
The comparison of thyroid function between the
control group and observation group indicated that the levels of
FT3, FT4, TT3 and TT4 in the observation group were significantly
elevated compared to these levels in the control group, with a
significant reduction in TSH level (P<0.05; Table II).
 | Table IIComparison of thyroid function between
the two groups. |
Table II
Comparison of thyroid function between
the two groups.
| Group | FT3 (pmol/l) | FT4 (pmol/l) | TSH (mIU/l) | TT3 (nmol/l) | TT4 (nmol/l) |
|---|
| Control group | 18.23±1.86 | 25.32±2.96 | 1.99±2.08 | 1.43±0.14 | 92.56±9.99 |
| Observation
group |
25.21±2.88a |
49.98±5.43a |
0.53±0.09a |
5.69±0.67a |
409.23±42.54a |
Bone mineral density was decreased in
patients with hyperthyroidism
Compared with the control group, BMD in the
observation group was significantly decreased at L1, L2, L3, L4,
the femoral neck, the Wards triangle as well as the total hip
(P<0.05; Table III).
 | Table IIIComparison of bone mineral density
(BMD) between the two groups. |
Table III
Comparison of bone mineral density
(BMD) between the two groups.
| Index | Control group | Observation
group |
|---|
| L1 | 0.89±0.05 |
0.80±0.09a |
| L2 | 0.99±0.08 |
0.85±0.08a |
| L3 | 0.98±0.08 |
0.90±0.09a |
| L4 | 0.99±0.09 | 0.97±0.08 |
| L1-4 | 0.97±0.09 |
0.91±0.09a |
| Femoral neck | 0.90±0.08 |
0.80±0.09a |
| Femoral great
trochanter | 0.69±0.08 | 0.67±0.08 |
| Wards triangle | 0.70±0.07 |
0.60±0.07a |
| Total hip | 0.89±0.09 |
0.80±0.09a |
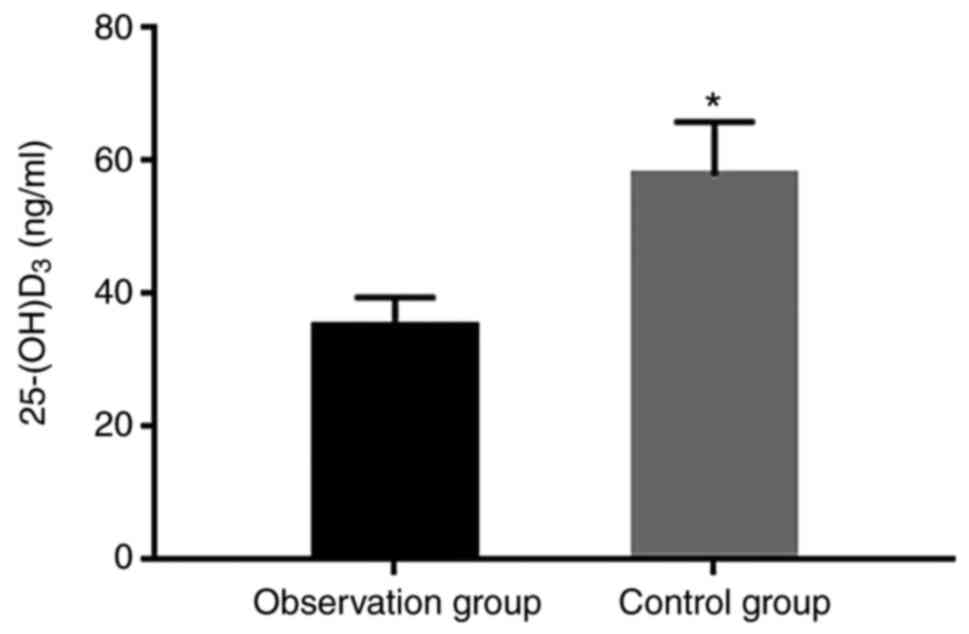
25-(OH)D3 is reduced in
patients with hyperthyroidism
Our ELISA result showed a significantly decreased
level of 25-(OH)D3 in the observation group compared
with the control group (P<0.05; Fig.
1).
Inflammatory factors are altered due
to hyperthyroidism
In the observation group, the levels of TGF-β and
IL-6 were significantly upregulated and the IL-2 level was
significantly decreased, compared with these levels in the control
group (P<0.05; Table IV).
 | Table IVA total of 55 patients with
hyperthyroidism (observation group) and 53 healthy patients
(control group) were enrolled. |
Table IV
A total of 55 patients with
hyperthyroidism (observation group) and 53 healthy patients
(control group) were enrolled.
| Group | TGF-β (ng/l) | IL-6 (ng/l) | IL-2 (ng/l) |
|---|
| Observation
group | 2.32±0.22 | 64.95±6.9 | 1.53±0.11 |
| Control group |
1.34±0.11a |
18.31±1.78a |
4.92±0.54a |
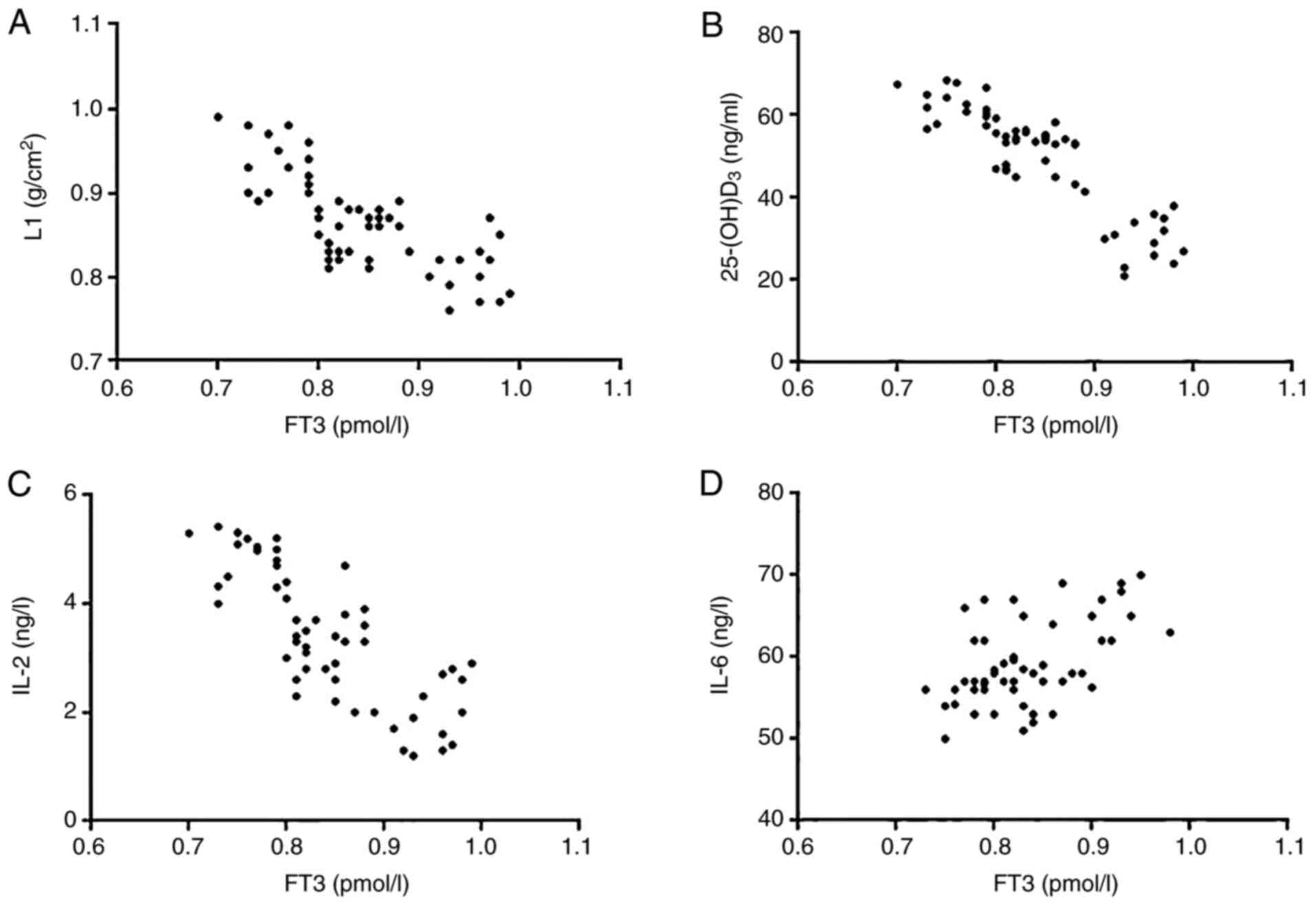
Correlations of FT3 with L1,
25-(OH)D3, IL-2 and IL-6
FT3 had a significantly negative correlation with L1
(r=-0.7435; P<0.001), 25-(OH)D3
(r=-0.8802; P<0.001) or IL-2 (r=-0.7854;
P<0.001) and a statistically positive correlation with IL-6
(r=-0.5420; P<0.001). This suggests that the aberrant
enhancement of thyroid function is associated with the reduction in
L1, 25-(OH)D3, IL-2, and an increase in IL-6 (Fig. 2).
Discussion
Hyperthyroidism is characterized as an endocrine
disorder, which results in abnormality of hormone secretion and
triggers various complications (13). In clinical practice, abnormal bone
metabolism is found in patients with hyperthyroidism, and a
majority of cases are accompanied with osteoporosis, thus it is
proposed that hyperthyroidism is associated with bone mineral
density (BMD) (14,15). In addition, hyperthyroidism is an
autoimmune disease, and the immune function is impaired with
abnormal secretion of inflammatory factors (16-18).
Previous research demonstrated changes in BMD, vitamin D and
inflammatory factors in hypothyroid patients (19), so as to provide clinical evidence
for the treatment and diagnosis of patients with hyperthyroidism.
In the present study, we further determined changes in BMD,
25-hydroxyvitamin D3 [25-(OH)D3] and
inflammatory factors in patients with hyperthyroidism, to explore
potential correlations with the pathogenesis of
hyperthyroidism.
It has been demonstrated that the excessive
secretion of thyroid hormone can accelerate the process of bone
metabolism (20). Thyroid hormone
is able to stimulate osteocytes and to enhance activity of these
cells (21). The overactivation of
bone metabolism suppresses the osteogenic function and the
imbalance between bone formation and resorption functions finally
resulting in osteopenia (22).
Furthermore, excessive thyroid hormone can induce the decomposition
of proteins in the body, retard the accumulation of calcium in the
body and decrease BMD (23). At the
molecular level, it has been revealed that thyroid hormone
interacts with interleukin (IL)-6, thus further improving the
production and secretion of osteoclasts and impairing the
osteogenic function (24).
Moreover, BMD is also associated with vitamin D in the body.
25-(OH)D3 serves as an important indicator to evaluate
the vitamin D level in vivo. Meanwhile, this factor
functions to maintain the stable states of calcium and phosphorus
in body and protect bone formation (25).
IL-6, a type of glycoprotein secreted from immune
cells such as T lymphocytes and B lymphocytes, mainly participates
in multiple inflammatory responses. It can promote the generation
and production of immune cells, induce the differentiation of
lymphocytes and favor the activity of immune cells at the same time
(26). Transforming growth factor-β
(TGF-β) is a type of cytokine with immunomodulatory functions,
which is involved in inflammation and tissue repair. According to
clinical research findings, this factor exerts relevant regulatory
effects mainly through suppressing differentiation of immune cells,
as well as generation of immunologic factors (27). IL-2 is produced by T lymphocytes and
is able to promote the differentiation and proliferation of B
lymphocytes, facilitating the immune response in the body (28). Previous study illustrated that the
content of IL-2 is decreased in patients with hyperthyroidism
(29), which plays a protective
role against autoimmune reactions. Clinically, it was discovered
that the IL-2 level is elevated in patients with hyperthyroidism
after treatment. It is also found that IL-6 can promote excessive
proliferation of B cells, induce hypersecretion of immunoglobulin G
(IgG) in the body and improve humoral immunity (30). Consistent with the present study,
previous data have demonstrated that the IL-6 level is
significantly increased and the IL-2 level is significantly reduced
in patients with hyperthyroidism (31).
Body mass index (BMI) is a person's weight in
kilograms divided by the square of height in meters. A high BMI can
be an indicator of high body fat. Consistent with a previous study,
no significant difference in BMI was found between the patients
with untreated hyperthyroidism and normal individuals (32). Of note, in this study, compared with
the control group, it was demonstrated that the levels of FT3, FT4,
TT3 and TT4 in the observation group were significantly increased,
with significantly decreasing level of TSH, indicating that the
thyroid function in patients with hyperthyroidism was abnormally
enhanced. Moreover, the BMD in the observation group was
significantly lower than that in the control group, which was in
line with previous findings (14,15),
indicating that in the case of excessive secretion of thyroid
hormone, both the BMD and osteogenic function in the body are
impaired. Importantly, in the observation group, the level of
inflammatory factor IL-2 was significantly lower than that in the
control group, while the levels of IL-6 and TGF-β were
significantly higher, suggesting that the immune function of
patients with hyperthyroidism is inhibited. It was also found that
FT3 had negative correlations with L1, 25-(OH)D3, IL-2
and IL-6, which implies that the bone formation of the patients
with hyperthyroidism was suppressed. However, one limitation of
this study was that the thyroid antibody was not detected in our
present study. Our initial study only focused on BMD and vitamin D
in hyperthyroidism. In the future, the expression of the thyroid
antibody will be evaluated to identify its relationship with BMD
and vitamin D in patients with hyperthyroidism. Importantly,
unexpectedly, in a study regarding the detection of serum levels of
osteotrophic cytokines in patients with various hyperthyroid
states, IL-6 was higher in the euthyroid control group, which was
in contrast with our data (33).
But another study showed that serum IL-6 values were significantly
higher in hyperthyroid patients when compared to a control group
(34). Therefore, a large number of
hypothyroid patients from different geographic regions ought to be
involve to further validate our result and ‘cocktail’ indicators of
the occurrence and development of hyperthyroidism require
additional evaluation. In addition, further investigation may focus
on specific therapy and evaluate its effect in clinical
practice.
In conclusion, our preliminary data demonstrated
that the abnormal enhancement of thyroid function is related to a
decrease in BMD, vitamin D and aggravated inflammation in patients
with hyperthyroidism, which provides insight into the development
of new markers for predicting the severity of hyperthyroidism.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analysed during the current
study available from the corresponding author on reasonable
request..
Authors' contributions
YZ substantially contributed to the experimentation
and acquisition of data, designed experiments, performed data
analysis and wrote the manuscript. XW contributed to the conception
of the study. MX helped perform the analysis with constructive
discussions. HZ contributed significantly to the data analysis and
manuscript preparation.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Weifang People's Hospital (Approval no. SPH20170206E)
(Weifang, Shandong, China) and informed consent from the subjects
was obtained prior to the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
De Leo S, Lee SY and Braverman LE:
Hyperthyroidism. Lancet. 388:906–918. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Vallabhajosula S, Radhi S, Cevik C,
Alalawi R, Raj R and Nugent K: Hyperthyroidism and pulmonary
hypertension: An important association. Am J Med Sci. 342:507–512.
2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Rao G, Verma R, Mukherjee A, Haldar C and
Agrawal NK: Melatonin alleviates hyperthyroidism induced oxidative
stress and neuronal cell death in hippocampus of aged female golden
hamster, mesocricetus auratus. Exp Gerontol. 82:125–130.
2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Karunakaran P, Maharajan C, Mohamed KN and
Rachamadugu SV: Rapid restoration of bone mass after surgical
management of hyperthyroidism: A prospective case control study in
Southern India. Surgery. 159:771–776. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Liu C, Zhang Y, Fu T, Liu Y, Wei S, Yang
Y, Zhao D, Zhao W, Song M, Tang X and Wu H: Effects of
electromagnetic fields on bone loss in hyperthyroidism rat model.
Bioelectromagnetics. 38:137–150. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Muscogiuri G, Palomba S, Caggiano M,
Tafuri D, Colao A and Orio F: Low 25 (OH) vitamin D levels are
associated with autoimmune thyroid disease in polycystic ovary
syndrome. Endocrine. 53:538–542. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Jahnsen J, Falch JA, Mowinckel P and
Aadland E: Vitamin D status, parathyroid hormone and bone mineral
density in patients with inflammatory bowel disease. Scand J
Gastroenterol. 37:192–199. 2002.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ogris E: Diagnostic criteria in
terminating therapy in basedow hyperthyroidism. Acta Med Austriaca.
14:77–84. 1987.PubMed/NCBI
|
|
9
|
Mihai S, Codrici E, Popescu ID, Enciu AM,
Albulescu L, Necula LG, Mambet C, Anton G and Tanase C:
Inflammation-related mechanisms in chronic kidney disease
prediction, progression, and outcome. J Immunol Res.
2018(2180373)2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Golia E, Limongelli G, Natale F, Fimiani
F, Maddaloni V, Pariggiano I, Bianchi R, Crisci M, D'Acierno L,
Giordano R, et al: Inflammation and cardiovascular disease: From
pathogenesis to therapeutic target. Curr Atheroscler Rep.
16(435)2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Choi YJ: Dual-Energy X-ray absorptiometry:
Beyond bone mineral density determination. Endocrinol Metab
(Seoul). 31:25–30. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhang Y, Liu F, Sun W, Huang Y, Zhang W,
Wang B, Su S, Gao Y, Gao Y, Yang H and Guo X: Establishment of
reference ranges for thyroid-related indicators in normal pregnant
women. Zhonghua Yi Xue Za Zhi. 96:339–343. 2016.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
13
|
Gunatilake SSC and Bulugahapitiya U:
Coexistence of primary hyperaldosteronism and graves' disease, a
rare combination of endocrine disorders: Is it beyond a
coincidence-A case report and review of the literature. Case Rep
Endocrinol. 2017(4050458)2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Parihar AS, Sood A, Lukose TT, Seam RK and
Mittal BR: Metabolic bone superscan in carcinoma breast with occult
graves' risease: Looking beyond skeletal metastases. Indian J Nucl
Med. 33:145–147. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yi HS, Kim JM, Ju SH, Lee Y, Kim HJ and
Kim KS: Multiple fractures in patient with graves' disease
accompanied by isolated hypogonadotropic hypogonadism. J Bone
Metab. 23:40–44. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Li LX, Deng K and Qu Y: Acupuncture
treatment for post-stroke dysphagia: An update meta-analysis of
randomized controlled trials. Chin J Integr Med. 24:686–695.
2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Graves KL and Vigerust DJ: Hp: An
inflammatory indicator in cardiovascular disease. Future Cardiol.
12:471–481. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Walker NF, Scriven J, Meintjes G and
Wilkinson RJ: Immune reconstitution inflammatory syndrome in
HIV-infected patients. HIV AIDS (Auckl). 7:49–64. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ahn HY, Chung YJ and Cho BY: Serum
25-hydroxyvitamin D might be an independent prognostic factor for
graves disease recurrence. Medicine (Baltimore).
96(e7700)2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Bassett JH and Williams GR: Role of
thyroid hormones in skeletal development and bone maintenance.
Endocr Rev. 37:135–187. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Williams GR: Thyroid hormone actions in
cartilage and bone. Eur Thyroid J. 2:3–13. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Feng X and McDonald JM: Disorders of bone
remodeling. Annu Rev Pathol. 6:121–145. 2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Mullur R, Liu YY and Brent GA: Thyroid
hormone regulation of metabolism. Physiol Rev. 94:355–382.
2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Alemu A, Terefe B, Abebe M and Biadgo B:
Thyroid hormone dysfunction during pregnancy: A review. Int J
Reprod Biomed (Yazd). 14:677–686. 2016.PubMed/NCBI
|
|
25
|
Zhou P, Cai J and Markowitz M: Absence of
a relationship between thyroid hormones and vitamin D levels. J
Pediatr Endocrinol Metab. 29:703–707. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Tanaka T, Narazaki M, Masuda K and
Kishimoto T: Regulation of IL-6 in immunity and diseases. Adv Exp
Med Biol. 941:79–88. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Yang L, Pang Y and Moses HL: TGF-Beta and
immune cells: An important regulatory axis in the tumor
microenvironment and progression. Trends Immunol. 31:220–227.
2010.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lagoo A, Tseng CK and Sell S: Interleukin
2 produced by activated B lymphocytes acts as an autocrine
proliferation-inducing lymphokine. Cytokine. 2:272–279.
1990.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ward LS and Fernandes GA: Serum cytokine
levels in autoimmune and non-autoimmune hyperthyroid states. Braz J
Med Biol Res. 33:65–69. 2000.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Maeda K, Mehta H, Drevets DA and
Coggeshall KM: IL-6 increases B-cell IgG production in a
feed-forward proinflammatory mechanism to skew hematopoiesis and
elevate myeloid production. Blood. 115:4699–4706. 2010.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Lv LF, Jia HY, Zhang HF and Hu YX:
Expression level and clinical significance of IL-2, IL-6 and TGF-β
in elderly patients with goiter and hyperthyroidism. Eur Rev Med
Pharmacol Sci. 21:4680–4686. 2017.PubMed/NCBI
|
|
32
|
Numbenjapon N, Costin G, Gilsanz V and
Pitukcheewanont P: Low cortical bone density measured by computed
tomography in children and adolescents with untreated
hyperthyroidism. J Pediatr. 150:527–530. 2007.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Senturk T, Kozaci LD, Kok F, Kadikoylu G
and Bolaman Z: Proinflammatory cytokine levels in hyperthyroidism.
Clin Invest Med. 26:58–63. 2003.PubMed/NCBI
|
|
34
|
Akalin A, Colak O, Alatas O and Efe B:
Bone remodelling markers and serum cytokines in patients with
hyperthyroidism. Clin Endocrinol (Oxf). 57:125–129. 2002.PubMed/NCBI View Article : Google Scholar
|