Introduction
Bone mass is maintained in a perfect balance between
osteoclastic bone resorption and osteoblastic bone formation in the
standard physiological situation (1). Overactivation of osteoclasts is a
significant cause of excessive bone loss in diseases, such as
osteoporosis (1). Studies on
osteoclast differentiation have revealed that several cytokines,
including macrophage colony-stimulating factor (M-CSF), receptor
activator of nuclear factor-κB ligand (RANKL), interleukin-1 (IL-1)
and tumor necrosis factor-α (TNF-α) regulate the differentiation
process subtly (2,3).
Osteoclasts are short-lived and terminally
differentiated cells that cannot be passaged and they are
relatively difficult to obtain (4).
To date, RAW264.7 cells and bone marrow-derived macrophages (BMMs)
are widely adopted in studies to differentiate into osteoclasts
(4-6).
RAW264.7 cells are murine macrophage cells that need RANKL to
complete osteoclast differentiation and are a type of osteoclast
precursors, which at a later stage of differentiation are
comparable with BMMs (7).
In vitro osteoclast generation
(osteoclastogenesis) consists of several steps: i) Hematopoietic
macrophage differentiation into osteoclast precursors induced by
M-CSF; ii) precursor cells development into mononuclear osteoclasts
in the presence of RANKL, IL-1, etc.; iii) mononuclear
preosteoclasts fusion into multinuclear osteoclasts; and iv)
activation and maturation of osteoclasts. The underlying mechanisms
of osteoclastogenesis have been partly unveiled, including the
RANKL-signaling pathway and RANKL-independent signaling pathway
(8).
RANKL/RANK interaction has been considered a
canonical pathway of osteoclastogenesis (9,10).
RANKL is a TNF ligand superfamily member (2). RANKL binds to the receptor nuclear
factor-κB (RANK) and recruits TNF receptor-related factors, such as
TRAF6, and initiates a downstream signaling cascade (11). This downstream signaling cascade
promotes expression of several osteoclastic transcriptional
factors, such as nuclear factor of activated T cells 1 (NFATc1) and
induces the expression of osteoclast-associated genes, including
matrix metalloprotein-9 (MMP-9), cathepsin K (CTSK),
tartrate-resistant acid phosphatase (TRAP), hence, the RANKL/RANK
axis is essential for osteoclastogenesis (12).
IL-1 has an essential role in various bone diseases
which are associated with overactivation of osteoclasts, including
osteoporosis, rheumatoid arthritis and periodontal disease
(13-15).
Besides indirectly stimulating osteoblast/stromal cells, IL-1α and
IL-1β can act on specific steps of osteoclast differentiation in
vitro by binding to IL-1RI with equal affinity (16). IL-1α and IL-1β facilitate the cell
fusion of mononuclear and activation of multinucleated osteoclasts,
but are not involved in the differentiation of osteoclast
precursors to mononuclear osteoclasts (17).
Previous published studies have revealed that
although IL-1 can activate osteoclast maturation and enhance bone
resorption, it alone cannot initiate the process of osteoclast
differentiation from osteoclast precursors (18,19).
Kim et al (20) however,
suggested that IL-1 can induce osteoclast differentiation without
the interaction of RANKL/RANK in the context of appropriate
microenvironmental conditions. The present study aimed to provide
stronger evidence for whether IL-1 could induce osteoclastogenesis
without the stimulation of RANKL by performing several qualitative
and quantitative experiments using the RAW264.7 macrophages cell
line.
Materials and methods
Cell culture and treatment
The RAW264.7 cell line, a murine macrophage cell
line, was obtained from the Zhong Qiao Xin Zhou Biotechnology Co.,
Ltd. The cells were cultured in α-MEM (Gibco; Thermo Fisher
Scientific Inc.) supplemented with antibiotics (1%
penicillin/streptomycin) and 10% fetal bovine serum (FBS; Gibco
Thermo Fisher Scientific Inc.). In every assay, the cells were
classified into 4 groups: i) Control (untreated cells); ii) IL-1
(10 ng/ml) (20,21); iii) RANKL (50 ng/ml); and iv) IL-1
(10 ng/ml)+RANKL (50 ng/ml). Soluble RANKL (PeproTech Inc.) or IL-1
(PeproTech Inc.) was added to the culture medium at room
temperature 12 h after RAW264.7 cells were seeded into wells.
First, the IL-1 and/or RANKL solution was added into fresh medium,
then it was used to replace the old medium. The cells were cultured
in a 5% CO2 humidified incubator at 37˚C and the medium
was refreshed every other day.
Cell viability assay
Cell viability was measured using the Cell Counting
Kit-8 (CCK-8; Dojindo Molecular Technologies Inc.) according to the
manufacturer's protocol. RAW 264.7 cells were cultured in a 96-well
plates at a density of 1x103 cells/well. The former
medium was replaced by 110 µl of fresh α-MEM containing 10 µl CCK-8
solution for 2 h prior to determination of cell viability.
Subsequently, a wavelength of 450 nm was used to determine the cell
viability. The CCK-8 assay was performed on the 4 previously
mentioned cell groups every 24 h (0, 24, 48, 72 and 96 h).
TRAP staining assay
RAW264.7 cells were seeded at a density of
1.5x104 cells/well into 24-well culture plate with a
matched cell slide in each well. After cell culture for 4 days, the
cells were first washed with PBS 3 times and then fixed for 45 sec
at 4˚C. The TRAP staining kit (Nanjing Fengfeng Biomedical
Technology Co., Ltd.) was used to count the number of mature
osteoclasts. The staining process was accomplished at 37˚C in the
dark for 45 min. The cell culture plate was observed under an
inverted light microscope and TRAP-positive cells (≥3 nuclei/cell)
were identified as mature osteoclasts. A total of 5 random views
were selected and the amount of mature osteoclasts was counted
manually. Subsequently, the measurement of TRAP activity was
detected at 540 nm wavelength and the results were presented as
expression related to control.
Western blotting
RAW264.7 cells were seeded into 6-well plates
(1.5x105 cells/well) and then incubated for 4 days after
stimulation with IL-1 and/or RANKL as aforementioned. Subsequently,
proteins were extracted with RIPA buffer (Nanjing Fengfeng
Biomedical Technology Co., Ltd.) and the protein concentration was
quantified using a bicinchoninic acid protein assay kit (Bioworld
Technology Inc.). Protein (30 µg/lane) was loaded onto 10% SDS PAGE
gels and was transferred onto the PVDF membrane (Merck & Co.,
Inc.). The membranes were blocked for 1 h at room temperature with
5% skimmed milk and then incubated with primary antibodies for
MMP-9 (cat. no. 10375-2-AP; 1:1,000; ProteinTech Group Inc.), TRAP
(cat. no. 10325-1-AP; 1:3,000; ProteinTech Group Inc.), anti-IL-1RI
(cat. no. orb499639; 1:2,000; ProteinTech Group Inc.), NFATc1 (cat.
no. 8032S; 1:1,000; Cell Signaling Technology, Inc.), β-actin (cat.
no. 20536-1-AP; 1:4,000; ProteinTech Group Inc.) or CTSK (cat. no.
11239-1-AP; 1:1,000; ProteinTech Group Inc.) at 4˚C overnight.
β-actin was used as the loading control. Subsequently, the membrane
was washed with TBS with 0.1% Tween-20 (TBST) 3 times and incubated
for 1 h at room temperature with horseradish peroxidase-conjugated
Affinipure Goat Anti-Rabbit IgG secondary antibody (cat. no.
SA00001-2; 1:6,000; ProteinTech Group Inc.). The membrane was
washed 3 times with TBST at room temperature and soaked in the
enhanced chemiluminescence kit (Santa Cruz Biotechnology Inc.).
Finally, the bands were detected by the Tanon Imaging System (Tanon
Science and Technology Co., Ltd.).
RNA extraction and reverse
transcription-quantitative (RT-q) PCR
RAW264.7 cells were seeded into 6-well plates
(1x105 cells/well). Following 4 days of stimulation with
IL-1 and/or RANKL as aforementioned, total intracellular RNA was
obtained by acid guanidinium thiocyanate-phenol-chloroform method
(22), the whole extraction process
was completed on ice to avoid degradation, and single-stranded cDNA
was synthesized using the Prime Script RT kit (Takara Biotechnology
Co, Ltd.) according to the manufacturer's protocol. qPCR was
performed using a SYBR Green-1 kit (Takara Biotechnology Co, Ltd.)
and the ABI 7500 real-time PCR system (Thermo Fisher Scientific
Inc.). The thermocycling conditions were as follows: Initial
denaturation for 30 sec at 95˚C; 40 cycles of denaturation for 10
sec at 95˚C and extension for 30 sec at 60˚C. The relative mRNA
expression of each gene was calculated using the 2-ΔΔCq
method (23) and was normalized to
GAPDH (6). The primers were
purchased from Generay Biotech Co., Ltd. The primer sequences used
were as follows: MMP-9 forward, 5'-GCAGAGGCATACTTGTACCG-3' and
reverse, 5'-TGATGTTATGATGGTCCCACTTG-3'; CTSK forward,
5'-GTTACTCCAGTCAAGAACCAGG-3' and reverse,
5'-TCTGCTGCACGTATTGGAAGG-3'; GAPDH forward,
5'-AGGTCGGTGTGAACGGATTTG-3' and reverse, 5'-GGGGTCGTTGATGGCAACA-3'
(24); TRAP forward,
5'-CTTGCGACCATTGTTAGC-3' and reverse, 5'-TTCTCGTCCTGAAGATACTG-3';
and NFATc1 forward, 5'-CAACGCCCTGACCACCGATAG-3' and reverse,
5'-GGCTGCCTTCCGTCTCATAGT-3' (25).
Resorption pit assay
Corning Osteo Assay Surface 96-well plates (Corning
Inc.) were used to examine the ability of bone resorption. The
plates are coated with inorganic polystyrene, which is a bone
biomimetic synthetic surface (26,27).
The RAW264.7 cells were seeded into a 96-well plate
(1x103 cells/well) and treated with IL-1 (10 ng/ml),
RANKL (50 ng/ml), or IL-1 (10 ng/ml)+RANKL (50 ng/ml) as
aforementioned. The IL-1/RANKL solution was re-added on the 4th day
when medium was refreshed. After 8 days of stimulation, cells were
removed using 10% sodium hypochlorite solution and stained with 1%
toluidine blue at room temperature for 30 min. Subsequently, the
plates were washed 3 times with distilled water and the area of
resorption pit was photographed using a light microscope. The
relative level of resorption area was measured though pixels area
analysis via ImageJ software (Version 1.8.0; National Institutes of
Health). The resorption area for the treatment groups was
normalized by using the pixel area in the control group (27,28).
Statistics
Each experiment was performed at least 3 times and
the data were presented as the mean ± standard deviation (SD). The
results were analyzed using one-way analysis of variance with
subsequent post hoc Tukey's tests using SPSS 26 software (IBM
Corp.). P<0.05 was considered to indicate a statistically
significant difference.
Results
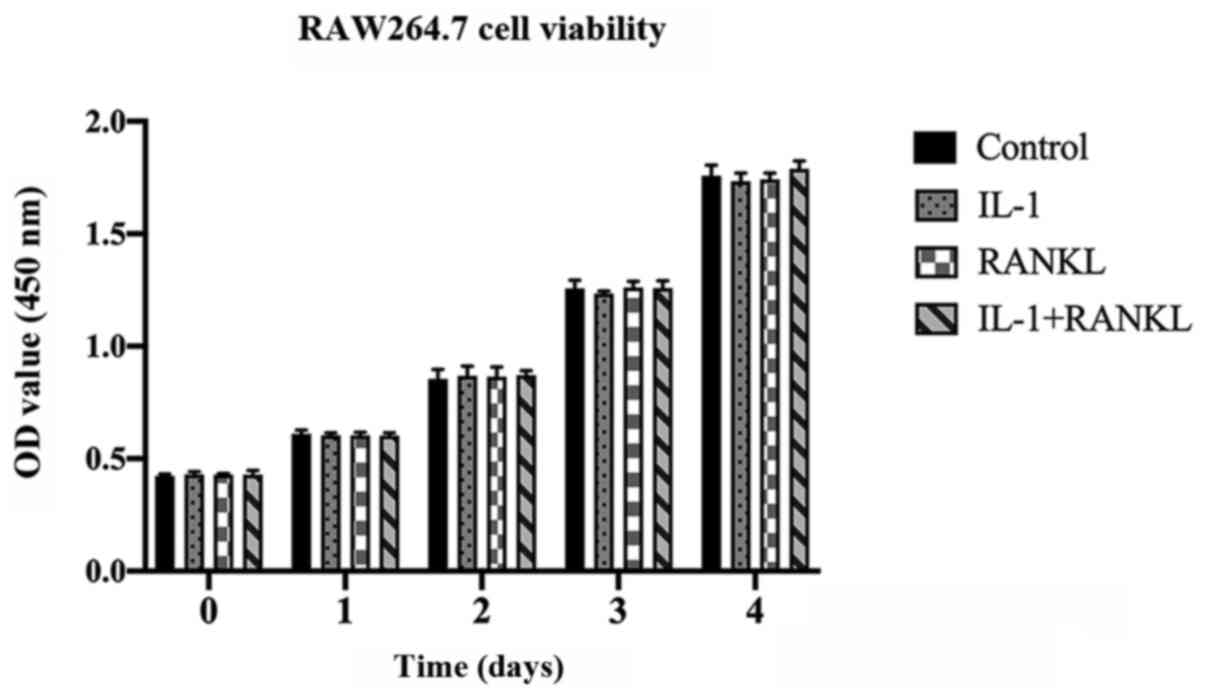
Cell viability of RAW264.7 cells is
not altered by IL-1/RANKL
Effects of IL-1 and/or RANKL on the cell viability
was examined by a CCK-8 assay following stimulation. The RAW264.7
cells were treated with or without IL-1 (10 ng/ml) and/or RANKL (50
ng/ml) for 4 days and no significant differences were found between
groups at the indicated days (P>0.05) (Fig. 1).
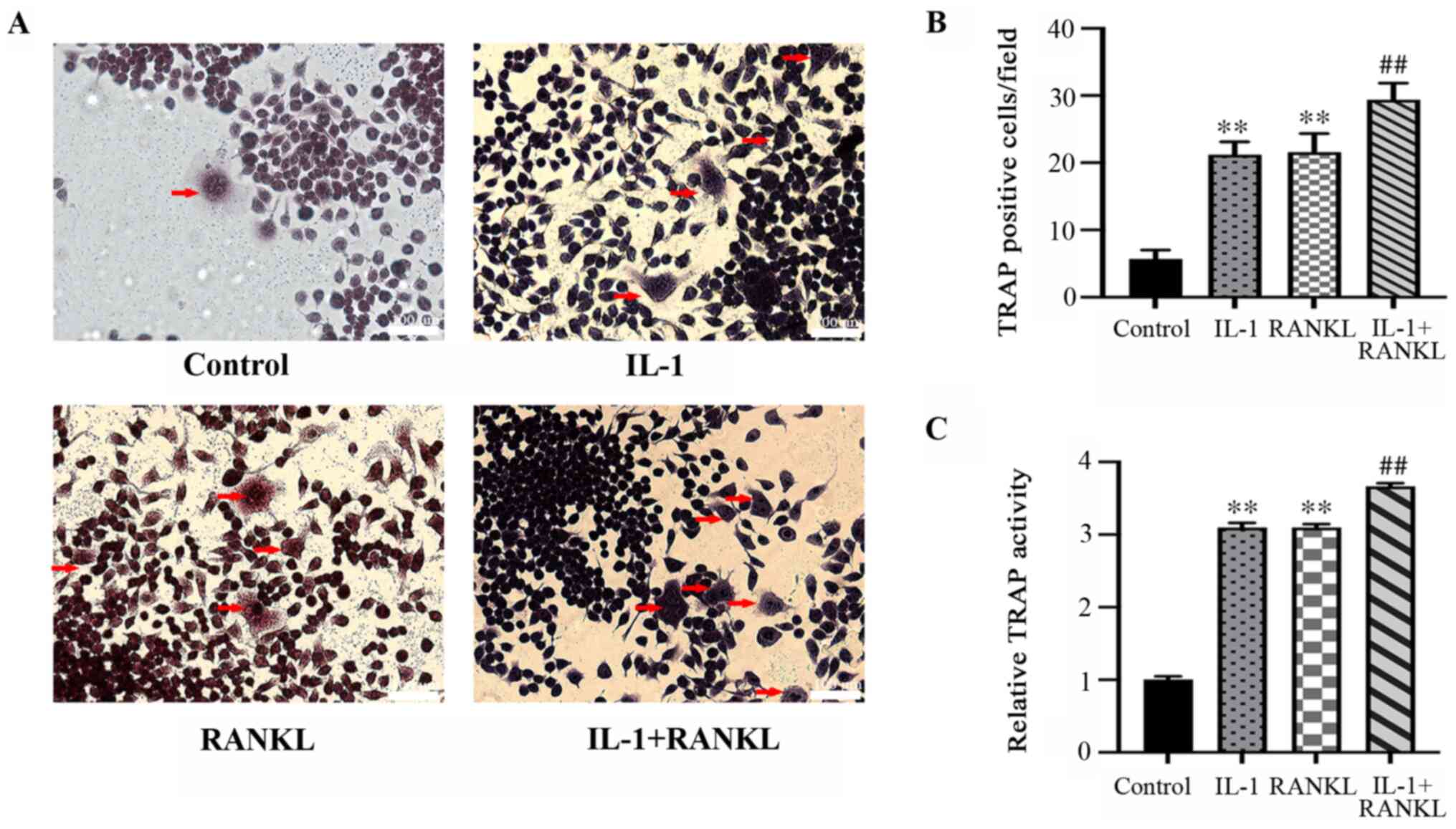
Osteoclast formation is promoted by
IL-1/RANKL
Osteoclast formation was evidenced by
TRAP+ multinuclear cells. Significantly more
TRAP+ cell were formed after stimulation of IL-1 or
RANKL compared with the control group (P<0.01), while there was
no statistically significant difference between the IL-1-treated
and RANKL-treated group (P>0.05) (Fig. 2A and B). The quantity of TRAP+
osteoclasts was significantly higher in the 2-stimulus group
(IL-1+RANKL) compared with the single-stimulus groups (IL-1/RANKL)
(P<0.01) (Fig. 2A and B). The results of TRAP activity (Fig. 2C) were in agreement with the
staining results (P<0.01) (Fig.
2A and B).
Expression of
osteoclastogenesis-specific genes and proteins are elevated in
IL-1/RANKL-treated cells
To further demonstrate the role of IL-1 in
osteoclast differentiation, the expressions of
osteoclastogenesis-related genes (NFATc1, MMP-9, CTSK and TRAP) and
IL-1RI were examined by western blotting and RT-qPCR analysis 4
days after stimulation. RAW264.7 cells exhibited substantial
protein expression of IL-1RI in the control group and compared with
other groups, the IL-1+RANKL group showed the highest expression of
IL-1RI (Fig. 3B). Compared with the
control group, IL-1 promoted the protein and gene expression of
MMP-9, NFATc1, CTSK and TRAP (P<0.01), which was comparable to
the results of RANKL-treated group (P<0.01) (Fig. 3A and C-G). In addition, compared with the
IL-1-treated group, the expressions of osteoclastogenesis-specific
genes were significantly higher in the 2-stimulus group (P<0.05
for TRAP and CTSK expression, P<0.01 for NFATc1 and MMP-9
expression) (Fig. 3A and C-G).
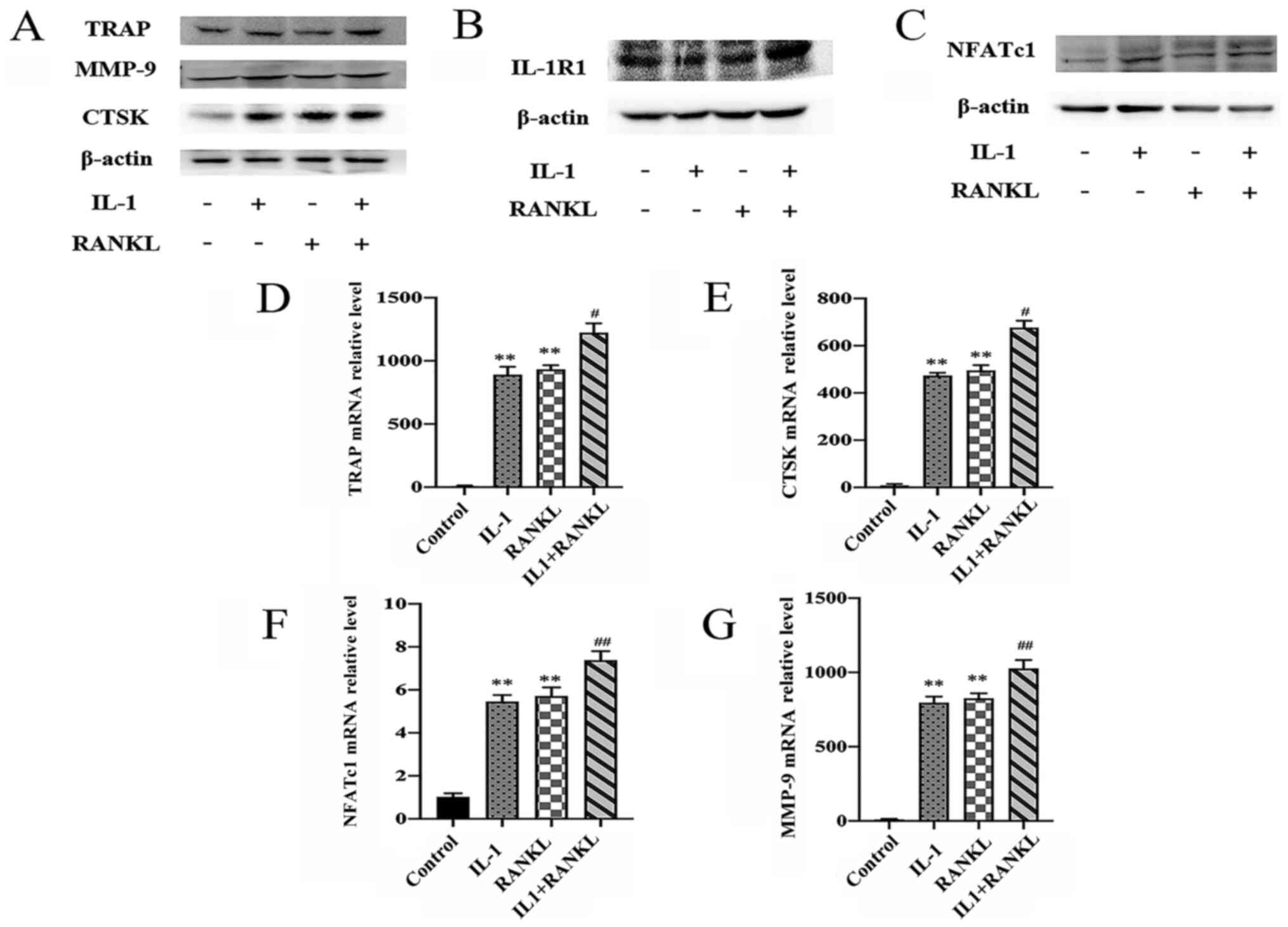 | Figure 3IL-1 promotes the expression of
osteoclastogenesis-specific genes. RAW264.7 cells were treated with
IL-1 (10 ng/ml) and/or RANKL (50 ng/ml) for 4 days. (A) Expressions
of osteoclastogenesis-specific genes [MMP-9, CTSK, TRAP and NFATc1
(C)] and IL-1RI (B) were determined by western blotting. The
exposure time of TRAP, IL-1RI and MMP-9 was 10 sec, the exposure
time of NFATc1 was 60 sec and the exposure time of other blots was
5 sec. IL-1RI and NFATc1 were from different gels, TRAP, MMP-9 and
CTSK were from the same gel. (D-G) RT-qPCR expression in the 4
groups of RAW 264.7 cells (control, IL-1, RANKL and IL-1+RANKL) of
(D) TRAP (E) NFATc1, (F) CTSK and (G) MMP-9. Relative gene
expression was normalized to GAPDH. **P<0.01 compared
with the control group, #P<0.05,
##P<0.01 compared with IL-1-treated or RANKL-treated
alone. IL, interleukin; RANKL, receptor activator of nuclear
factor-κB ligand-independent; control, untreated cells; RT-q,
reverse transcription-quantitative; NFATc1, nuclear factor of
activated T cells cytoplasmic 1; MMP-9, matrix metalloprotein-9;
CTSK, cathepsin K; TRAP, Tartrate-resistant acid phosphatase;
IL-1RI, IL-1 receptor I. |
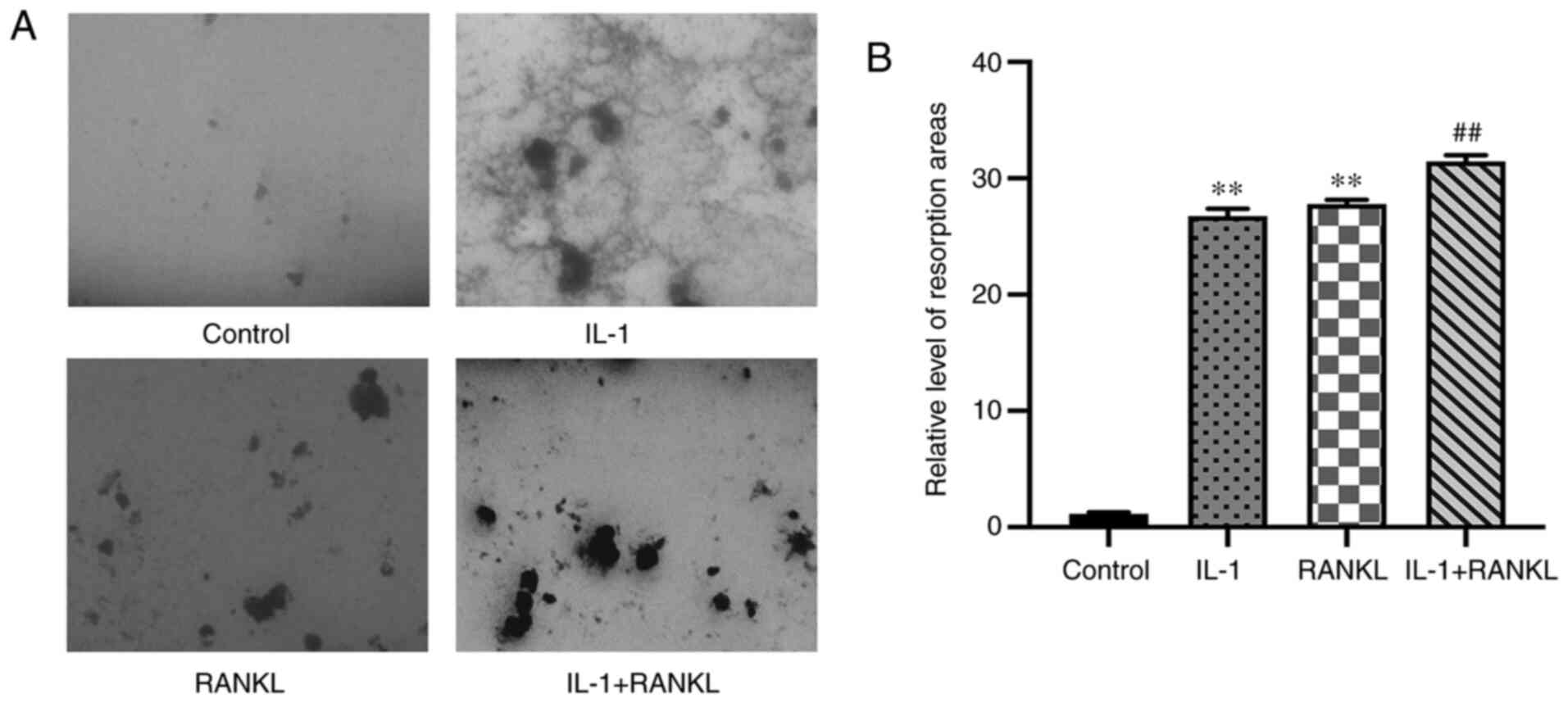
The area of bone resorption is
increased in IL-1/RANKL-treated cells compared with the control
group
IL-1 significantly increased the bone resorption
area compared with the control group (P<0.01), meanwhile, no
significant difference was found in the area of resorption between
the IL-1-treated and RANKL-treated groups (P>0.05) (Fig. 4A and B). In addition, the bone resorption area
in the IL-1+RANKL group was also significantly increased compared
with that in the single stimulus groups (P<0.01) (Fig. 4).
Discussion
As one of the highly important factors that causes
osteoporosis, the excessive activation of osteoclasts has been
studied in vivo and in vitro for a long time
(1-3).
RAW264.7 cells are widely used as osteoclast precursors, because
like BMMs, they also originate from a hematopoietic lineage
(29). In addition, compared with
BMMs, RAW264.7 cells are easily accessible and sensitive to the
stimulation of RANKL (30). In the
present study, to clarify the direct effect of IL-1 on osteoclast
differentiation, RAW264.7 cells were used as osteoclast precursors
and TRAP staining and bone resorption assay were used to examine
the osteoclast differentiation and activity, respectively. In the
present study, a significantly increased quantity of osteoclasts
was not only observed in the IL-1 group compared with the control
group, but also in the IL-1+RANKL group compared with the
IL-1/RANKL group. The present study demonstrated that IL-1 can
induce osteoclastogenesis in a RANKL-independent way and upregulate
the osteoclast differentiation in RAW264.7 cells in the presence of
RANKL.
It is now acknowledged that activation of RANK
pathway is essential for osteoclast differentiation (1). Substitutes for RANKL include IL-1,
transforming growth factor β and IL-6(8). However, the function of IL-1 in
osteoclast differentiation remains controversial. IL-1 can enhance
the capacity of mature osteoclasts in bone resorption, which is
supported by previous studies (8,31) and
the results of the present study. However, IL-1 could not induce
osteoclast differentiation from BMMs partly due to the insufficient
expression of IL-1RI (20). In the
present study, RAW264.7 cells exhibited substantial protein
expression of IL-1RI.
Osteoclasts are the unique cells which can resorb
bone. An excessive increase in osteoclast differentiation leads to
several bone-resorptive diseases, such as osteoporosis (1). Jimi et al (32) concluded that IL-1 can bind to
putative IL-1 receptors on osteoclast-like cells leading to an
induction of a NF-κB-like factor. Wei et al (31) discovered IL-1 can enhance the
expression of RANKL in bone marrow stromal cells and directly
stimulate differentiation of osteoclast precursors. However,
Watanabe et al (33) found
that the formation of osteoclasts was suppressed by IL-1β via
decreasing M-CSF production and increasing osteoprotegerin
production. The cause of conflicting results regarding the effect
of IL-1 on the differentiation and proliferation of osteoclasts may
lie in the different cells and induction methods that were used in
each study.
Lorenzo et al (34) demonstrated that there was no
significant bone loss in IL-1RI-deficient mice after ovariectomy,
which is a widely used osteoporosis model relevant to menopause
(35,36). Osteoclast formation and bone
resorption area are decreased in ovariectomized mice treated with
IL-1 receptor antagonist compared with the sham control group
(36). The results suggested that
IL-1 is an important cytokine in bone loss associated with a
decline in estrogen (34-36).
However, studies using IL-1RI-deficient mice to explore the effects
of IL-1 on bone metabolism have revealed controversial results.
Bajayo et al (37) reported
the loss of bone mass in IL-1RI-deficient mice, indicating that
IL-1 receptor signaling pathway is also an important regulator of
bone mass and bone remodeling. On the contrary, Vargas et al
(38) demonstrated that the bone
volume and number of osteoclasts of humeri in IL-1RI-deficient mice
are normal compared with the control group.
RANKL induces the gene and protein expression of
NFATc1 by activating Ca2+ signals from the
immunoreceptor tyrosine-based activation motif pathway (39). The expression of NFATc1 is
significantly increased by auto-amplification (40) and subsequently, the expression of
osteoclast-related genes, such as TRAP, CTSK and MMP-9 are induced
by NFATc1(41). It was also
reported in the aforementioned study, that NFATc1-deficient stem
cells stimulated with RANKL did not differentiate into osteoclasts,
while the upregulation of NFATc1 led to efficient osteoclast
differentiation without the stimulation of RANKL (41). Hence, NFATc1 may function as a
master switch in activating target genes expression of downstream
of RANKL in the terminal stage of osteoclast differentiation. In
the present study, RAW264.7 cells expressed nearly no NFATc1
without the stimulation of IL-1 and RANKL, which suggested that
IL-1 or RANKL is important for initiating the expression of NFATc1
during the process of osteoclast differentiation. It has been
reported that IL-1 can upregulate the induction of osteoclasts in
the presence of RANKL (20,42), and the present study found that
RAW264.7 cells expressed NFATc1 on stimulation with IL-1 and that
there was a potential interaction between IL-1RI, NFATc1 and RANKL.
Further studies are needed for investigating the interaction
between IL-1RI, NFATc1 and RANKL and to confirm that IL-1 is a
requisite for bone remodeling.
Normally, dentine or bone slices are used to
evaluate osteoclast differentiation and function (20,43),
however, they are difficult to handle and easily damaged. The
Corning Osteo Assay Surface represents a convenient and
reproducible substitute for slices (26,27).
Hence, in the present study the bone resorption area was observed
and calculated using the Corning Osteo Assay Surface. The present
study demonstrated that there was no statistically significant
difference between osteoclasts and the area of the resorption
induced from IL-1 and RANKL-treated cells. In contrast, Kim et
al (20) reported that the
number of osteoclasts and the relative intensity of resorption pit
on dentine slices formed in IL-1/IL-1RI-treated cells was less
compared with RANKL-treated cells. The aforementioned results
suggested that the activation of both signaling pathways are
important in osteoclast formation and function and the difference
in their findings may be due to the different osteoclast precursors
used in the 2 studies.
There are several limitations of the present study.
Firstly, the osteoclast precursors used, although RAW264.7 cells
have been widely used as osteoclast precursors to study osteoclast
differentiation they are not identical to BMMs in the human body.
Secondly, the optimum in vitro concentration of IL-1 was not
determined in the present study. Hence, the results of the present
study are not completely applicable to osteoclasts derived from
human body. Lastly, the pathways involved and the potential
interaction between IL-1RI, NFATc1 and RANKL were not investigated
in the present study. Further studies are needed to investigate all
the aforementioned points and to verify the finding of the present
study.
In conclusion, the present study to the best of our
knowledge for the first time demonstrated that IL-1 can induce
osteoclast differentiation from RAW264.7 macrophages and the
results may provide groundwork for the study of diseases involving
in the bone loss associated with inflammation.
Acknowledgements
The authors are grateful to Professor Ming Zhao
(Department of Pathophysiology, Key Laboratory for Shock and
Microcirculation Research of Guangdong, Southern Medical
University, China) for providing laboratory access and technical
assistance during the experiment.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JT conceived and designed this study, RoL, ZF, WL,
RuL, XX and SY performed the experiments. RoL and ZF confirmed the
authenticity of all the raw data and drafted the manuscript. WL,
RuL, XX and SY reviewed the manuscript for important intellectual
content. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Roodman GD: Mechanisms of bone metastasis.
N Engl J Med. 350:1655–1664. 2004.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Suda T, Takahashi N, Udagawa N, Jimi E,
Gillespie MT and Martin TJ: Modulation of osteoclast
differentiation and function by the new members of the tumor
necrosis factor receptor and ligand families. Endocr Rev.
20:345–357. 1999.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lacey DL, Timms E, Tan HL, Kelley MJ,
Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S,
et al: Osteoprotegerin ligand is a cytokine that regulates
osteoclast differentiation and activation. Cell. 93:165–176.
1998.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wang Y, Brooks PJ, Jang JJ, Silver AS,
Arora PD, Mcculloch CA and Glogauer M: Role of actin filaments in
fusopod formation and osteoclastogenesis. Biochim Biophys Acta.
1853:1715–1724. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Pi Y, Liang H, Yu Q, Yin Y, Xu H, Lei Y,
Han Z and Tian J: Low-frequency pulsed electromagnetic field
inhibits RANKL-induced osteoclastic differentiation in RAW264.7
cells by scavenging reactive oxygen species. Mol Med Rep.
49:4129–4136. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Park KH, Park B, Yoon DS, Kwon SH, Shin
DM, Lee JW, Lee HG, Shim JH, Park JH and Lee JM: Zinc inhibits
osteoclast differentiation by suppression of
Ca2+-Calcineurin-NFATc1 signaling pathway. Cell Commun
Signal. 11(74)2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Xu J, Wang C, Han R, Pavlos N, Phan T,
Steer JH, Bakker AJ, Joyce DA and Zheng MH: Evidence of reciprocal
regulation between the high extracellular calcium and RANKL signal
transduction pathways in RAW cell derived osteoclasts. J Cell
Physiol. 202:554–562. 2005.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Feng W, Guo J and Li M: RANKL-independent
modulation of osteoclastogenesis. J Oral Biosci. 61:16–21.
2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kong Y, Yoshida H, Sarosi I, Tan HL, Timms
E, Capparelli C, Morony S, Van G, Itie A, Khoo W, et al: OPGL is a
key regulator of osteoclastogenesis, lymphocyte development and
lymph-node organogenesis. Nature. 397:315–323. 1999.PubMed/NCBI View
Article : Google Scholar
|
|
10
|
Fuller K, Wong B, Fox S, Choi Y and
Chambers TJ: TRANCE is necessary and sufficient for
osteoblast-mediated activation of bone resorption in osteoclasts. J
Exp Med. 188:997–1001. 1998.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Takayanagi H: Osteoimmunology: Shared
mechanisms and crosstalk between the immune and bone systems. Nat
Rev Immunol. 7:292–304. 2007.PubMed/NCBI View
Article : Google Scholar
|
|
12
|
Ikebuchi Y, Aoki S, Honma M, Hayashi M,
Sugamori Y, Khan M, Kariya Y, Kato G, Tabata Y, Penninger JM, et
al: Coupling of bone resorption and formation by RANKL reverse
signalling. Nature. 561:195–200. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Dinarello CA: The interleuldn-1 family: 10
years of discovery. FASEB J. 8:1314–1325. 1994.PubMed/NCBI
|
|
14
|
Yang J, Wang J, Liang X, Zhao H, Lu J, Ma
Q, Jing B and Tian F: IL-1β increases the expression of
inflammatory factors in synovial fluid-derived fibroblast-like
synoviocytes via activation of the NF-κB-mediated ERK-STAT1
signaling pathway. Mol Med Rep. 20:4993–5001. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hanazawa S, Nakada K, Ohmori Y, Miyoshi T,
Amano S and Kitano S: Functional role of interleukin 1 in
periodontal disease: Induction of interleukin 1 production by
Bacteroides gingivalis lipopolysaccharide in peritoneal macrophages
from C3H/HeN and C3H/HeJ mice. Infect Immun. 50:262–270.
1985.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lee YM, Fujikado N, Manaka H, Yasuda H and
Iwakura Y: IL-1 plays an important role in the bone metabolism
under physiological conditions. Int Immunol. 22:805–816.
2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Nakamura I and Jimi E: Regulation of
osteoclast differentiation and function by interleukin-1. Vitam
Horm. 74:357–370. 2006.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wang Y, Galli M, Shade Silver A, Lee W,
Song Y, Mei Y, Bachus C, Glogauer M and McCulloch CA: IL1β and TNFα
promote RANKL-dependent adseverin expression and
osteoclastogenesis. J Cell Sci. 131(jcs213967)2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kudo O, Fujikawa Y, Itonaga I, Sabokbar A,
Torisu T and Athanasou NA: Proinflammatory cytokine (TNFα/IL-Iα)
induction of human osteoclast formation. J Pathol. 198:220–227.
2002.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kim JH, Jin HM, Kim K, Song I, Youn BU,
Matsuo K and Kim N: The mechanism of osteoclast differentiation
induced by IL-1. J Immunol. 183:1862–1870. 2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Nakamura I, Kadono Y, Takayanagi H, Jimi
E, Miyazaki T, Oda H, Nakamura K, Tanaka S, Rodan GA and Duong LT:
IL-1 regulates cytoskeletal organization in osteoclasts via TNF
receptor-associated factor 6/c-Src complex. J Immunol.
168:5103–5109. 2002.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Chomczynski P and Sacchi N: Single-step
method of RNA isolation by acid guanidinium
thiocyanate-phenol-chloroform extraction. Anal Biochem.
162:156–159. 1987.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Xiaowei W and Seed B: A PCR primer bank
for quantitative gene expression analysis. Nucleic Acids Res.
31(e154)2003.PubMed/NCBI View Article : Google Scholar
|
|
25
|
He JQ, Zhang YS, Chen J, Zheng S, Huang H
and Dong X: Effects of pulsed electromagnetic fields on the
expression of NFATc1 and CAII in mouse osteoclast-like cells. Aging
Clin Exp Res. 27:13–19. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Guo YQ, Xie CZ, Li XY, Yang J, Yu T, Zhang
RH, Zhang TQ, Saxena D, Snyder M, Wu YJ and Li X: Succinate and its
G-protein-coupled receptor stimulates osteoclastogenesis. Nat
Commun. 8(15621)2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Liu TH, Tsai TY and Pan TM: The
anti-periodontitis effects of ethanol extract prepared using
Lactobacillus paracasei subsp. paracasei NTU 101. Nutrients.
10(472)2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Takeshita S, Kaji K and Kudo A:
Identification and characterization of the new osteoclast
progenitor with macrophage phenotypes being able to differentiate
into mature osteoclasts. J Bone Miner Res. 15:1477–1488.
2000.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ono T and Nakashima T: Recent advances in
osteoclast biology. Histochem Cell Biol. 149:325–341.
2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Collin-Osdoby P and Osdoby P:
RANKL-mediated osteoclast formation from murine RAW 264.7 cells.
Methods Mol Biol. 816:187–202. 2012.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wei S, Kitaura H, Zhou P, Ross FP and
Teitelbaum SL: IL-1 mediates TNF-induced osteoclastogenesis. J Clin
Invest. 115:282–290. 2005.PubMed/NCBI View
Article : Google Scholar
|
|
32
|
Jimi E, Ikebe T, Takahashi N, Hirata M,
Suda T and Koga T: Interleukin-1 alpha activates an NF-kappaB-like
factor in osteoclast-like cells. J Biol Chem. 271:4605–4608.
1996.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Watanabe Y, Namba A, Aida Y, Honda K,
Tanaka H, Suzuki N, Matsumura H and Maeno M: IL-1β suppresses the
formation of osteoclasts by increasing OPG production via an
autocrine mechanism involving celecoxib-related prostaglandins in
chondrocytes. Mediators Inflamm. 2009(308596)2009.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Lorenzo JA, Naprta A, Rao Y, Alander C,
Glaccum M, Widmer M, Gronowicz G, Kalinowski J and Pilbeam CC: Mice
lacking the type I interleukin1 receptor do not lose bone mass
after ovariectomy. Endocrinology. 139:3022–3025. 1998.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Lee EJ, Kim JL, Gong JH, Park SH and Kang
YH: Inhibition of osteoclast activation by phloretin through
disturbing αvβ3 integrin-c-Src pathway. Biomed Res Int.
2015(680145)2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kitazawa R, Kimble RB, Vannice JL, Kung VT
and Pacifici R: Interleukin-1 receptor antagonist and tumor
necrosis factor binding protein decrease osteoclast formation and
bone resorption in ovariectomized mice. J Clin Invest.
94:2397–2406. 1994.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Bajayo A, Goshen I, Feldman S, Csernus V,
Iverfeldt K, Shohami E, Yirmiya R and Bab I: Central IL-1 receptor
signaling regulates bone growth and mass. Proc Natl Acad Sci USA.
102:12956–12961. 2005.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Vargas SJ, Naprta A, Glaccum M, Lee SK,
Kalinowski J and Lorenzo JA: Interleukin-6 expression and
histomorphometry of bones from mice deficient in receptors for
interleukin-1 or tumor necrosis factor. J Bone Miner Res.
11:1736–1744. 1996.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Shaw AT and Gravallese EM: Mediators of
inflammation and bone remodeling in rheumatic disease. Semin Cell
Dev Biol. 49:2–10. 2016.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Asagiri M, Sato K, Usami T, Ochi S,
Nishina H, Yoshida H, Morita I, Wagner EF, Mak TW, Serfling E and
Takayanagi H: Autoamplification of NFATc1 expression determines its
essential role in bone homeostasis. J Exp Med. 202:1261–1269.
2005.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Takayanagi H, Kim S, Koga T, Nishina H,
Isshiki M, Yoshida H, Saiura A, Isobe M, Yokochi T, Inoue J, et al:
Induction and activation of the transcription factor NFATc1 (NFAT2)
integrate RANKL signaling in terminal differentiation of
osteoclasts. Dev Cell. 3:889–901. 2002.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Ma T, Miyanishi K, Suen A, Epstein NJ,
Tomita T, Smith RL and Goodman SB: Human interleukin-1-induced
murine osteoclastogenesis is dependent on RANKL, but independent of
TNF-alpha. Cytokine. 26:138–144. 2004.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Lei Y, Su J, Xu H, Yu Q, Zhao M and Tian
J: Pulsed electromagnetic fields inhibit osteoclast differentiation
in RAW264.7 macrophages via suppression of the protein kinase
B/mammalian target of rapamycin signaling pathway. Mol Med Rep.
18:447–454. 2018.PubMed/NCBI View Article : Google Scholar
|