Introduction
Heat shock protein 90 (Hsp90), a molecular
chaperone, is present in the cytoplasm of human cells in the form
of homodimers (αα, ββ), which are essential for the regulation of
stability and function of client proteins (1). Human Hsp90 has four subtypes:
Cytoplasmic chaperone Hsp90α (inducible/major), Hsp90β
(constitutive/minor), homologous endoplasmic reticulum partner
glucose-associated protein 94 and mitochondrial homolog Hsp75/tumor
necrosis factor receptor-associated protein 1 (2,3).
Hsp90 is composed of an N-terminal adenosine
triphosphate (ATP) binding domain, an intermediate domain and a
C-terminal dimerization domain containing a tetrachloropeptide
repeat binding motif (4,5). There are numerous classical Hsp90
inhibitors, including 17-dimethy
laminoethylamino-17-demethoxygeldanamycin (6), radicicol (7), PU24FCl (8), PH-H71(9), CNF-2024/BIIB021 (10,11)
and geldanamycin and its derivative 17-allylamino geldanamycin
(12). These classical Hsp90
inhibitors prevent the maturation of the super-chaperone complex by
interfering with ATP binding to the N-terminal pocket of Hsp90,
which in turn inhibits the activation of client proteins and
ultimately leads to their degradation through the
ubiquitin-proteasome pathway (12,13).
Research indicates that the majority of these
compounds are associated with poor water solubility, high toxicity,
drug resistance and other disadvantages. Therefore, novel Hsp90
inhibitors would be beneficial alternatives. Representative
candidate small molecule inhibitors of Hsp90 are artificially
synthesized and include BJ-B11, AT-760 (SNX-2112) and AT-533
(SNX-25a). These structurally novel compounds are different
derivatives with benzamide as the basic nucleus (14). X-ray diffraction has confirmed that
these small molecule inhibitors can competitively bind to the ATP
site on Hsp90(15).
In our previous studies, the wide application of
novel Hsp90 inhibitors for anti-tumor, anti-viral and
anti-inflammatory purposes was demonstrated. AT-533 was revealed to
exhibit high selectivity in inhibiting the tumorigenic properties
of various cancer cell lines, including K562 (leukemia), Hep-2
(laryngeal cancer), A549 (lung cancer), Hep-G2 (liver cancer),
SW620 (colon carcinoma), A375 (melanoma), MCF-7 (breast cancer) and
Hela (cervical cancer) cell lines (16). In addition, AT-533 exerted a potent
inhibitory effect on human herpes simplex virus type 1 (HSV-1) by
blocking HSV-1 nuclear egress and assembly in vitro
(17,18). Additionally, AT-533 gel efficiently
inhibited keratitis caused by HSV-1 infection in a rabbit keratitis
model (19) and ameliorated
HSV-1-induced skin lesions in C57BL/6 mouse zosteriform (18) and guinea pig skin herpes models
(data not shown). AT-533 also suppressed HSV-1-induced inflammation
through inhibition of the nuclear factor κ-light-chain-enhancer of
activated B cells signaling pathway and the cleavage of
pro-interleukin-1β in an NLR family pyrin domain containing
3-independent manner in vitro (20). Furthermore, AT-533 has been
demonstrated to attenuate angiogenesis in breast cancer through the
hypoxia inducible factor-1α/vascular endothelial growth
factor/vascular endothelial growth factor receptor-2 signaling
pathway in vitro and in vivo (21). Although the potential bioactivities
of AT-533 and AT-533 gel have been extensively investigated, their
possible side-effects and toxicities remain to be determined.
In the present study, subacute toxicological
experiments were performed with AT-533 gel and its active
pharmaceutical ingredient AT-533, in order to determine the
non-toxic dosage and potential toxicity in rats, in accordance with
the relevant guiding principles (22). The present study provides an
important reference for further preclinical research into the use
of AT-533 gel in animals and its clinical application as a skin
medication for humans.
Materials and methods
Test substance
AT-533 was acquired as previously described
(20). AT-533 (batch no. 20180520)
was extracted and purified by our laboratory in conjunction with
Kunming Jisheng Biotechnology Co., Ltd. (Kunming, China). The
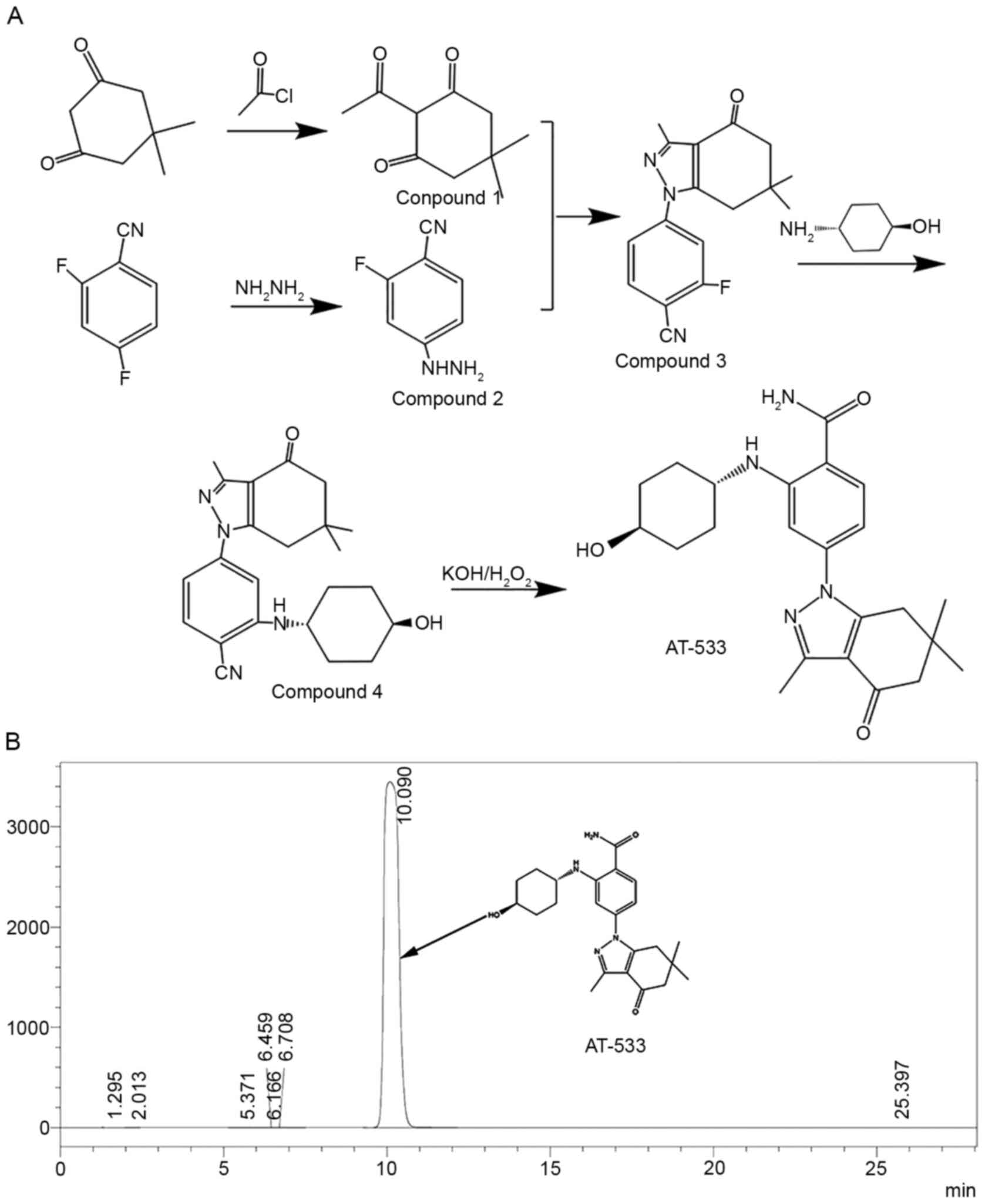
synthesis of AT-533 was as follows and as shown in Fig. 1A. First,
2-acetyl-5,5-dimethyl-1,3-cyclohexanedione (Compound 1) and
2-fluoro-4-hydrazino-benzonitrile (Compound 2) were synthesized in
parallel, and then condensed to obtain
2-fluoro-4-(3,6,6-trimethyl-4-oxo-4,5,6,7-tetrahydro-1H-indazol-1-yl)benzonitrile
(Compound 3). Compound 3 further reacted with trans
4-hydroxycyclohexylamine to obtain
2-(4-hydroxycyclohexylamino)-4-(3,6,6-trimethyl-4-oxo-4,5,6,7-tetrahydro-1H-indazol-1-yl)benzonitrile
(Compound 4). Finally, compound 4 and hydrogen peroxide were
reacted under alkaline conditions to get the product
2-[(4-hydroxycyclohexyl)
amino]-4-(4,5,6,7-tetrahydro-3,6,6-trimethyl-4-oxo-1H-indazol-1-yl)-benzamide
(AT-533).
Purity analysis was performed using high performance
liquid chromatography (HPLC) on a Shimadzu LC-16 instrument
(Shimadzu Corporation) with a GL WondaCract ODS-2 (5 µm, 4.6x150
mm). The mobile phase was prepared by a 55/45 (v/v) mixture of
methanol/water. The injection volume was 20 µl. The UV detector was
set at a wavelength of 254 nm. The HPLC purity of AT-533 was
determined to be >99.9%. The HPLC chromatogram and chemical
structure of AT-533 are shown in Fig.
1B. AT-533 was dissolved in propylene glycol, PEG400 and
sterile water for skin absorption. AT-533 gel (batch no. 20180913;
drug content, 0.04%) was prepared by Guangzhou (Jinan) Biomedicine
Research and Development Center. The preparation process was as
follows: AT-533 was dissolved in propylene glycol and dispersed in
carbomer 940. Following dispersing, sterile water was added to the
mixture, which was stirring until the carbomer was completely
swollen. Finally, triethanolamine was added and the mixture was
stirred evenly to form a gel. All solvents met the requirements of
the Chinese pharmacopoeia (23).
Animals
Specific pathogen-free male and female
Sprague-Dawley rats (age, 6-8 weeks; weight, 180-220 g), were
purchased from the Laboratory Animal Center of Southern Medical
University (Guangzhou, China). All rats were housed separately in
groups of 5 rats per cage with labels attached for identification.
The animal code, test code, sex and date of the first test were
marked on the label. The rats were kept in an animal room with a
12/12 h light/dark cycle and under standard conditions of
temperature (22±2˚C) and humidity (70±10%). All rats had free
access to food and water. After 7 days of acclimation, the fur
(about 20 cm2) of the rats from the back to the abdomen
was shaved. Depilatory cream was then uniformly applied to ensure
thorough fur removal. Rats were then returned to the cage overnight
to recover from the stimulation of the depilatory cream. All animal
experiments were performed using protocols approved by the
Institutional Animal Care and Use Committee of Jinan
University.
Grouping and administration
A total of 140 rats (70 male and 70 female) were
randomly assigned to the control, vehicle, AT-533 (1, 2 and 4
mg/kg), blank gel or AT-533 gel (5 g/kg, maximum capacity) groups.
A total of 10 female rats and 10 male rats were assigned to each
group. All rats were transdermally treated with solvent or test
substance once per day for 30 days. The test substance was applied
directly to the skin and exposure was for 2 h per day. After 2 h
the test substance was wiped off using warm water.
Observation and detection
indicators
The food consumption of the rats was recorded daily,
and their body weight was measured three times per week. Daily
observation parameters included body weight, skin, hair color,
eyes, legs, genitals, autonomic activity, diet, defecation,
survival and overall appearance of mice. After 30 days of
treatment, all rats were anesthetized with pentobarbital sodium (60
mg/kg interperitoneally) and dissected for blood sampling and
exsanguination from the abdominal aorta. Routine and biochemical
examinations were performed on the blood samples. Hematological
tests were performed for white blood cell count (WBC) and
differential, erythrocyte count (RBC), hemoglobin concentration
(HGB), hematocrit (HCT), mean corpuscular volume (MCV), mean
corpuscular hemoglobin (MCH), MCH concentration (MCHC), red cell
distribution width (RDW), platelet count (PLT), mean platelet
volume (MPV), reticulocyte count (RET) and differential,
prothrombin time (PT) and thrombin time (TT). Biochemical indexes
included total serum protein (TP; cat. no. K134), albumin (ALB;
cat. no. K135), alanine-aminotransferase (ALT; cat. no. K114),
serum aspartate aminotransferase (AST; cat. no. K118), total
bilirubin (T-Bil; cat. no. S219), alkaline phosphatase (ALP; cat.
no. K115), creatinine (CRE; cat. no. K131), urea nitrogen (BUN;
cat. no. K132), uric acid (UA; cat. no. K122), triglycerides (TG;
cat. no. K119), blood glucose (GLU; cat. no. K121), total
cholesterol (CHOL; cat. no. K120), high-density lipoprotein
cholesterol (HDL; cat. no. K116), low density
lipoprotein-cholesterol (LDL; cat. no. K117), creatine kinase (CK;
cat. no. K119), creatine kinase isoenzyme (CK-MB; cat. no. K110),
sodium (Na; cat. no. K170), potassium (K; cat. no. K169), and
chloride (Cl; cat. no. K171). All the kits were purchased from
Shanghai Kehua Bio-engineering Co., Ltd., and used in accordance
with the manufacturer's protocol. Hematological examination was
performed using an automatic hematology analyzer (BC-3000plus;
Shenzhen Mindray Bio-Medical Electronics Co., Ltd.). Blood
biochemical indexes were detected by automatic biochemical analyzer
(7020; Hitachi, Ltd.). Gross necropsy and anatomy analyses were
performed. The tissues and organs requiring pathological
examination included the heart, liver, pancreas, spleen, lungs,
adrenal glands, kidneys, brain, esophagus, stomach, duodenum,
mesenteric lymphoid node, colon, spinal cord (cervical, thoracic
and lumbar), bone marrow, prostate, epididymis, testis, ovaries,
uterus, mammary gland, sciatic nerve, bladder, pituitary gland,
trachea, thyroids, thymus, salivary glands, optic nerve and dosing
site (skin). All of these tissues and organs were fixed with 4%
paraformaldehyde for 8 h at room temperature, dehydrated
successively using 50, 75, 90 and 100% ethanol and embedded in
paraffin. The tissues were then sectioned into 3-µm slices and
stained with hematoxylin and eosin (H&E) for 1-3 min at room
temperature. Prior to fixation, the heart, liver, spleen, lung,
adrenal glands, kidneys, brain, thymus, epididymis, testis, ovaries
and uterus were weighed and the organ coefficients
(organ-to-final-body-weight and organ-to-brain-weight ratios) were
calculated.
Statistical analysis
All data were analyzed using Graph Pad Prism 5.0
(GraphPad Software, Inc.) and Microsoft Excel 2013 (Microsoft
Corporation) and are presented as the mean ± SD. Statistical
significance was determined using one-way ANOVA followed by
Dunnett's multiple comparison test. P<0.05 was considered to be
statistically significant.
Results
Laboratory observation, food
consumption and body weight
During continuous administration of treatment no
mortality was observed in the control, vehicle, 1 mg/kg AT-533,
blank gel and AT-533 gel group rats. In the 2 mg/kg AT-533 group
two animal deaths occurred (1 male and 1 female) and in the 4 mg/kg
AT-533 group 6 deaths (3 males and 3 females) were recorded during
the drug exposure period. The symptoms presented following
transdermal administration of AT-533 and AT-533 gel included a
decreased appetite, skin irritation and hypersensitivity
(manifested as scratching and bleeding), particularly in the rats
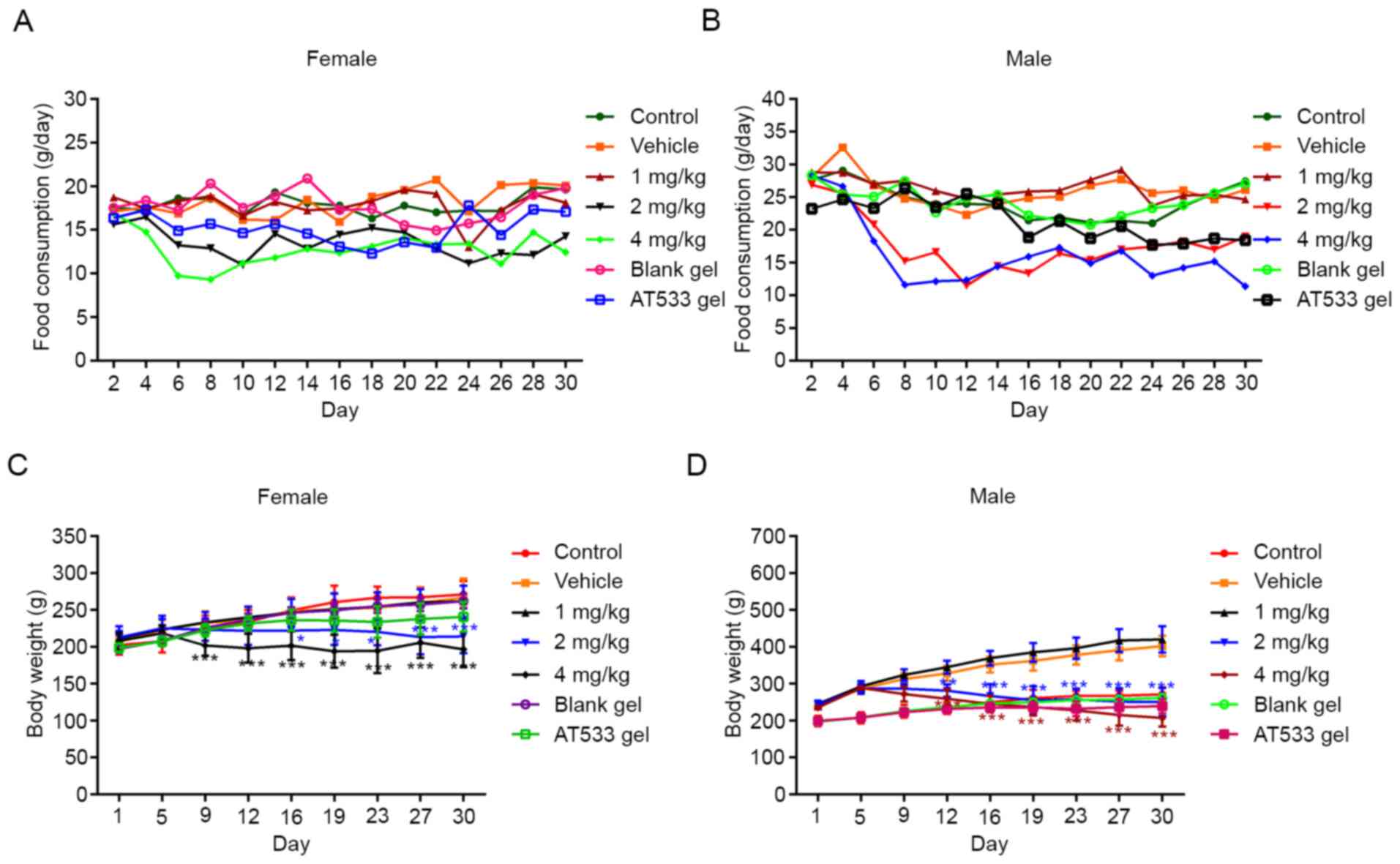
from the 2 and 4 mg/kg AT-533 groups. As shown in Fig. 2A and B, food consumption in female and male rats
from the 2 and 4 mg/kg AT-533 group decreased sharply from day 4,
as compared with the vehicle group, though this did not occur in
the 1 mg/kg group female and male rats. There was no statistically
significant difference in food consumption and body weight between
the blank gel and AT-533 gel groups. There was no statistically
significant difference in body weight between the normal control
and vehicle/blank gel groups in both female and male rats,
indicating that the vehicle/blank gel was safe for rats. As
compared with the vehicle group, the body weight of female rats in
the 4 mg/kg group decreased sharply from day 9 and in the 2 mg/kg
AT-533 group from day 16 (Fig. 2C).
Fig. 2D revealed that the body
weight of male rats in the 2 and 4 mg/kg AT-533 groups decreased
significantly from day 12. Compared with the vehicle/blank gel
group, there was no statistically significant body weight change in
the rats of the 1 mg/kg AT-533 and AT-533 gel groups during a
continuous 30-day administration.
Hematology and biochemical
examination
Following consecutive administration of AT-533 for
30 days, hematology and serum biochemistry was performed in the
rats. As shown in Table I, the WBC,
MCHC and neutrophil (NE) counts and the NE, lymphocyte (LY) and
monocyte (MO) percentages of female rats in the 2 and 4 mg/kg
AT-533 groups were significantly increased as compared with the
vehicle group, whereas the MCV and MCH levels and the HCT,
eosinophil (EO) and basophil percentages were reduced. For the
AT-533 gel experiment, NE % and LY % in female rats were increased
and thrombin time (TT) was decreased at an AT-533 gel dose of 5
g/kg in comparison with vehicle while other hematological
parameters were not obviously changed after 30 days of treatment.
However, except for the counts of WBC and NE, other alterations
were not considered to be toxicologically significant, as the
markers in question stayed within the normal reference ranges
(24,25).
 | Table IHematological values of female rats
treated with AT-533 or AT-533 gel for 30 days. |
Table I
Hematological values of female rats
treated with AT-533 or AT-533 gel for 30 days.
| Parameters | Control | Vehicle | 1 mg/kg | 2 mg/kg | 4 mg/kg | Blank gel | AT-533 gel |
|---|
| WBC
(x109/l) | 4.69±2.49 | 5.59±0.85 | 8.63±3.83 | 9.96±5.64 |
17.32±4.40b | 5.53±1.73 | 4.74±2.42 |
| RBC
(x1012/l) | 7.59±0.62 | 7.71±0.36 | 7.92±0.57 | 7.48±0.42 | 7.98±0.79 | 7.94±0.50 | 7.50±0.46 |
| HGB (g/l) | 151.33±13.49 | 148.50±5.58 | 149.67±11.09 | 136.67±6.98 | 145.20±12.34 | 156.50±9.33 | 143.67±7.74 |
| HCT (%) | 42.53±3.59 | 43.63±1.44 | 43.32±2.76 |
39.53±1.24a | 41.28±2.49 | 44.42±2.48 | 40.70±1.77 |
| MCV (fl) | 56.03±1.59 | 56.68±2.31 | 54.75±1.63 |
52.97±1.77b |
51.88±2.10b | 55.97±0.66 | 54.33±1.46 |
| MCH (pg) | 19.92±0.45 | 19.27±0.73 | 18.90±0.60 |
18.30±0.48a |
18.24±0.3a | 19.73±0.35 | 19.17±0.35 |
| MCHC (g/l) | 355.83±5.23 | 340.33±4.72 | 345.33±6.31 | 345.50±8.71 |
351.20±8.50a | 352.33±5.39 | 353.00±9.30 |
| PLT
(x109/l) |
1,201.33±157.57 |
1,105.50±132.27 |
1,117.33±175.16 |
1,316.67±155.22 |
1,337.25±174.21 |
1,258.60±139.33 |
1,212.00±154.72 |
| RDW-SD (fl) | 27.38±1.42 | 28.28±0.70 | 28.30±0.83 | 27.45±0.97 | 28.12±0.62 | 27.88±1.78 | 25.97±1.02 |
| RDW-CV (%) | 15.10±1.33 | 15.62±1.33 | 16.52±1.05 | 16.42±1.55 | 17.62±1.68 | 16.08±1.62 | 14.77±1.40 |
| PDW (fl) | 9.65±0.52 | 9.47±0.60 | 9.42±0.52 | 8.70±0.37 | 9.72±0.73 | 9.50±0.21 | 9.32±0.47 |
| MPV (fl) | 8.67±0.41 | 8.57±0.46 | 8.43±0.37 |
8.02±0.23a | 8.40±0.21 | 8.57±0.29 | 8.32±0.23 |
| P-LCR (%) | 15.57±3.06 | 14.78±3.32 | 14.42±2.62 | 10.92±1.77 | 14.32±2.17 | 14.73±1.94 | 13.32±1.95 |
| PCT (%) | 1.04±0.11 | 0.95±0.12 | 0.94±0.14 | 1.05±0.12 | 1.05±0.18 | 1.07±0.09 | 0.95±0.19 |
| NE
(x109/l) | 0.64±0.24 | 0.73±0.23 | 1.06±0.50 |
3.53±2.22a |
5.85±1.54c | 0.74±0.38 | 1.22±0.55 |
| LY
(x109/l) | 4.01±2.54 | 4.66±0.84 | 7.13±3.65 | 5.84±3.53 | 10.37±3.48 | 4.68±1.37 | 4.31±3.10 |
| MO
(x109/l) | 0.13±0.03 | 0.20±0.05 | 0.16±0.07 | 0.47±0.25 | 0.98±0.33 | 0.18±0.09 | 0.25±0.15 |
| EO
(x109/l) | 0.08±0.06 | 0.09±0.02 | 0.10±0.07 | 0.12±0.08 | 0.09±0.07 | 0.13±0.09 | 0.08±0.03 |
| BA
(x109/l) | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | 0.01±0.00 | 0.00±0.00 | 0.00±0.00 |
| NE (%) | 11.70±6.46 | 13.34±3.86 | 18.22±8.64 |
37.76±8.94b | 34.65±7.94 | 9.87±2.53 |
27.00±6.34e |
| LY (%) | 83.98±7.82 | 83.08±5.70 | 79.62±8.94 |
59.03±9.67b |
64.84±15.21a | 85.42±3.72 |
67.15±6.38e |
| MO (%) | 3.07±1.19 | 3.50±0.76 | 2.02±0.28 | 4.24±1.30 |
6.66±2.41a | 2.70±0.73 | 4.12±1.00 |
| EO (%) | 1.25±0.28 | 1.63±0.34 | 1.37±0.40 | 1.18±0.57 |
0.54±0.43c | 2.26±0.87 | 1.36±0.59 |
| BA (%) | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | 0.02±0.04 |
0.10±0.12a | 0.00±0.00 | 0.02±0.04 |
| RET
(x109/l) | 233.42±43.40 | 243.38±30.01 | 257.95±41.78 | 197.48±50.31 | 227.48±75.54 | 282.12±37.05 | 270.95±78.70 |
| RET (%) | 3.09±0.59 | 3.16±0.42 | 3.29±0.70 | 3.02±1.12 | 2.86±0.95 | 3.55±0.40 | 3.62±1.01 |
| PT (s) | 9.43±0.34 | 9.02±0.30 | 8.68±0.25 | 8.60±0.33 | 9.36±2.00 | 9.62±1.40 | 9.13±0.52 |
| TT (s) | 45.47±4.15 | 40.77±6.06 | 49.33±5.12 | 37.75±4.21 | 44.74±6.95 | 47.28±4.19 |
42.05±4.19d |
In male rats, the WBC, PLT, NE and MO counts, and NE
% and MO % of the 2 and 4 mg/kg AT-533 groups increased, as
compared with the vehicle group, while the HGB, MCV, MPV, RET, PT
and TT levels and HCT, LY and EO percentages decreased. The HGB
level and MO percentage increased and the number and percentage of
RET decreased following treatment with 5 g/kg AT-533 gel in
comparison with vehicle, while other hematological parameters were
not obviously changed after 30 days of treatment (Table II). Except for the PLT, NE and MO
counts, other alterations were not considered to be toxicologically
significant, as the markers in question stayed within the normal
reference ranges (24,25).
 | Table IIHematological values of male rats
treated with AT-533 or AT-533 gel for 30 days. |
Table II
Hematological values of male rats
treated with AT-533 or AT-533 gel for 30 days.
| Parameters | Control | Vehicle | 1 mg/kg | 2 mg/kg | 4 mg/kg | Blank gel | AT-533 gel |
|---|
| WBC
(x109/l) | 5.27±4.29 | 7.34±2.66 | 10.83±3.44 | 12.74±3.92 |
14.45±4.06a | 8.56±1.21 | 11.77±1.55 |
| RBC
(x1012/l) | 7.65±1.02 | 8.32±0.51 | 8.10±0.35 | 7.66±0.78 | 7.08±0.22 | 7.89±0.35 | 8.67±0.48 |
| HGB (g/l) | 154.00±6.38 | 160.33±8.12 | 156.83±5.91 |
141.00±13.10a | 130.33±1.53 | 154.43±9.50 |
166.00±7.21a |
| HCT (%) | 41.90±5.31 | 46.72±2.53 | 45.82±1.68 |
40.00±3.27b |
36.83±1.08a | 44.14±2.51 | 46.72±2.07 |
| MCV (fl) | 54.86±1.29 | 56.18±1.64 | 56.58±1.66 | 53.60±3.42 |
52.10±2.91a | 55.94±2.10 | 53.92±2.64 |
| MCH (pg) | 18.36±1.54 | 19.28±0.33 | 19.35±0.38 | 18.47±0.27 | 18.47±0.81 | 19.57±0.75 | 19.18±0.56 |
| MCHC (g/l) | 335.00±30.94 | 343.50±6.09 | 342.33±4.03 | 345.17±18.65 | 354.00±7.00 | 349.86±2.54 | 355.40±10.24 |
| PLT
(x109/l) | 1,144.50±78.86 |
1,188.67±104.82 | 1,240.17±88.11 |
1,488.50±114.31c |
1,511.00±109.08c | 1,165.00±69.38 |
1,131.25±138.69 |
| RDW-SD (fl) | 29.26±1.90 | 29.32±1.23 | 30.17±0.83 | 29.30±1.22 | 28.80±1.61 | 29.39±1.32 | 28.48±1.54 |
| RDW-CV (%) | 17.00±1.98 | 17.30±0.87 | 17.35±0.87 | 17.73±1.44 | 16.97±2.60 | 17.19±0.63 | 17.92±1.10 |
| PDW (fl) | 10.42±1.64 | 9.30±0.20 | 9.43±0.27 | 9.55±0.68 | 8.43±0.15 | 9.80±0.77 | 9.86±0.59 |
| MPV (fl) | 8.96±0.57 | 8.33±0.16 | 8.42±0.27 | 8.57±0.39 |
7.73±0.06a | 8.63±0.48 | 8.64±0.42 |
| P-LCR (%) | 18.28±5.03 | 13.35±1.33 | 14.30±1.81 | 15.32±3.08 | 9.33±0.21 | 15.83±3.85 | 15.76±3.22 |
| PCT (%) | 1.00±0.08 | 0.99±0.07 | 1.05±0.06 | 1.28±0.07 | 1.17±0.09 | 0.97±0.06 | 0.91±0.15 |
| NE
(x109/l) | 0.71±0.48 | 1.20±0.50 | 2.83±0.71 |
4.45±1.35c |
8.61±1.58c | 2.26±0.59 | 1.90±1.47 |
| LY
(x109/l) | 3.70±2.80 | 6.23±2.31 | 6.53±1.76 | 8.19±1.96 | 4.85±2.34 | 6.48±0.61 | 7.99±2.59 |
| MO
(x109/l) | 0.12±0.08 | 0.29±0.17 | 0.37±0.16 |
0.81±0.32b |
0.91±0.30c | 0.37±0.15 | 0.54±0.32 |
| EO
(x109/l) | 0.08±0.05 | 0.14±0.06 | 0.13±0.07 | 0.08±0.05 | 0.07±0.05 | 0.13±0.03 | 0.09±0.08 |
| BA
(x109/l) | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | 0.01±0.01 | 0.00±0.01 | 0.00±0.00 |
0.01±0.01e |
| NE (%) | 22.68±11.89 | 17.85±5.43 | 27.17±7.74 |
35.63±5.99b |
60.60±5.93c | 19.00±8.57 | 30.93±13.14 |
| LY (%) | 72.32±11.22 | 77.12±5.96 | 68.35±8.16 |
57.48±5.62c |
32.40±6.15c | 76.14±9.76 | 67.60±14.28 |
| MO (%) | 3.16±0.74 | 3.64±1.22 | 3.12±0.58 |
6.23±0.90c |
6.47±2.42b | 3.54±1.07 |
5.24±1.3d |
| EO (%) | 1.84±0.72 | 1.70±0.47 | 1.13±0.35 |
0.68±0.20c |
0.50±0.26c | 1.30±0.34 | 0.92±0.55 |
| BA (%) | 0.00±0.00 | 0.00±0.00 | 0.02±0.04 | 0.05±0.05 | 0.03±0.06 | 0.03±0.05 | 0.16±0.21f |
| RET
(x109/l) | 276.10±75.90 | 284.65±52.34 | 269.70±55.99 | 309.68±65.48 |
161.27±6.10a | 301.59±33.05 |
141.52±122.70e |
| RET (%) | 3.56±0.64 | 3.41±0.49 | 3.34±0.74 | 4.58±2.08 | 2.28±0.11 | 3.83±0.42 |
1.03±0.61f |
| PT (s) | 9.74±0.46 | 9.48±0.39 | 9.70±0.34 |
8.75±0.43a |
8.50±0.50b | 9.37±0.26 | 9.72±0.54 |
| TT (s) | 47.74±5.39 | 51.35±7.93 | 51.87±7.05 |
35.62±5.71b |
37.17±6.57b | 48.62±8.52 | 50.50±5.94 |
Serum biochemistry of female rats showed that the
level of ALB was lower and the level of AST higher in rats treated
with 4 mg/kg AT-533, as compared with the vehicle group, while
other biochemical indicators were not obviously changed following
30 days of treatment. The levels of AST and LDL increased and the
level of ALB decreased in female rats following treatment with 5
g/kg AT-533 gel in comparison with vehicle, while other biochemical
indicators were not obviously changed following 30 days of
treatment (Table III). However,
the changes of female rats in the 5 g/kg AT-533 gel group were not
considered to be toxicologically significant, as the markers in
question stayed within the normal reference ranges (26,27).
 | Table IIISerum biochemical values of female
rats treated with AT-533 or AT-533 gel for 30 days. |
Table III
Serum biochemical values of female
rats treated with AT-533 or AT-533 gel for 30 days.
| Parameters | Control | Vehicle | 1 mg/kg | 2 mg/kg | 4 mg/kg | Blank gel | AT-533 gel |
|---|
| TP (g/l) | 62.85±4.06 | 55.87±4.46 | 64.40±11.77 | 51.58±9.53 | 50.90±7.73 | 63.58±3.78 | 59.25±7.33 |
| ALB (g/l) | 29.82±1.38 | 27.40±2.34 | 29.90±5.81 | 22.15±5.22 |
16.84±3.74c | 30.70±1.32 |
27.72±2.82a |
| ALT (U/l) | 57.67±21.34 | 42.17±6.31 | 39.17±11.69 | 57.50±20.04 | 79.60±25.83 | 53.83±10.25 | 62.25±7.63 |
| AST (U/l) | 126.80±18.67 | 83.17±5.34 | 102.33±43.57 | 182.50±94.05 |
316.50±229.56a | 103.50±11.43 |
165.00±45.21b |
| T-Bil (umol/l) | 0.12±0.09 | 0.12±0.05 | 0.24±0.13 | 0.19±0.11 | 0.14±0.07 | 0.10±0.10 | 0.15±0.16 |
| ALP (U/l) | 145.83±31.57 | 135.40±29.05 | 167.80±63.15 | 158.80±4.55 | 307.20±245.60 | 175.50±1.73 | 189.00±39.17 |
| BUN (mmol/l) | 7.03±1.72 | 5.85±0.70 | 5.89±0.98 | 6.19±1.16 | 8.20±3.79 | 6.56±0.86 | 6.43±0.70 |
| CRE (umol/l) | 35.05±10.73 | 23.77±3.69 | 30.88±8.51 | 33.35±6.96 | 28.24±10.03 | 33.27±6.67 | 27.55±4.18 |
| UA (umol/l) | 110.55±34.80 | 93.38±8.14 | 121.86±11.47 | 104.70±34.12 | 85.53±46.07 | 79.25±13.48 | 64.92±18.43 |
| GLU (mmol/l) | 19.88±4.06 | 15.93±3.38 | 17.12±1.93 | 13.65±2.55 | 12.36±4.07 | 16.88±2.16 | 15.69±3.89 |
| TG (mmol/l) | 0.97±0.55 | 1.30±0.84 | 0.99±0.53 | 1.02±0.48 | 0.49±0.07 | 0.75±0.34 | 1.01±0.44 |
| CHO (mmol/l) | 1.95±0.22 | 1.63±0.28 | 2.07±0.45 | 1.73±0.43 | 1.20±0.62 | 1.85±0.22 | 1.73±0.32 |
| HDL (mmol/l) | 0.77±0.11 | 0.66±0.15 | 0.81±0.18 | 0.74±0.20 | 0.52±0.29 | 0.74±0.09 | 0.72±0.15 |
| LDL (mmol/l) | 0.24±0.03 | 0.19±0.04 | 0.21±0.05 | 0.25±0.07 | 0.22±0.04 | 0.20±0.04 |
0.25±0.04d |
| CK (U/l) |
1,326.50±190.12 | 579.60±338.26 |
1,544.50±391.89 | 899.00±301.61 |
2,246.60±1528.21 |
1,594.83±679.49 |
1,627.20±784.94 |
| CK-MB (U/l) | 391.50±140.6 | 303.20±54.87 | 505.80±190.8 | 271.50±39.26 | 341.33±38.53 | 415.20±43.64 | 434.40±106.4 |
| K+
(mmol/l) | 5.50±0.66 | 5.22±0.90 | 5.81±1.35 | 6.18±1.80 | 5.34±1.04 | 5.05±0.83 | 5.87±1.42 |
| Na+
(mmol/l) | 141.32±6.21 | 125.38±6.51 | 132.37±18.12 | 129.52±18.95 | 130.88±10.78 | 141.48±7.59 | 141.92±5.21 |
| Cl-
(mmol/l) | 109.62±2.78 | 100.88±4.85 | 105.02±15.9 | 101.28±18.31 | 104.50±7.69 | 109.65±3.25 | 111.07±5.67 |
Table IV revealed
that the levels of ALB, GLU, TG, CHO, HDL and CK of male rats in
the 4 mg/kg AT-533 group decreased, while the level of BUN was
markedly increased in comparison with vehicle. Male rats treated
with 1 mg/kg AT-533 exhibited a slight reduction of TG. The levels
of ALB and TG decreased and the levels of CRE, LDL, Na+,
and Cl- increased in male rats at an AT-533 gel dose of
5 g/kg in comparison with vehicle, while other biochemical
indicators were not obviously changed after 30 days of treatment.
Except for the levels of ALB, CHO, HDL and BUN in the 4 mg/kg
AT-533 group male rats, other alterations that stayed within the
normal reference ranges were not considered to be toxicological
(26,27). It may be hypothesized that the
slightly low levels of GLU, TG and CK could be partly relevant to
the decrease in food consumption.
 | Table IVSerum biochemical values of male rats
treated with AT-533 or AT-533 gel for 30 days. |
Table IV
Serum biochemical values of male rats
treated with AT-533 or AT-533 gel for 30 days.
| Parameters | Control | Vehicle | 1 mg/kg | 2 mg/kg | 4 mg/kg | Blank gel | AT-533 gel |
|---|
| TP (g/l) | 61.30±6.22 | 59.12±8.13 | 57.15±9.59 | 50.88±9.44 | 48.78±4.55 | 60.78±3.86 | 62.98±3.86 |
| ALB (g/l) | 27.52±1.74 | 27.93±3.65 | 25.53±4.26 |
17.73±2.64c |
14.22±2.28c | 28.23±1.65 |
23.44±4.70d |
| ALT (U/l) | 53.00±5.35 | 52.80±13.65 | 42.50±9.65 | 38.80±6.26 | 133.83±143.50 | 55.00±16.49 | 54.50±14.84 |
| AST (U/l) | 124.50±16.8 | 138.80±30.7 | 100.17±21.4 | 94.33±32.6 | 272.50±156.2 | 116.00±18.6 | 120.50±46.3 |
| T-Bil (umol/l) | 0.11±0.05 | 0.44±0.14 | 0.23±0.18 | 0.06±0.07 | 0.24±0.20 | 0.13±0.06 | 0.07±0.06 |
| ALP (U/l) | 268.2±33.5 | 252.2±49.2 | 195.2±43.5 | 272.5±119.7 | 289.8±131.2 | 249.2±22.02 | 259.5±25.38 |
| BUN (mmol/l) | 6.60±0.96 | 5.86±1.30 | 5.30±0.73 | 5.43±2.08 |
15.46±9.61a | 6.78±1.28 | 6.42±0.45 |
| CRE (umol/l) | 27.34±5.90 | 25.90±5.68 | 23.92±7.13 | 20.10±8.60 | 23.03±10.80 | 29.47±3.91 |
40.88±8.23e |
| UA (umol/l) | 112.8±19.8 | 111.4±28.0 | 114.4±37.7 | 81.2±27.9 | 75.8±24.1 | 90.1±20.8 | 93.5±16.8 |
| GLU (mmol/l) | 17.24±1.84 | 16.69±3.03 | 14.28±2.23 | 13.06±3.09 |
9.84±2.36c | 20.56±2.89 | 17.60±5.92 |
| TG (mmol/l) | 1.84±0.35 | 2.16±0.67 |
1.52±0.42a |
1.03±0.08c |
0.35±0.18c | 1.71±0.22 |
0.96±0.56d |
| CHO (mmol/l) | 1.45±0.14 | 1.62±0.36 | 1.52±0.35 | 1.22±0.46 |
0.92±0.23b | 1.82±0.22 | 1.47±0.52 |
| HDL (mmol/l) | 0.60±0.04 | 0.68±0.16 | 0.61±0.14 | 0.55±0.20 |
0.37±0.12b | 0.74±0.11 | 0.58±0.21 |
| LDL (mmol/l) | 0.26±0.02 | 0.23±0.04 | 0.22±0.03 | 0.24±0.07 | 0.25±0.08 | 0.26±0.03 |
0.32±0.01f |
| CK (U/l) | 2,059.0±584.5 | 2,541.6±811.2 | 1,816.8±575.2 |
966.3±617.5b |
815.7±689.7b | 1,929.3±1224.6 | 1,681.0±787.1 |
| CK-MB (U/l) | 486.0±114.4 | 470.8±111.9 | 642.40±216.13 | 271.33±161.47 | 358.20±350.24 | 452.50±68.25 | 370.80±52.08 |
| K+
(mmol/l) | 6.61±0.99 | 5.68±1.46 | 5.59±1.69 | 6.10±2.82 | 4.86±2.10 | 5.72±0.53 | 5.18±0.94 |
| Na+
(mmol/l) | 140.64±3.74 | 132.28±11.46 | 127.92±16.86 | 119.87±23.25 | 120.10±22.52 | 143.08±4.79 |
149.24±3.35d |
| Cl-
(mmol/l) | 109.88±4.43 | 105.68±8.89 | 101.83±13.63 | 98.44±16.09 | 93.42±23.34 | 111.67±4.55 |
117.60±3.10d |
Absolute and relative weight of
organs
As shown in Table V,
in female rats treated with 4 mg/kg AT-533, the absolute heart,
thymus and ovary weight was decreased in comparison with that in
the vehicle group. The absolute spleen weight decreased in female
rats at an AT-533 gel dose of 5 g/kg; however, this change was not
significant, as the value stayed within the normal reference ranges
(28,29). The brain-to-body, heart-to-body,
spleen-to-body, lung-to-body, kidney-to-body and adrenals-to-body
weight ratios were elevated, while the thymus-to-body weight ratio
was slightly decreased in female rats from the 4 mg/kg treatment
group in comparison with vehicle. As compared with the vehicle
group, the thymus-to-brain and ovary-to-brain weight ratios of
female rats in the 4 mg/kg treatment were slightly decreased.
 | Table VAbsolute and relative weights of
organs of female treated with AT-533 or AT-533 gel for 30 days. |
Table V
Absolute and relative weights of
organs of female treated with AT-533 or AT-533 gel for 30 days.
| A, Absolute
weights |
|---|
| Parameters | Control | Vehicle | 1 mg/kg | 2 mg/kg | 4 mg/kg | Blank gel | AT-533 gel |
|---|
| Body weight
(g) | 275.2±22.0 | 269.4±30.8 | 261.2±24.8 |
219.7±18.3a |
185.2±35.1c | 261.9±12.1 | 250.2±15.5 |
| Brain | 1.93±0.09 | 1.97±0.07 | 1.92±0.12 | 1.89±0.09 | 1.83±0.04 | 2.08±0.35 | 1.96±0.13 |
| Heart | 0.94±0.10 | 0.97±0.11 | 0.98±0.13 | 0.89±0.11 |
0.82±0.14b | 0.97±0.06 | 0.98±0.10 |
| Liver | 9.34±1.39 | 9.35±1.25 | 8.62±1.04 | 8.50±0.40 | 6.86±1.24 | 9.69±0.40 | 8.80±0.59 |
| Spleen | 0.60±0.07 | 0.54±0.08 | 0.56±0.06 | 0.46±0.04 | 0.44±0.08 | 0.62±0.09 |
0.50±0.06d |
| Lung | 1.20±0.16 | 1.12±0.06 | 1.20±0.09 | 1.06±0.13 | 1.08±0.10 | 1.18±0.04 | 1.24±0.18 |
| Kidneys | 1.83±0.40 | 1.71±0.19 | 1.72±0.24 | 1.51±0.18 | 1.57±0.21 | 1.96±0.10 | 1.71±0.10 |
| Adrenals | 0.068±0.015 | 0.080±0.013 | 0.081±0.006 | 0.066±0.010 | 0.073±0.008 | 0.090±0.012 | 0.066±0.012 |
| Thymus | 0.43±0.14 | 0.40±0.08 | 0.45±0.12 |
0.24±0.06a |
0.20±0.10a | 0.45±0.02 | 0.38±0.13 |
| Ovaries | 0.16±0.02 | 0.16±0.03 | 0.16±0.03 | 0.14±0.02 |
0.11±0.01b | 0.17±0.02 | 0.15±0.02 |
| Uterus | 0.52±0.11 | 0.57±0.12 | 0.64±0.12 | 0.48±0.16 | 0.40±0.24 | 0.61±0.16 | 0.58±0.22 |
| B, Organ-to-body
weight ratio (%) |
| Parameters | Control | Vehicle | 1 mg/kg | 2 mg/kg | 4 mg/kg | Blank gel | AT-533 gel |
| Brain | 0.71±0.07 | 0.74±0.08 | 0.74±0.09 | 0.87±0.10 |
1.02±0.21b | 0.79±0.15 | 0.79±0.07 |
| Heart | 0.34±0.03 | 0.36±0.01 | 0.37±0.03 |
0.41±0.02a |
0.44±0.03c | 0.37±0.02 | 0.39±0.04 |
| Liver | 3.39±0.42 | 3.48±0.03 | 3.31±0.04 | 3.88±0.27 | 3.71±0.27 | 3.70±0.12 | 3.53±0.28 |
| Spleen | 0.22±0.02 | 0.20±0.01 | 0.22±0.04 | 0.21±0.01 |
0.24±0.01b | 0.24±0.04 | 0.20±0.02 |
| Lung | 0.43±0.03 | 0.42±0.03 | 0.46±0.05 | 0.48±0.06 |
0.60±0.11c | 0.45±0.03 | 0.50±0.07 |
| Kidneys | 0.66±0.12 | 0.64±0.02 | 0.66±0.04 | 0.69±0.08 |
0.86±0.12c | 0.75±0.02 | 0.69±0.04 |
| Adrenals | 0.025±0.006 | 0.030±0.003 | 0.031±0.004 | 0.030±0.003 |
0.041±0.012a | 0.035±0.006 | 0.027±0.005 |
| Thymus | 0.16±0.06 | 0.15±0.02 | 0.17±0.03 |
0.11±0.02a |
0.10±0.04a | 0.17±0.01 | 0.15±0.05 |
| Ovaries | 0.060±0.009 | 0.061±0.009 | 0.062±0.012 | 0.062±0.009 | 0.061±0.014 | 0.062±0.009 | 0.058±0.009 |
| Uterus | 0.19±0.04 | 0.22±0.06 | 0.25±0.04 | 0.22±0.08 | 0.21±0.10 | 0.24±0.07 | 0.23±0.08 |
| C, Organ-to-body
weight ratio (g/g) |
| Parameters | Control | Vehicle | 1 mg/kg | 2 mg/kg | 4 mg/kg | Blank gel | AT-533 gel |
| Heart | 0.49±0.05 | 0.49±0.05 | 0.51±0.09 | 0.48±0.08 | 0.45±0.08 | 0.48±0.08 | 0.50±0.05 |
| Liver | 4.85±0.76 | 4.75±0.64 | 4.54±0.87 | 4.51±0.28 | 3.74±0.66 | 4.76±0.73 | 4.51±0.53 |
| Spleen | 0.31±0.04 | 0.27±0.04 | 0.30±0.04 | 0.25±0.03 | 0.24±0.04 | 0.31±0.07 | 0.26±0.04 |
| Lung | 0.62±0.10 | 0.57±0.02 | 0.63±0.08 | 0.56±0.08 | 0.59±0.05 | 0.58±0.07 | 0.63±0.08 |
| Kidneys | 0.95±0.21 | 0.87±0.08 | 0.90±0.15 | 0.80±0.10 | 0.85±0.11 | 0.96±0.15 | 0.88±0.04 |
| Adrenals | 0.035±0.006 | 0.041±0.006 | 0.042±0.005 | 0.035±0.006 | 0.040±0.004 | 0.045±0.010 | 0.034±0.007 |
| Thymus | 0.22±0.08 | 0.20±0.04 | 0.23±0.06 | 0.13±0.03 |
0.11±0.06a | 0.19±0.10 | 0.19±0.07 |
| Ovaries | 0.085±0.012 | 0.083±0.014 | 0.085±0.020 | 0.072±0.011 |
0.059±0.05a | 0.083±0.017 | 0.074±0.008 |
| Uterus | 0.27±0.05 | 0.29±0.06 | 1.92±0.12 | 1.89±0.09 | 1.83±0.04 | 0.29±0.04 | 0.29±0.11 |
The male rats exhibited a sharp drop in food
consumption and body weight in the 2 and 4 mg/kg AT-533 groups
compared with the vehicle group and the absolute weight of the main
organs, except for adrenal weight decreased; however, such
differences were not significant, as values stayed within the
normal reference ranges (28,29).
The absolute liver weight decreased in male rats at an AT-533 gel
dose of 5 g/kg in comparison with the vehicle while the weight of
other organs was not obviously changed after 30 days of
treatment.
For male rats treated with 2 mg/kg AT-533, the
brain-to-body, heart-to-body, spleen-to-body, and lung-to-body
weight ratios increased, while the thymus-to-body-weight ratio
decreased, as compared with the vehicle group. In comparison with
male rats in the vehicle group, the brain-to-body, heart-to-body,
lung-to-body, kidney-to-body, and testis-to-body weight ratios were
elevated, while the thymus-to-body weight ratio was decreased in
male rats treated with 4 mg/kg AT-533. Heart-to-body and
lung-to-body weight ratios were increased in male rats at an AT-533
gel dose of 5 g/kg in comparison with the vehicle. As compared with
their corresponding control groups, heart-to-brain (for male rats
treated with 2 and 4 mg/kg AT-533), liver-to-brain (for male rats
treated with 2, 4 mg/kg AT-533 and 5 g/kg AT-533 gel),
spleen-to-brain (for male rats treated with 4 mg/kg AT-533 and 5
g/kg AT-533 gel) and thymus-to-brain (for male rats treated with 2
and 4 mg/kg AT-533) weight ratios decreased (Table VI).
 | Table VIAbsolute and relative weights of
organs of male treated with AT-533 or AT-533 gel for 30 days. |
Table VI
Absolute and relative weights of
organs of male treated with AT-533 or AT-533 gel for 30 days.
| A, Absolute
weights |
|---|
| Parameters | Control | Vehicle | 1 mg/kg | 2 mg/kg | 4 mg/kg | Blank gel | AT-533 gel |
|---|
| Body weight
(g) | 379.5±36.1 | 400.5±24.0 | 421.2±32.0 |
250.4±47.7c |
198.7±24.0c | 388.3±32.1 |
317.5±68.9d |
| Brain | 1.97±0.17 | 2.05±0.10 | 2.12±0.10 | 1.99±0.05 | 1.87±0.13 | 2.07±0.11 | 1.93±0.16 |
| Heart | 1.26±0.15 | 1.41±0.22 | 1.41±0.13 |
1.10±0.11b |
0.91±0.15c | 1.29±0.12 | 1.20±0.11 |
| Liver | 13.70±1.26 | 14.29±1.71 | 14.95±2.23 |
9.17±0.73c |
7.20±1.83c | 14.87±1.90 |
11.01±2.66e |
| Spleen | 0.72±0.18 | 0.68±0.03 | 0.72±0.10 | 0.61±0.07 |
0.38±0.16c | 0.75±0.12 | 0.56±0.09 |
| Lung | 1.44±0.15 | 1.52±0.16 | 1.62±0.16 | 1.27±0.31 |
1.15±0.11b | 1.43±0.17 | 1.45±0.31 |
| Kidneys | 2.65±0.20 | 2.59±0.34 | 2.85±0.24 |
2.09±0.20a |
2.02±0.40b | 2.67±0.27 | 2.42±0.34 |
| Adrenals | 0.056±0.013 | 0.059±0.008 | 0.059±0.012 | 0.193±0.324 | 0.074±0.011 | 0.063±0.025 | 0.065±0.011 |
| Thymus | 0.47±0.06 | 0.45±0.06 | 0.55±0.10 |
0.11±0.09c |
0.10±0.06c | 0.41±0.11 | 0.29±0.13 |
| Testis | 2.93±0.35 | 3.09±0.31 | 3.21±0.43 | 2.57±0.43 |
2.21±0.64b | 3.15±0.38 | 3.14±0.18 |
| Epididymis | 1.35±0.23 | 1.10±0.20 | 1.12±0.19 | 1.10±0.76 | 0.64±0.27 | 1.49±0.78 | 1.31±0.26 |
| B, Organ-to-body
weight ratio (%) |
| Parameters | Control | Vehicle | 1 mg/kg | 2 mg/kg | 4 mg/kg | Blank gel | AT-533 gel |
| Brain | 0.52±0.03 | 0.51±0.05 | 0.51±0.05 |
0.82±0.14c |
0.95±0.10c | 0.53±0.04 | 0.63±0.12 |
| Heart | 0.33±0.02 | 0.35±0.04 | 0.34±0.03 |
0.45±0.07a |
0.46±0.08b | 0.33±0.02 |
0.39±0.05d |
| Liver | 3.62±0.23 | 3.56±0.29 | 3.54±0.35 | 3.73±0.48 | 3.59±0.62 | 3.82±0.31 | 3.46±0.28 |
| Spleen | 0.19±0.04 | 0.17±0.01 | 0.17±0.02 |
0.25±0.05b | 0.19±0.06 | 0.19±0.03 | 0.18±0.02 |
| Lung | 0.38±0.05 | 0.38±0.04 | 0.39±0.05 |
0.52±0.12a |
0.59±0.09c | 0.37±0.03 |
0.46±0.05d |
| Kidneys | 0.70±0.06 | 0.65±0.05 | 0.68±0.07 | 0.85±0.13 |
1.03±0.28c | 0.69±0.06 | 0.78±0.12 |
| Adrenals | 0.015±0.004 | 0.015±0.002 | 0.014±0.003 | 0.090±0.160 | 0.038±0.010 | 0.016±0.006 | 0.022±0.007 |
| Thymus | 0.12±0.02 | 0.11±0.02 | 0.13±0.02 |
0.04±0.03c |
0.03±0.03c | 0.11±0.03 | 0.09±0.02 |
| Testis | 0.77±0.03 | 0.77±0.09 | 0.76±0.07 | 1.03±0.10 |
1.13±0.39a | 0.83±0.09 | 1.02±0.18 |
| Epididymis | 0.36±0.05 | 0.28±0.05 | 0.26±0.04 | 0.42±0.21 | 0.32±0.10 | 0.39±0.28 | 0.42±0.10 |
| C, Organ-to-body
weight ratio (g/g) |
| Parameters | Control | Vehicle | 1 mg/kg | 2 mg/kg | 4 mg/kg | Blank gel | AT-533 gel |
| Heart | 0.64±0.06 | 0.69±0.12 | 0.67±0.07 |
0.55±0.05a |
0.48±0.06c | 0.62±0.04 | 0.63±0.08 |
| Liver | 6.96±0.60 | 6.99±0.90 | 7.07±1.10 |
4.61±0.31c |
3.83±0.87c | 7.17±0.74 |
5.71±1.40d |
| Spleen | 0.36±0.07 | 0.33±0.02 | 0.34±0.05 | 0.31±0.04 |
0.20±0.08b | 0.36±0.05 |
0.29±0.05a |
| Lung | 0.73±0.08 | 0.74±0.07 | 0.77±0.08 | 0.64±0.14 | 0.62±0.09 | 0.69±0.05 | 0.75±0.16 |
| Kidneys | 1.35±0.10 | 1.27±0.18 | 1.35±0.13 | 1.05±0.08 | 1.09±0.28 | 1.29±0.15 | 1.26±0.22 |
| Adrenals | 0.029±0.008 | 0.029±0.003 | 0.028±0.006 | 0.097±0.163 | 0.040±0.008 | 0.030±0.012 | 0.034±0.006 |
| Thymus | 0.24±0.03 | 0.22±0.02 | 0.26±0.05 |
0.06±0.05c |
0.03±0.03c | 0.17±0.09 | 0.15±0.07 |
| Testis | 1.48±0.11 | 1.51±0.15 | 1.52±0.20 | 1.29±0.19 | 1.20±0.41 | 1.52±0.15 | 1.63±0.10 |
| Epididymis | 0.69±0.11 | 0.54±0.10 | 0.53±0.08 | 0.55±0.37 | 0.35±0.16 | 0.73±0.42 | 0.68±0.12 |
Macroscopic and histopathological
examination
Prior to gross necropsy and anatomy analyses, the
appearance and morphology of rats were observed. In addition to the
symptoms described above, gaseous distention was observed in the
treatment groups (2, 4 mg/kg AT-533 and 5 g/kg AT-533 gel)
following anatomy analysis. Histopathological observations were
conducted for organs and tissues of all 30-day subacute toxicity
portion rats. Although some of the absolute and relative weights of
organs in rats from the treatment groups showed significant
differences, no obvious pathological lesions were found in the
organs described above, except for the liver. H&E staining
results indicated that no evident pathological lesions were
observed in the skin tissue (dosing site) of female and male rats
treated with saline, blank gel, vehicle or 1 mg/kg AT-533 (Fig. 3A, B,
D, E, H,
I, K and L).
However, male and female rats exhibited skin irritation and
infiltration of a few inflammatory cells following continuous 2
mg/kg AT-533 and 5 g/kg AT-533 gel administration, respectively. No
twisting and breaking of dermal elastic fibers was identified
(Fig. 3C, F, J and
M). In addition, the skin tissue of
female and male rats from the 4 mg/kg AT-533 group was
characterized by epidermal keratinization, thickening, twisting and
breaking of dermal elastic fibers, as well as infiltration of a
large number of inflammatory cells (Fig. 3G and N). Furthermore, liver tissue section
results indicated that a few hepatocytes exhibited signs of
vacuolar degeneration, while no hepatic edema, hyperemia or
necrotic cells were observed in the liver of female and male rats
treated with saline, vehicle, blank gel, 5 g/kg AT-533 gel or 1
mg/kg AT-533 (Fig. 4A-E and
H-L). In the 2 and 4 mg/kg AT-533
groups, liver edema, cytoplasmic vacuolar degeneration and slight
dilatation of blood vessels was observed, but no necrotic or
inflammatory cell infiltration was identified in the rat liver
(Fig. 4F, G, M and
N). Apart from these, there were no
pathological lesions in the heart, pancreas, spleen, lungs, adrenal
glands, kidneys, brain, esophagus, stomach, duodenum, mesenteric
lymphoid node, colon, spinal cord (cervical, thoracic and lumbar),
bone marrow, prostate, epididymis, testis, ovaries, uterus, mammary
gland, sciatic nerve, bladder, pituitary gland, trachea, thyroids,
thymus, salivary glands and optic nerve of rats (data no shown). In
combination, these results suggested that the AT-533 and AT-533 gel
used in this test did little damage to organs and tissues.
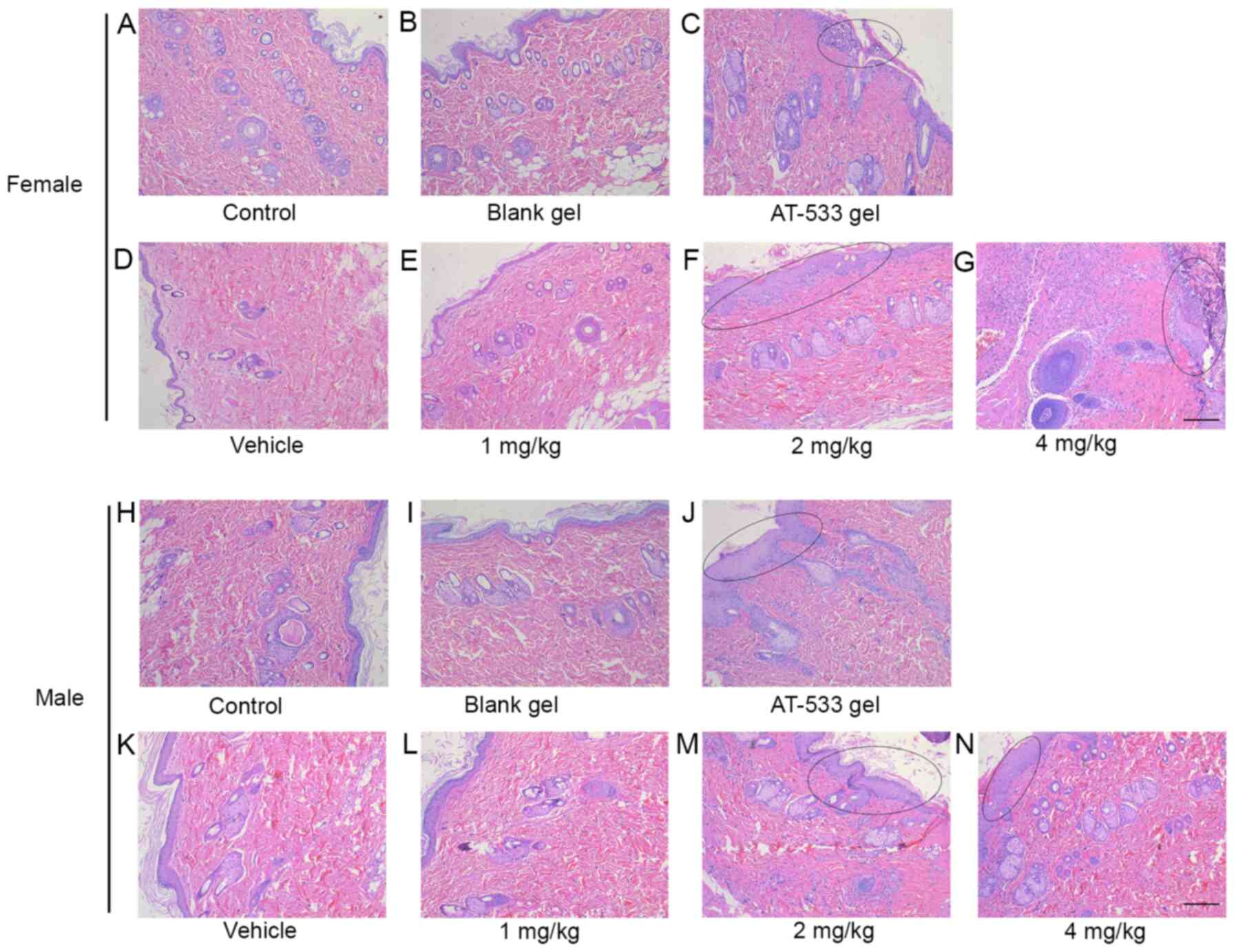 | Figure 3Histological analysis of rat skin
treated with AT-533 or AT-533 gel in the 30-day subacute toxicity.
Representative micrographs of skin sections from female rats under
the conditions of (A) control, (B) blank, (C) AT-533 gel, (D)
vehicle, (E) 1 mg/kg AT-533, (F) 2 mg/kg AT-533 and (G) 4 mg/kg
AT-533 stained with hematoxylin and eosin. Representative
micrographs of skin sections from male rats under the conditions of
(H) control, (I) blank, (J) AT-533 gel, (K) vehicle, (L) 1 mg/kg
AT-533, (M) 2 mg/kg AT-533 and (N) 4 mg/kg AT-533 hematoxylin and
eosin. The elliptical circle represents epidermal keratinization,
thickening and inflammatory cell infiltration. Magnification,
x10. |
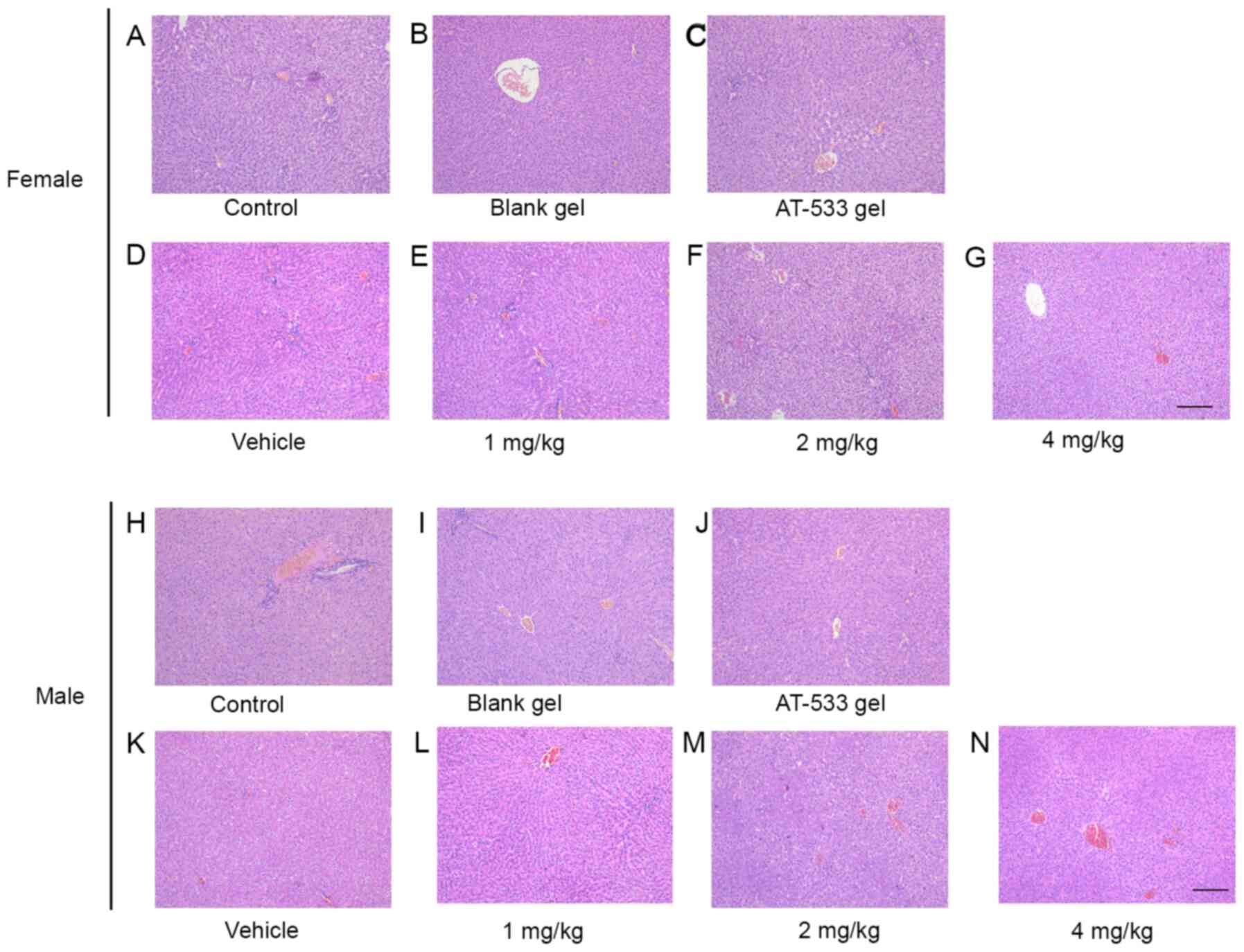 | Figure 4Histological analysis of rat livers
treated with AT-533 or AT-533 gel in the 30-day subacute toxicity
test. Representative micrographs from female rats under the
conditions of (A) control, (B) blank, (C) AT-533 gel, (D) vehicle,
(E) 1 mg/kg AT-533, (F) 2 mg/kg AT-533 and (G) 4 mg/kg AT-533
stained with hematoxylin and eosin. Representative micrographs from
male rats under the conditions of (H) control, (I) blank, (J)
AT-533 gel, (K) vehicle, (L) 1 mg/kg AT-533, (M) 2 mg/kg AT-533 and
(N) 4 mg/kg AT-533 hematoxylin and eosin. Magnification, x10. |
Discussion
Drug safety evaluation is an essential part of the
development of new drugs (22,30).
In the present study, subacute toxicity tests were conducted in
order to examine whether AT-533 or AT-533 gel produced adverse
reactions. To the best of our knowledge, this is the first report
demonstrating the safety of AT-533 and AT-533 gel through in
vivo toxicological evaluation. In the present study, the most
common symptoms resulting from the dermal administration of AT-533
and AT-533 gel were loss of appetite, skin irritation and
hypersensitivity, particularly in rats from the 2 and 4 mg/kg
AT-533 group rats. The weight loss in these rats may have been
induced by nutrient absorption inhibition. Unexpectedly, dermic
exposure to 5 g/kg AT-533 gel over a period of 30 days did not
influence food consumption. Although the body weight of rats from
the 5 g/kg AT-533 gel group was slightly lighter than that in rats
from the blank gel group, there were no significant differences
between them. It is known that blood parameters are key indicators
of physiological and pathological status, and therefore alterations
in them predict toxic effects of the test substances (31). The hematopoietic system is
considered to be the most sensitive target for toxicities, and its
index of physiological and pathological status is direct evidence
for toxicology studies (32).
Hematological analysis data suggested that, except for the WBC, NE
and PLT counts, other hematological parameters showed no
toxicologically significant differences. In rats from the 4 mg/kg
AT-533 group, the reason for the abnormal increase in the WBC and
NE counts was likely skin irritation caused by the dermal
administration of AT-533. During the drug exposure period, some
rats may have been uncomfortable, due to the stimulation of AT-533,
and used their front paws to scratch the skin continuously in order
to feel comfortable. As a result, continuous scratching caused skin
damage and bleeding, leading to inflammation, as characterized by
the abnormal increased numbers of WBC and NE (33). This phenomenon was
concentration-dependent and rats in the 4 mg/kg AT-533 group were
more severely affected than other medication-administration groups.
Similarly, the abnormal increase in the number of PLT was related
to the skin bleeding in male rats at an AT-533 dose of 2 and 4
mg/kg.
To evaluate the influence of AT-533 and AT-533 gel
on organ (heart, liver and kidney) functions, blood biochemical
examinations were carried out on day 30 of the drug exposure and
recovery periods (data not shown). The level of ALB was abnormally
decreased and the liver enzyme AST was relatively increased when
female rats were administered 4 mg/kg AT-533. Furthermore, the
levels of ALB, CHO and HDL were abnormally decreased when male rats
were administered 4 mg/kg AT-533. The reason for the abnormal
increase of the BUN level may be either an excessive production of
BUN (due to high protein absorption from diet, diabetes, severe
liver disease or high fever) or excretion disorder of BUN (such as
mild renal dysfunction, hypertension, gout, multiple myeloma,
urinary tract occlusion or postoperative oliguria Wait) (34). In addition to BUN, CRE is another
biomarker of renal function (35)
and the abnormal increase of the CRE level indicates excretory
dysfunction associated with renal failure. However, there was no
abnormal change in the level of CRE. Furthermore, no abnormalities
were identified in the kidney, heart and spleen tissue sections by
H&E staining (Fig. S1,
Fig. S2 and S3). These conflicting results between the
blood biochemical indexes and histopathological examinations may
have been due to several reasons; a main one may be a decrease in
food consumption. Following the administration of 2 or 4 mg/kg
AT-533 to rats, they showed loss of appetite and flatulence.
Long-term malnutrition would lead to reduced levels of ALB and CHO
to some extent.
Organ weight and organ coefficient are important
indicators for evaluating the toxic effects of test substances in
preclinical toxicology studies of new drugs, and have a high
reference value for target organ confirming drug toxicity (22,30).
The absolute weight of the major organs was altered in rats from
the high-dose (2 or 4 mg/kg) AT-533 groups, but stayed within the
normal reference ranges (28,29).
The relative weight of most organs (brain, heart, spleen, lung,
kidney, and thymus) in the 4 mg/kg group was changed, but no
abnormalities were observed by pathological examination. These
conditions may have been due to the weight loss of the rats at an
AT-533 dose of 4 mg/kg.
In conclusion, the lowest observed adverse effect
level was induced by 1 mg/kg daily exposure to AT-533 during a
30-day subacute toxicity test; thus, the
no-observed-adverse-effect-level (NOAEL) was <1 mg/kg. The
results of the 30-day subacute toxicity test of AT-533 gel
indicated that 5 g/kg AT-533 gel did not cause lethality in either
male or female rats; it only caused a slight body weight decrease
and skin irritation. Therefore, the NOAEL of AT-533 gel was <5
g/kg. The effective dose of AT-533 gel is <5 g/kg and AT-533 gel
at a therapeutic dose was not toxic to the mice (18,19).
In the future work, whether AT-533 gel at a therapeutic dose will
have toxic effects on rats will be confirmed. The aforementioned
results provide a reference for subsequent subchronic and chronic
toxicity tests of AT-533 and AT-533 gel. In our next subchronic and
chronic toxicological study, considering the differences
hematological values and organ weights between male and female rat,
the sex of animals will be taken into consideration.
Supplementary Material
Histological analysis of rat kidneys
treated with AT-533 or AT-533 gel in the 30-day subacute toxicity.
Representative micrographs from female rats under the conditions of
(A) control, (B) blank, (C) AT-533 gel, (D) vehicle, (E) 1 mg/kg
AT-533, (F) 2 mg/kg AT-533 and (G) 4 mg/kg AT-533 stained with
hematoxylin and eosin. Representative micrographs from male rats
under the conditions of (H) control, (I) blank, (J) AT-533 gel, (K)
vehicle, (L) 1 mg/kg AT-533, (M) 2 mg/kg AT-533 and (N) 4 mg/kg
AT-533 hematoxylin and eosin. Magnification, x20.
Histological analysis of rat hearts
treated with AT-533 or AT-533 gel in the 30-day subacute toxicity.
Representative micrographs from female rats under the conditions of
(A) control, (B) blank, (C) AT-533 gel, (D) vehicle, (E) 1 mg/kg
AT-533, (F) 2 mg/kg AT-533 and (G) 4 mg/kg AT-533 stained with
hematoxylin and eosin. Representative micrographs from male rats
under the conditions of (H) control, (I) blank, (J) AT-533 gel, (K)
vehicle, (L) 1 mg/kg AT-533, (M) 2 mg/kg AT-533 and (N) 4 mg/kg
AT-533 hematoxylin and eosin. Magnification, x20.
Histological analysis of rat spleen
treated with AT-533 or AT-533 gel in the 30-day subacute toxicity.
Representative micrographs from female rats under the conditions of
(A) control, (B) blank, (C) AT-533 gel, (D) vehicle, (E) 1 mg/kg
AT-533, (F) 2 mg/kg AT-533 and (G) 4 mg/kg AT-533 stained with
hematoxylin and eosin. Representative micrographs from male rats
under the conditions of (H) control, (I) blank, (J) AT-533 gel, (K)
vehicle, (L) 1 mg/kg AT-533, (M) 2 mg/kg AT-533 and (N) 4 mg/kg
AT-533 hematoxylin and eosin. Magnification, x20.
Acknowledgements
The authors would like to thank Mr. Xinlu Fu (Sun
Yat-sen University Experimental Animal Center) for technical
guidance during the animal experiments, Dr Kai Zheng (Shenzhen
University) for revising the manuscript spelling and grammar and
Mr. Hui Chen (Guangzhou University of Chinese Medicine) for the
care and encouragement of the experimental progress.
Funding
Funding: This work was supported by grants from the Key
Laboratory of Virology of Guangzhou (grant no. 201705030003) and
Guangzhou Major Program of the Industry-University-Research
collaborative innovation (grant nos. 201604020178 and
201704030087).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YH and YiW designed the study. YaW, XS, SQ, PC, LH,
QW, TS, FL, XL and QL performed the experiments. YaW and XS
performed the statistical analysis. YH, YiW, YaW and XS confirmed
the legitimacy and authenticity of all raw data. YaW drafted the
manuscript. SQ, PC and LH made significant conceptual contributions
to the manuscript. YH and YiW reviewed the final version of the
paper. All the authors provided intellectual content and approved
the final version of the manuscript.
Ethics approval and consent to
participate
All applicable international, national, and/or
institutional guidelines for the care and use of animals were
followed and the study protocols were approved by The Institutional
Animal Care and Use Committee of Jinan University (approval no.
IACUC-2020-000038).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pearl LH and Prodromou C: Structure and
mechanism of the Hsp90 molecular chaperone machinery. Annu Rev
Biochem. 75:271–294. 2006.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Goetz MP, Toft DO, Ames MM and Erlichman
C: The Hsp90 chaperone complex as a novel target for cancer
therapy. Ann Oncol. 14:1169–1176. 2003.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zininga T, Ramatsui L and Shonhai A: Heat
shock proteins as immunomodulants. Molecules.
23(2846)2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Whitesell L and Lindquist SL: HSP90 and
the chaperoning of cancer. Nat Rev Cancer. 5:761–772.
2005.PubMed/NCBI View
Article : Google Scholar
|
|
5
|
Hoter A, El-Sabban ME and Naim HY: The
HSP90 family: Structure, regulation, function, and implications in
health and disease. Int J Mol Sci. 19(2560)2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Workman P: Pharmacogenomics in cancer drug
discovery and development: Inhibitors of the Hsp90 molecular
chaperone. Cancer Detect Prev. 26:405–410. 2002.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Schulte TW, Akinaga S, Soga S, Sullivan W,
Stensgard B, Toft D and Neckers LM: Antibiotic radicicol binds to
the N-terminal domain of Hsp90 and shares important biologic
activities with geldanamycin. Cell Stress Chaperones. 3:100–108.
1998.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Vilenchik M, Solit D, Basso A, Huezo H,
Lucas B, He H, Rosen N, Spampinato C, Modrich P and Chiosis G:
Targeting wide-range oncogenic transformation via PU24FCl, a
specific inhibitor of tumor Hsp90. Chem Biol. 11:787–797.
2004.PubMed/NCBI View Article : Google Scholar
|
|
9
|
He H, Zatorska D, Kim J, Aguirre J,
Llauger L, She Y, Wu N, Immormino RM, Gewirth DT and Chiosis G:
Identification of potent water soluble purine-scaffold inhibitors
of the heat shock protein 90. J Med Chem. 49:381–390.
2006.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Llauger L, He H, Kim J, Aguirre J, Rosen
N, Peters U, Davies P and Chiosis G: Evaluation of 8-arylsulfanyl,
8-arylsulfoxyl, and 8-arylsulfonyl adenine derivatives as
inhibitors of the heat shock protein 90. J Med Chem. 48:2892–2905.
2005.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kasibhatla SR, Hong K, Biamonte MA, Busch
DJ, Karjian PL, Sensintaffar JL, Kamal A, Lough RE, Brekken J,
Lundgren K, et al: Rationally designed high-affinity
2-amino-6-halopurine heat shock protein 90 inhibitors that exhibit
potent antitumor activity. J Med Chem. 50:2767–2778.
2007.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Pearl LH: Review: The HSP90 molecular
chaperone-an enigmatic ATPase. Biopolymers. 105:594–607.
2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Prodromou C and Pearl LH: Structure and
functional relationships of Hsp90. Curr Cancer Drug Targets.
3:301–323. 2003.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Huang KH, Veal JM, Fadden RP, Rice JW,
Eaves J, Strachan JP, Barabasz AF, Foley BE, Barta TE, Ma W, et al:
Discovery of novel 2-aminobenzamide inhibitors of heat shock
protein 90 as potent, selective and orally active antitumor agents.
J Med Chem. 52:4288–4305. 2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Barta TE, Veal JM, Rice JW, Partridge JM,
Fadden RP, Ma W, Jenks M, Geng L, Hanson GJ, Huang KH, et al:
Discovery of benzamide tetrahydro-4H-carbazol-4-ones as novel small
molecule inhibitors of Hsp90. Bioorg Med Chem Lett. 18:3517–3521.
2008.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wang S, Wang X, Du Z, Liu Y, Huang D,
Zheng K, Liu K, Zhang Y, Zhong X and Wang Y: SNX-25a, a novel Hsp90
inhibitor, inhibited human cancer growth more potently than 17-AAG.
Biochem Biophys Res Commun. 450:73–80. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Li F, Jin F and Wang Y, Zheng D, Liu J,
Zhang Z, Wang R, Dong D, Zheng K and Wang Y: Hsp90 inhibitor AT-533
blocks HSV-1 nuclear egress and assembly. J Biochem. 164:397–406.
2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wang Y, Wang R, Li F and Wang Y, Zhang Z,
Wang Q, Ren Z, Jin F, Kitazato K and Wang Y: Heat-shock protein 90α
is involved in maintaining the stability of VP16 and VP16-mediated
transactivation of α genes from herpes simplex virus-1. Mol Med.
24(65)2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Xiang YF, Qian CW, Xing GW, Hao J, Xia M
and Wang YF: Anti-herpes simplex virus efficacies of
2-aminobenzamide derivatives as novel HSP90 inhibitors. Bioorg Med
Chem Lett. 22:4703–4706. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Li F, Song X, Su G and Wang Y, Wang Z,
Qing S, Jia J and Wang Y, Huang L, Zheng K and Wang Y: AT-533, a
Hsp90 inhibitor, attenuates HSV-1-induced inflammation. Biochem
Pharmacol. 166:82–92. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhang X, Cheng Y, Zhou Q, Huang H, Dong Y,
Yang Y, Zhao M and He Q: The effect of Chinese traditional medicine
huaiqihuang (HQH) on the protection of nephropathy. Oxid Med Cell
Longev. 2020(2153912)2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
SFDA: Technical guidelines of chronic
toxicity for chemical drugs. SFDA Guidelines No. [H]
GPT2-12005.
|
|
23
|
Chinese Pharmacopoeia Commission: Chinese
pharmacopoeia. China Medical Science and Technology Press, Beijing,
pp291-353, 2015.
|
|
24
|
Li CL, Shu XJ, Chen XQ, Liu YW, Yi HL and
Ma BM: Determination and comparison of blood physiological and
biochemical indicators in SPF SD rats of different ages and
genders. J Jianghan Univ (Nat Sci Ed). 44:58–63. 2016.
|
|
25
|
Wang R, Wen XT, Wang H, Tang JL, Guan B
and Zeng YL: Establishment of blood routine reference interval in
SD rats. Med Equip. 28:11–15. 2015.
|
|
26
|
Wang LM, Wang YH, Han XF, Yang RH and Cao
X: Determination and analysis of blood biochemical indicators in SD
rats of different ages and genders. Heilongjiang Anim Sci Vet Med.
214-216:268–269. 2018.
|
|
27
|
He XY, Wang H, Tang JL, Guan B, Zeng YL
and Shi JF: Preliminary establishment of normal interval of blood
biochemical indicators in SD rats. Med Equip. 28:13–15. 2015.
|
|
28
|
Dong YS, Ying JY, Chen C, Zhai WS, Yuan
BL, Gao XX, Ding RG and Wang HM: Establishment and application of
the normal reference values of organ masses and organ/body
coefficients in SD rats. Mil Med Sci. 36:351–353. 2012.
|
|
29
|
Liu YE, Yao BY, Yang DH and Wang J:
Analysis on organ weights and their changes trend in Sprague Dawley
rats with different ages. Chin J Comp Med. 22:22–27. 2012.
|
|
30
|
SFDA: Technical guidelines of acute
toxicity testing for chemical drugs. SFDA Guidelines No. [H]
GPT1-12005.
|
|
31
|
Bigoniya P, Sahu T and Tiwari V:
Hematological and biochemical effects of sub-chronic artesunate
exposure in rats. Toxicol Rep. 2:280–288. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Tan YJ, Ren YS, Gao L, Li LF, Cui LJ, Li
B, Li X, Yang J, Wang MZ, Lv YY, et al: 28-Day oral chronic
toxicity study of arctigenin in rats. Front Pharmacol.
9(1077)2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Yao Q, Sun R, Bao S, Chen R and Kou L:
Bilirubin protects transplanted islets by targeting ferroptosis.
Front Pharmacol. 11(907)2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Maher PA: Using plants as a source of
potential therapeutics for the treatment of Alzheimer's disease.
Yale J Biol Med. 93:365–373. 2020.PubMed/NCBI
|
|
35
|
Charoonratana T, Puntarat J,
Vinyoocharoenkul S, Sudsai T and Bunluepuech K: Innocuousness of a
polyherbal formulation: A case study using a traditional Thai
antihypertensive herbal recipe in rodents. Food Chem Toxicol.
112:458–465. 2018.PubMed/NCBI View Article : Google Scholar
|