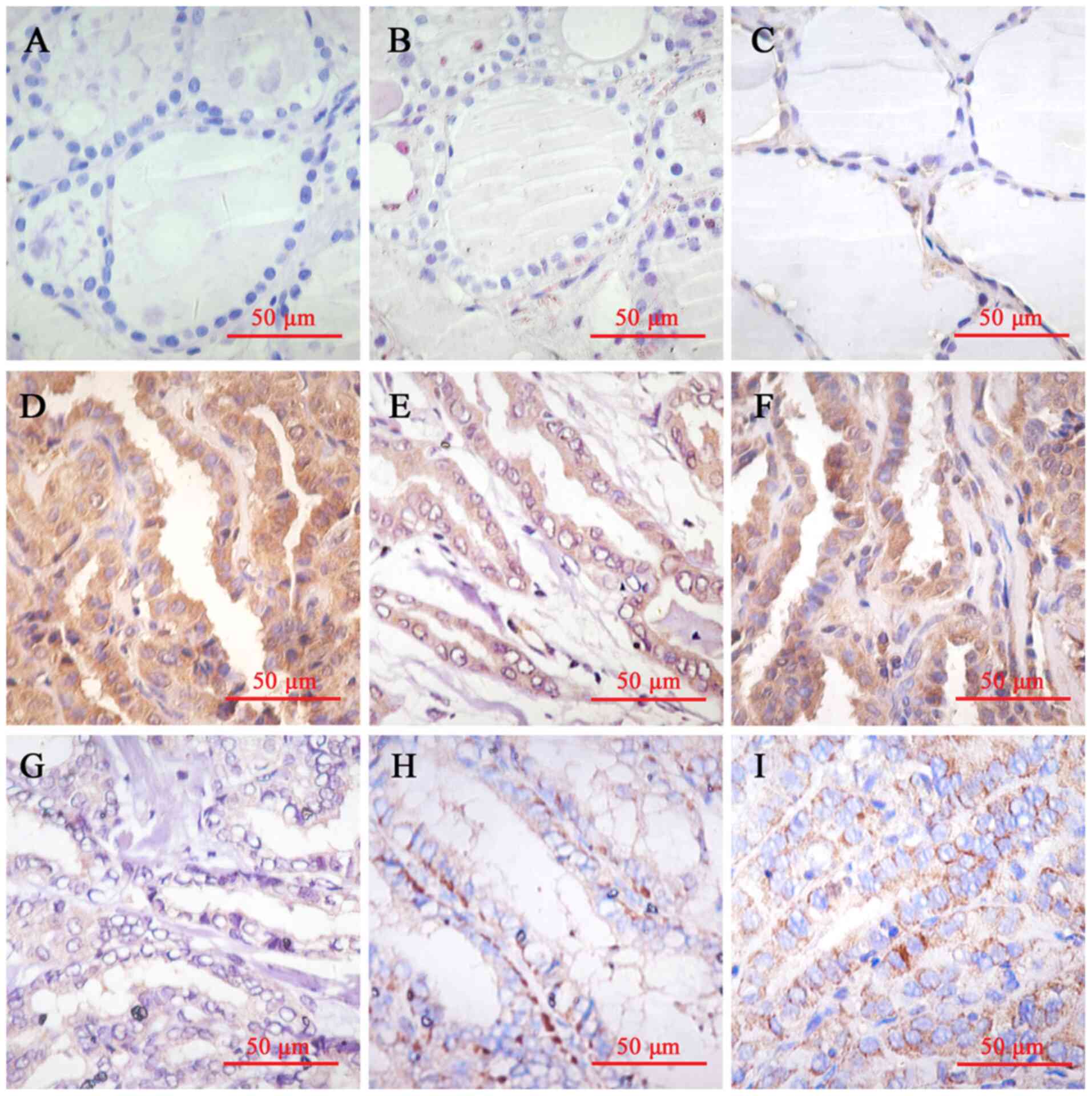
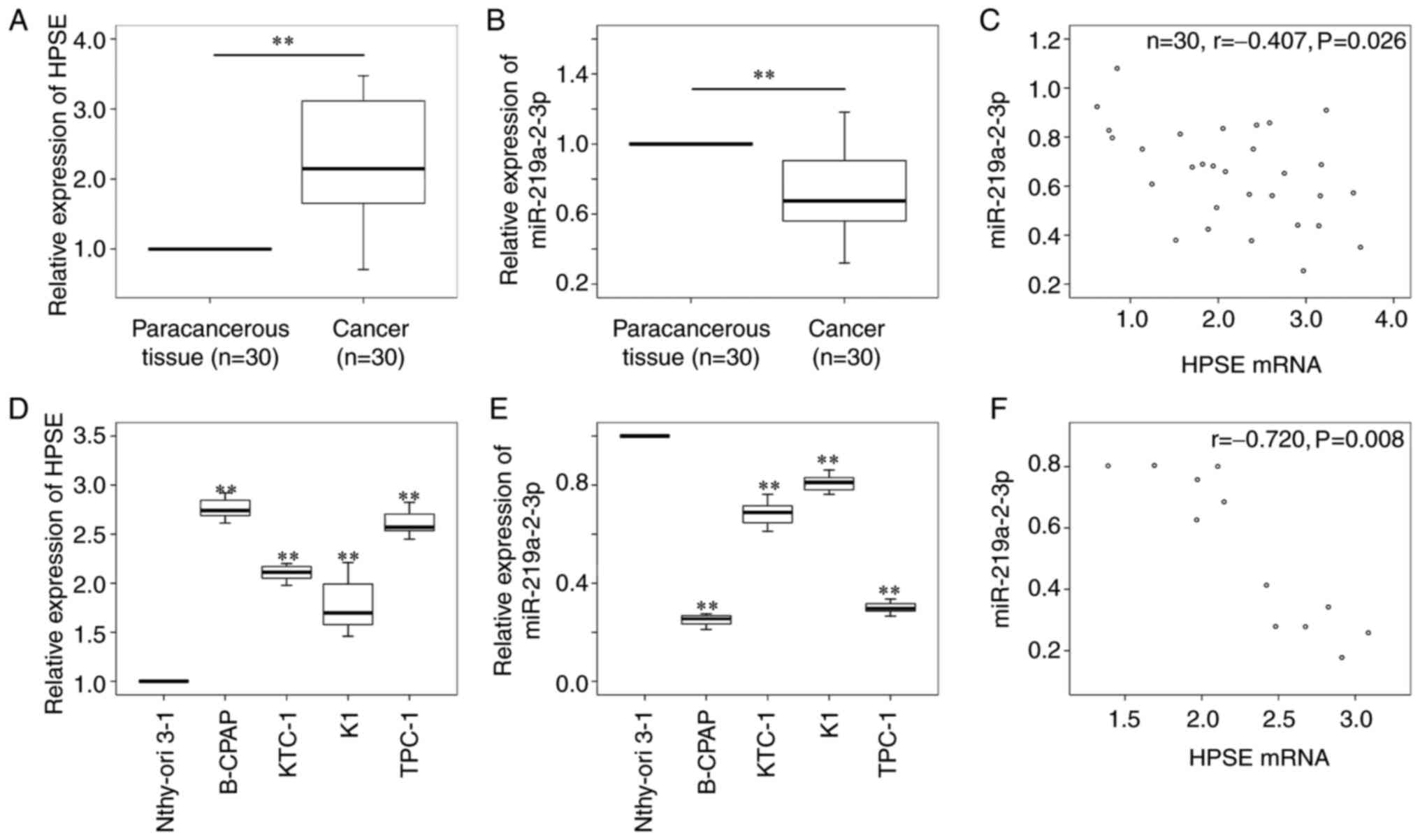
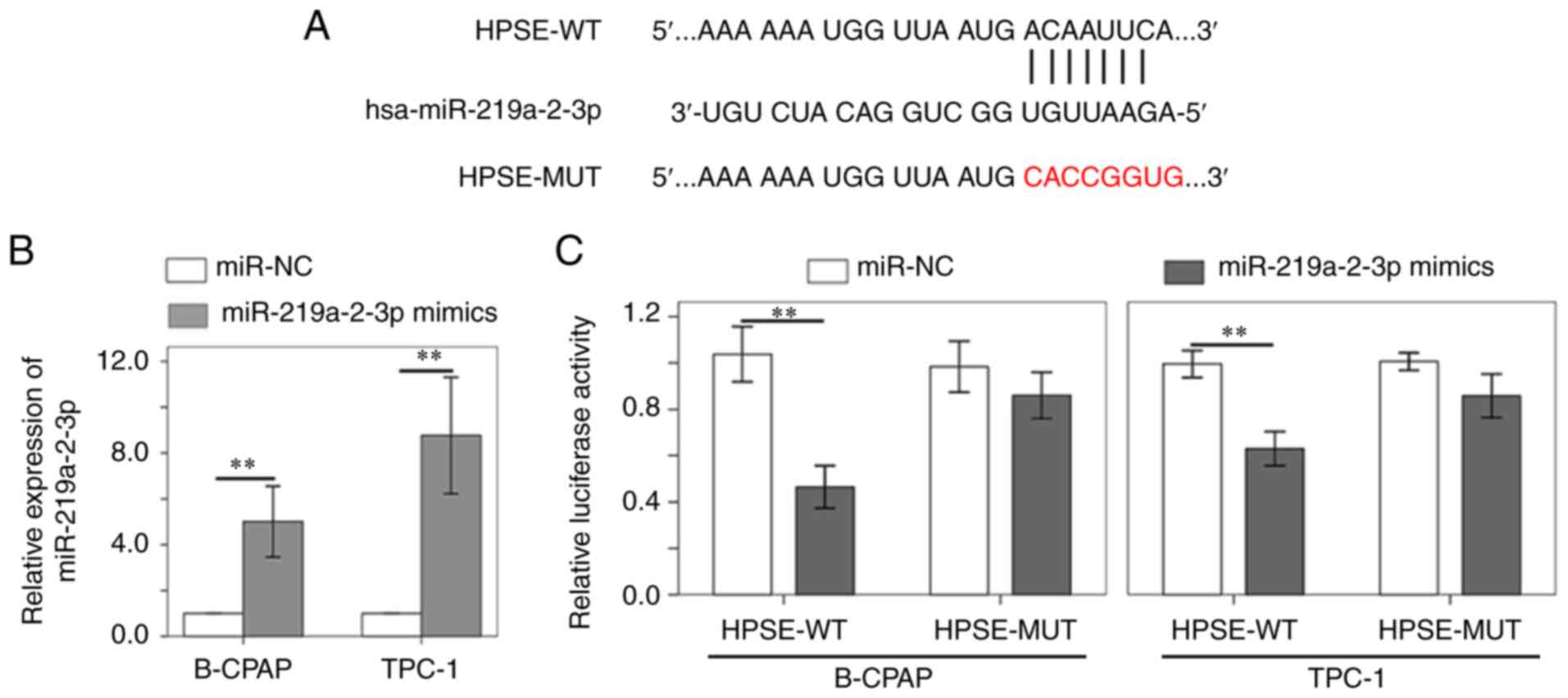
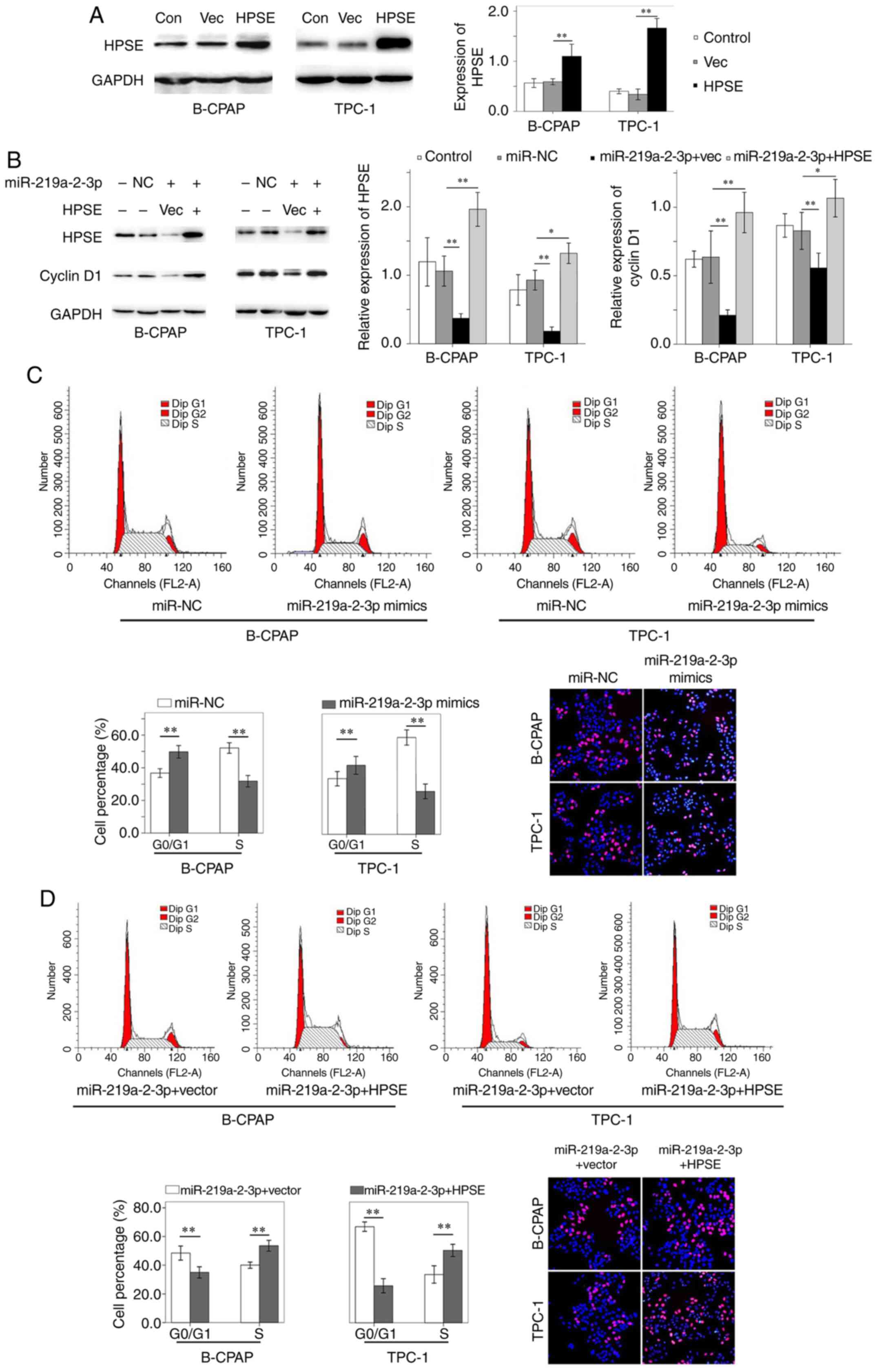
|
1
|
Masola V, Zaza G, Gambaro G, Franchi M and
Onisto M: Role of heparanase in tumor progression: Molecular
aspects and therapeutic options. Semin Cancer Biol. 62:86–98.
2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zheng HY, Ruan J, Zhao P, Chen SP, Pan LL
and Liu JQ: Heparanase is involved in proliferation and invasion of
ovarian cancer cells. Cancer Biomark. 15:525–534. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Chen XP, Luo JS, Tian Y, Nie CL, Cui W and
Zhang WD: Downregulation of heparanase expression results in
suppression of invasion, migration, and adhesion abilities of
hepatocellular carcinoma cells. Biomed Res Int.
2015(241983)2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lv B, Zhang B, Hu XY and Zeng QD:
Heparanase regulates in vitro VEGF-C expression and its clinical
significance to pancreatic ductal cell adenocarcinoma. Oncol Lett.
11:1327–1334. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lv QY, Wu KJ, Liu FL, Wu WR, Chen YR and
Zhang W: Interleukin-17A and heparanase promote angiogenesis and
cell proliferation and invasion in cervical cancer. Int J Oncol.
53:1809–1817. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Matos LL, Suarez ER, Theodoro TR, Trufelli
DC, Melo CM, Garcia LF, Oliveira OC, Matos MG, Kanda JL, Nader HB,
et al: The profile of heparanase expression distinguishes
differentiated thyroid carcinoma from benign neoplasms. PLoS One.
10(e0141139)2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chen XP, Jiang W, Yue CF, Zhang WJ, Tong
CG, Dai DF, Cheng B, Huang C and Lu L: Heparanase contributes to
trans-endothelial migration of hepatocellular carcinoma cells. J
Cancer. 8:3309–3317. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zheng LD, Jiao WJ, Song HJ, Qu HX, Li D,
Mei H, Chen YJ, Yang F, Li HH, Huang K and Tong QG: miRNA-558
promotes gastric cancer progression through attenuating
Smad4-mediated repression of heparanase expression. Cell Death Dis.
7(e2382)2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ma K, Xu HY, Zhang J, Zhao F, Liang HQ,
Sun HT, Li P, Zhang S, Wang RJ and Chen XY: Insulin-like growth
factor-1 enhances neuroprotective effects of neural stem cell
exosomes after spinal cord injury via an miR-219a-2-3p/YY1
mechanism. Aging (Albany NY). 11:12278–12294. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wang YB, Zhao JN, Zhang CC, Wang PC, Huang
CX and Peng H: MiR-219a-2-3p suppresses cell proliferation and
promotes apoptosis by targeting MDM2/p53 in pituitary adenomas
cells. Biosci Biotechnol Biochem. 84:911–918. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yang Z, Dong XH, Pu ML, Yang HW, Chang WL,
Ji FH, Liu T, Wei CQ, Zhang XF and Qiu XG:
LBX2-AS1/miR-219a-2-3p/FUS/LBX2 positive feedback loop contributes
to the proliferation of gastric cancer. Gastric Cancer. 23:449–463.
2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lloyd RV, Osamura RY, Klöppel G and Rosai
J (eds): WHO Classification of Tumours of Endocrine Organs. 4th
edition. IARC, Lyon, 2017.
|
|
13
|
Amin MB, Edge S, Greene F, Byrd DR,
Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR,
Sullivan DC, et al: AJCC Cancer Staging Manual. 8th edition.
Springer, New York, NY, 2017.
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wang C, Wei YJ, Wang G, Zhou YM, Zhang JC
and Xu K: Heparanase potentiates the invasion and migration of
pancreatic cancer cells via epithelial-to-mesenchymal transition
through the Wnt/β-catenin pathway. Oncol Rep. 44:711–721.
2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chen XP, Cheng B, Dai DF, Wu YH, Feng ZW,
Tong CG, Wang XM and Zhao J: Heparanase induces necroptosis of
microvascular endothelial cells to promote the metastasis of
hepatocellular carcinoma. Cell Death Discov. 7(33)2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Tang DB, Piao Y, Zhao S, Mu XD, Li S, Ma
WJ, Song Y, Wang JX, Zhao WH and Zhang QY: Expression and
correlation of matrix metalloproteinase-9 and heparanase in
patients with breast cancer. Med Oncol. 31(26)2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhang W, Chan H, Wei LW, Pan ZM, Zhang JQ
and Li L: Overexpression of heparanase in ovarian cancer and its
clinical significance. Oncol Rep. 30:2279–2287. 2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kundu S, Xiong AQ, Spyrou A, Wicher G,
Marinescu VD, Edqvist PHD, Zhang L, Essand M, Dimberg A, Smits A,
et al: Heparanase promotes glioma progression and is inversely
correlated with patient survival. Mol Cancer Res. 14:1243–1253.
2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Sheng N, Zhang L and Yang SF: MicroRNA-429
decreases the invasion ability of gastric cancer cell line BGC-823
by downregulating the expression of heparanase. Exp Ther Med.
15:1927–1933. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Fleming J, Bell EH, Tong ZY, Grozdic I,
McElroy J, Beyer S, Fabian D, Cui TT, Popp I, Staszewski O, et al:
CSIG-21. The role of miR-219a-2-3p as a tumor suppressor in
IDH1/2-wild-type grade II/III gliomas. Neuro Oncol. 20 (Suppl
6)(vi47)2018.
|