Introduction
Helicobacter pylori (HP) infection is one of
the most frequent bacterial infections in humans, and approximately
half of the global population are infected with HP (1,2). HP is
associated with the pathogenesis of chronic gastritis, peptic
ulcers and gastric cancer (3), and
also with gastric motility disorders such as functional dyspepsia
and delayed gastric emptying (DGE) (4,5). The
effect of HP infection on gastric emptying has been an enduring
topic of study and most researchers consider that HP infection
delays gastric emptying, but there has been little research on the
associated mechanism (5-8).
Gastric motility involves interactions among
gastrointestinal hormones, smooth muscle, enteric and extrinsic
autonomic nerves and interstitial cells of Cajal (ICCs) (9-11).
ICCs are a specific type of interstitial cells in the
gastrointestinal tract (12); they
play an important role in gastrointestinal motility and are
acknowledged to act as physiological pacemakers for the
gastrointestinal tract (13-16).
The proliferation, differentiation and function of ICCs is
associated with activation of c-kit receptors on their surfaces
(17,18). The activation of c-kit is dependent
on the natural ligand stem cell factor (SCF) (19,20).
Previous studies have shown that a mutation or loss of expression
of SCF leads to a reduction in the number of ICCs or alters the
integrity of the ICC network, which contributes to gastrointestinal
dysmotility, including chronic idiopathic intestinal
pseudo-obstruction, achalasia, afferent loop syndrome and chronic
constipation (21-23).
Therefore, the SCF/c-kit pathway is important for the development
and maintenance of ICC phenotype and function (24,25).
In the present study, an HP-infected C57BL/6 mouse
model was established with the aim of studying the effect of HP
infection on gastric emptying function and the underlying
mechanism.
Materials and methods
Animals and HP infection
All experiments and procedures in the present study
were approved by the Ethics Committee of Shandong Cancer Hospital.
A total of 24 female specific pathogen-free (SPF) C57BL/6 mice,
aged 6-8 weeks and weighing 16-18 g were purchased from Beijing
Vital River Laboratory Animal Technology Co., Ltd. To establish a
model of HP infection, 12 mice (the HP+ group) were
infected with HP Sydney strain 1 (SS1) at a concentration of
1x109 colony-forming units/ml by oral gavage. Another 12
mice remained uninfected and were used as controls (the
HP- group). All the mice were housed at constant
temperature (23±2˚C) and humidity (50±10%) in a SPF facility with a
12-h light/dark cycle and unconditional access to water and food.
The mice were monitored twice each day to evaluate their health. If
they were extremely weak, unable to drink and eat by themselves, or
suffered from cachexia, they would be euthanized by an
intraperitoneal injection of barbiturate (150 mg/kg). After 6
weeks, all 24 mice were sacrificed using cervical dislocation. The
criteria used to confirm death included immobility, respiratory
arrest and pupil dilation. The stomachs were dissected from the
mice and subjected to histological examination and the extraction
of protein. The gastric emptying of the HP-infected mice (n=12) and
control mice (n=12) was evaluated prior to sacrifice as described
below. HP infection was confirmed in the stomachs of all the
HP-infected mice by the microaerobic bacterial culture of stomach
homogenates on tryptic soy agar medium (cat. no. T8650; Beijing
Solarbio Science & Technology Co., Ltd.) supplemented with 5%
sheep blood (cat. no. TX0030; Beijing Solarbio Science &
Technology Co., Ltd.) and HP selective supplement (cat. no.
HB8646a; Qingdao Hope Bio-Technology Co., Ltd.) at 37˚C in an
atmosphere containing 5% O2, 10% CO2 and 85%
N2 and by immunohistochemical (IHC) examination.
Evaluation of gastric emptying and
specimen collection
Distilled water (0.5 ml/mouse) containing 40 resin
beads (diameter, 0.4 mm) was administered to each mouse via oral
gavage. The stomach was removed 1 h after the gavage of beads and
cut along the greater curvature. The contents were washed out into
a Petri dish and the number of beads in the dish was counted.
Gastric emptying was calculated using the following formula:
Gastric emptying (%)= (40-number of beads in the stomach at 1 h)/40
x100 (26,27).
Each gastric tissue specimen was divided into two
pieces: One piece was washed with PBS and fixed with 4%
paraformaldehyde at room temperature for 48 h for
immunohistochemistry, while the other piece was frozen quickly with
liquid nitrogen and placed in a refrigerator at -70˚C until
required for protein extraction.
Confirmation of HP infection by
immunohistochemistry
Each gastric tissue specimen in 4% paraformaldehyde
was encased in a paraffin block and sectioned. The sections (~5 µm)
were mounted on slides and baked at 70˚C for 10 min. Xylene
solution was used for dewaxing.
The gastric tissue specimens were also examined by
hematoxylin and eosin (H&E) staining as follows. The sections
were hydrated for 15 min in anhydrous ethanol, 95% ethanol, 80%
ethanol and 70% ethanol and then washed with water for 2 min.
Hematoxylin staining was performed at room temperature for 3 min,
after which the sections were washed with flowing water for 5 min,
1% hydrochloric acid-ethanol for 5 sec, flowing water for 5 min and
0.5% aqueous ammonia for 20 sec. The sections were then stained
with 1% eosin at room temperature for 2 min and washed with water
for 2 min. After this, they were dehydrated in an ethanol gradient,
made transparent in xylene for 20 min, washed with PBS three times
and sealed with neutral gum.
The procedures used for the IHC staining of HP were
as follows. The sections were dewaxed, hydrated and then washed
with PBS for 5 min. After this, they were incubated with 3%
hydrogen peroxide solution at room temperature for 15 min to block
endogenous peroxidase activity and washed with PBS three times for
5 min each. Sodium citrate was used for antigen retrieval at 100˚C
for 20 min, and the sections were allowed to cool naturally. The
sections were washed with PBS three times for 3 min each and then
incubated in 5% BSA blocking solution (cat. no. SW3015; Beijing
Solarbio Science & Technology Co., Ltd.) at room temperature
for 20 min. The excess liquid was removed without washing. Anti-HP
antibody (dilution 1:500; ab140128; Abcam) was added and the
sections were incubated at 4˚C overnight and at room temperature
for 45 min. After washing the sections with PBS for 5 min four
times each, the sections were incubated with biotin-labeled goat
anti-rabbit secondary antibody (dilution 1:200; cat. no. BA1003;
Wuhan Boster Biological Technology, Ltd.) at room temperature for
30 min and then washed with PBS for 5 min three times each. The
sections were subsequently incubated with
streptavidin-biotin-peroxidase complex (cat. no. SA1029; Wuhan
Boster Biological Technology, Ltd.) at room temperature for 20 min
and washed with PBS for 5 min four times each. They were then
incubated with diaminobenzidine detection reagent solution at room
temperature for 3 min, washed with distilled water, counterstained
with hematoxylin at room temperature for 2 min and washed with
flowing water for 5 min. The stained sections were immersed in 1%
hydrochloric acid-ethanol for 10 sec, washed with flowing water for
5 min, immersed in 0.5% aqueous ammonia for 20 sec and washed with
flowing water for 5 min (all at room temperature). Finally, the
sections were dehydrated in an ethanol gradient, made transparent
in xylene for 20 min, washed with PBS three times and sealed with
neutral gum.
Specimens were observed under a light microscope
(magnification, x400). A yellow or brown bacterial colony visible
on the surface of the gastric mucosa was considered positive, which
indicated the success of the model.
Measurement of c-kit-positive ICCs and
c-kit expression in the gastric mucosa
The procedures for c-kit protein IHC staining were
the same as those used for HP. The primary antibody used was a
rabbit anti-mouse c-kit monoclonal antibody (dilution 1:200;
sc-365504; Santa Cruz Biotechnology, Inc.).
Specimens were observed under a light microscope
(magnification, x400). The c-kit-positive cells, which were
indicated to be ICCs, exhibited blue nuclei and brown cytoplasm.
Five high-magnification views (x400) were randomly selected in each
section and the number of ICCs was calculated in each field; the
sections were collected from different groups in a blinded manner
by different examiners (9,28). The number of ICCs was calculated in
the intramuscular layer (ICC-IM subtype) and submucosal layer
(ICC-SM subtype).
Western blotting of SCF
The protein expression levels of SCF in gastric
samples were evaluated by western blotting. Each sample (~50 mg)
was harvested and homogenized. The homogenate was added to lysis
buffer (cat. no. AR0101; Wuhan Boster Biological Technology, Ltd.)
for 30 min at 4˚C and then subjected to centrifugation at 10,000 x
g for 10 min at 4˚C. The supernatants were collected and the
protein concentrations determined using a BCA protein concentration
determination kit. Total protein samples (~40 µg/lane) were
separated by SDS-PAGE (12% gel by weight) and transferred to PVDF
membranes. Following blocking with 5% skimmed milk on a shaker at
room temperature for 1 h, the membranes were incubated with primary
antibodies against SCF (dilution 1:200; rabbit polyclonal antibody;
ab83866; Abcam) and glyceraldehyde 3 phosphate dehydrogenase
(GAPDH; dilution 1:500; rabbit polyclonal antibody; BA2913; Wuhan
Boster Biological Technology, Ltd.) at 4˚C overnight. The membranes
were then washed with TBS containing 0.05% Tween-20 three times
prior to incubation with the HRP-conjugated secondary antibody
(dilution 1:5,000; goat anti-rabbit IgG; BA1054; Wuhan Boster
Biological Technology, Ltd.) at room temperature for 1 h.
Hypersensitive ECL Chemiluminescence Ready-to-Use Substrate (cat.
no. AR1170; Wuhan Boster Biological Technology, Ltd.) was used to
reveal the bands and the Odyssey Infrared Imaging System (LI-COR
Biosciences) was used for chemiluminescence detection. The
reference protein GAPDH was used as a loading control. The amount
of protein expression relative to that of GAPDH was calculated
using ImageJ software version 1.8.0 (National Institutes of
Health).
Statistical analysis
SPSS software version 20.0 (IBM Corp.) was used for
data analysis. Measurement data are presented as the mean ± SD. For
the normally distributed data, differences between two groups were
evaluated using a Student's t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Confirmation of HP infection by
histological staining and IHC analysis
Tissue sections were examined to confirm the HP
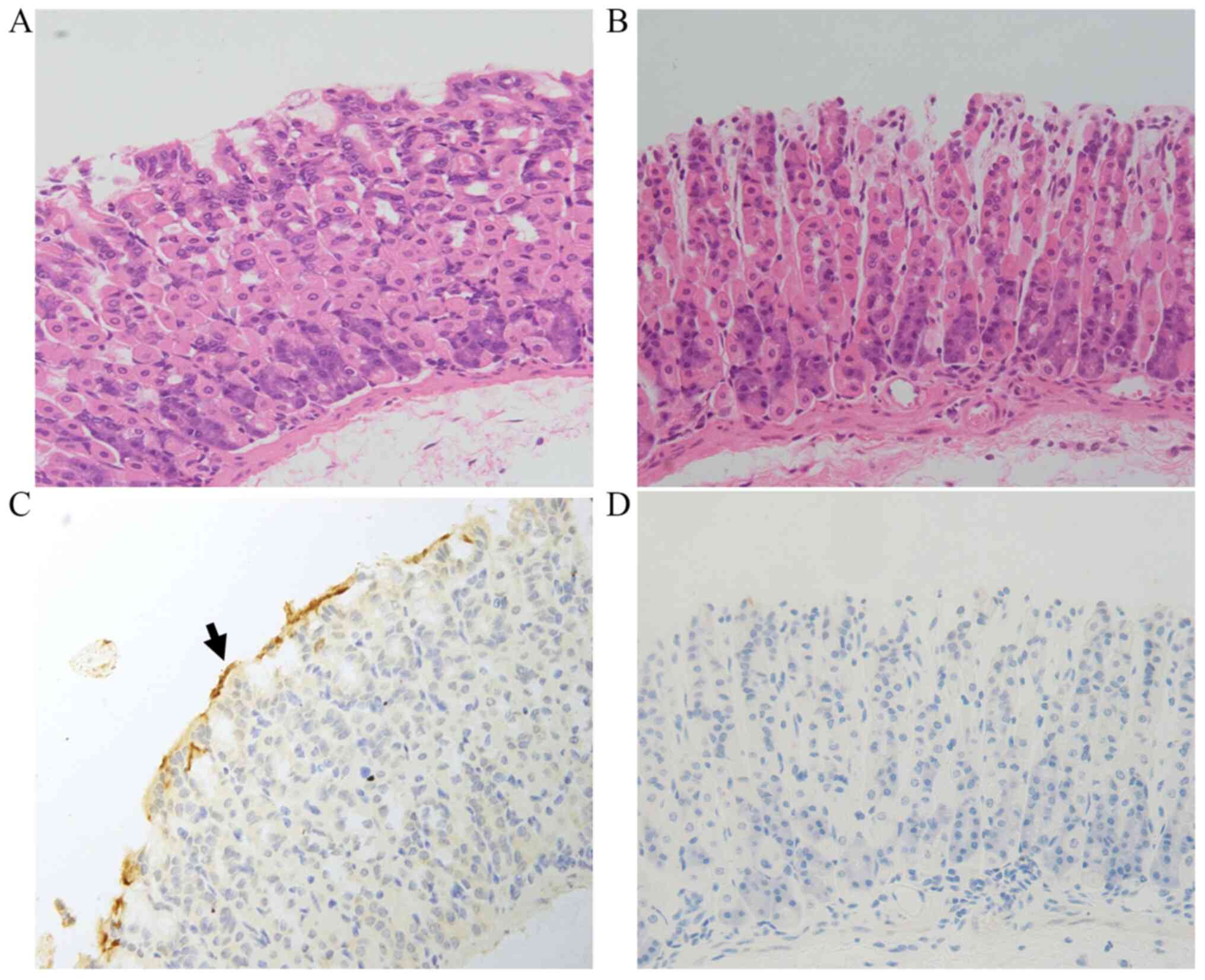
infection in the HP-treated mice. As shown in Fig. 1, no visible differences between
groups were detected by H&E staining. IHC analysis revealed
that in the HP+ group, yellow or brown HP colonies were
present on the surface of the tissue, while no such HP colonies
were detected in the HP- group (Fig. 1).
Influence of HP infection on gastric
emptying
The gastric emptying rate in the HP+
group was 65.8±5.6%, which was significantly lower compared with
that in the HP- group (83.8±6.4%) (P<0.001).
Number of ICCs is decreased in the
gastric tissues of C57BL/6 mice with HP infection
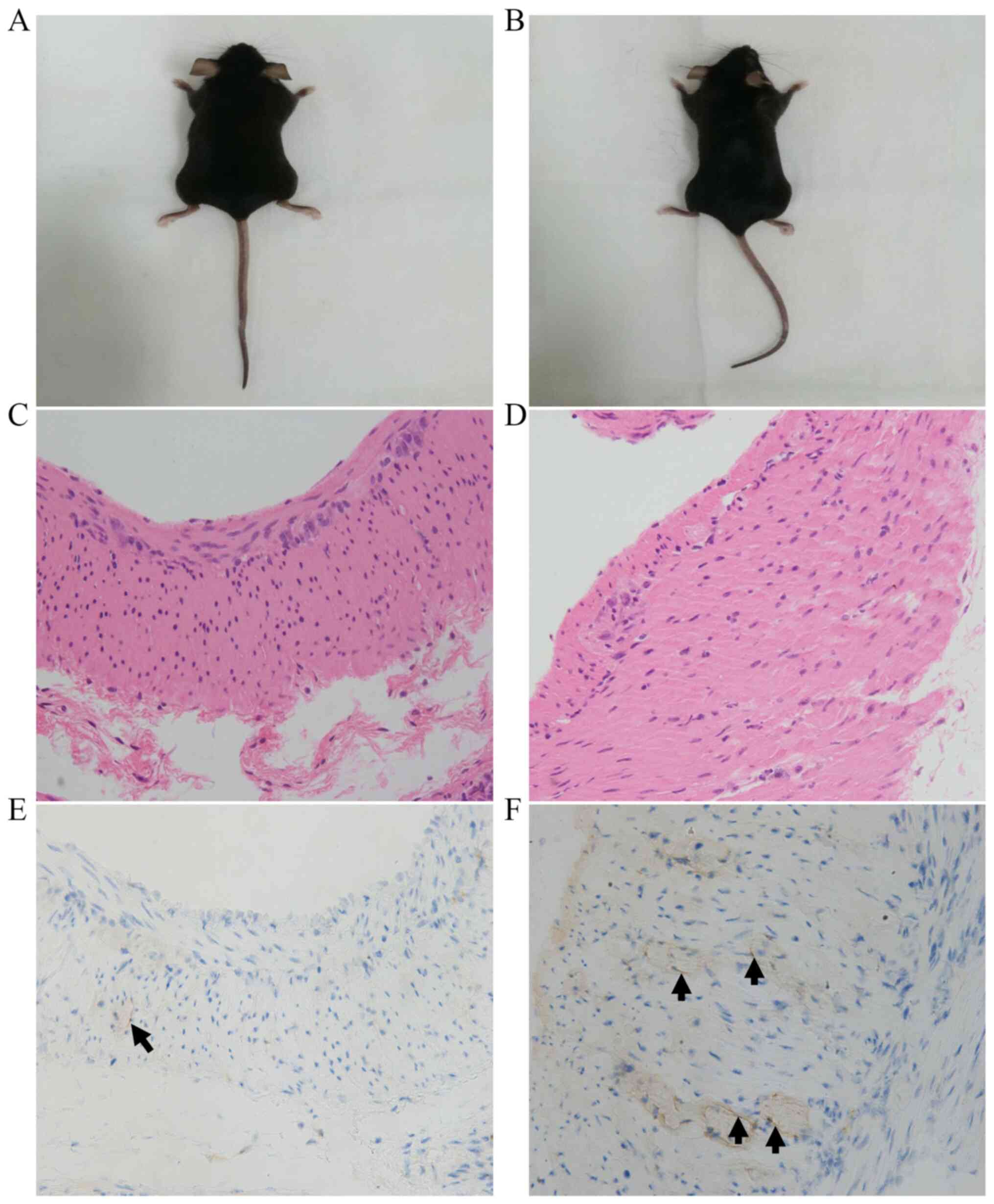
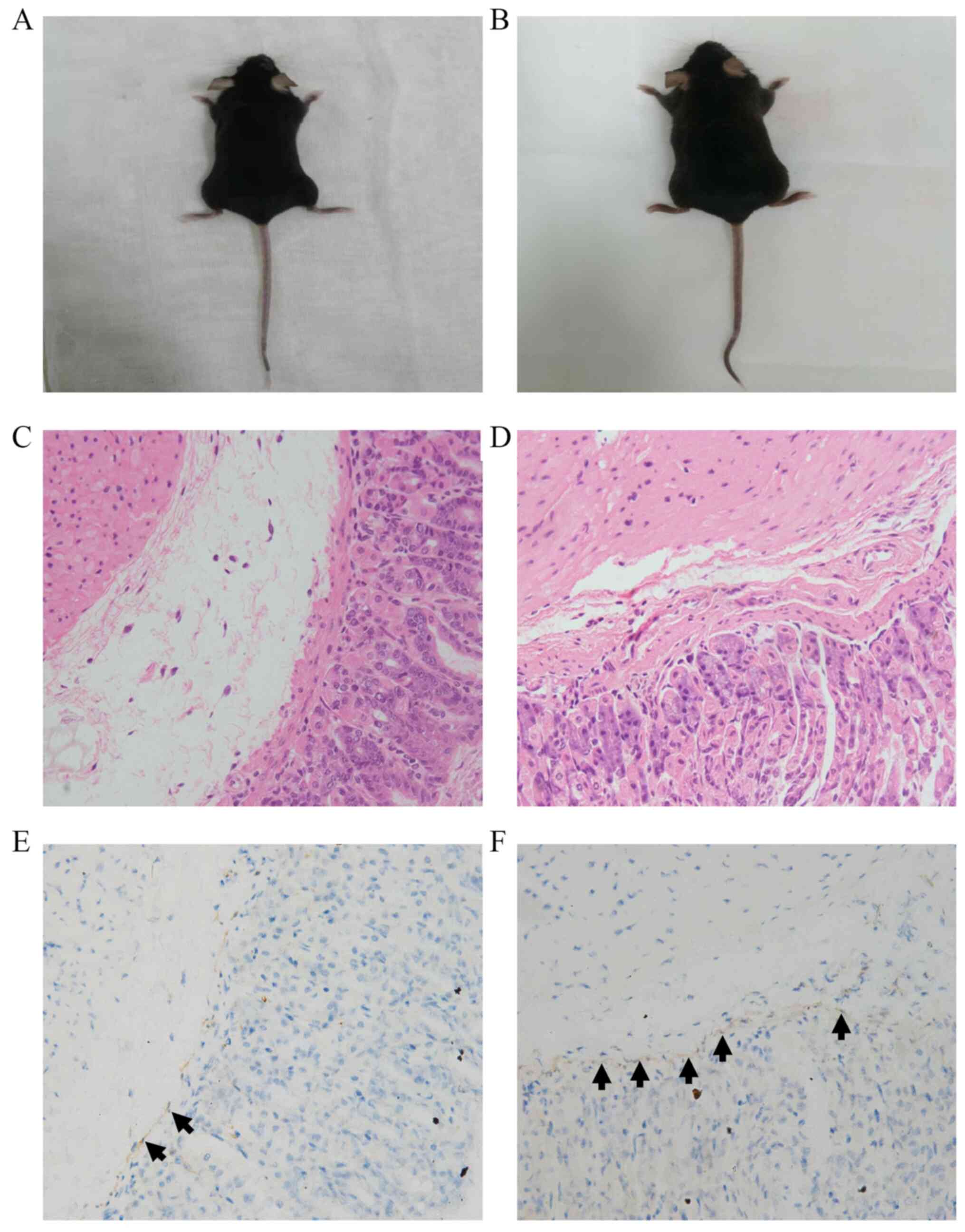
As shown in Figs. 2
and 3, there was no significant
difference in the appearance between the two groups of mice, and no
visible differences between groups were detected by H&E
staining. As shown in Fig. 2 and
Table I, the number of ICCs in the
intramuscular layer in the HP+ group was significantly
lower compared with that in the HP- group (3.32±1.60 vs.
5.52±2.17, respectively; P=0.011). Furthermore, as shown in
Fig. 3 and Table II, the number of ICCs in the
submucosal layer in the HP+ group was also significantly
lower compared with that in the HP- group (6.29±2.46 vs.
14.00±5.18, respectively; P<0.001).
 | Table IICC count in the gastric
intramuscular layer. |
Table I
ICC count in the gastric
intramuscular layer.
| Groups | Number of ICCs | P-value |
|---|
| HP+
group | 3.32±1.60 | 0.011 |
| HP-
group | 5.52±2.17 | |
 | Table IIICC count in the gastric submucosal
layer. |
Table II
ICC count in the gastric submucosal
layer.
| Groups | Number of ICCs | P-value |
|---|
| HP+
group | 6.29±2.46 | <0.001 |
| HP-
group | 14.00±5.18 | |
Protein expression of SCF in the
gastric tissues of C57BL/6 mice after HP infection
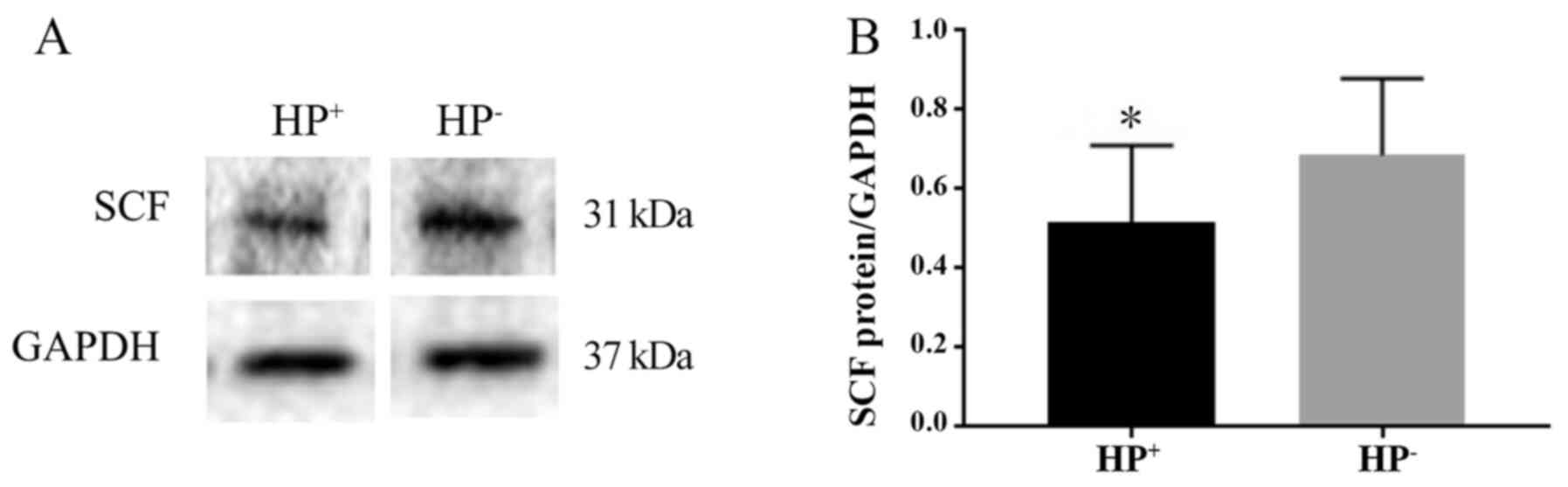
The protein expression level of SCF was determined
by western blotting and normalized to that of the internal control
GAPDH. The presence of the 31-kDa SCF protein was revealed by
western blot analysis (Fig. 4A).
The expression level of SCF in the HP+ group was
significantly lower compared with that in the HP- group
(P<0.05). The SCF/GAPDH ratio was 0.52±0.19 in the
HP+ group and 0.69±0.19 in the HP- group
(Fig. 4B).
Discussion
HP infection is a highly prevalent bacterial
infection in humans worldwide (1,2), and
is a cause of organic diseases, including gastritis, gastric ulcer
and gastric cancer, and also gastrointestinal functional diseases
such as functional dyspepsia (3,4).
DGE is defined as the delayed emptying of gastric
contents in the absence of mechanical obstruction. The major
symptoms of DGE include nausea, vomiting, bloating, early satiety
and abdominal pain (29-31).
DGE is a common complication following gastrointestinal surgery,
particularly pancreatoduodenectomy (32,33).
Among patients with DGE who have not undergone surgery, diabetes is
the most common cause of the condition (11,34).
Patients with DGE suffer from nutritional deficiencies and
metabolic consequences as well as impairment of social activities
and quality of life (18,35). There have been a number of studies
on the relationship between HP infection and DGE, and it has been
reported that HP infection can retard the gastric emptying function
and induce DGE, but the specific mechanism has rarely been studied
(5,6).
The present study was conducted to investigate the
mechanism underlying the effect of HP infection on DGE in mice. The
SS1 strain of HP was selected because it is the most widely used
strain for establishing the mouse model of HP infection.
Furthermore, the use of this strain is convenient for comparing the
results of the present study with those of other studies using the
same strain.
The results of the present study showed that the
gastric emptying rate of the mice infected with HP was
significantly lower than that of noninfected mice, which is
consistent with the results of previous studies (5,6).
Gastric motility involves interactions between
smooth muscle, enteric and extrinsic autonomic nerves and ICCs,
with the role of ICC considered to be critical for proper
gastrointestinal motility; several gastrointestinal motility
disorders have been confirmed to be caused by ICC damage (21-23).
ICCs are mesenchymal cells that act as pacemakers for the
generation of slow waves in the gastrointestinal tract. The
electrical activity of ICCs defines the frequency of the rhythmic
contraction of smooth muscles in the tract (13-16).
ICCs are distributed throughout the gastrointestinal tract from the
esophagus to the internal anal sphincter (36). They are involved in motor activities
as conduits for muscular innervation and may also provide sensory
innervation to the gastrointestinal tract (37). It has been identified that subtypes
of ICC exist according to their location in the body and certain
morphological and functional criteria; these include ICCs located
in muscle bundles and between muscle cells (the ICC-IM subtype) and
ICCs located in submucosal layers (the ICC-SM subtype) (38-40).
Smooth muscle cells (SMCs), ICC-IMs and platelet-derived growth
factor α-positive cells form the SIP syncytium, and ICC-IMs are
considered to integrate neuronal signals in the SMC syncytium to
ensure functional gastrointestinal motility (41). ICC-SMs are able to generate slow
waves and induce gastrointestinal activity (42). ICCs express the proto-oncogene c-kit
and its natural ligand SCF, which are associated with the
proliferation, differentiation and function of ICCs (17-20,43).
Changes in SCF levels have been shown to lead to changes in the
numbers of ICCs, which contributes to gastrointestinal dysmotility
(12).
In the present study, western blot analysis revealed
that the expression levels of SCF were significantly lower in
gastric tissue from the HP-infected group in comparison with that
from the HP-uninfected group. The numbers of ICC-SMs and ICC-IMs
were also significantly reduced in the HP-infected group. From
these results, it may be concluded that HP infection caused a
reduction in the SCF level, leading to a reduction in the number of
ICCs in gastric tissue and resulting in DGE. Specifically, this
suggests that HP infection causes DGE by reducing the number of
ICCs in gastric tissue. At present, there is no literature
describing the mechanism by which HP causes SCF protein expression
to be reduced. As protein expression requires transcription and
translation processes, any factors that affect the process of
transcription and translation could potentially affect protein
expression. Therefore, it may be inferred that HP infection changed
the environment in the stomach so that it was less suitable for SCF
protein expression, which caused the reduction in SCF protein
expression.
At present, the rate of antibiotic resistance to HP
is worrying and continues to increase each year, leading to
difficulties in eradicating the infection (44,45).
The rates of resistance of HP to various antibiotics are rising
(44). Therefore, it may become
increasingly difficult to eradicate HP and thereby resolve
associated diseases, including DGE caused by HP. Thus, it is
necessary to explore novel methods for treating DGE, such as
methods for increasing the number of ICCs in gastric tissue.
Previous studies have shown that electroacupuncture
at ST36(25), aqueous extracts of
Herba Cistanche (13) and Yangyin
Runchang decoction (14) can
increase the number of ICCs by increasing the expression of the SCF
protein. Therefore, we hypothesize that the reduction in the number
of ICCs caused by HP infection may be rescued by the overexpression
of SCF. However, this additional animal experiment requires ethical
approval and fund applications to acquire financing. Therefore,
this experiment was not performed as part of the present study,
which is a limitation of the study; however, it will be conducted
in the future.
Although the effect of HP infection on solid gastric
emptying in mice was studied in the present study, its effect of
liquid gastric emptying was not investigated, which is another
limitation of the study. The gastric emptying of liquid is
physiologically different from that of a solid meal; therefore, the
effect of HP infection on liquid gastric emptying requires
investigation in another study.
In summary, the present study investigated whether
HP causes DGE by reducing the number of ICCs. However, whether the
increased expression of ICCs can be targeted for the treatment of
DGE remains to be explored, and other mechanisms of HP that affect
gastric emptying require further study.
Acknowledgements
The authors would like to thank Professor Wenjuan Li
at Shandong University for her gift of HP SS1.
Funding
Funding: Funding was received from the National Natural Science
Fund of China (grant nos. 81272375 and 81872400) and from the
Shandong Provincial Health and Family Planning Commission (grant
no. 2018WS218).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BL analyzed the data and drafted the manuscript. JD
established the model and collected samples from the mice. SW and
HY performed IHC staining. ZL and PS performed western blotting. LZ
designed the study and revised the paper. BL and LZ confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
All experiments and procedures in the present study
were approved by the Ethics Committee of Shandong Cancer
Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Polyzos SA, Zeglinas C, Artemaki F,
Doulberis M, Kazakos E, Katsinelos P and Kountouras J:
Helicobacter pylori infection and esophageal adenocarcinoma:
A review and a personal view. Ann Gastroenterol. 31:8–13.
2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Dolak W, Bilgilier C, Stadlmann A, Leiner
J, Püspök A, Plieschnegger W, Siebert F, Wewalka F, Schöfl R,
Huber-Schönauer U, et al: A multicenter prospective study on the
diagnostic performance of a new liquid rapid urease test for the
diagnosis of Helicobacter pylori infection. Gut Pathog.
9(78)2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Gressot P, Frossard JL, Grosgurin O and
Marti C: First line eradication treatment of Helicobacter
pylori in 2019. Rev Med Suisse. 15:1854–1858. 2019.PubMed/NCBI View Article : Google Scholar : (In French).
|
|
4
|
Saito Y, Suzuki H, Tsugawa H, Suzuki S,
Matsuzaki J, Hirata K and Hibi T: Dysfunctional gastric emptying
with down-regulation of muscle-specific microRNAs in
Helicobacter pylori-infected mice. Gastroenterology.
140:189–198. 2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Huang J: Analysis of the relationship
between Helicobacter pylori infection and diabetic
gastroparesis. Chin Med J (Engl). 130:2680–2685. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhang CL, Geng CH, Yang ZW, Li YL, Tong
LQ, Gao P and Gao YQ: Changes in patients' symptoms and gastric
emptying after Helicobacter pylori treatment. World J
Gastroenterol. 22:4585–4593. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Verdu EF, Bercik P, Huang XX, Lu J,
Al-Mutawaly N, Sakai H, Tompkins TA, Croitoru K, Tsuchida E, Perdue
M and Collins SM: The role of luminal factors in the recovery of
gastric function and behavioral changes after chronic
Helicobacter pylori infection. Am J Physiol Gastrointest
Liver Physiol. 295:G664–G670. 2008.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Leontiadis GI, Minopoulos GI, Maltezos E,
Kotsiou S, Manolas KI, Simopoulos K and Hatseras D: Effects of
Helicobacter pylori infection on gastric emptying rate in
patients with non-ulcer dyspepsia. World J Gastroenterol.
10:1750–1754. 2004.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Park KS, Cho KB, Hwang IS, Park JH, Jang
BI, Kim KO, Jeon SW, Kim ES, Park CS and Kwon JG: Characterization
of smooth muscle, enteric nerve, interstitial cells of Cajal, and
fibroblast-like cells in the gastric musculature of patients with
diabetes mellitus. World J Gastroenterol. 22:10131–10139.
2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Tong L, Ao JP, Lu HL, Huang X, Zang JY,
Liu SH, Song NN, Huang SQ, Lu C, Chen J and Xu WX: Tyrosine kinase
Pyk2 is involved in colonic smooth muscle contraction via the
RhoA/ROCK pathway. Physiol Res. 68:89–98. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Neshatian L, Gibbons SJ and Farrugia G:
Macrophages in diabetic gastroparesis-the missing link?
Neurogastroenterol Motil. 27:7–18. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Feng J, Gao J, Zhou S, Liu Y, Zhong Y, Shu
Y, Meng MS, Yan J and Sun D, Fang Q and Sun D: Role of stem cell
factor in the regulation of ICC proliferation and detrusor
contraction in rats with an underactive bladder. Mol Med Rep.
16:1516–1522. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yan S, Yue YZ, Wang XP, Dong HL, Zhen SG,
Wu BS and Qian HH: Aqueous extracts of Herba Cistanche promoted
intestinal motility in loperamide-induced constipation rats by
ameliorating the interstitial cells of cajal. Evid Based Complement
Alternat Med. 2017(6236904)2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Jiang F, Zhou JY, Wu J, Tian F, Zhu XX,
Zhu CL, Yang BL and Chen YG: Yangyin Runchang Decoction improves
intestinal motility in mice with atropine/diphenoxylate-induced
slow-transit constipation. Evid Based Complement Alternat Med.
2017(4249016)2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hwang SJ, Pardo DM, Zheng H, Bayguinov Y,
Blair PJ, Fortune-Grant R, Cook RS, Hennig GW, Shonnard MC,
Grainger N, et al: Differential sensitivity of gastric and small
intestinal muscles to inducible knockdown of anoctamin 1 and the
effects on gastrointestinal motility. J Physiol. 597:2337–2360.
2019.PubMed/NCBI View
Article : Google Scholar
|
|
16
|
Blair PJ, Hwang SJ, Shonnard MC, Peri LE,
Bayguinov Y, Sanders KM and Ward SM: The role of prostaglandins in
disrupted gastric motor activity associated with type 2 diabetes.
Diabetes. 68:637–647. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yang S, Wu B, Sun H, Sun T, Han K, Li D,
Ji F, Zhang G and Zhou D: Impaired insulin/IGF-1 is responsible for
diabetic gastroparesis by damaging myenteric cholinergic neurones
and interstitial cells of Cajal. Biosci Rep.
37(BSR20170776)2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhao J, An J and Liu S: Electroacupuncture
at ST36 increases bone Marrow-Derived interstitial cells of Cajal
via the SDF-1/CXCR4 and mSCF/Kit-ETV1 pathways in the stomach of
diabetic mice. Evid Based Complement Alternat Med.
2018(7878053)2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Muangchan N, Kooptiwut S, Tapechum S,
Akarasereenont P, Vongsopanagul N, Pongwattanapakin K and Chaikomin
R: 13C-Acetic Acid Breath test monitoring of gastric
emptying during disease progression in diabetic rats. Biol Pharm
Bull. 40:1506–1514. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Jin QH, Shen HX, Wang H, Shou QY and Liu
Q: Curcumin improves expression of SCF/c-kit through attenuating
oxidative stress and NF-κB activation in gastric tissues of
diabetic gastroparesis rats. Diabetol Metab Syndr.
5(12)2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhou J, O'Connor MD and Ho V: The
potential for Gut Organoid derived interstitial cells of Cajal in
replacement therapy. Int J Mol Sci. 18(2059)2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Tan YY, Ji ZL, Zhao G, Jiang JR, Wang D
and Wang JM: Decreased SCF/c-kit signaling pathway contributes to
loss of interstitial cells of Cajal in gallstone disease. Int J
Clin Exp Med. 7:4099–4106, eCollection 2014. 2014.PubMed/NCBI
|
|
23
|
Bekkelund M, Sangnes DA, Gunnar Hatlebakk
J and Aabakken L: Pathophysiology of idiopathic gastroparesis and
implications for therapy. Scand J Gastroenterol. 54:8–17.
2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yadak R, Breur M and Bugiani M:
Gastrointestinal Dysmotility in MNGIE: From thymidine phosphorylase
enzyme deficiency to altered interstitial cells of Cajal. Orphanet
J Rare Dis. 14(33)2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Tian L, Zhu B and Liu S:
Electroacupuncture at ST36 Protects ICC Networks via mSCF/Kit-ETV1
Signaling in the stomach of diabetic mice. Evid Based Complement
Alternat Med. 2017(3980870)2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ando K and Takagi K: Solid gastric
emptying mediated by the serotonin (5-HT)3 receptor in mice is a
simple marker to predict emesis. J Toxicol Sci. 36:23–29.
2011.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ando K, Takagi K and Tsubone H: Enhanced
gastric retention of solid resin beads as a marker for emetic
potential of agents in rats. J Toxicol Sci. 37:549–553.
2012.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Bhatia Y, Singh S, Rattan KN, Parmar P,
Sahni D and Sen R: Anorectal malformations: Histomorphological and
immunohistochemical evaluation of neuronal dysfunction. J Neonatal
Surg. 6(29)2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kim BJ and Kuo B: Gastroparesis and
functional dyspepsia: A blurring distinction of pathophysiology and
treatment. J Neurogastroenterol Motil. 25:27–35. 2019.PubMed/NCBI View
Article : Google Scholar
|
|
30
|
Sanger GJ and Pasricha PJ: Investigational
drug therapies for the treatment of gastroparesis. Expert Opin
Investig Drugs. 26:331–342. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Choi KM, Gibbons SJ, Sha L, Beyder A,
Verhulst PJ, Cipriani G, Phillips JE, Bauer AJ, Ordog T, Camp JJ,
et al: Interleukin 10 restores gastric emptying, electrical
activity, and interstitial cells of Cajal networks in diabetic
mice. Cell Mol Gastroenterol Hepatol. 2:454–467. 2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Khan AS, Williams G, Woolsey C, Liu J,
Fields RC, Doyle MMB, Hawkins WG and Strasberg SM: Flange
gastroenterostomy results in reduction in delayed gastric emptying
after standard pancreaticoduodenectomy: A prospective cohort study.
J Am Coll Surg. 225:498–507. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Ahn JY, Jung HY, Bae SE, Jung JH, Choi JY,
Kim MY, Lee JH, Choi KS, Kim DH, Choi KD, et al: Proper preparation
to reduce endoscopic reexamination due to food residue after distal
gastrectomy for gastric cancer. Surg Endosc. 27:910–917.
2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Othman MO, Davis B, Saroseik I, Torabi A
and McCallum RW: EUS-guided FNA biopsy of the muscularis propria of
the antrum in patients with gastroparesis is feasible and safe.
Gastrointest Endosc. 83:327–333. 2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Hayashi Y, Toyomasu Y, Saravanaperumal SA,
Bardsley MR, Smestad JA, Lorincz A, Eisenman ST, Cipriani G, Nelson
Holte MH, Al Khazal FJ, et al: Hyperglycemia increases interstitial
cells of Cajal via MAPK1 and MAPK3 Signaling to ETV1 and KIT,
leading to rapid gastric emptying. Gastroenterology.
153:521–535.e20. 2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Chen J, Du L, Xiao YT and Cai W:
Disruption of interstitial cells of Cajal networks after massive
small bowel resection. World J Gastroenterol. 19:3415–3422.
2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zhu F, Xu S, Zhang Y, Chen F, Ji J and Xie
G: Total Glucosides of Paeony promote intestinal motility in slow
transit constipation rats through amelioration of interstitial
cells of Cajal. PLoS One. 11(e0160398)2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Tian L, Song S, Zhu B and Liu S:
Electroacupuncture at ST-36 protects interstitial cells of Cajal
via Sustaining Heme Oxygenase-1 Positive M2 Macrophages in the
stomach of diabetic mice. Oxid Med Cell Longev.
2018(3987134)2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Bashashati M and McCallum RW: Is
interstitial cells of Cajal-opathy present in gastroparesis? J
Neurogastroenterol Motil. 21:486–493. 2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Chen Y, Xu JJ, Liu S and Hou XH:
Electroacupuncture at ST36 ameliorates gastric emptying and rescues
networks of interstitial cells of Cajal in the stomach of diabetic
rats. PLoS One. 8(e83904)2013.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Lu C, Lu H, Huang X, Liu S, Zang J, Li Y,
Chen J and Xu W: Colonic transit disorder mediated by
downregulation of interstitial cells of Cajal/Anoctamin-1 in
dextran sodium sulfate-induced colitis mice. J Neurogastroenterol
Motil. 25:316–331. 2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Wang L, Liang Y, Chen Q, Ahmed N, Wang F,
Hu B and Yang P: Identification and distribution of the
interstitial cells of Cajal in the abomasum of goats. Cell
Transplant. 27:335–344. 2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Kwon YH, Kim N, Nam RH, Park JH, Lee SM,
Kim SK, Lee HS, Kim YS and Lee DH: Change in the interstitial cells
of Cajal and nNOS positive neuronal cells with aging in the stomach
of F344 Rats. PLoS One. 12(e0169113)2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
An B, Moon BS, Kim H, Lim HC, Lee YC, Lee
G, Kim SH, Park M and Kim JB: Antibiotic resistance in
Helicobacter pylori strains and its effect on H.
pylori eradication rates in a single center in Korea. Ann Lab
Med. 33:415–419. 2013.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Diaconu S, Predescu A, Moldoveanu A, Pop
CS and Fierbinteanu-Braticevici C: Helicobacter pylori
infection: Old and new. J Med Life. 10:112–117. 2017.PubMed/NCBI
|