Introduction
Hyperemesis gravidarum (HG) is defined as
intractable vomiting and nausea during pregnancy. Ptyalism,
fatigue, weakness and dizziness are frequent symptoms, whilst rare
symptoms also include hyperolfaction, dysgeusia, decreased
gustatory discernment, sleep disturbance, depression, anxiety,
irritability and mood changes (1-4).
Although >75% of pregnant women suffer from nausea or vomiting
during pregnancy, only 0.3-2% pregnant women are diagnosed with HG.
The most frequent reasons for hospital admission in women diagnosed
with HG include weight loss (>5% pre-pregnancy weight),
ketonuria, dehydration, electrolyte imbalance, acid-base imbalances
and arrhythmias (1-4).
Although the pathogenesis of HG remains widely
unknown, pregnancy in the first trimester, first pregnancy,
multiple pregnancies, obesity, family history of HG, trophoblastic
disorder, hyperthyroid disorders, psychiatric illness, previous
molar pregnancy, preexisting diabetes, gastrointestinal disorders,
allergies prior to pregnancy and a history of eating disorders are
known risk factors (2-5).
The list of complications noted in HG are classified as
complications associated with pregnancy [malnutrition, anemia,
hyponatremia, Wernicke's encephalopathy (WE), kidney failure,
central pontine myelinolysis (CPM), stroke, vasospasms of cerebral
arteries, seizures, coagulopathy, hypoglycemia, esophageal rupture
or perforation, hepatic disease, jaundice, pancreatitis, deep vein
thrombosis, pulmonary embolism, pneumothorax, pneumomediastinum,
rhabdomyolysis, vitamin K deficiency and coagulopathy, splenic
avulsion, depression and post-traumatic stress disorder],
complications associated with central nutrition (sepsis, fungemia,
tamponade, local infection, venous thrombosis, fatty infiltration
of the placenta and transaminitis) and infant complications (lower
weight at birth, small for gestational age and birth before 37
weeks of gestation) (6-9).
The aim of the present systematic review was to
summarize the available evidence regarding severe complications in
HG with a heightened risk of fatality.
Materials and methods
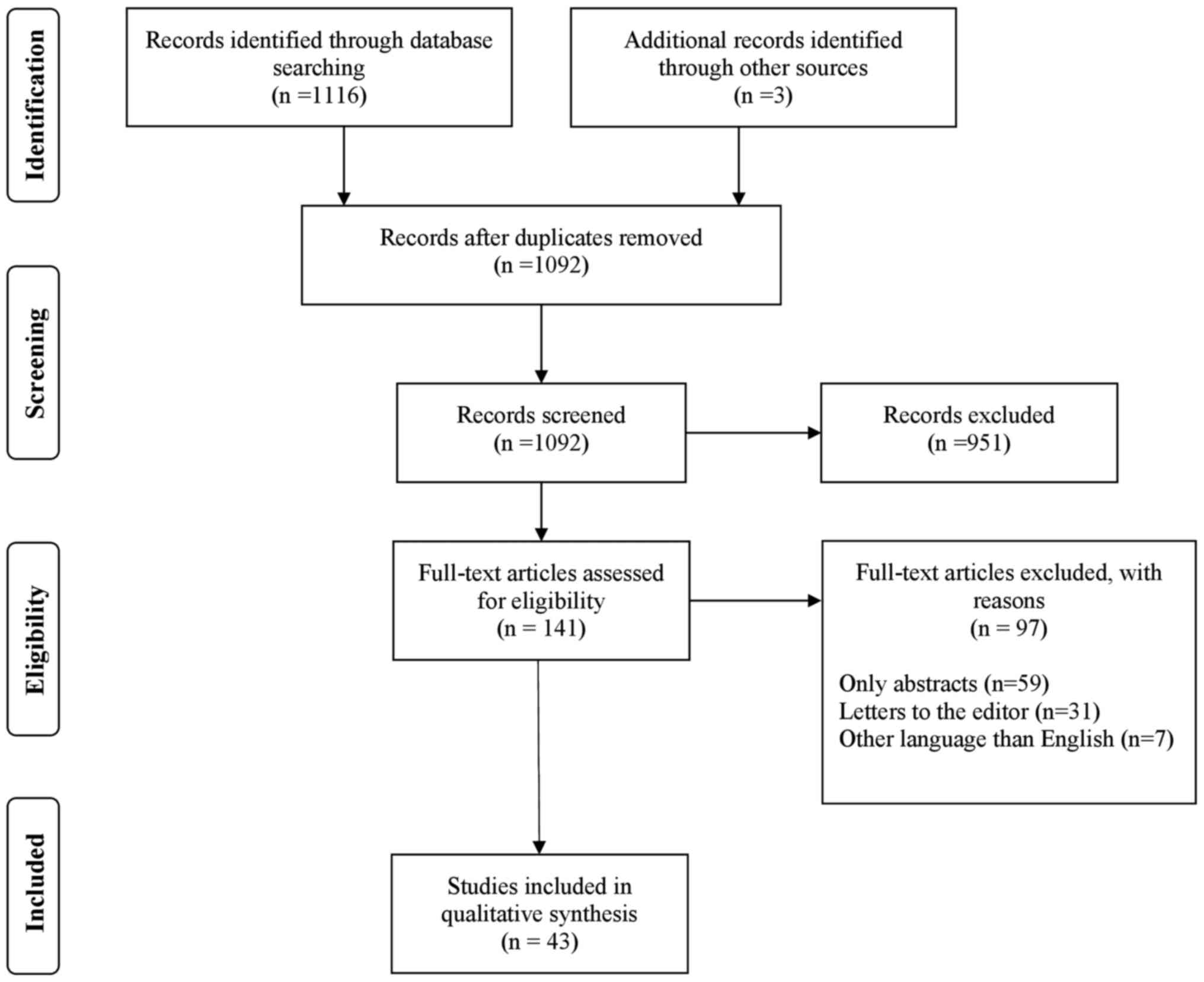
PubMed (https://pubmed.ncbi.nlm.nih.gov/), Cochrane Library
(https://www.cochranelibrary.com/),
EMBASE (https://www.elsevier.com/solutions/embase-biomedical-research)
and WILEY (https://onlinelibrary.wiley.com/) databases were
screened for relevant publications regarding severe and
life-threatening complications of HG. The search terms used were as
follows: ‘(Hyperemesis gravidarum)’ AND (‘complications’ OR
‘severe’ OR ‘adverse pregnancy outcomes’ OR ‘stroke’ OR ‘seizures’
OR ‘Wernicke's encephalopathy’ OR ‘arrhythmias’ OR
‘pneumomediastinum’ OR ‘coagulopathy’ OR ‘electrolytic imbalance’).
The exclusion criteria were abstracts, conference presentations,
letters to the editor, studies written in languages other than
English and editorials (Fig. 1).
Two independent authors (SLP and CA) reviewed the studies for
eligibility titles, abstracts and full text of eligible articles.
Disagreements between the two authors were resolved by discussion.
The search strategy using the PRISMA flow diagram is shown in
Fig. 1.
Results
Neurological complications
The search identified 11 articles regarding severe
HG neurological complications, of which four articles examined
stroke (10-13),
two articles focused on seizures (14,15),
three focused on CPM (16-18),
of which two case reports with associated WE (17,18),
and two articles focused only on WE (19,20)
(Table I). A study performed by
Lanska and Kryscio (10) analyzed
the incidence of peripartum stroke and cerebral venous thrombosis
(CVT) in the United States from 1993 to 1994. The aim of their
study was to identify potential risk factors for peripartum or
postpartum stroke and IVT (10).
The results reported that 183 cases of peripartum stroke and 170
cases of peripartum intracranial venous thrombosis (IVT) were
identified out of 1,408,015 sampled deliveries (10). Furthermore, 975 cases of stroke and
864 cases of IVT during pregnancy, where puerperium was observed
among 7,463,712 deliveries. Statistical analysis demonstrated that
the following conditions exerted a significant association with
peripartum and postpartum stroke: Cesarean delivery, fluid,
electrolyte and acid-base disorders and hypertension (10). The risk of stroke and CVT in
patients with HG was statistically significant (P=0.009). Similar
findings were noted for fluid, electrolyte and acid-base disorders
(P<0.001) (10). The authors
concluded that the risk of severe, life-threatening neurological
complications was low in patients with HG (10).
 | Table INeurologic complications of
hyperemesis gravidarum. |
Table I
Neurologic complications of
hyperemesis gravidarum.
| Author | Year | Evidence type | Age of patient (s),
years | Country | Gestational period
on presentation, weeks | Complication | Symptoms | Treatment |
|---|
| Lanska and Kryscio
(10) | 2000 | Cohort study | 15-44 | USA | Not specified | Peripartum and
postpartum stroke and intracranial venous thrombosis | Not specified | Conservative |
| Seki et al
(11) | 2015 | Case report | 26 | Japan | 8 weeks | Intracerebral
hemorrhage due to venous thrombosis | Sudden generalized
seizures | Conservative |
| Kennelly et
al (12) | 2008 | Case report | 26 | UK | 11 weeks | Sagittal sinus
thrombosis | A history of
vomiting, headaches and tonic clonic seizures. Drowsy with a left
homonymous hemianopia and brisk tendon reflexes in the left upper
and lower limbs. | Conservative
intravenous unfractionated heparin and dexamethasone direct
catheter thrombolysis with tissue plasminogen activator |
| Kanayama et
al (13) | 1998 | Case report | 29, 26 | Japan | 10 and 8 weeks | Vasospasms of
cerebral arteries | Frequent vomiting,
general fatigue and weight loss | Conservative |
| Beach and Kaplan
(14) | 2008 | Review | Not specified | Not specified | Not specified | Seizures | Not specified | Conservative |
| O'Brien et
al (15) | 2004 | Review | Not specified | Not specified | Not specified | Epilepsy | Not specified | Conservative |
| Sinn et al
(16) | 2013 | Case report | 16 | USA | 20 weeks | Simultaneous optic
neuropathy and osmotic demyelinating syndrome | Blurry vision | Conservative |
| Bergin and Harvey
(17) | 1992 | Case report | 25 | India | 9 weeks | Wernicke
encephalopathy and central pontine myelinolysis | Confusion and
ataxia | Concentrated
intravenous injections of the vitamin B complex and ascorbic acid
and parenteral feeding. |
| Sutamnartpong et
al (18) | 2013 | Case report | 21 | Thailand | 16 weeks | Wernicke
encephalopathy and central pontine myelinolysis | Progressive
difficulty in walking | Conservative |
| Zara et al
(19) | 2012 | Case report | 29 | Italy | 20 weeks | Wernicke
encephalopathy | Weight loss (14
kg), hematemesis and episodes of bilious vomiting, diarrhea,
weakness, drowsiness and increased body temperature (39˚C) | Thiamine was
administered (100 mg/day intravenously for 10 days, then 300 mg/day
orally) |
| Oudman et al
(20) | 2019 | Systematic
review | 26.9±5.5 | Not specified | 15-25 weeks | Wernicke
encephalopathy | Mental status
change | Thiamine
supplementation |
A total of three articles were case reports. In the
first case report (11), a
26-year-old woman with intracerebral hemorrhage due to the venous
thrombosis of a developmental venous anomaly (DVA) was described.
Although DVA is the most common cerebral vascular malformation,
diagnosis is frequently made incidentally on routine brain imaging
due to the lack of symptoms (11).
The patient was diagnosed with hemorrhagic stroke and epilepsy
secondary to thrombosis of the DVA during week 8 of pregnancy. The
authors concluded that HG and the resulting intravascular
dehydration increased the risk of thrombosis in this patient with
previously undiagnosed DVA (11).
The second case report analyzed the severe effects of sinus
thrombosis in patients with HG whereas the third case report was
regarding transient ischemic attack (12,13).
Although the majority of pregnant women with seizures exhibited
epilepsy prior to pregnancy, HG was a risk factor for pregnant
women without prior diagnosis of epilepsy to develop seizures
(14,15). It is important to note that limited
evidence is present regarding the risk of seizures in patients with
HG. In addition, although brain damage is responsible for their
onset, other factors may also be involved, including variations in
blood pressure, metabolic disorders and infections (14,15).
CPM is one of the rarest but potentially fatal
complications of HG (4). HG may be
a risk factor when CPM is mainly caused by the following
conditions: Rapid correction of hyponatremia, alcoholism,
malnutrition, severe burns, hypokalemia, psychogenic polydipsia
(patients with schizophrenia), liver cirrhosis and severe
electrolyte and acid-base disorders (4). However, limited evidence has been
found to support this notion. A number of case reports were
published (16-19),
each of these illustrating patients that were diagnosed with CPM in
an HG context.
WE is an important type of encephalopathy that is
caused by a single vitamin B1 deficiency (21). This disease is clinically
characterized by the classic triad of ocular findings, cerebellar
dysfunction and confusion (20,21).
Epidemiological studies are rare and unreliable, since >80%
patients with WE are either not diagnosed or misdiagnosed, making
it impossible to calculate the morbidity and mortality rates
(20,21). WE is the most frequent neurological
complication of HG, with over 70 papers reporting this over the
past 6 decades. Those papers were not included in the current
review, because they all were case reports. At present, WE can be
readily diagnosed, treated, prevented and reversed even in severe
cases due to new pharmacological agents and tailored therapies
(20,21).
Cardiovascular complications
Existing literature regarding cardiovascular
complications of HG is scarce and the majority of the articles
published are case reports. The search strategy revealed the
following seven articles related to cardiovascular complications of
HG: Three case reports (22-24)
related to ventricular arrhythmias, all discussing consequences of
serum electrolyte imbalance, mainly hypokalemia, among which one
case report described a case of QT prolongation (22) and two case reports involved
ventricular tachycardia (23,24);
one population-based cohort study evaluating, among other placental
disorders, the risk of developing pre-eclampsia (25); one nationwide cohort study
evaluating the subsequent long-term risk of maternal cardiovascular
morbidity (26); one case report on
right atrial thrombus with a central venous catheter placement
complication (27) and one case
report regarding important arterial blood pressure variations
(28) (Table II).
 | Table IICardiovascular and thoracic
complications of hyperemesis gravidarum. |
Table II
Cardiovascular and thoracic
complications of hyperemesis gravidarum.
| Author | Year | Evidence type | Age of patient (s),
years | Country | Gestational age,
weeks | Complication | Symptoms | Treatment |
|---|
| Mitchell and Cox
(22) | 2016 | Case report | 30 | UK | 24 weeks | Long QTc | Severe epigastric
pain, long QTc on ECG. | Conservative |
| Kochhar and Ghosh
(23) | 2018 | Case report | 26 | India | 7 weeks | Ventricular
tachycardia | Shortness of
breath, palpitations and atypical chest tightness | Intravenous
lidocaine, isotonic saline and parenteral potassium and magnesium
supplementation, metoprolol at 25 mg twice daily |
| Jadhav et al
(24) | 2010 | Case report | 25 | India | 13 weeks | Ventricular
tachycardia and seizure | Recurrent
generalized clonic tonic convulsions and sustained ventricular
tachycardia with hypotension and evidence of Torsade de pointes on
cardiac monitor | Conservative |
| Bolin et al
(25) | 2013 | Population- based
cohort study | <25 (172,336
subjects), 25-29.9 (358,454 subjects), 30-34.9 (400,752 subjects),
>35 (221,216 subjects), | Sweden | First or second
trimester | Placental
dysfunction disorders (preeclampsia, placental abruption,
stillbirth and small for gestational age) | Not specified | Not specified |
| Fossum et al
(26) | 2019 | Nationwide cohort
study | 24 (with HG), 25
(without HG) | Norway | Not specified | Long-term
cardiovascular morbidity (nonfatal stroke, myocardial infarction,
or angina pectoris, or cardiovascular death) | Not specified | Not specified |
| Turrentine et
al (27) | 1994 | Case report | 23 | USA | 26 weeks | Right atrial
thrombus | Left-side chest
pain | Removal of central
catheter, i.v. heparinization at 33,000 U/day |
| Salmon (28) | 2009 | Case report | 25 | Australia | 18 weeks | Postural
hypotension and autonomic neuropathy | Labile blood
pressure | Fludrocortisone 0.1
mg daily |
| Schwartz and
Rossoff (34) | 1994 | Case report | 26 | USA | 10 weeks | Pneumomediastinum
and bilateral pneumothorax | Hematemesis
followed by severe nonpleuritic chest pain without dyspnea.
Swelling of the head, neck, and anterior chest | Total parenteral
nutrition and systemic antibiotic therapy |
| Gorbach et
al (35) | 1997 | Case report | 21 | USA | 9.5 weeks | Spontaneous
pneumomediastinum | Sore throat, sharp
pain in the middle of the chest at deep inspiration and a ‘squishy’
sensation when the patient rubbed the outside of her throat | Conservative |
| Liang et al
(36) | 2002 | Case report | 25 | Japan | 15 weeks | Pneumomediastinum
following esophageal rupture | Disturbance of
consciousness | Conservative |
| Yamamoto et
al (37) | 2001 | Case report | 29 | Japan | 6 weeks |
Pneumo-mediastinum | Face swelling,
severe toothache | Conservative |
| Germes-Piña et
al (38) | 2016 | Case report | 21 | Mexico | 15 weeks |
Pneumo-mediastinum | Neck swelling and
pain, odynophagia, dysphonia | Conservative |
| Chen et al
(39) | 2012 | Case report | 18 | China | 13 weeks | Diaphragmatic
tear | Upper abdomen
discomfort | Glucose and saline,
antiemetic therapy, and parenteral nutrition with 3-4 l input
volume per day |
| Fiaschi et
al (40) | 2017 | Population- based
cohort study | All ages | England | Not specified | Subjects grouped in
patients experiencing no HG; only one or at least one hospital
admission due to HG and complications grouped in antenatal,
perinatal and postnatal complications, delivery and birth
factors | Not specified | Not specified |
QT interval prolongation, with or without subsequent
malignant ventricular arrhythmias (generally torsade de pointes),
is a condition caused by serum electrolyte imbalance (mainly
hypokalemia, but also hypomagnesemia and hypocalcemia) that can be
treated by the administration of antiemetics, including
metoclopramide, ondansetron and domperidone (29). Severe episodes of nausea and
vomiting in patients with HG can easily lead to dehydration,
hypokalemia, hypomagnesemia and hypocalcemia (4). Nausea is treated with antiemetics,
which can prolong the QT interval further on the 12-lead
electrocardiogram (ECG) and favor the appearance of malignant
ventricular arrhythmias (22).
Mitchel and Cox 22) described a case of QT prolongation in a
patient at 24-week primiparous pregnancy presenting due to
hyperemesis, severe vomiting and loss of appetite for 1 week prior
to hospital admission. These events led to hypokalemia,
hypomagnesemia and hypocalcemia, with subsequent QT prolongation on
the ECG with a QTc interval of 510 msec. This increase in the QTc
interval was most likely aggravated by antiemetic drug
administration (metoclopramide and ondansetron). Patient management
consisted of intravenous fluid resuscitation, electrolyte
administration (K+, Mg+, phosphates and
Ca2+), antalgics, thromboembolism prophylaxis, gastric
protection with intravenous proton pump inhibitors, nasojejunal
tube nutrition and vitamin (B and C) supplements. The patient's
condition was improved and she was discharged from the hospital
following a reduction in the QTc interval to normal values with
corrected electrolyte levels. Prompt treatment of prolonged QTc is
essential, since a QTc interval >500 msec increases the risk of
malignant ventricular arrhythmias, notably torsades de pointes
(30), which can result in adverse
events, including syncope and sudden cardiac arrest. Kochhar and
Ghosh (23) described a case of
ventricular arrhythmia due to hypokalemia and hypomagnesaemia in a
patient with HG and structurally normal heart. The patient was 7
weeks pregnant and developed ventricular bigeminy in a context of
hypokalemia (2.3 mEq/l; normal 3.5 to 5.5 mEq/l) and hypomagnesemia
(107 mEq/l; normal- 130-145 mEq/l) due to frequent episodes of
vomiting, who was treated with antiemetics (metoclopramide and
ondansetron). The arrhythmia was treated with an intravenous
administration of lidocaine, magnesium sulfate, potassium
supplements and oral metoprolol in combination with supportive
treatment (23). Her condition
improved and she was discharged 5 days later. Although the authors
state that the patient developed short-term ventricular tachycardia
and episodes of polymorphic ventricular tachycardia, the ECG data
obtained in this previous study demonstrated that only isolated
premature ventricular complexes and monomorphic ventricular
bigeminism were present with an outflow tract origin, which is
frequently presented in individuals with a normal heart (23). Therefore, their conclusions should
be interpreted with caution. Jadhav et al (24) presented a case of a 25-year-old
female patient, who was 13 weeks pregnant and developed ventricular
tachycardia due to severe hypokalemia (2.4 mmol/l; normal 3.5 to
5.5 mEq/l) as a consequence of repeated episodes of nausea and
vomiting and lack of appetite, with severely reduced intake of both
solids and fluids. Her nausea was also treated with metoclopramide.
On day 4 of admission, she developed torsade de pointes and was
treated with electrical cardioversion, lidocaine and magnesium
sulfate. During hospitalization, she experienced recurrent episodes
of polymorphic ventricular tachycardia and was treated successfully
with electrical cardioversion. She aborted spontaneously on day 5
of admission. The authors focused on this potential severe
cardiovascular complication of HG. The mechanism of torsade de
pointes was not discussed further in the case report (24). This ventricular tachyarrhythmia,
usually referred to as torsade de pointes, was associated with an
increased QT interval and in the majority of the cases, aggravated
by hypokalemia and drug administration, such as metoclopramide
(31). In addition, Jadhav et
al (24) did not present ECG
data in patients with or without torsade de pointes in their
article, which was an important limitation of the study, since this
type of ventricular arrhythmia was the key object of discussion of
this case report.
The association between HG and pre-eclampsia was
discussed by a study by Bolin et al (25), who assessed the possible association
between HG during the first or second trimester of pregnancy and
placental dysfunction disorders, including pre-eclampsia, placental
abruption, stillbirth and small for gestational age births. During
a period of 13 years 1,156,050 pregnancies were included and the
data indicated that individuals with HG in the first trimester of
pregnancy exhibited a slightly higher risk of pre-eclampsia
compared with that noted in individuals with HG in the second
trimester of pregnancy, who exhibited increased risk of preterm
(<37 weeks) pre-eclampsia by >2-fold (25), with an odds ratio of 2.09 and 95%
confidence interval (CI) of 1.38-3.16. Pre-eclampsia may have a
negative impact on the fetus (fetal hypoxia, premature birth,
placental abruption, fetal death in utero, hypertension,
abnormal endothelial dilation, arterial thickening, reduced
microvascular density, increased LV wall thickness and reduced left
ventricular end-diastolic volume), the offspring (8% increase in
mortality risk from ischemic heart disease and 12% increase in the
risk of stroke) and on the mother (eclampsia, hypertension,
ischemic heart disease, thromboembolic events, kidney or liver
failure, stroke and an increased risk of mortality) (32). The impact of HG on the long-term
risk of maternal cardiovascular morbidity was assessed by Fossum
et al (26), which is the
largest study that assessed more concrete cardiovascular endpoints
in women with HG. This Norwegian cohort study included births from
1967 to 2002 from individuals with and without HG. These cases were
followed-up from 1994 to 2009, where the following cardiovascular
outcomes were recorded: Non-fatal stroke, myocardial infarction or
angina pectoris and cardiovascular death. The authors highlighted
that the prevalence of HG in a cohort of 989,473 women was 1.3%. At
least one adverse cardiovascular event was experienced by 4.4%
individuals. However, no association was found between HG and the
risk of fatal or non-fatal cardiovascular events [adjusted hazards
ratio (HR), 1.08; 95% CI, 0.99-1.18]. Only the risk of
hospitalization for angina pectoris was found to be higher in
patients with HG (adjusted HR, 1.28; 95% CI, 1.15-1.44). The risk
of mortality due to a cardiovascular event was not significantly
different in patients with and without HG (HR, 0.73; 95% CI,
0.59-0.91) following adjustment for age, whilst the association was
not significant following adjustment for other possible confounders
data pertaining the mother: age at first pregnancy, year of birth,
highest education obtained, country of birth, hypertensive
disorders during pregnancy, placental abruption, pre-gestational
hypertension and diabetes) (26).
In terms of vascular complications of HG, Turrentine
et al (27) described a case
of right atrial thrombus as a complication of central venous
catheter placement in a patient with HG that required parenteral
nutrition. The thrombus was successfully treated with heparin,
which led to its resolution and therefore did not exhibit a
negative outcome on pregnancy. Venous thrombosis with or without
subsequent pulmonary embolism is a known complication of central
catheter placement (27). Pregnancy
is associated with a hypercoagulable state and an increased risk of
venous thrombosis (27). Should a
central venous catheter be deemed necessary for a patient who is
also pregnant, measures should be taken to minimize the risk of
thromboembolic complications, including limiting the duration of
placement and anticoagulant treatment. In this aforementioned case,
the right atrial thrombus most likely developed as a complication
of the central catheter per se and not the direct presence
of HG (27).
Salmon (28)
described a case of a 25-year-old woman with arterial blood
pressure variations and HG, with a personal history of
pre-eclampsia. These values varied between 60/30 mmHg and 180/118
throughout the pregnancy. A high value of 220/130 mmHg was recorded
immediately after spontaneous vaginal delivery, where postpartum
vomiting was also observed. She was treated with chlorpromazine and
her blood pressure stabilized. The author attributed the marked
blood pressure variations to autonomic nervous system dysfunction,
causing subsequent postural hypotension. In this particular
patient, HG caused repeated episodes of vomiting, resulting in
significant hypovolemia and aggravated postural hypotension due to
autonomic dysfunction, which may explain the cause of the low blood
pressure values (28). The high
blood pressure values could be explained by her personal history of
pre-eclampsia. Given the unique nature of this case report, firm
conclusions could be drawn regarding the association between HG and
arterial blood pressure.
Thoracic complications
This search strategy yielded eight articles
(33-40)
related to thoracic complications of HG, most of which were case
reports. Among the selected articles, evidence regarding
pneumothorax, pneumomediastinum, diaphragmatic tears and
thromboembolic events was presented (Table II). Spontaneous pneumomediastinum
and pneumothorax occur most frequently during the second stage of
labor (33). Although
pneumomediastinum during pregnancy is rare, it can be lethal
(35). The case reports presented
in the literature regarding this complication are exceptionally
rare. Therefore, at present a thorough study is not possible. The
etiology for pneumomediastinum is characterized by esophageal tear
and spontaneous alveolar ruptures (33-38).
Esophageal tear can be determined by increased intraluminal
esophageal pressure due to the vomiting hyperextending the tensile
strength of its wall (34). In
addition, abdominal muscles contracting against a closed glottis
may rupture the alveoli and bronchovascular sheaths (34). It is important to differentiate
between these two causes since the treatment option must be
selected based on the precise cause of pneumomediastinum. Risk
factors include nulliparity, pregnancy at a young age, increased
estrogen levels, excessive emotional response to stress, excessive
coughing, drug use via inhalation and alcohol abuse (34-36).
The studies included in the present review demonstrated that all
patients with pneumothorax and pneumomediastinum were <30 years
of age, multiparous and presented with an extended history of
emesis (33-39).
The majority of patients were admitted for hematemesis, chest pain
and subcutaneous emphysema (Mackler's triad). Yamamoto et al
(37) presented a case with unusual
symptomatology (toothache, face swelling), which rendered
appropriate diagnosis and treatment difficult. Abnormal results in
esophageal barium examination, fever and leukocytosis can aid the
diagnosis of an esophageal tear (35). However, establishing the appropriate
selection of treatment, whether it is conservative, or surgical,
remains difficult. Although esophageal rupture is a surgical
emergency, in all cited cases, any esophageal lacerations reported
were limited without extensive signs of sepsis (36,39)
(Table II). All patients received
only supportive care, antibiotics and nihil per os for ≥7
days. High flow oxygen was also administered, since it was thought
to result in the increased rapid reabsorption of mediastinal air
(35). The association between
pneumomediastinum and pneumothorax was presented in case reports
(33,34). However, Schwartz and Rossoff
(34) demonstrated that the
bilateral pneumothorax described in the radiological findings may
in fact represent ‘extrapleural air’, as air may outline the tissue
planes of the neck, pectoral muscles and axilla. Lateral decubitus
exposure may be helpful in differential diagnosis. Elevation of the
thymus by underlying air or ‘thymic sail sign’ may assist the
diagnosis of pneumomediastinum (34). If correctly diagnosed and treated,
the prognosis for spontaneous pneumomediastinum during pregnancy is
favorable (33).
The diaphragm is vulnerable during pregnancy due to
increased intra-abdominal pressure (mass effect of the gravid
uterus, vomiting) and high progesterone levels, which can lead to
muscle relaxation and diaphragmatic hernia (DH). Chen et al
(39) described a rare case of
diaphragmatic tear secondary to an enlargement of a preexisting DH
at a young (18-year-old) nulliparous patient. In the majority of
the cases of DH, gastric decompression surgery was recommended in
the second trimester (39). In
terms of delivery following DH repair, the vaginal alternative
remained preferable to the cesarean (39).
Another possible cardiovascular complication, venous
thromboembolism (VTE) can appear during pregnancy, at delivery and
during the first 12 weeks postpartum. The distinction between deep
vein thrombosis and pulmonary thromboembolism is possible. A higher
risk was described for women with > one admission for HG
(40).
Systemic complications
The search strategy yielded 17 articles related to
systemic complications of HG, of which two articles were on
rhabdomyolysis (41,42), one on porphyria (43), three on electrolyte imbalance
(44-46),
seven on vitamin K deficiency (47-53),
two on endocrine complications (54,55)
and two on infectious complications (56,57)
(Table III). Rhabdomyolysis is
the destruction of a significant amount of strained muscle, leading
to disruptions in fluid balance, electrolytes and renal function
(41). Diagnosis is made through
serum creatine kinase determination and main symptoms include
fatigue, weakness, myalgia and swelling, although it is possible
that this condition remains completely asymptomatic (41,42).
 | Table IIISystemic complications of hyperemesis
gravidarum. |
Table III
Systemic complications of hyperemesis
gravidarum.
| Author | Year | Evidence type | Age of patient (s),
years | Country | Gestational age,
weeks | Complication | Symptoms | Treatment |
|---|
| Lassey et al
(41) | 2016 | Case report | 20 | USA | 19 weeks | Rhabdomyolysis | Fatigue, with
general muscle weakness in upper and lower extremities. Frequent
falls. | Aggressive
rehydration and a phosphorous binder. |
| Fukada et al
(42) | 1999 | Case report | 29 | Japan | 12 weeks | Rhabdomyolysis | Severe muscle
weakness of extremities | Conservative |
| Shenhav et
al (43) | 1997 | Case report | 29 | Israel | 13 weeks | Acute intermittent
porphyria | Abdominal pain,
constipation, and weakness of the lower extremities.
Neuro-psychiatric syndrome: irritability, memory loss,
concentration difficulties, hallucinations and depression. | Metoclopramide was
stopped, and concentrated glucose was commenced i.v., at the rate
of 20 ml/kg per day (600 ml 50% glucose). Supplemented with a high
carbohydrate diet. |
| Kondo et al
(44) | 2018 | Case report | 34 | Japan | 17 weeks | Electrolytic
imbalance inducing rhabdomyolysis and diabetes insipidus | General fatigue,
myalgia, muscle weakness and appetite loss, polyuria | Conservative |
| Walch et al
(45) | 2018 | Case report | 39 | Australia | 15+5 weeks | Cardiac arrest | Cardiac arrest | Conservative |
| Daskalakis et
al (46) | 2009 | Case report | 20 | Greece | 10 weeks | Gitelman syndrome-
associated severe hypokalemia and hypomagnesemia | Tiredness and
muscle weakness | Conservative |
| Lane et al
(47) | 2015 | Case report | 21 | USA | 21 weeks | Vitamin K
deficiency embryopathy | Nasal hypoplasia,
flat facial profile, and prominent forehead | Conservative with
vitamin K supplementation |
| Shigemi et
al (48) | 2015 | Case report | 39 | Japan | 8 weeks | Vitamin K
deficiency | Recurrent vomiting,
no food or drink for 1 week | Conservative with
vitamin K supplementation |
| Kawamura et
al (49) | 2008 | Case report | 33 | Japan | 9 weeks | Vitamin K
deficiency- induced fetal intracranial hemorrhage and
hydrocephalus | Persisting
vomiting | Conservative |
| Brunetti-Pierri
et al (50) | 2007 | Case report | Not specified | USA | 20 weeks | Brachytelephalangic
chondro-dysplasia punctata and gray matter heterotopias | Loss of
appetite | Conservative |
| Toriello et
al (51) | 2012 | Clinical
reports | Case 1: 22 years,
Case 2: Not specified, Case 3: 27 years, Case 4: 25 years, Case 5:
Not specified, Case 6: Not specified | USA | Case 1:
103/7 weeks, Case 2: Not specified, Case 3: 18 weeks,
Case 4: 11 weeks, Case 5: 8 weeks, Case 6: 6 weeks | Vitamin K
deficiency embryopathy | Midfacial
hypoplasia, absence of nasal spine, wide and flat nasal bridge | Conservative |
| Eventov-Friedman
et al (52) | 2009 | Case report | 41 | Israel | 16 weeks | Fetal intracranial
hemorrhage associated with vitamin K deficiency | Infant at birth was
pale, not breathing, bradycardic, and hypotonic | Conservative |
| Robinson et
al (53) | 1998 | Case report | 22 | USA | 15 weeks | Coagulopathy
secondary to vitamin K deficiency | Sudden onset of
severe right-sided epistaxis | Cauterization with
topical silver nitrate and surgical packing. Vitamin K
supplementation. |
| Yilmaz et al
(54) | 2014 | Case report | 22 | Turkey | 11 weeks | Hyper-parathyroid
crisis | Lethargy,
responding to noise and somatosensory stimulations with
vocalization, eye opening and limb movement |
Parathyroidectomy |
| Sun et al
(55) | 2014 | Clinical
analysis | 25.8 | Japan | | Transient
thyrotoxicosis | | Conservative |
| Katz et al
(56) | 2000 | Case report | 38 | USA | 30 weeks | Mycobacterium
chelonae sepsis associated with long-term use of an intravenous
catheter | Development of
tender, erythematous nodules on legs and arms | Clarithromycin |
| Paranyuk et
al (57) | 2006 | Case report | 33 | USA | Not specified | Candida
septicemia | Fever | Intravenous
fluconazole |
Severe hyperemesis can result in hypovolemia and
electrolyte abnormalities, in turn causing rhabdomyolysis (41). A total of two case reports described
this complication in the first trimester of pregnancy (41,42).
One case described acute intermittent porphyria (43). All patients received aggressive
fluid resuscitation and repletion. Administration of oxygen was
used as prophylaxis against extreme hypokalemia and renal failure
(41,42). HG is frequently associated with
weight loss, acetonuria and electrolytic imbalance with dehydration
(40). Hyponatremia, hypokalemia,
hypochloremia, hypophosphatemia and dehydration caused by HG can
influence other parameters, including QRS prolongation, hematocrit
increase, liver cholestasis and cytolysis with increased
transaminases, liver steatosis and hypoalbuminemia (4,23).
Hypokalemia is usually caused by nutritional deficiencies as a
result of electrolyte wasting, extracellular fluid volume reduction
and activation of the renin-angiotensin-aldosterone axis (44). In addition, physiological changes
that promote potassium wasting during pregnancy, such as volume
expansion, increased renal blood flow, increased glomerular
filtration rate and increased cortisol levels can all contribute to
reduced total body potassium levels (44). Kondo et al (44) reported a case of nephrogenic
diabetes insipidus (characterized by polyuria with impaired urine
concentration) and rhabdomyolysis (with increases in creatine
kinase increase) as a consequence of electrolytic imbalance, with
hypokalemia noted during prolonged HG (44). In addition, profound hypokalemia was
reported by Walch et al (45), which caused cardiac arrest and
spontaneous miscarriage. Cardiopulmonary resuscitation,
stabilization and electrolyte repletion was performed. The patient
suffered after 4 h a reversible episode of ventricular fibrillation
(venous blood potassium levels were decreased compared with the
time of hospital admission). The final outcome was favorable, and
the patient was released. Gitelman syndrome is a genetic disorder
caused by a defect in the solute carrier family 12 member 3
gene, which leads to the impaired function of thiazide-sensitive
sodium-chloride co-transporter (46). This is a condition that predisposes
the patient to electrolytic imbalance, notably in the context of HG
(46). However, appropriate
treatment with antiemetics, fluid and electrolyte supplementation
with restorations in nutritional balance may lead to full recovery
of the majority of patients in a few days (44-46).
Vitamin K deficiency has been rarely associated with
HG but can present with possible severe complications. Lane et
al (47) reported that
embryopathy with nasal hypoplasia was causally associated with HG.
In addition, Shigemi et al (48) reported a case of HG associated with
fetal intracranial hemorrhage due to severe HG. Vitamin K
deficiency is a complication of malnutrition and liver dysfunction
associated with prolonged HG (48).
In exceedingly rare cases, vitamin K deficiency can cause
coagulopathy and fetal intracranial hemorrhage resulting in
hydrocephalus and miscarriage (49). Several reports have concluded a
possible association between HG and severe fetal complications,
including gray matter heterotopias associated with seizures and
various types of bone dysplasias, such as brachytelephalangic
chondrodysplasia punctata, consistent with the Binder phenotype
(50,51). Vitamin K deficiency can cause fetal
intracranial hemorrhage associated with seizures even in the
absence of fetal morphological complications (52). Complications of HG
associated-vitamin K deficiency can also affect the progression of
pregnancy in women. Robinson et al (53) reported a severe case of epistaxis in
a patient at 15 weeks of gestation. When detected early and treated
with vitamin K replacement, complete correction of all clotting
factors was achieved (53).
Endocrine complications caused by HG are also relatively rare.
However, at least one report of primary hyperparathyroidism has
been published in pregnant women diagnosed with HG (54). Yilmaz et al (54) reported a case of severe
hypercalcemia associated with a parathyroid crisis, which was
resolved following urgent parathyroidectomy, without evidence of
neonatal hypocalcemia or tetany. Gestational transient
thyrotoxicosis in HG is highly prevalent, with an incidence of ~48%
and its severity correlating with serum hCG values (55). By the second trimester, thyroid
function was normalized without antithyroid treatment for all
patients with clinical gestational transient thyrotoxicosis
(55). Prolonged parenteral therapy
is required for pregnant women who develop HG and is associated
with an increased risk of infection development. Katz et al
(56) reported a case of
Mycobacterium chelonae-induced sepsis associated with the
long-term use of an intravenous catheter for HG treatment, which
was resolved slowly following treatment with clarithromycin without
any adverse effects on the fetus. In addition, Candida septicemia
was reported in a pregnant woman who underwent catheterization for
parenteral nutrition (57). The
patient recovered fully and gave birth to a healthy infant.
Discussion
The aim of the present systematic review was to
summarize the available evidence regarding severe, life-threatening
complications in HG. Observations from the majority of the studies
included in the present review demonstrated that certain
complications of HG could occur in cases of inadequate therapy or
even lack of medical support (58).
Nevertheless, some complications occurred even after the patient
received the appropriate therapy. The most frequent
life-threatening complication of HG was WE, which was demonstrated
by >70 studies over the last six decades. However, these papers
were not included in the present review since they were case
reports and to avoid redundancy of information. The main
limitations of the present study were the insufficient number of
studies assessing each complication and the fact that the majority
of the articles included were case reports. Another limitation was
the fact that the pathogenesis of endocrine complications was
insufficiently analyzed and inadequately clarified. Overall, the
current available data regarding the cardiovascular complications
of HG are limited, with most case reports being of low quality.
Based on the present evidence, it can be concluded that cardiac
complications in women with HG are rare but can be severe. These
mostly refer to ventricular arrhythmias, which are caused by QTc
prolongation due to electrolyte imbalance (hypokalemia,
hypomagnesemia, hypocalcemia) and are provoked by repeated episodes
of vomiting. Pre-eclampsia is another potential complication of HG,
which usually occurs during the second trimester of pregnancy which
if present, should be promptly managed. Data regarding vascular
complications are also limited. Although complications, including
thromboembolic episodes and marked arterial blood pressure
variations have been described, no conclusion can be drawn. In
addition, a high-quality study included in the present review,
which assessed the impact on HG on the long-term risk of maternal
cardiovascular morbidity (26)
demonstrated no evidence suggesting an increased risk of mortality
among women with HG and those without this condition.
The present systematic review exhibits several
important strengths. The topic of this systematic review is of
important clinical relevance due to the rapid increase in the
prevalence of teenage pregnancy in addition to pregnant women with
a history of long-term substance abuse (5). Both parameters are considered risk
factors for HG. An innovation of this review is that the data
provided by the present study, which analyses life-threatening
complications in HG, have not been previously published in this
form and can assist clinicians for developing an efficient tailored
therapy.
In conclusion, life-threatening complications are
exceedingly rare in HG. The most frequent severe complications are
WE, electrolyte imbalance and vitamin K deficiency. The low
mortality rate for patients with HG over the last decade is
explained by the high efficiency of modern therapy, where and the
precise management of every complication can be addressed by
current guidelines.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Data sharing is not applicable to this article, as
no datasets were generated or analyzed during the current
study.
Authors' contributions
SLP suggested the selection of the methodology,
searched the literature and made substantial contributions to the
writing of the manuscript by confirming the authenticity of the
studies used. MB analyzed the results, revised the manuscript and
made contribution to the preparation of the manuscript, confirming
the authenticity of the studies. AC made contributions to the
preparation of the thoracic complications chapter. CP made
contributions to the writing of the systemic complications chapter
and revised the manuscript. LM made contributions to the
preparation of the cardiovascular complications chapter and revised
the manuscript. LCP made contributions to the writing of the
neurological complications chapter. LPD made contribution to the
writing of the neurological complications chapter and revised the
manuscript. Data sharing is not applicable. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
McParlin C, O'Donnell A, Robson SC, Beyer
F, Moloney E, Bryant A, Bradley J, Muirhead CR, Nelson-Piercy C,
Newbury-Birch D, et al: Treatments for hyperemesis gravidarum and
nausea and vomiting in pregnancy: A systematic review. JAMA.
316:1392–1401. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Grooten IJ, Vinke ME, Roseboom TJ and
Painter RC: A systematic review and meta-analysis of the utility of
corticosteroids in the treatment of hyperemesis gravidarum. Nutr
Metab Insights. 8 (Suppl 1):S23–S32. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Matthews A, Haas DM, O'Mathúna DP and
Dowswell T: Interventions for nausea and vomiting in early
pregnancy. Cochrane Database Syst Rev. 9(CD007575)2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Mullin PM, Ching C, Schoenberg F,
MacGibbon K, Romero R, Goodwin TM and Fejzo MS: Risk factors,
treatments, and outcomes associated with prolonged hyperemesis
gravidarum. J Matern Fetal Neonatal Med. 25:632–636.
2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Fell DB, Dodds L, Joseph KS, Allen VM and
Butler B: Risk factors for hyperemesis gravidarum requiring
hospital admission during pregnancy. Obstet Gynecol. 107:277–284.
2006.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Summers A: Emergency management of
hyperemesis gravidarum. Emerg Nurse. 20:24–28. 2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ahmed KT, Almashhrawi AA, Rahman RN,
Hammoud GM and Ibdah JA: Liver diseases in pregnancy: Diseases
unique to pregnancy. World J Gastroenterol. 19:7639–7646.
2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Erick M, Cox JT and Mogensen KM: ACOG
practice bulletin 189: Nausea and vomiting of pregnancy. Obstet
Gynecol. 131(935)2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Koudijs HM, Savitri AI, Browne JL, Amelia
D, Baharuddin M, Grobbee DE and Uiterwaal CS: Hyperemesis
gravidarum and placental dysfunction disorders. BMC Pregnancy
Childbirth. 16(374)2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lanska DJ and Kryscio RJ: Risk factors for
peripartum and postpartum stroke and intracranial venous
thrombosis. Stroke. 31:1274–1282. 2000.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Seki M, Shibata M, Itoh Y and Suzuki N:
Intracerebral hemorrhage due to venous thrombosis of developmental
venous anomaly during pregnancy. J Stroke Cerebrovasc Dis.
24:e185–187. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kennelly MM, Baker MR, Birchall D, Hanley
JP, Turnbull DM and Loughney AD: Hyperemesis gravidarum and first
trimester sagittal sinus thrombosis. J Obstet Gynaecol. 28:453–454.
2008.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kanayama N, Khatun S, Belayet HM,
Yamashita M, Yonezawa M, Kobayashi T and Terao T: Vasospasms of
cerebral arteries in hyperemesis gravidarum. Gynecol Obstet Invest.
46:139–141. 1998.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Beach RL and Kaplan PW: Seizures in
pregnancy: Diagnosis and management. Int Rev Neurobiol. 83:259–271.
2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
O'Brien MD and Gilmour-White SK:
Management of epilepsy in women. Postgrad Med J. 81:278–285.
2005.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sinn DI, Bachman D and Feng W:
Simultaneous optic neuropathy and osmotic demyelinating syndrome in
hyperemesis gravidarum. Am J Med Sci. 347:88–90. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Bergin PS and Harvey P: Wernicke's
encephalopathy and central pontine myelinolysis associated with
hyperemesis gravidarum. BMJ. 6852:517–518. 1992.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Sutamnartpong P, Muengtaweepongsa S and
Kulkantrakorn K: Wernicke's encephalopathy and central pontine
myelinolysis in hyperemesis gravidarum. J Neurosci Rural Pract.
4:39–41. 2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zara G, Codemo V, Palmieri A, Schiff S,
Cagnin A, Citton V and Manara R: Neurological complications in
hyperemesis gravidarum. Neurol Sci. 33:133–135. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Oudman E, Wijnia JW, Oey M, van Dam M,
Painter RC and Postma A: Wernicke's encephalopathy in hyperemesis
gravidarum: A systematic review. Eur J Obstet Gynecol Reprod Biol.
236:84–93. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Azim W and Walker R: Wernicke's
encephalopathy: A frequently missed problem. Hosp Med. 64:326–327.
2003.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Mitchell SJ and Cox P: ECG changes in
hyperemesis gravidarum. BMJ Case Rep.
2017(bcr2016217158)2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kochhar PK and Ghosh P: Ventricular
tachycardia in a primigravida with Hyperemesis Gravidarum. J Obstet
Gynaecol Res. 44:1308–1312. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Jadhav RS, Sushil K, Anupam K and Pragati
T: Ventricular tachycardia and seizure in hyperemesis gravidarum. J
Obstet Gynaecol India. 60:339–340. 2010.
|
|
25
|
Bolin M, Åkerud H, Cnattingius S,
Stephansson O and Wikström AK: Hyperemesis gravidarum and risks of
placental dysfunction disorders: A population-based cohort study.
BJOG. 120:541–547. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Fossum S, Næss Ø, Halvorsen S, Tell GS and
Vikanes ÅV: Long-term cardiovascular morbidity following
hyperemesis gravidarum: A Norwegian nationwide cohort study. PLoS
One. 14(e0218051)2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Turrentine MA, Smalling RW and Parisi VM:
Right atrial thrombus as a complication of total parenteral
nutrition in pregnancy. Obstet Gynecol. 84:675–677. 1994.PubMed/NCBI
|
|
28
|
Salmon JR: Severe autonomic dysfunction
complicated by hyperemesis gravidarum causing unstable blood
pressure in pregnancy. Aust N Z J Obstet Gynaecol. 49:699–700.
2009.PubMed/NCBI View Article : Google Scholar
|
|
29
|
van Noord C, Eijgelsheim M and Stricker
BH: Drug- and non-drug-associated QT interval prolongation. Br J
Clin Pharmacol. 70:16–23. 2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Johnson JN and Ackerman MJ: QTc: How long
is too long? Br J Sports Med. 43:657–662. 2009.PubMed/NCBI View Article : Google Scholar
|
|
31
|
El-Sherif N, Turitto G and Boutjdir M:
Acquired long qt syndrome and electrophysiology of torsade de
pointes. Arrhythm Electrophysiol Rev. 8:122–130. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Fox R, Kitt J, Leeson P, Aye CYL and
Lewandowski AJ: Preeclampsia: Risk factors, diagnosis, management,
and the cardiovascular impact on the offspring. J Clin Med.
10(1625)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Karson EM, Saltzman D and Davis MR:
Pneumomediastinum in pregnancy: Two case reports and a review of
the literature, pathophysiology, and management. Obstet Gynecol. 64
(3 Suppl):39S–43S. 1984.PubMed/NCBI
|
|
34
|
Schwartz M and Rossoff L:
Pneumomediastinum and bilateral pneumothoraces in a patient with
hyperemesis gravidarum. Chest. 106:1904–1906. 1994.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Gorbach JS, Counselman FL and Mendelson
MH: Spontaneous pneumomediastinum secondary to hyperemesis
gravidarum. J Emerg Med. 15:639–643. 1997.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Liang SG, Ooka F, Santo A and Kaibara M:
Pneumomediastinum following esophageal rupture associated with
hyperemesis gravidarum. J Obstet Gynaecol Res. 28:172–175.
2002.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Yamamoto T, Suzuki Y, Kojima K, Sato T,
Tanemura M, Kaji M, Yamakawa Y, Yokoi M and Suzumori K:
Pneumomediastinum secondary to hyperemesis gravidarum during early
pregnancy. Acta Obstet Gynecol Scand. 80:1143–1145. 2001.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Germes-Piña F, Acosta-Orozco DM,
Flores-Franco RA and Verdugo-Castro PN: Pneumomediastinum
associated with hyperemesis gravidarum: A case report. Ginecol
Obstet Mex. 84:586–592. 2016.PubMed/NCBI(In Spanish).
|
|
39
|
Chen X, Yang X and Cheng W: Diaphragmatic
tear in pregnancy induced by intractable vomiting: A case report
and review of the literature. J Matern Fetal Neonatal Med.
25:1822–1824. 2012.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Fiaschi L, Nelson-Piercy C, Gibson J,
Szatkowski L and Tata LJ: Adverse maternal and birth outcomes in
women admitted to hospital for hyperemesis gravidarum: A
population-based cohort study. Paediatr Perinat Epidemiol.
32:40–51. 2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Lassey SC and Robinson JN: Rhabdomyolysis
after hyperemesis gravidarum. Obstet Gynecol. 128:195–196.
2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Fukada Y, Ohta S, Mizuno K and Hoshi K:
Rhabdomyolysis secondary to hyperemesis gravidarum. Acta Obstet
Gynecol Scand. 78(71)1999.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Shenhav S, Gemer O, Sassoon E and Segal S:
Acute intermittent porphyria precipitated by hyperemesis and
metoclopramide treatment in pregnancy. Acta Obstet Gynecol Scand.
76:484–485. 1997.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Kondo T, Nakamura M, Kawashima J,
Matsumura T, Ohba T, Yamaguchi M, Katabuchi H and Araki E:
Hyperemesis gravidarum followed by refeeding syndrome causes
electrolyte abnormalities induced rhabdomyolysis and diabetes
insipidus. Endocr J. 66:253–258. 2019.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Walch A, Duke M, Auty T and Wong A:
Profound hypokalaemia resulting in maternal cardiac arrest: A
catastrophic complication of hyperemesis gravidarum? Case Rep
Obstet Gynecol. 29(4687587)2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Daskalakis G, Marinopoulos S, Mousiolis A,
Mesogitis S, Papantoniou N and Antsaklis A: Gitelman
syndrome-associated severe hypokalemia and hypomagnesemia: Case
report and review of the literature. J Matern Fetal Neonatal Med.
23:1301–1304. 2010.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Lane AS, Stallworth JL, Eichelberger KY
and Trofatter KF: Vitamin K deficiency embryopathy from hyperemesis
gravidarum. Case Rep Obstet Gynecol. 2015(324173)2015.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Shigemi D, Nakanishi K, Miyazaki M,
Shibata Y and Suzuki S: A case of maternal vitamin K deficiency
associated with hyperemesis gravidarum: Its potential impact on
fetal blood coagulability. J Nippon Med Sch. 82:54–58.
2015.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Kawamura Y, Kawamata K, Shinya M, Higashi
M, Niiro M and Douchi T: Vitamin K deficiency in hyperemesis
gravidarum as a potential cause of fetal intracranial hemorrhage
and hydrocephalus. Prenat Diagn. 28:59–61. 2008.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Brunetti-Pierri N, Hunter JV and Boerkoel
CF: Gray matter heterotopias and brachytelephalangic
chondrodysplasia punctata: A complication of hyperemesis gravidarum
induced vitamin K deficiency? Am J Med Genet A. 143:200–204.
2007.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Toriello HV, Erick M, Alessandri JL,
Bailey D, Brunetti-Pierri N, Cox H, Fryer A, Marty D, McCurdy C,
Mulliken JB, et al: Maternal vitamin K deficient embryopathy:
Association with hyperemesis gravidarum and Crohn disease. Am J Med
Genet A. 161:417–429. 2013.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Eventov-Friedman S, Klinger G and Shinwell
ES: Third trimester fetal intracranial hemorrhage owing to vitamin
K deficiency associated with hyperemesis gravidarum. J Pediatr
Hematol Oncol. 31:985–988. 2009.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Robinson JN, Banerjee R and Thiet MP:
Coagulopathy secondary to vitamin K deficiency in hyperemesis
gravidarum. Obstet Gynecol. 92:673–675. 1998.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Yilmaz BA, Altay M, Değertekin CK, Çimen
AR, Iyidir ÖT, Biri A, Yüksel O, Törüner FB and Arslan M:
Hyperparathyroid crisis presenting with hyperemesis gravidarum.
Arch Gynecol Obstet. 290:811–814. 2014.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Sun S, Qiu X and Zhou J: Clinical analysis
of 65 cases of hyperemesis gravidarum with gestational transient
thyrotoxicosis. J Obstet Gynaecol Res. 40:1567–1572.
2014.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Katz VL, Farmer R, York J and Wilson JD:
Mycobacterium chelonae sepsis associated with long-term use of an
intravenous catheter for treatment of hyperemesis gravidarum. A
case report. J Reprod Med. 45:581–584. 2000.PubMed/NCBI
|
|
57
|
Paranyuk Y, Levine G and Figueroa R:
Candida septicemia in a pregnant woman with hyperemesis receiving
parenteral nutrition. Obstet Gynecol. 107:535–537. 2006.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Fejzo MS, MacGibbon K and Mullin PM: Why
are womenstill dying from nausea and vomiting of pregnancy? Gynecol
Obstet Case Rep: Jul 4, 2016 (Epub ahead of print).
|