Introduction
Periodontitis is the most common dental disease
globally and one of the major threats for tooth loss worldwide
(1). Periodontitis can damage the
supporting tissues of the teeth, which can promote alveolar bone
resorption, and periodontitis can be worsened by the alveolar bone
loss (2). Periodontitis progression
has been attributed to hyperlipidemia and particularly to
hypercholesterolemia (3).
Hyperlipidemia is associated with the development of severe
periodontitis and high degrees of alveolar bone loss in animal
models (4). However, little
information is available about whether hyperlipidemia is related to
the severity of alveolar bone resorption in Chinese patients with
periodontitis. Additionally, it is unclear how hyperlipidemia
promotes periodontitis-related alveolar bone resorption.
Understanding the mechanisms by which hyperlipidemia promotes
alveolar bone resorption will be of significance in uncovering new
therapeutic targets for the development of novel periodontitis
treatments.
Hyperlipidemia can cause chronic inflammation, as
lipids can engage toll-like receptors (TLRs)-2 and 4 to activate
the nuclear factor-κB (NF-κB) signaling to produce pro-inflammatory
cytokines, including tumor necrosis factor (TNF)-α, interleukin
(IL)-1β, IL-6 and receptor activator of NF-κB ligand (RANKL). These
pro-inflammatory cytokines can enhance osteoclastogenesis and lead
to bone resorption, which is suppressed by osteoprotegerin (OPG)
produced by osteoblasts. Osteoclastogenesis is also regulated by
autophagic components, including beclin1 and p62, as well as
inflammatory signaling (5,6). Given that autophagy is crucial for the
survival and function of many types of cells, including osteoclasts
(7), modulation of
hypercholesterolemia, inflammation and osteoclastogenesis, as well
as osteoclast autophagy, may be valuable for the management of
hyperlipidemia-induced alveolar bone resorption and
periodontitis.
Statins can inhibit 3-hydroxy-3-methylglutaryl
coenzyme A (HMG-CoA) reductase to lower serum lipid levels and have
been demonstrated to benefit patients with hyperlipidemia in the
clinic (8). Previous studies have
shown that statins not only reduce serum lipid levels, but also
reduce inflammation and benefit bone metabolism (9). A recent study indicated that treatment
with statins promoted bone formation and increased bone mineral
density (BMD) in patients with hyperlipidemia and ameliorated
hypercholesterolemia-related microstructure changes in the jaw bone
of animals (10). However, whether
and how treatment with simvastatin could modulate
hypercholesterolemia-related alveolar bone resorption has not been
clarified.
The present study aimed to investigate how
hyperlipidemia was related to the severity of alveolar bone
resorption in Chinese patients with periodontitis and examined the
therapeutic efficacy and potential mechanisms of simvastatin
intervention for alveolar bone resorption in hypercholesterolemic
rats.
Materials and methods
Subjects
The present study was conducted in accordance with
the guidelines in the Helsinki Declaration (11) and the experimental protocol was
approved by the Ethics Committee of Shandong Provincial Hospital
(approval no. SWYX: No. 2021-013). This study enrolled 100 patients
(age range, 28 to 65 years) with periodontitis at the Shandong
Provincial Hospital between March and June 2018. Individual
patients with periodontitis were diagnosed on the basis of
periodontal examinations, which included assessment of plaque,
tartar buildup and easy bleeding, measurement of pocket depth and
teeth X-rays by oral panoramic radiograph using Promax 3D (Planmeca
OY; tube type D-054SB-C, 84 kV, 16 mA, filtration 2,5 mm AI). The
exclusion criteria included periodontal treatment in the past six
months, a history of diabetes mellitus, metabolic syndrome, other
endocrine diseases, cardiovascular and cerebrovascular diseases,
rheumatic diseases, chronic renal failure, nephrotic syndrome,
obesity [body mass index (BMI) ≥30 kg/m2], recent
diagnosis with an infectious disease, pregnancy, lactation, hormone
replacement therapy, chronic treatment with non-steroidal
anti-inflammatory drugs or bisphosphonate or glucocorticoids,
smokers and ex-smokers. All subjects were divided into the normal
group (NC-h) and the hyperlipidemia group (HC-h). Their body
weights (kg) and heights (m) were measured to calculate body mass
index (BMI) as (kg/m2). Fasting peripheral venous blood
samples were collected into anticoagulant tubes and centrifuged at
2,200 x g for 5 min at 25°C to prepare individuals serum
samples. The levels of serum triglycerides (TG; mg/dl), total
cholesterol (TC; mg/dl), high-density lipoprotein (HDL; mg/dl) and
low-density lipoprotein (LDL; mg/dl) were measured using Biobase
reagent kit (BIOBASE LLC) in an autobiochemical analyzer (cat. no.
AU5400; Olympus Corporation). Patients were diagnosed with
hyperlipidemia, based on their serum levels of TC >200 mg/dl, TG
>200 mg/dl, LDL-C >130 mg/dl and HDL-C <35 mg/dl.
Periodontal examination
Periodontal examination was performed by an
experienced dentist. All teeth in the mouth of individual patients
were examined for their looseness (L), sulcus bleeding index (SBI),
clinical attachment level (CAL), probing depth (PD) at six sites
and the distance from the gingival margin to the cementoenamel
junction (CEJ) at six sites (mesiobuccal, mid buccal, distobuccal,
distolingual, mid lingual and mesiolingual). In addition, missing
teeth were recorded (not including the third molar). The distance
between the near and middle CEJ-alveolar bone crest (ABC) of each
tooth was measured three times using Planmeca Romexis 3.0.1.R
software (Planmeca OY; accurate to 0.1 mm).
Animal experiments
The animal experimental study was approved by The
Animal Ethics Committee of the Shandong Provincial Hospital
(approval no. 2016-06) and the experimental procedures were in
accordance with the Guide for the Care and Use of Laboratory
Animals (12). Male Sprague-Dawley
(SD) rats (n=30; age, 6 weeks; weight, 170-190 g) were obtained
from Beijing Vital River Laboratory Animal Technology Co., Ltd..
Animals were housed in a specific pathogen-free room under a
consistent temperature (23±2°C) and humidity (55±10%)
with a cycle of 12-h light/dark and free access to water and chow.
The rats were randomized and fed with standard rodent chow [normal
control (NC) group; n=10], or high cholesterol rodent chow
(Table I, Beijing Vital River
Laboratory Animal Technology Co., Ltd.) containing 2% cholesterol
(n=20) for 32 weeks. Beginning from the 25th week of a high
cholesterol diet, the high cholesterol-fed rats were randomized to
treatment with vehicle saline [hypercholesterolemia (HC) group;
n=10] or with 5 mg/kg simvastatin by gavage daily for 8 weeks
[simvastatin (SIM) group; n=10]. During the last 8-week period, the
NC rats were treated with vehicle saline. Their body weights were
measured weekly. At the end of the experiment, abdominal aorta
blood samples (5-10 ml) were obtained from individual rats and
their serum samples were prepared. The rats were euthanized
following excessive anesthesia with 200 mg/kg pentobarbital
sodium.
 | Table IComposition of the high cholesterol
diet. |
Table I
Composition of the high cholesterol
diet.
| Component | Normal control
diet | High cholesterol
diet |
|---|
| Protein (g/100
g) | 20 | 17 |
| Carbohydrate (g/100
g) | 58 | 49 |
| Fat (g/100 g) | 6 | 21 |
| Selenium (g/100
g) |
1.4x10-5 |
1.6x10-5 |
| Cholesterol (g/100
g) | 0 | 2 |
| Fatty acids (g/100
g) | | |
|
C14:0 | 0.02 | 0.02 |
|
C16:0 | 0.97 | 0.97 |
|
C16:1 | 0.02 | 0.02 |
|
C18:0 | 0.21 | 0.21 |
|
C18:1 | 1.23 | 1.23 |
|
C18:2 | 2.57 | 2.57 |
|
C18:3 | 0.17 | 0.17 |
| Total
saturated | 1.11 | 1.11 |
| Total
monounsaturated | 1.22 | 1.22 |
| Total
polyunsaturated | 2.93 | 2.93 |
| Total kcal/g | 3.4 | 3.4 |
Laboratory examinations
The levels of serum TG, TC, HDL and LDL in
individual rats were determined by enzymatic methods in an
autobiochemical analyzer (cat. no. AU5400; Olympus).
Analysis of alveolar bone
resorption
The maxillae from individual rats were dissected and
separated as right and left sides. The semi-maxillary bone was
stained with 1% methylene blue dye at 25°C for 1 min to
demarcate the cement-enamel junction (CEJ). Both buccal and lingual
root surfaces of all molar teeth were imaged using a digital camera
(Canon). Alveolar bone loss was evaluated using Image-Pro Plus
version 6.0 (Media Cybernetics, Inc.) in a blinded manner and was
expressed in mm. The mean of 3 buccal and 3 lingual surfaces on
each molar was calculated as the total alveolar bone loss in each
animal.
Histology
The semi-maxillae were fixed in 4% paraformaldehyde
at 25°C for 48 h and decalcified in 10% EDTA solution
(pH 7.2) at 25°C for 2 weeks, followed by
paraffin-embedding. The bone longitudinal sections (5 µm) were
stained with hematoxylin and eosin (H&E). From each section 3-5
fields were randomly selected and imaged under an Olympus light
microscope (model BX51TRF; Olympus Corporation).
Immunohistochemistry
The expression levels of NF-κB, LC3 and p62 proteins
in the bone tissues were determined by immunohistochemistry. The
bone tissue sections (4 µm) were dewaxed in xylene I and II
solution at 25˚C for 10 min each and rehydrated by serial decreased
concentrations of ethanol [100 (I), 100 (II), 95, 80 and 70%] for 5
min each. The tissue sections were subjected to antigen retrieval
in 0.125% pancreatin at 37˚C for 30 min and treated with 3%
H2O2 in methanol at 25°C for 30
min. The tissue sections were blocked with 3% bovine serum albumin
(Shanghai Yeasen Biotechnology Co., Ltd.; cat. no. 36104ES25) in
TBST at 25°C for 1 h and incubated with rabbit
anti-NF-κB p65 (1:200; cat. no. ab16502), anti-LC3B (1:100; cat.
no. ab63817), anti-p62 (1:200; cat. no. ab91526; all, Abcam) at
4ºC overnight. After washing with PBS, the sections were
incubated with biotinylated goat anti-rabbit IgG (1:400; cat. no.
ab150077; Abcam). Bound antibodies were detected with
peroxidase-labeled streptavidin (Shanghai Herochem Co., Ltd.) at
37˚C for 30 min, followed by development with Diaminobenzidine
(OriGene Technologies, Inc.) and counterstained with haematoxylin
at 25°C for 1 min. The stained signals in six fields of
view were imaged under a light microscope (Olympus CX21; Olympus
Corporation). The stained intensity of density (IOD) and the ratio
of the stained areas to total areas (sum/area sum) in each image
were analyzed using Image-Pro Plus software version 6.0 (Media
Cybernetics, Inc.) in a blinded manner, as described previously
(13).
Reverse transcription-quantitative
PCR
The levels of OPG, RANKL, NF-κB, LC3 and p62 mRNA
transcripts, relative to the control GAPDH, were determined in the
bone tissues by RT-qPCR. Briefly, total RNA was extracted from each
alveolar bone tissue using Trizol® reagent (Thermo
Fisher Scientific, Inc.) and reverse transcribed into cDNA using
the PrimeScript™ RT reagent kit (Takara Bio., Inc.)
according to the manufacturer's protocol. Subsequently, qPCR was
performed using the FastStart DNA Master SYBR Green I kit (Takara
Bio) with specific primers in a LightCycler System 480 (Roche
Diagnostics). The PCR reactions were performed in duplicate at 95˚C
for 30 sec and subjected to 40 cycles of 95˚C for 5 sec and 60˚C
for 30 sec. Data were analyzed using the 2-ΔΔCq method
(14). The primer sequences were
forward 5'CCCTCTCTCTGCTCACTCTGCT3' and reverse
5'CTTACTGCCCTCCTGCTTGG3' for GAPDH; forward 5'TCAGTTCCATGGCCCAGAC3'
and reverse 5'GTTGTCTTTGAGATCCATGCCATT3' for TNF-α; forward
5'CCCTGAACTCAACTGTGAAATAGCA3' and reverse 5'CCCAAGTCAAGGGCTTGGAA3'
for IL-1β; forward 5'TGTGGAATAGATGTCACCCTGTGC3' and reverse
5'CACAGAGGTCAATGTCTTGGATGATC3' for OPG; forward
5'GCTTCTCAGGAGTTCCAGCTATGAT3' and reverse
5'CGTTGCTTAACGTCATGTTAGAGATCT3' for RANKL; forward
5'GCTATAATCCTGGACTTCTG3' and reverse 5'GAGGAAGGCTGTGAACATGA3' for
NF-κB; forward 5'GAGTGGAAGATGTCCGGCTC3' and reverse
5'CCAGGAGGAAGAAGGCTTGG3' for LC3; and forward
5'GCTGCTCTCTTCAGGCTTACAG3' and reverse 5'CCTGCTTCACAGTAGACGAAAG3'
for p62.
Statistical analysis
All experiments were independently performed in
triplicate. Data are presented as the mean ± SD. The difference
between two experimental groups was analyzed by the unpaired
Student's t-test using the SPSS, version 17.0 (SPSS, Inc.). The
correlation between blood lipid concentrations and periodontal
index was analyzed by Spearman's rank correlation analysis.
P<0.05 was considered statistically significant.
Results
Hyperlipidemia is associated with
severe alveolar bone resorption in patients with periodontitis
To determine the potential effect of hyperlipidemia
on alveolar bone loss, 100 patients with periodontitis were
recruited and stratified into the hyperlipidemia and normal lipid
level groups, according to their serum TC, TG, LDL-C and HDL-C
levels. Their demographic and laboratory characteristics are
reported in Table II. The levels
of serum TC, TG and LDL-C in the hyperlipidemia group were
significantly higher than that in the normal lipid group, while the
serum HDL-C levels were significantly lower in the hyperlipidemia
group than in the normal lipid group. Clinically, periodontal
examination revealed that the levels of SBI, CAL, PD and CEJ-ABC,
but not tooth looseness (L), in the hyperlipidemia group were
significantly higher than that in the normal lipid level group. The
levels of TC, TG and LDL-C were positively correlated with the
values of SBI, CAL, PD, and CEJ-ABC, while the levels of HDL-C were
negatively correlated with the values of SBI, CAL, PD and CEJ-ABC
in this population (Table III).
These data indicated that hyperlipidemia was correlated positively
with the severity of alveolar bone loss during the process of
periodontitis in humans.
 | Table IICharacteristics of the study
population (mean ± standard deviation). |
Table II
Characteristics of the study
population (mean ± standard deviation).
| Characteristic | NC-h (n=52) | HC-h (n=48) | P-value |
|---|
| Age (years) | 42.56±10.39 | 44.12±9.87 | 0.348 |
| Male/female | 28/24 | 27/21 | 0.164 |
| BMI
(kg/m2) | 24.73±2.5 | 26.12±3.4 | 0.083 |
| TC (mg/dl) | 135.92±14.07 | 242.41±17.89 | <0.001 |
| TG (mg/dl) | 127.13±16.54 | 248.70±16.25 | <0.001 |
| LDL-C (mg/dl) | 81.24±12.09 | 167.95±13.13 | <0.001 |
| HDL-C (mg/dl) | 55.79±6.29 | 21.87±3.21 | <0.001 |
| L | 0.19±0.15 | 0.26±0.21 | 0.449 |
| SBI | 0.94±0.37 | 2.59±0.42 | <0.001 |
| CAL (mm) | 0.58±0.46 | 2.72±0.89 | <0.001 |
| PD (mm) | 1.29±0.56 | 3.24±0.75 | <0.001 |
| CEJ-ABC (mm) | 1.09±0.80 | 2.32±0.81 | <0.001 |
 | Table IIIPartial correlation after controlling
for confounding variables. |
Table III
Partial correlation after controlling
for confounding variables.
| | TC | TG | LDL-C | HDL-C | SBI | CAL | PD | CEJ-ABC |
|---|
| TC | | | | | | | | |
|
Correlation | 1 | 0.752a | 0.702a | -0.909a | 0.490a | 0.560a | 0.489a | 0.525a |
|
Significance
(two-tailed) | | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| TG | | | | | | | | |
|
Correlation | | 1 | 0.762a | -0.712a | 0.595a | 0.591a | 0.445a | 0.593a |
|
Significance
(two-tailed) | | | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| LDL-C | | | | | | | | |
|
Correlation | | | 1 | -0.761a | 0.529a | 0.557a | 0.439a | 0.587a |
|
Significance
(two-tailed) | | | | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| HDL-C | | | | | | | | |
|
Correlation | | | | 1 | -0.484a | -0.561a | -0.429a | -0.562a |
|
Significance
(two-tailed) | | | | | <0.001 | <0.001 | <0.001 | <0.001 |
| SBI | | | | | | | | |
|
Correlation | | | | | 1 | 0.360a | 0.250a | 0.414a |
|
Significance
(two-tailed) | | | | | | <0.001 | 0.012 | <0.001 |
| CAL | | | | | | | | |
|
Correlation | | | | | | 1 | 0.333a | 0.550a |
|
Significance
(two-tailed) | | | | | | | 0.001 | <0.001 |
| PD | | | | | | | | |
|
Correlation | | | | | | | 1 | 0.357a |
|
Significance
(two-tailed) | | | | | | | | <0.001 |
| CEJ-ABC | | | | | | | | |
|
Correlation | | | | | | | | 1 |
|
Significance
(two-tailed) | | | | | | | | |
Simvastatin intervention reduces
hypercholesterolemia and its-related alveolar bone resorption in
rats
Next, we tested whether treatment with simvastatin
could mitigate the hypercholesterolemia-related alveolar bone
resorption in rats. After fed with high cholesterol food for 24
weeks, the high cholesterol-fed rats were randomized and treated
with vehicle saline (HC group) or simvastatin (SIM group) by gavage
daily for 8 weeks under continual high cholesterol diet. Another
group of rats were fed with normal chow and received vehicle
treatment as the normal control (NC). In comparison with the NC,
although we observed gradually increased body weights with time in
the high cholesterol-fed rats there was no significant difference
among the groups (P>0.05, Fig.
1A). As expected, we detected significantly increased levels of
serum TC, and LDL-C, but not TG and HDL-C in the high
cholesterol-fed rats (P<0.001 for both, Fig. 1B). The levels of serum TC, and LDL-C
in the SIM group were significantly lower than that in the HC group
(P<0.001, P<0.05).
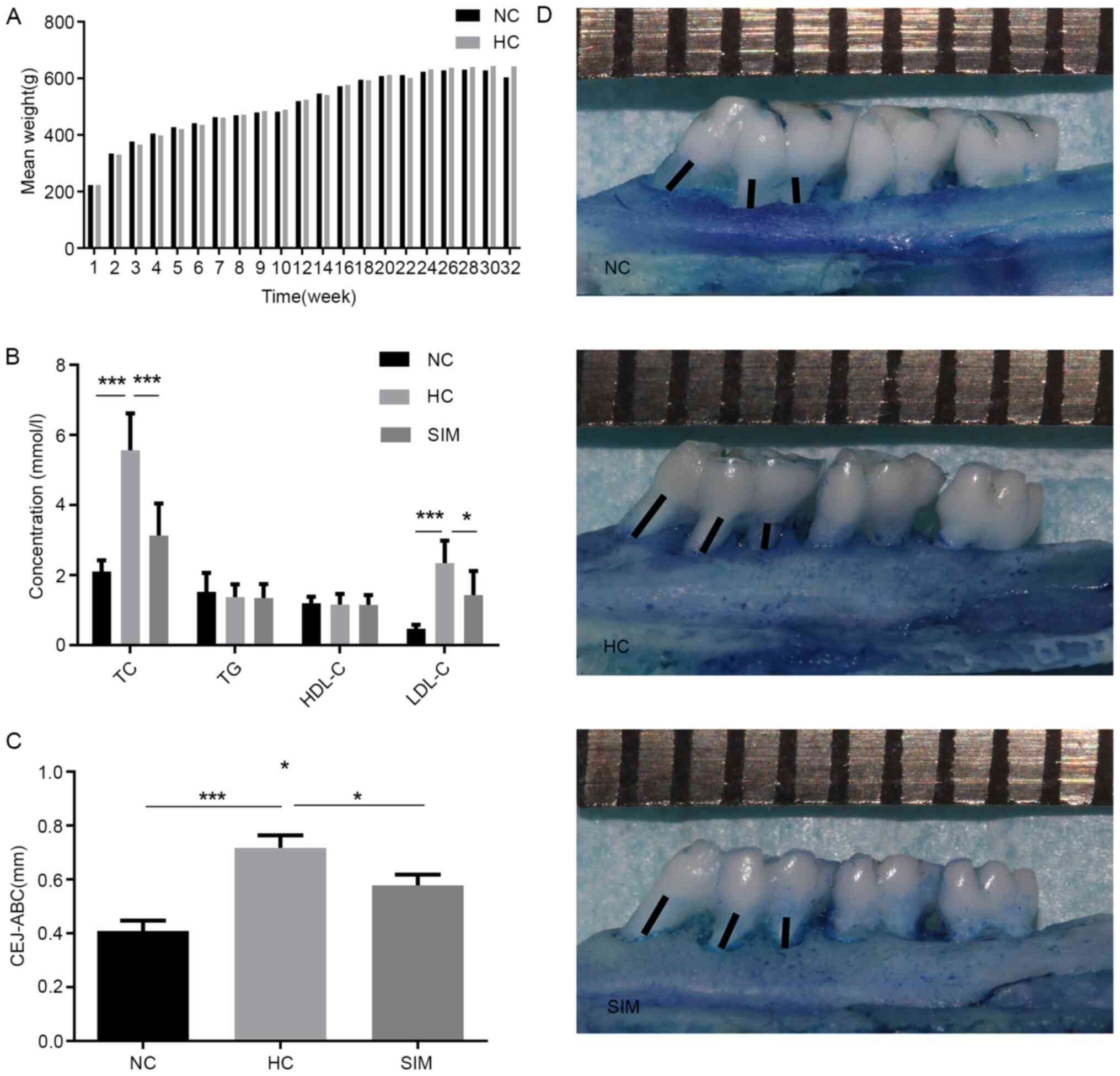 | Figure 1Simvastatin reduces the
hypercholesterolemia-induced alveolar bone loss in rats. Rats were
fed with normal rat chow for 32 weeks or with chow containing 2%
cholesterol for 32 weeks. Beginning at the 25th weeks of high
cholesterol diet, the high cholesterol-fed rats were randomized,
continually fed with high cholesterol diet, and treated with
vehicle saline or simvastatin by gavage daily for 8 weeks. (A) Rat
body weights were measured weekly and (B) their TG, TC, HDL and
low-density LDL levels were quantified. (C) The linear distance
between the cement-enamel CEJ and the ABC was measured in (D)
images of the buccal and lingual root surfaces. Each small grid on
the upper ruler represents 1 mm. The black lines represent the
distance of alveolar bone resorption. Results are presented as the
mean ± SD of each group (n=10 per group). *P<0.05;
***P<0.001. NC, normal control group; HC,
hypercholesterolemia group; SIM, simvastatin group; TC, total
cholesterol; TG, plasma triglyceride; LDL, low density lipoprotein;
HDL, high density lipoprotein; CEJ, cement-enamel junction; ABC,
alveolar bone crest. |
Next, we measured the linear distance between the
CEJ and ABC to evaluate the levels of alveolar bone loss in
individual groups of rats (Fig. 1C
and D). We found that the linear
distance between the CEJ and ABC in the SIM group of rats was
significantly shorter than that in the HC group (P<0.05), but
remained significantly longer than that in the NC group of rats
(P<0.05, Fig. 1C). Hence, such
data indicated that high cholesterol feeding induced
hypercholesterolemia and alveolar bone loss, where were
significantly mitigated by simvastatin treatment in rats.
Simvastatin intervention mitigates
hypercholesterolemia-modulated
OPG, RANKL and NF-κB expression in the alveolar bone
of rats. Given that OPG and RANKL are crucial for
osteoclastogenesis and hyperlipidemia can activate the NF-κB
signaling though engagement of TLR2/4(15), the potential mechanisms underlying
the action of simvastatin in regulating
hypercholesterolemia-induced alveolar bone loss in rats were
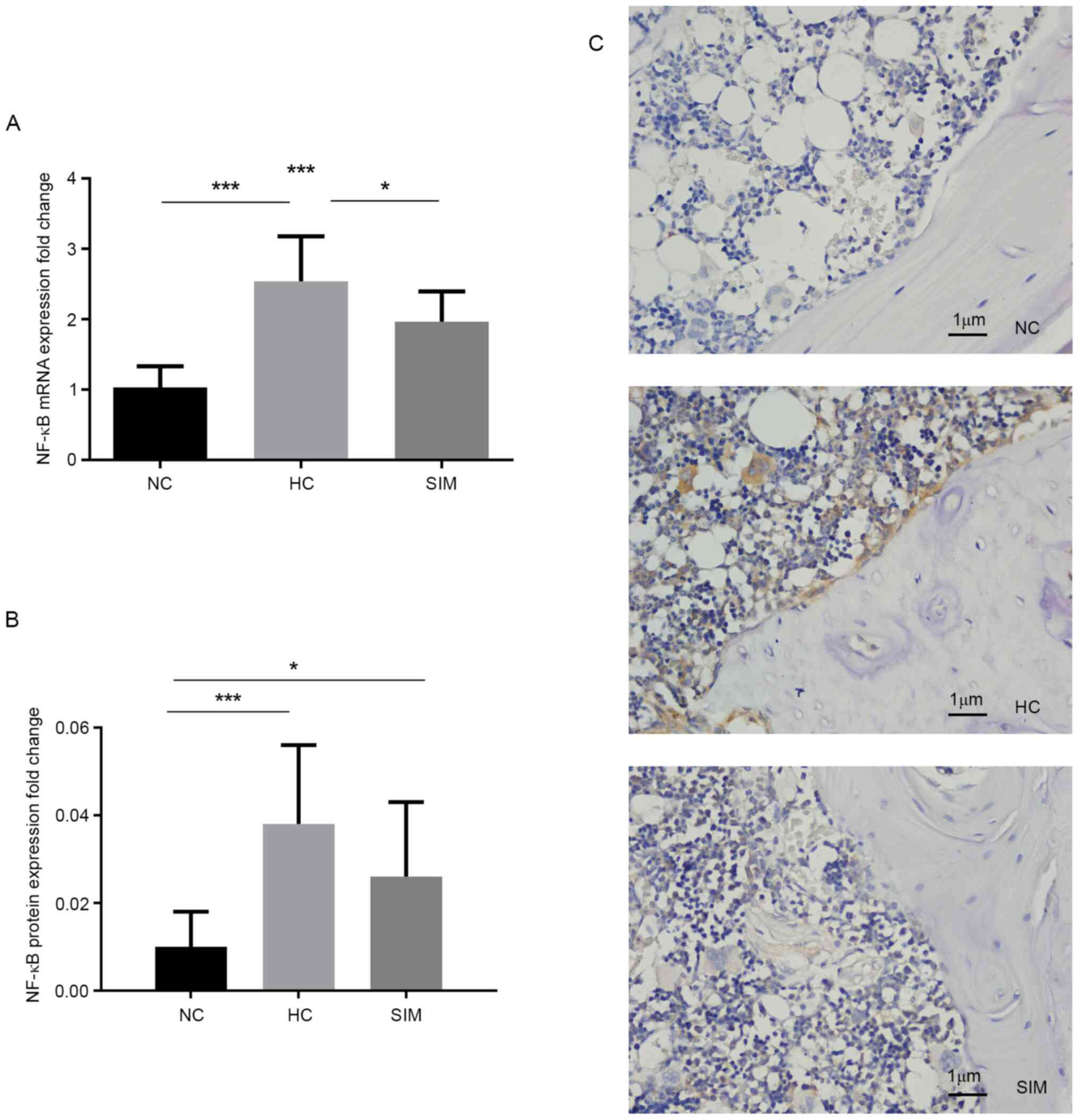
explored. The relative levels of NF-κB expression in the alveolar
bone tissues of the different groups of rats were measured. Levels
of NF-κB mRNA transcripts in the SIM group were significantly lower
than those in the HC group (P<0.05), but still significantly
higher than those in the NC group of rats (P<0.001; Fig. 2A). Further immunohistochemistry
revealed anti-NF-κB+ granules in the nucleus and cytoplasm of
osteoclast-like cells and that the intensity of anti-NF-κBp65
staining in in the SIM group was obviously lower than in the HC
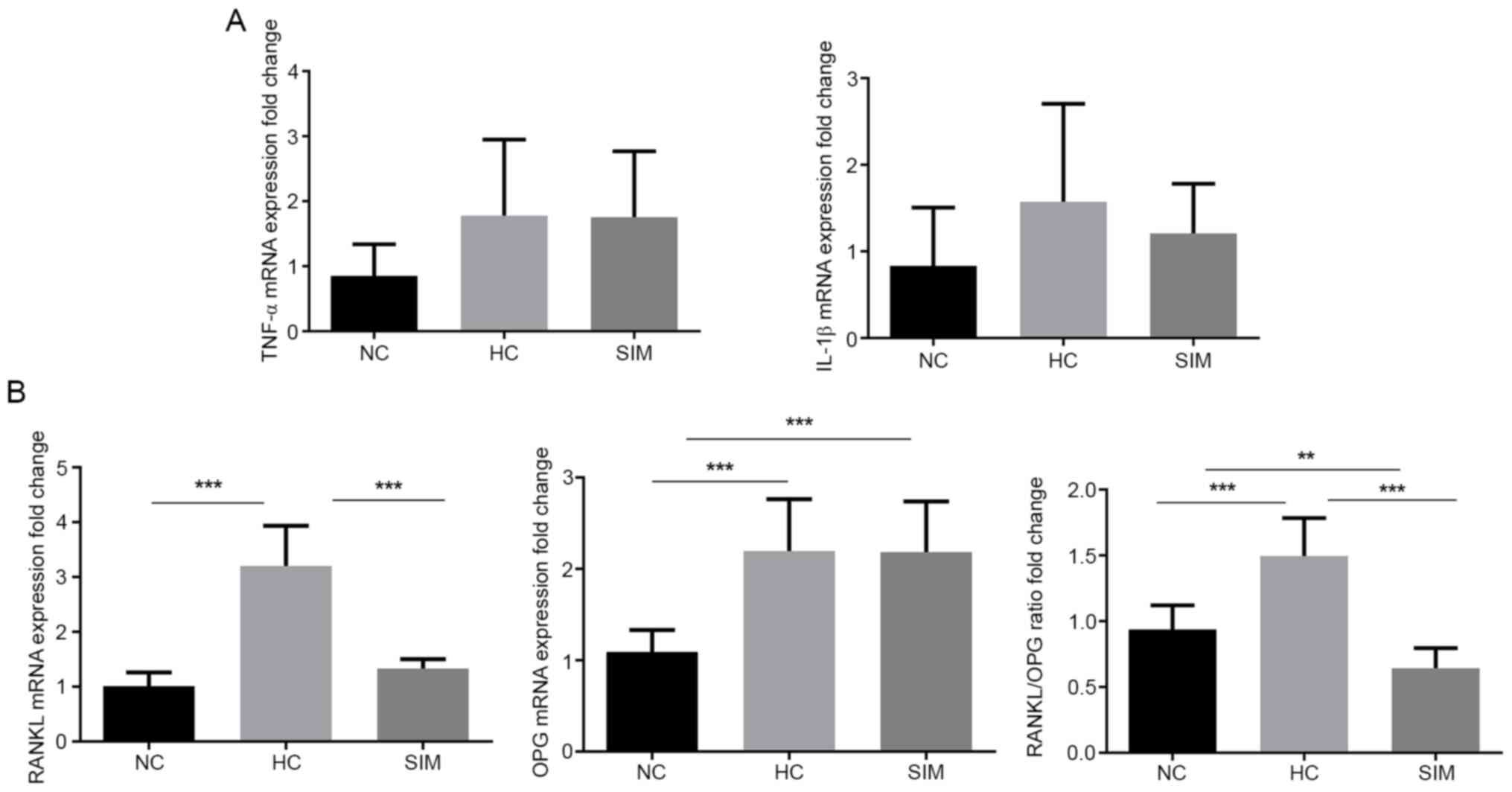
group, but still higher than that in the NC group (Fig. 2B and C). Although a significant difference in
the relative levels of TNF-α and IL-1β mRNA transcripts in the
alveolar bone tissues from different groups of rats was not
detected (P>0.05 for all; Fig.
3A), significantly higher levels of RANKL and OPG mRNA
transcripts, leading to high ratios of RANKL to OPG in the HC group
relative to the NC group were observed (Fig. 3B). However, simvastatin treatment
significantly reduced the levels of RANKL, but not OPG, mRNA
transcripts, decreasing the ratios of RANKL to OPG mRNA transcripts
in rats. Collectively, such data indicated that simvastatin
intervention minimized hypercholesterolemia-enhanced NF-κB
expression and modulated RANKL and OPG transcription, ameliorating
hypercholesterolemia-related alveolar bone loss in rats.
Simvastatin intervention modulates LC3
and p62 expression in the alveolar bone of hypercholesterolemia
rats
Hyperlipidemia can enhance oxidative stress and
increase autophagy during the process of osteoclastogenesis. The
possible effect of simvastatin intervention on
hypercholesterolemia-enhanced LC3 and p62 expression in the
alveolar bone tissues was assessed by RT-qPCR and
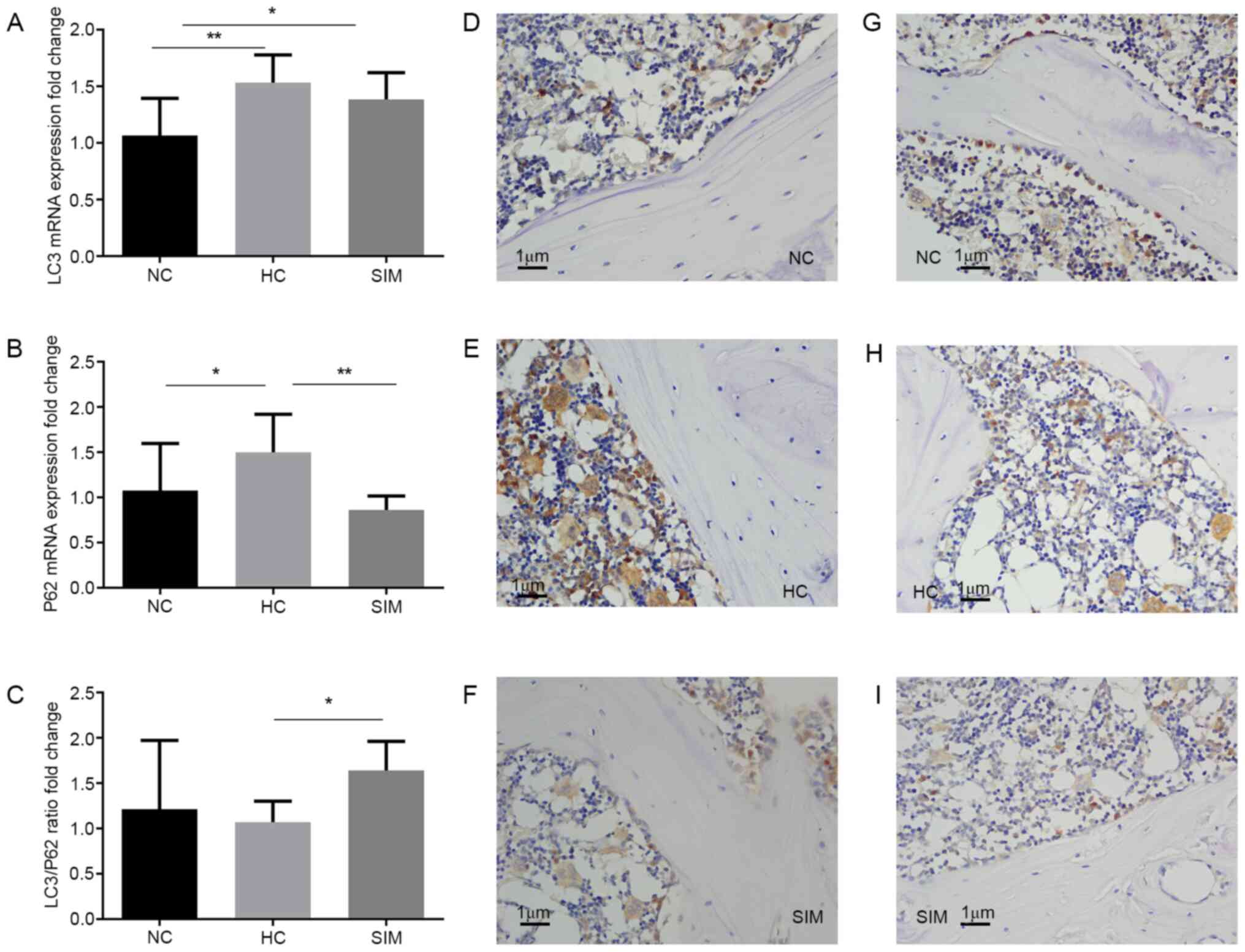
immunohistochemistry. Compared with the NC group, significantly
increased levels of LC3 and p62 mRNA transcripts were detected in
the alveolar bone tissues of the HC group (P<0.01, P<0.05,
Fig. 4A). Simvastatin intervention
did not significantly change the relative levels of LC3 mRNA
transcripts, but did significantly reduce the levels of p62 mRNA
transcripts in the alveolar bone tissues of the SIM group of rats,
relative to that the HC group (P<0.01 Fig. 4A and B). As a result, simvastatin intervention
increased the ratios of LC3 to p62 mRNA transcripts in the alveolar
bone tissues of the SIM group of rats (P<0.05 Fig. 4C). Immunohistochemistry revealed
that the intensity of anti-LC3 staining in the SIM group was
similar to that in the NC group, but less than that in the HC group
of rats (Fig. 4D-F). A similar
pattern of anti-p62 staining was observed in the alveolar bone
tissues from the different groups of rats (Fig. 4G-I). Simvastatin intervention may
therefore modulate LC3 and p62 expression and increase the ratios
of LC3 to p62 expression in the alveolar bone tissues of
hypercholesterolemia rats.
Discussion
A previous study demonstrated a positive correlation
between deep periodontal pockets and elevated blood lipid levels
(16). In the present study,
patients with periodontitis and hyperlipidemia displayed severe
periodontal tissue damage and alveolar bone resorption in
comparison to those with normal lipid levels, consistent with
previous observations (16). The
level of hyperlipidemia was positively correlated with the severity
of periodontal tissue damage in this population, supporting the
hypothesis that hyperlipidemia is associated with the progression
of periodontitis-related alveolar bone loss (17). Hyperlipidemia can increase innate
immune cell activity (18) and
activate innate immune cells, including macrophages and natural
killer cells, which can secrete pro-inflammatory cytokines and
migrate into the inflammatory lesion, worsening periodontitis and
promoting alveolar bone resorption (19). Assessing the degree of
hyperlipidemia may therefore be beneficial when evaluating the
severity of periodontitis.
Simvastatin can promote bone formation and increase
BMD in rats with hyperlipidemia, and ameliorate
hypercholesterolemia-related microstructure changes in the jaw bone
of animals (10). A study suggested
that simvastatin may promote bone formation and reduce alveolar
bone loss in the maxillary following ovariectomy and ligature
placement in rats (20). To
understand the therapeutic effect and potential mechanisms
underlying the action of simvastatin, a rat model of
hypercholesterolemia-related periodontitis was established in the
present study. High cholesterol feeding was demonstrated not only
to induce hypercholesterolemia, but also to promote severe alveolar
bone loss in rats. By contrast, treatment with simvastatin did not
significantly alter rat body weights, but significantly ameliorated
hypercholesterolemia and alveolar bone loss, extending previous
observations (10,20). These findings suggest that treatment
with simvastatin may benefit patients with periodontitis by
preventing and inhibiting alveolar bone loss.
The RANKL/RANK/OPG system is crucial for bone
metabolism and its imbalance is associated with promoting
osteoclastogenesis by activating c-Fos, NF-κB and nuclear factor of
activated T cells 1(21). NF-κB
activation can promote the early differentiation of osteoclast
precursors, while inhibition of NF-κB activation prevents RANKL and
TNFα-induced bone resorption (22).
NF-κB signaling is also involved in the progression of
periodontitis, damaging the alveolar bone and periodontal ligament
(23,24). In the present study, simvastatin
intervention significantly mitigated hypercholesterolemia-induced
NF-κB and RANKL expression, but not that of OPG, TNF-α and IL-1β,
in the alveolar bone tissues of rats, consistent with a previous
report (25,26). These findings extended previous
observations that simvastatin inhibits NF-κB signaling and
RANKL-induced osteoclastogenesis in RAW 264.7 cells and alveolar
bone loss induced by lipopolysaccharide in rats (27,28).
Inhibition of NF-κB signaling and related RANKL expression by
simvastatin may therefore be crucial for the control of
hypercholesterolemia-related periodontitis and alveolar bone loss
in rats.
Autophagy can regulate bone resorption by
osteoclasts (7). In the present
study, while hypercholesterolemia enhanced LC3 and p62 expression,
simvastatin intervention did not significantly alter the levels of
LC3 expression, but significantly reduced the levels of p62
expression, leading to an increase in the ratios of LC3 to p62
expression in the alveolar bone tissues of rats. It may be
hypothesized that increased ratios of LC3 to p62 expression
predominantly occur in osteoclasts and enhance their autophagic
flux, which may contribute to the simvastatin-induced inhibition of
alveolar bone loss in rats (29).
Given that p62 is the most specific adaptor protein to regulate the
formation of protein aggregates and is continuously degraded by the
autophagolysosome (30,31) the increased ratios of LC3 to p62
expression by simvastatin may at least partially explain its
therapeutic role in the inhibition of hypercholesterolemia-mediated
bone loss in the alveolar bone of rats (32-34).
Modulation of autophagy may therefore be a viable strategy for the
inhibition of hypercholesterolemia-related alveolar bone loss.
The present study had numerous limitations,
including a relatively small sample size for a human study without
prospective simvastatin treatment; measuring cytokine expression at
the level of mRNA transcripts and not their proteins; assessing
NF-κB, p62 expression but not its phosphorylation level; measuring
LC3 but not LC3 I and II expression; the lack of specific markers
for identification of osteoclasts in bone tissues; and the lack of
more valuable measures for determining autophagy. Hence, further
investigation of the therapeutic effect and potential mechanisms
underlying the action of simvastatin in regulating
hyperlipidemia-related alveolar bone loss in a larger population is
warranted.
In conclusion, the present results indicated that
hyperlipidemia was associated with alveolar bone loss in patients
with periodontitis. Simvastatin intervention not only reduced
hypercholesterolemia, but also mitigated
hypercholesterolemia-related alveolar bone loss by reducing NF-κB
and RANKL expression and modulating LC3 and p62 expression in the
alveolar bone tissues of hypercholesterolemic rats. These data may
suggest new mechanisms underlying the therapeutic action of
simvastatin in intervention for periodontitis-related bone
loss.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by grants from the
Shandong Provincial Natural Science Foundation, China (grant nos.
2016ZRB1462 and ZR2017BH005) and Shandong Provincial Health
Commission Foundation (grant no. 2016WS0410).
Availability of data and materials
The datasets used and/or analyzed in the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XG, SH and DZ participated the study design. XG and
JZ performed the experiments. XG and YB collected and analyzed the
data. XG drafted the manuscript. XG and DZ confirmed the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The experimental protocols were approved by the
Ethics Committee of Shandong Provincial Hospital (approval no.
SWYX:NO.2021-013) and the Animal Ethics Committee of Shandong
Provincial Hospital (approval no. 2016-06). Written informed
consent for participation was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Acharya A, VanWormer JJ, Waring SC, Miller
AW, Fuehrer JT and Nycz GR: Regional epidemiologic assessment of
prevalent periodontitis using an electronic health record system.
Am J Epidemiol. 177:700–707. 2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kinane DF: Causation and pathogenesis of
periodontal disease. Periodontol 2000. 25:8–20. 2001.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kaye EK: n-3 fatty acid intake and
periodontal disease. J Am Diet Assoc. 110:1650–1652.
2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Macri E, Lifshitz F, Ramos C, Orzuza R,
Costa O, Zago V, Boyer P and Friedman S: Atherogenic
cholesterol-rich diet and periodontal disease. Arch Oral Biol.
59:679–686. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Li RF, Chen G, Ren JG, Zhang W, Wu ZX, Liu
B, Zhao Y and Zhao YF: The adaptor protein p62 is involved in
RANKL-induced autophagy and osteoclastogenesis. J Histochem
Cytochem. 62:879–888. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Chung YH, Jang Y, Choi B, Song DH, Lee EJ,
Kim SM, Song Y, Kang SW, Yoon SY and Chang EJ: Beclin-1 is required
for RANKL-induced osteoclast differentiation. J Cell Physiol.
229:1963–1971. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
DeSelm CJ, Miller BC, Zou W, Beatty WL,
van Meel E, Takahata Y, Klumperman J, Tooze SA, Teitelbaum SL and
Virgin HW: Autophagy proteins regulate the secretory component of
osteoclastic bone resorption. Dev Cell. 21:966–974. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Towne SP and Thara E: Do statins reduce
events in patients with metabolic syndrome? Curr Atheroscler Rep.
10:39–44. 2008.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sadowitz B, Maier KG and Gahtan V: Basic
science review: Statin therapy-Part I: The pleiotropic effects of
statins in cardiovascular disease. Vasc Endovascular Surg.
44:241–251. 2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhou J, Gao X, Huang S, Ma L, Cui Y, Wang
H, Qiu J, Wang L, Dong Q, Chen Z, et al: Simvastatin improves the
jaw bone microstructural defect induced by high cholesterol diet in
rats by regulating autophagic flux. Biomed Res Int.
2018(4147932)2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Snaedal J: The Helsinki declaration.
Laeknabladid. 100(135)2014.PubMed/NCBI View Article : Google Scholar : (In Icelandic).
|
|
12
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals: Guide for the Care and use of Laboratory Animals. 8th
edition. National Academies Press, Washington, DC, 2011.
|
|
13
|
Wang CJ, Zhou ZG, Holmqvist A, Zhang H, Li
Y, Adell G and Sun XF: Survivin expression quantified by image
pro-plus compared with visual assessment. Appl Immunohistochem Mol
Morphol. 17:530–535. 2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhang P, Liu J, Xu Q, Harber G, Feng X,
Michalek SM and Katz J: TLR2-dependent modulation of
osteoclastogenesis by Porphyromonas gingivalis through differential
induction of NFATc1 and NF-kappaB. J Biol Chem. 286:24159–24169.
2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Katz J, Flugelman MY, Goldberg A and Heft
M: Association between periodontal pockets and elevated cholesterol
and low density lipoprotein cholesterol levels. J Periodontol.
73:494–500. 2002.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Scardina GA, Pisano T, Cacioppo A and
Messina P: Periodontal alteration of the microcirculation and
hypercholesterolemia: A possible correlation? South Med J.
104:116–120. 2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Croft KD, Beilin LJ, Vandongen R, Rouse I
and Masarei J: Leukocyte and platelet function and eicosanoid
production in subjects with hypercholesterolaemia. Atherosclerosis.
83:101–109. 1990.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Gustafsson A and Asman B: Increased
release of free oxygen radicals from peripheral neutrophils in
adult periodontitis after Fc delta-receptor stimulation. J Clin
Periodontol. 23:38–44. 1996.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Xu XC, Chen H, Zhang X, Zhai ZJ, Liu XQ,
Qin A and Lu EY: Simvastatin prevents alveolar bone loss in an
experimental rat model of periodontitis after ovariectomy. J Transl
Med. 12(284)2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wada T, Nakashima T, Hiroshi N and
Penninger JM: RANKL-RANK signaling in osteoclastogenesis and bone
disease. Trends Mol Med. 12:17–25. 2006.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Yamashita T, Yao Z, Li F, Zhang Q, Badell
IR, Schwarz EM, Takeshita S, Wagner EF, Noda M, Matsuo K, et al:
NF-kappaB p50 and p52 regulate receptor activator of NF-kappaB
ligand (RANKL) and tumor necrosis factor-induced osteoclast
precursor differentiation by activating c-Fos and NFATc1. J Biol
Chem. 282:18245–18253. 2007.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Cochran DL: Inflammation and bone loss in
periodontal disease. J Periodontol. 79 (Suppl 8):S1569–S1576.
2008.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Xu J, Wu HF, Ang ES, Yip K, Woloszyn M,
Zheng MH and Tan RX: NF-kappaB modulators in osteolytic bone
diseases. Cytokine Growth Factor Rev. 20:7–17. 2009.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kaji H, Kanatani M, Sugimoto T and Chihara
K: Statins modulate the levels of osteoprotegerin/receptor
activator of NFkappaB ligand mRNA in mouse bone-cell cultures. Horm
Metab Res. 37:589–592. 2005.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Estanislau IM, Terceiro IR, Lisboa MR,
Teles Pde B, Carvalho Rde S, Martins RS and Moreira MM: Pleiotropic
effects of statins on the treatment of chronic periodontitis - a
systematic review. Br J Clin Pharmacol. 79:877–885. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ahn KS, Sethi G, Chaturvedi MM and
Aggarwal BB: Simvastatin, 3-hydroxy-3-methylglutaryl coenzyme A
reductase inhibitor, suppresses osteoclastogenesis induced by
receptor activator of nuclear factor-kappaB ligand through
modulation of NF-kappaB pathway. Int J Cancer. 123:1733–1740.
2008.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Jin J, Machado ER, Yu H, Zhang X, Lu Z, Li
Y, Lopes-Virella MF, Kirkwood KL and Huang Y: Simvastatin inhibits
LPS-induced alveolar bone loss during metabolic syndrome. J Dent
Res. 93:294–299. 2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Maynard AA, Dvorak K, Khailova L, Dobrenen
H, Arganbright KM, Halpern MD, Kurundkar AR, Maheshwari A and
Dvorak B: Epidermal growth factor reduces autophagy in intestinal
epithelium and in the rat model of necrotizing enterocolitis. Am J
Physiol Gastrointest Liver Physiol. 299:G614–G622. 2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Komatsu M, Kurokawa H, Waguri S, Taguchi
K, Kobayashi A, Ichimura Y, Sou YS, Ueno I, Sakamoto A, Tong KI, et
al: The selective autophagy substrate p62 activates the stress
responsive transcription factor Nrf2 through inactivation of Keap1.
Nat Cell Biol. 12:213–223. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
31
|
Johansen T and Lamark T: Selective
autophagy mediated by autophagic adapter proteins. Autophagy.
7:279–296. 2011.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Chen K, Yang YH, Jiang SD and Jiang LS:
Decreased activity of osteocyte autophagy with aging may contribute
to the bone loss in senile population. Histochem Cell Biol.
142:285–295. 2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Luo D, Ren H, Li T, Lian K and Lin D:
Rapamycin reduces severity of senile osteoporosis by activating
osteocyte autophagy. Osteoporos Int. 27:1093–1101. 2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Onal M, Piemontese M, Xiong J, Wang Y, Han
L, Ye S, Komatsu M, Selig M, Weinstein RS, Zhao H, et al:
Suppression of autophagy in osteocytes mimics skeletal aging. J
Biol Chem. 288:17432–17440. 2013.PubMed/NCBI View Article : Google Scholar
|