Introduction
Dental erosion (DE) represents a subject of interest
and concern for dentists because of its increasing prevalence in
the last few years (1). Beside the
medical significance, the quality of the teeth has a tremendous
aesthetic effect involving psychosocial and artistic significance.
DE leads to both alterations in physiognomy and to health issues
(2). A common cause of DE includes
an increase in oral cavity acidity by gastroesophageal reflux
disease (GERD) (3). GERD is
diagnosed as a result of heartburn and is assessed by
intra-esophageal pH-impedance monitoring; guidelines mention
extraesophageal complications, including DE (4,5).
Unlike GERD, there are no diagnostic, prevention and treatment
guidelines for DE at present. The mechanisms of action of gastric
juice in the oral cavity are known; salivary pH decreases with
individual variations according to the buffer capacity and the
chemical composition (calcium and phosphorus) of the saliva
(6-14).
DE is the result of the repeated or continuous
exposure of hard dental tissues to acidic pH condition and it
includes three consecutive stages: Loss of the protective matrix on
the enamel, its demineralization at a salivary pH below 5.5 and
alteration of the dentin structure, with opening of the dentinal
tubules (15-20).
The duration and frequency of reflux episodes is relative. Many
studies report different values, from 1 min to 2 h, one to 15
episodes within 24 h (21-27).
GERD may be asymptomatic but can still induce DE
(28). Therefore, it is important
to complete a dental exam for DE with a gastroenterological
examination that would confirm a diagnosis of GERD. Of the GERD
patients who present with DE, 31-56% have distal GE reflux.
Patients with more than 2 reflux episodes per week were found to
have a longer distal esophageal exposure time to pH values below
4.0 and 5.5 according to a previous study (28).
Atomic force microscopy (AFM) enables surface
scanning and 3D presentation of its aspect. Such details can
provide important information on the mechanism of erosion. The
images may be explored and analyzed by the dentist in a new and
interactive manner.
Atomic force microscopy differs from classic
microscopy by enabling a 3D view of the surface studied. The high
cost of the equipment makes it available in only a few medical
centers; therefore, the number of studies is also small (29-32).
Given the alterations induced by gastric acid on
dental surfaces, we analyzed the physical modifications and their
evolution by a multidisciplinary team including physicians and
physicists. The aim of this study was to assess, by AFM, the
roughness value of enamel, dentine, and the materials used in
minimal invasive treatments for DE such as composites and ceramics,
exposed to a low pH level in the oral cavity as a consequence of
gastric reflux.
Materials and methods
An in vitro experiment on enamel, dentin,
dental composite Nexco and Emax ceramic was designed. The present
study included dental surfaces submitted to acid erosion in the
mouth, namely enamel and dentin. The behavior of ceramic and
composite materials used in the prosthetic restorative treatments
of erosion was also assessed. Preparations of the samples were
conducted in the ‘ArtChrys Dental Lab’ in Cluj-Napoca, Romania by a
dental technician and a dentist. Four dentin and 4 enamel surfaces
were prepared in the shape of a parallelepiped, one side 5 and 1 mm
thickness, polished with a disk, from 8 central extracted incisors
that presented no signs of erosion. The 4 ceramic surfaces Emax IPS
(Ivoclar), one of the most frequently used ceramic facets, were
made to be square shaped, 5 mm in size, 1 mm in thickness. The
composite surfaces, Nexco (Ivoclar), used in direct dental
reconstruction were the same size, photopolymerized for 40 sec and
polished with abrasive disks and special rubbers. All the samples
were maintained in the saliva harvested after meals from patients
without GERD, medium buffer capacity, in order to prevent
dehydration and realize a protective layer similar to the
conditions in the mouth.
In all, there were 16 surfaces divided into 4 groups
of 4 samples each: Enamel, dentin, Emax IPS ceramic and Nexco
(Ivoclar) composite. For each group, one surface, not exposed to
acid, was studied by AFM and served as a normal control, attributed
to control patients without GERD.
From one patient diagnosed with GERD, as confirmed
by endoscopy, and admitted to the 2nd Department of Internal
Medicine, ‘Iuliu Haţieganu’ University of Medicine and Pharmacy in
Cluj Napoca, 120 ml gastric juice was collected by endoscopy. Each
sample was immersed in 10 ml gastric juice mixed with saliva, pH of
6.0, as tested by GC Saliva Check (33).
All the surfaces, following immersion, were examined
by AFM at 24, 120 and 240 h. These intervals were chosen as being
equivalent to 1, 5 and 10 years of exposure of dental tissues and
prosthetic materials to an oral acid pH for 15 min - the time of a
GE reflux (salivary buffer restores the pH to normal within about
15 min after a sudden drop to acid). We chose a frequency of 2
gastroesophageal episodes per week, a threshold value most used in
studies (28).
Preparation of the samples for AFM analysis was
conducted after their extraction from the immersion solution. This
preparation included intense washing with double distilled water
and natural drying on paper. They were then mounted onto a specific
support, with the studied surface upward.
The effect of the acid on the teeth was assessed and
approved by a professional visual artist working with image
processing.
For each sample, we examined two surfaces by AFM to
confirm the results. Examination was conducted in tapping mode, in
environmental conditions at 20̊C, using microscope JSPM 4210 (Jeol
Co. Japan). Minikit used was NSC15 (MikroMasch Co.) with a silicone
tip and 300 kHz resonance frequency. Topographic images were
obtained at approximately x1 Hz on surfaces from 5x5 to 1x1 µm. All
images were processed using WinSPM 2.0 software (JEOL), which
provided data on the sample surface, diameter of morphological
changes and roughness values.
The patient gave informed consent. The study was
conducted according to the Helsinki Declaration on Human and Animal
Studies.
Results
Gastric reflux has a complex acid profile as it
includes not only acids from ingested food, but also gastric juice
containing diluted hydrochloric acid among other components with a
role in digestion. Long-term exposure of teeth to GE reflux leads
to severe alterations in the morphology and structure of enamel and
dentin. We recorded, using AFM, the evolution in time of enamel and
dentin after immersion in acidic solution, using as controls
healthy enamel and dentin samples. The roughness values in this
study are the means of 3 measurements per each sample.
Enamel
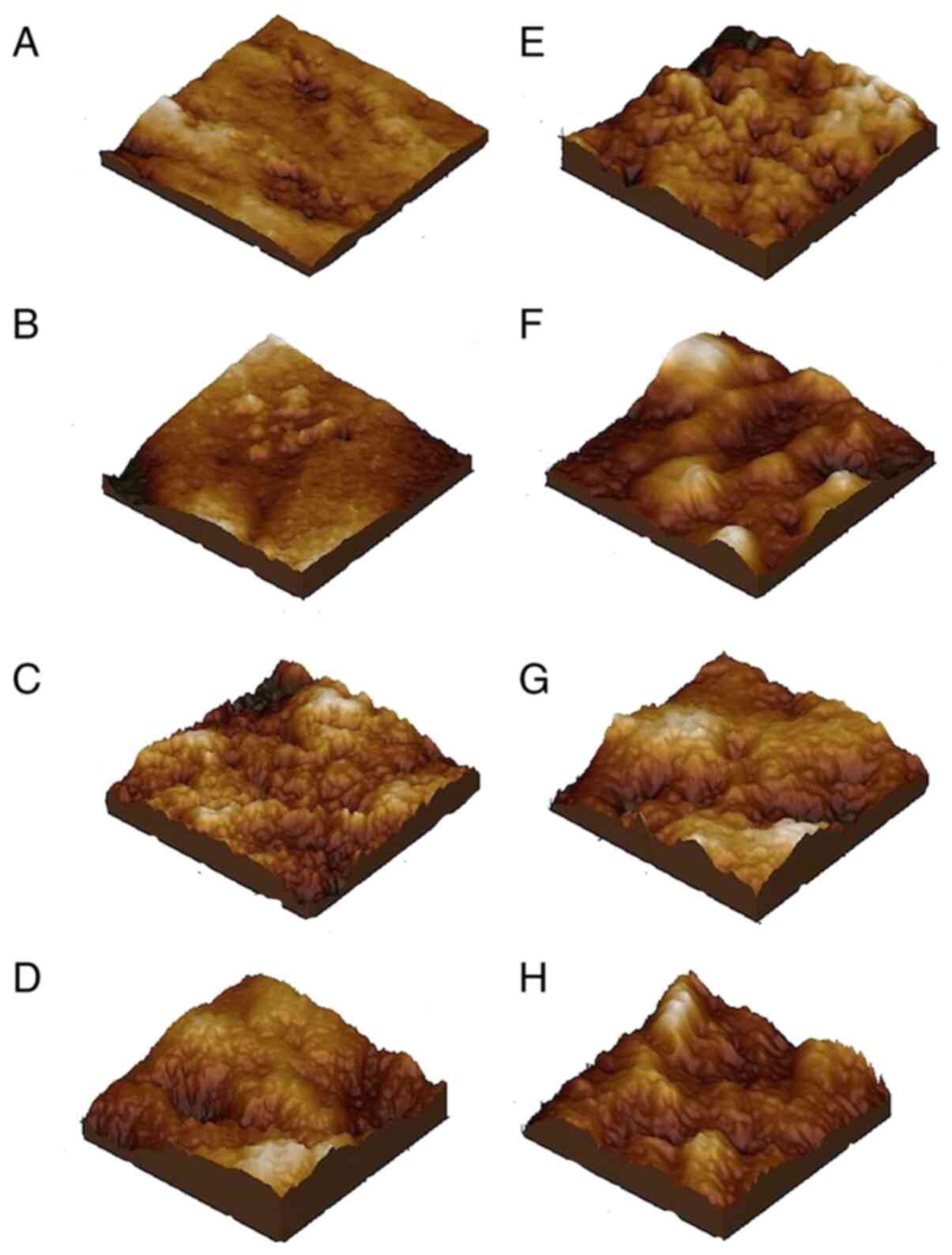
The AFM examination of the state of the enamel
evidenced 2 structure areas: The microstructural area, best
observed at a scan area of 5x5 µm, and the nanostructural area, at
an area of 1x1 µm.
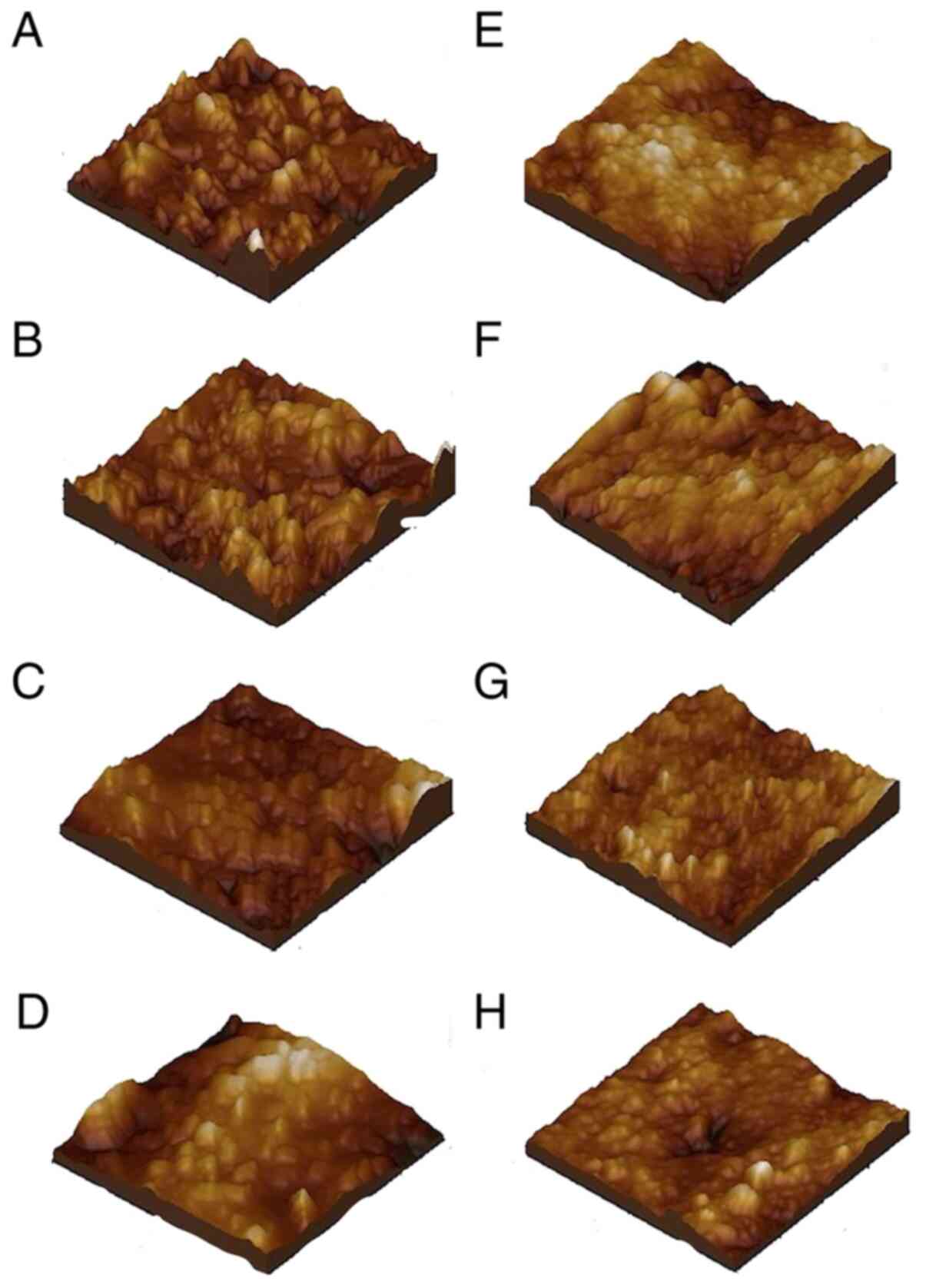
Microstructure of the healthy
enamel
Microstructure of the healthy enamel presented with
a smooth and even surface of crystallites and hydroxyapatite (HAP)
well bonded together. We note some small dips on this surface (the
darker spots), which are normal for healthy enamel. In these
conditions, the microstructural roughness was Rq=9.69 nm (Fig. 1A).
Nanostructure of the healthy
enamel
The nanostructure of the healthy enamel is presented
in Fig. 1E. It shows a compact
crystallite and HAP structure, well bonded, 40 nm in diameter. The
surface is very smooth, roughness Rq=2.14 nm. However, some HAP
crystallites have higher positions in the surface topography, while
others are lower. This led to local depressions (darker spots) of
10-50 nm in diameter. Still, these represent a natural
characteristic of healthy enamel, becoming vulnerable at a lengthy
exposure to acid-erosive conditions.
Evolution of the microstructure
Evolution of the microstructure of the enamel
surface in relation to time of exposure is presented in Fig. 1B-D. Evolution of the microstructure
of the enamel is presented after 1 year of aggression (Fig. 1B), after 5 years (Fig. 1C), and after 10 years (Fig. 1D). After 1 year of acid erosion the
microstructure of the enamel surface was barely changed, with only
some well-defined uneven areas being visible at the top of the
image (Fig. 1B). This caused a
slight increase in the Rq value to 9.75 nm. We noted that the small
natural dips became slightly more visible. After 5 years of acid
erosion, the microstructure morphology was drastically altered; the
small dips progressed to a diameter of >200 nm and with
considerable depths (Fig. 1C).
These were integrated into the surface topography by depth, while
the local irregularities observed at 1 year extended to the whole
surface after 5 years, causing some local pikes (lighter spots
almost white). This affected the roughness significantly, reaching
Rq=23.7 nm. Ten years of acid-erosive aggression was found to lead
to a totally destroyed enamel surface, resembling that noted at 5
years but very uneven, with depressions of 100-200 µm in diameter
and variable depths reaching even 100 nm. This caused heavily
roughness, with Rq=42.7 nm.
Evolution of the nanostructure
The evolution of the enamel surface nanostructure
according to the GE reflux aggression is presented in Fig. 1F-H. After one year of acid
aggression by GE reflux the enamel nanostructure underwent
significant alterations (Fig. 1F).
Entire nanostructural areas became demineralized, namely the HAP
crystallites were dissolved from the superficial layer, forming
depressed zones, while the remaining ones formed higher islets. The
diameter of the HAP crystallites on the aggressed surface was
markedly increased to 60 nm. This led to more roughness, Rq=4.21
nm. After 5 years (Fig. 1G) the
nanostructure progressed to deeper areas and erosion of the margins
of resisting islets, forming a rugged surface of Rq=11.0 nm. The
HAP crystallites diameter was 80 nm, almost double of the initial
phase, which indicates evidence of erosive-acid decay. After 10
years (Fig. 1H) the nanostructure
was deeply affected, evidenced by the deep areas associated with
the flattening of HAP formations, Rq=16.0 nm.
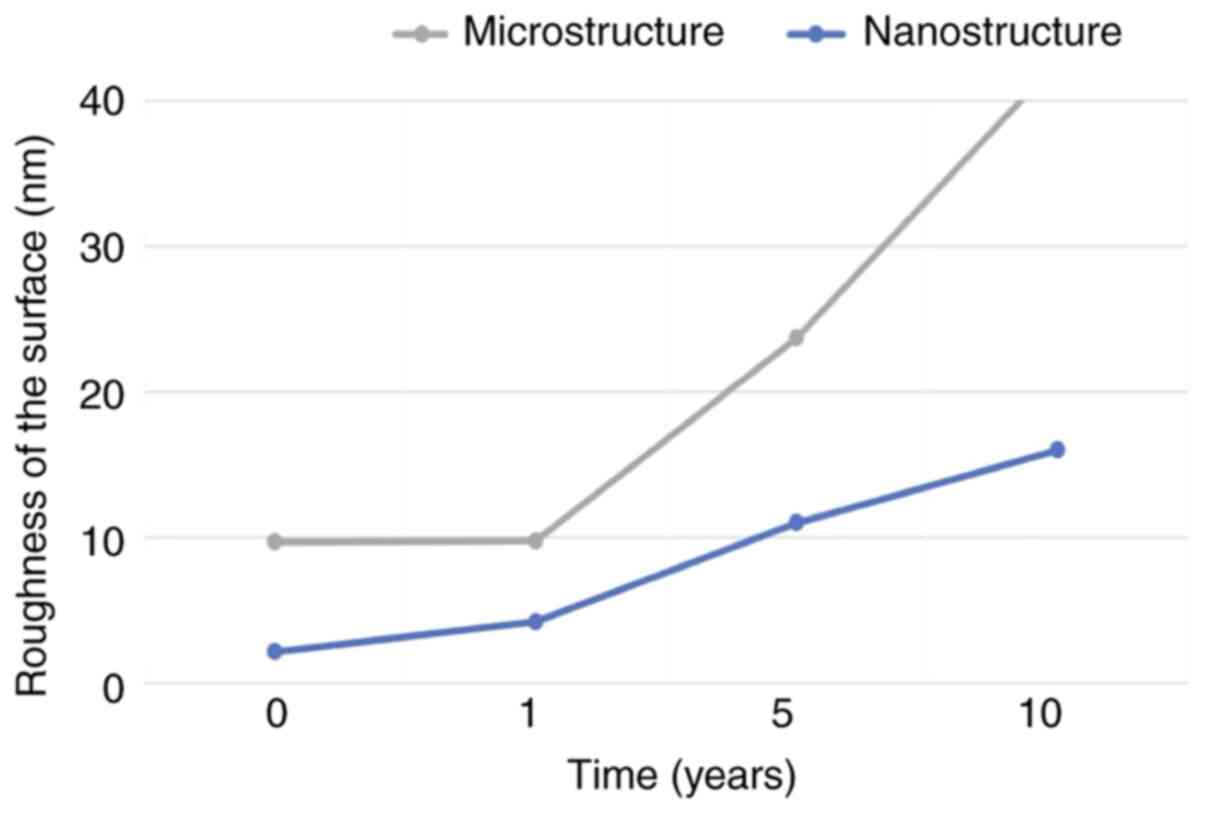
Values of roughness
The 3D surface roughness of enamel alterations are
presented in Fig. 1. The values of
roughness that resulted at the microstructural and nanostructural
levels are presented in Fig. 2. The
complex aspect of the acid erosion on the dental enamel is
evidenced. We noted that surface erosion started at a
nanostructural level after 1-year exposure, with the microstructure
being less affected. Beyond one year, alterations become more
obvious. At 5 years, the nanostructural roughness reached the
initial microstructural values and surpassing them after a period
of 10 years (Fig. 2).
Summing up the findings, we may conclude that after
5 years and later, GE reflux has severe effects on the dental
enamel, manifested by decay, which may reach the dentin in patients
with thin enamel or dental surface injuries. Consequently, this led
us to investigate how dentin reacts to acid aggression.
Dentin
Dentin is a bio-composite formed of HAP
crystallites, about 40 nm in diameter, well bonded by an organic
network of collagen fibers. It is more heterogeneous in structure
than enamel and more susceptible to surface alterations. Data in
the literature show that dentin demineralization under acid
circumstances is caused by progressive depletion of HAP
crystallites, while the collagen network remains in place. Acid
mineral loss may leave dentin totally depleted of HAP, in which
case only collagen fibers are seen (34).
Dentin, the matter of which teeth are made of,
cannot be visualized by AFM; thus, the samples needing adequate
sectioning and preparation. Dental samples in our study were
sectioned from healthy teeth, so that the plane-parallel facets
could be polished to be shiny for optical visualization.
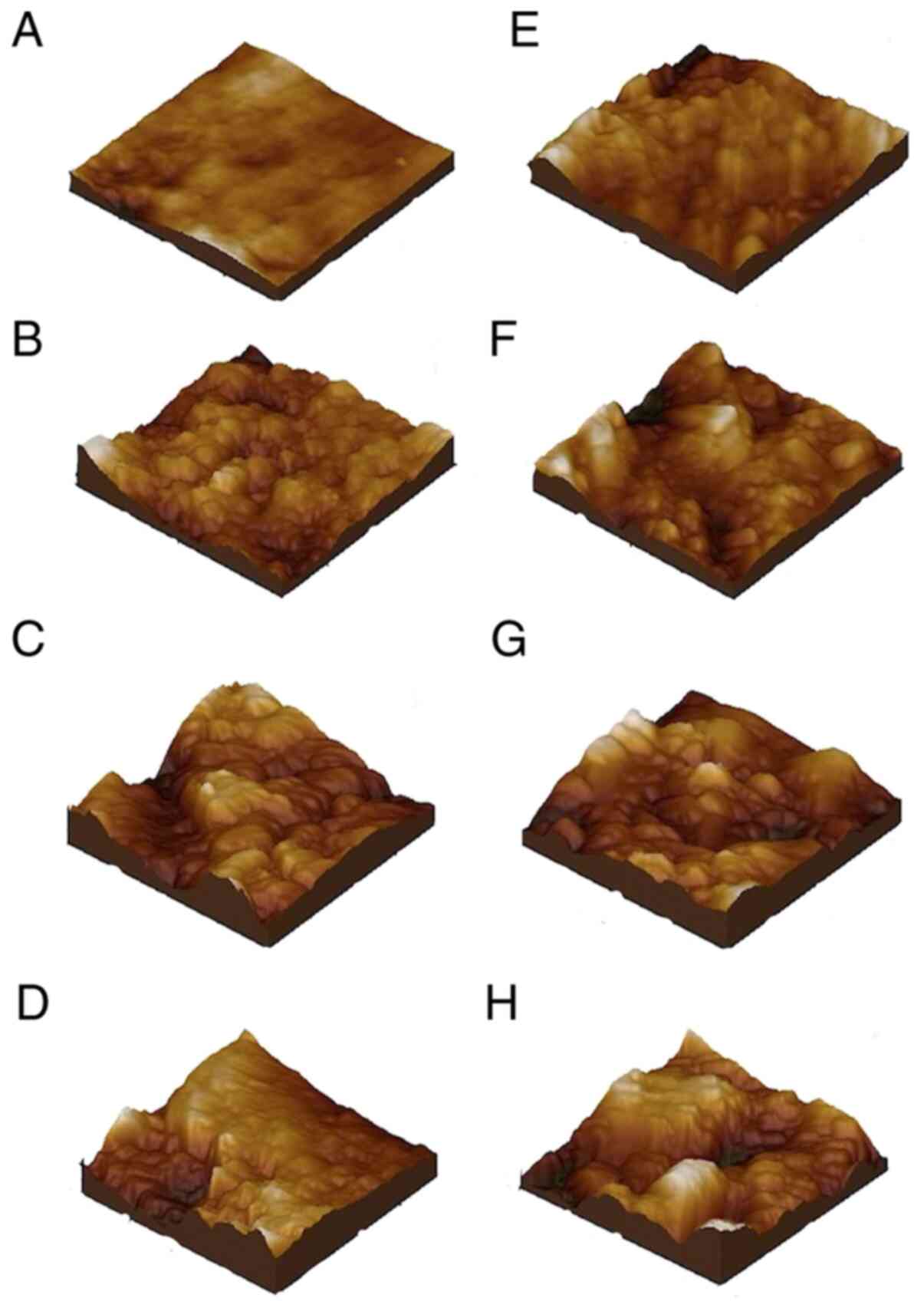
Microstructure of the healthy
dentin
Considering these aspects, microstructure of the
healthy dentin is shown in Fig. 3A
at a scan area of 5x5 µm. It presents a smooth and compact surface
with a topography characterized by densely mineralized HAP.
Microstructural ruggedness of the healthy dentin was Rq=30.7
nm.
Nanostructure of the healthy
dentin
The nanostructure of the healthy dentin may be well
visualized at 1x1 µm scan. The surface texture included collagen
fibers densely mineralized with HAP crystallites, 40 nm in
diameter. The roughness was approximately Rq=4.59 nm (Fig. 3E).
Evolution of the microstructure
After one year of GE reflux aggression, the dentin
microstructure underwent significant changes (Fig. 3B). Numerous HAP crystallites were
dislodged from the surface, which became uneven, with depressions
of approximately 200-700 nm. This doubled the roughness index to
Rq=62.7. After 5 years, the dentin microstructure was seriously
damaged. Its surface was an alteration of well delimited pikes with
grooves and dips in which collagen strings may be observed
(Fig. 3C). Roughness reached a
value Rq=137 nm. The degree of erosion was even higher after 10
years of GE reflux aggression (Fig.
3D), with a roughness value Rq=322 nm.
Evolution of the nanostructure
At the nanostructure level, after 1-year exposure
the dentin surface presented with partially demineralized collagen
strings and dips formed by the elimination of surface HAP
crystallites (Fig. 3F). There was
also an effect of surface crystallite wear; diameter of
approximately 80 nm. Roughness at the nanostructural level reached
Rq=22.9 nm. Surface alteration and erosion became more marked after
5 years (Fig. 3G), leading to a
roughness value Rq=44.0 nm. After 10 years of acid aggression on
the dentin, the decay was advanced with an alteration of
nanostructural and submicron pikes and dips, Rq=51.3 nm (Fig. 3H).
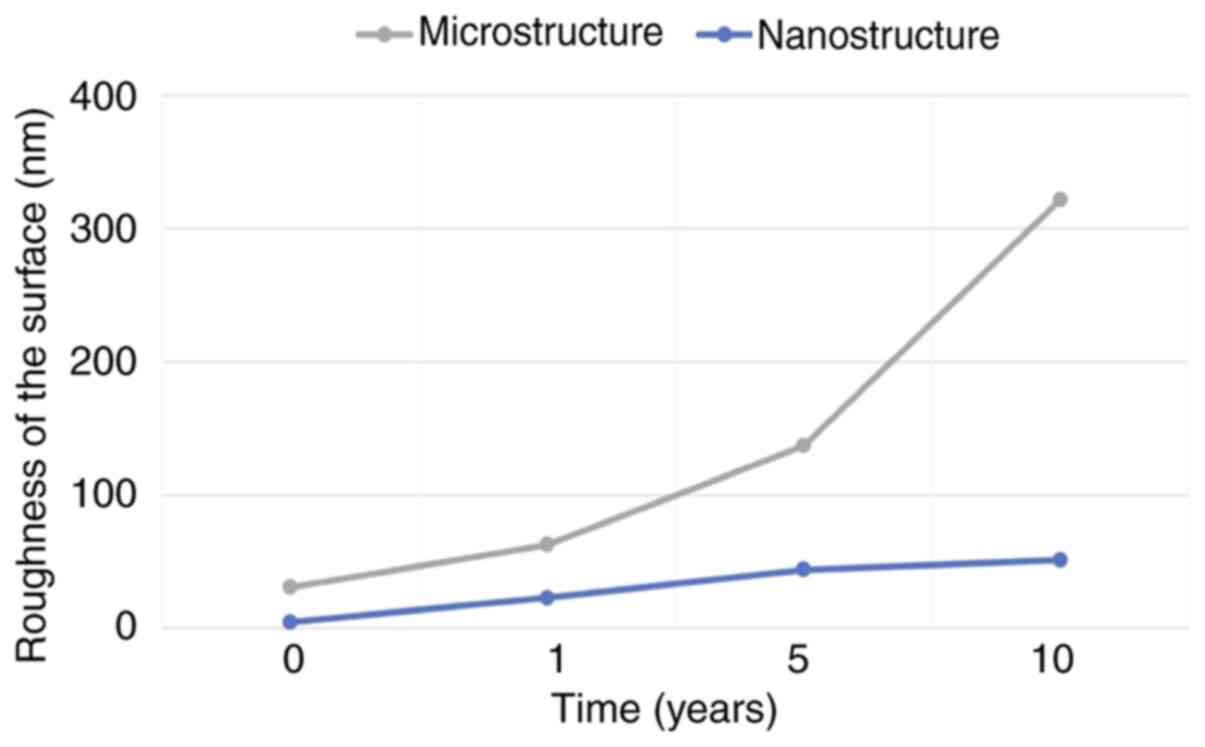
The values of roughness in the dentin samples
submitted to acid aggression by GE reflux can be seen in Fig. 4. It may be evidenced that as soon as
dentin is exposed to acid attack, at a microstructural level
roughness increases progressively to 10 times more than the initial
value after 10 years. At a nanostructural level, it progresses
rapidly, reaching the microstructural initial value after 5 years,
and also after 10 years (Fig.
4).
Ceramic and composite materials
Ceramic and composite materials were also tested for
gastric acid aggression. In their case, the optimal scanning area
was 2.5x2.5 µm. AFM images are presented in Fig. 5.
Ceramic material unexposed to gastric
reflux
The ceramic material presented with a heterogeneous
submicronic structure based on tabular polyhedric crystals, 150 nm
wide and approximately 300 nm long, included into a very compact
mass (Fig. 5A). Surface roughness
was Rq=20.0 nm.
Ceramic material exposed to gastric
reflux
Exposure to gastric reflux for 1 year (Fig. 5B), 5 years (Fig. 5C) and 10 years (Fig. 5D) did not alter ceramic
microstructure, which preserved its shape and size. The roughness
increased slowly, doubling its value only after 10 years of acid
attack. Given the preserved shape and size, the mild increase in
roughness may be explained by the relative flattening of the lower
areas, while ceramic crystals preserved their initial value. The
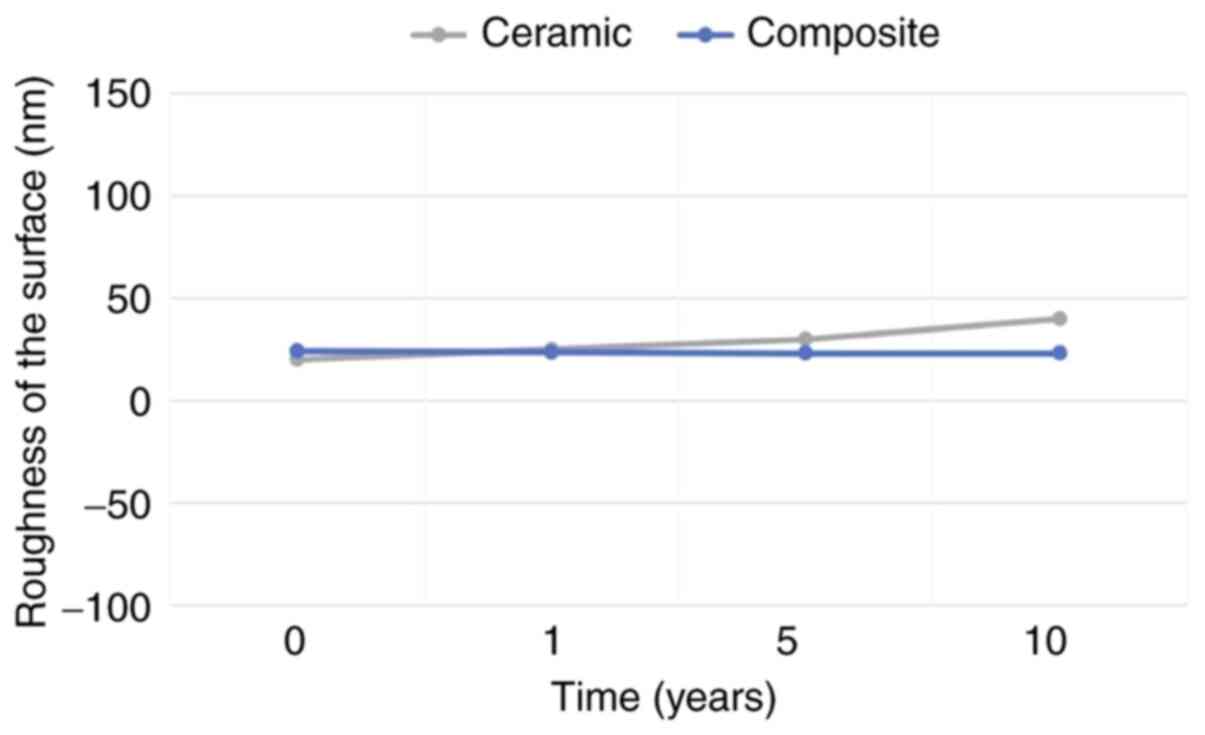
evolution of roughness may be observed in Fig. 6. Overall, we demonstrated good
resistance of the ceramic material to erosion by GE reflux for 10
years.
Composite material unexposed to
gastric reflux
The unexposed composite material presented a
granular structure with an average diameter of 80 nm, well
compacted by the bonding matter (Fig.
5E). Its surface was quite smooth, with roughness of 24.1
nm.
Composite material exposed to gastric
reflux
We found that the morphology, dimensions, and
roughness of the surface were preserved at 1 year (Fig. 5F), 5 years (Fig. 5G) and 10 years (Fig. 5H) of acid aggression by GE reflux.
The evolution of roughness is also presented in Fig. 6.
There are numerous studies (4,6,10) in
the literature on the demineralization of dental enamel under the
acid action from different components in food, mainly phosphoric
and citric acids. A modern approach of enamel demineralization uses
AFM to monitor the changes in surface morphology and size. The
method allows a follow-up of these changes at high resolution
(35).
The understanding of the etiopathogenetic mechanism
of dental wear and awareness regarding prevention and early
treatment by minimally invasive methods can help the patient
maintain healthy hard dental structures and enjoy a good quality of
life.
Summing up our findings at the microstructural and
nanostructural levels, we may state that the decay of dentin
surface is a process starting at the nanostructural level by
progressive loss of surface HAP crystallites, which rapidly
influences microstructure. We noted a weaker resistance of dentin
to acid action and a more marked decay of its surface in comparison
to enamel (Table I).
 | Table ISurface roughness of the analyzed
material before and after exposure to acidic environment. |
Table I
Surface roughness of the analyzed
material before and after exposure to acidic environment.
| | Surface roughness
of material in nm |
|---|
| | Enamel | Dentine | |
|---|
| Acidic exposure
simulation in years | Microstructure | Nanostructure | Microstructure | Nanostructure | Ceramic
Microstructure | Composite
Microstructure |
|---|
| 0 | 9.69 | 2.14 | 30.70 | 4.59 | 20.00 | 24.10 |
| 1 | 9.75 | 4.21 | 62.70 | 22.90 | 27.30 | 22.30 |
| 5 | 23.70 | 11.00 | 137.00 | 44.00 | 45.30 | 15.50 |
| 10 | 42.70 | 16.00 | 322.00 | 51.30 | 58.80 | 20.00 |
Analyzing the AFM results on the ceramic and
composite materials, we found that they maintain their morphology
and size in time, which confirms good resistance (Table I). It is difficult to conclude which
of the two materials, ceramic or composite is more indicated as the
therapeutic choice, as both performed well under the given
conditions. The morphologic-topographic factor cannot be decisive
in choosing one or the other. The choice should be based on the
requirements of the dental appliance. Only in this case would it
matter that for the ceramic material a slow but progressive
roughness was found in time, while the composite roughness was
constant. It is known that surface roughness is important in the
retention of oral bacterial plaque, involved in the onset of dental
caries by local demineralization and action of cariogenic bacteria
(Streptococcus mutans, Lactobacillus) and maintenance of
local acid pH. Prevention in dentistry may ensure the control of
dental plaque by periodical professional cleaning and topic
fluoridation (36-39).
Discussion
In the present study, the atomic force microscopy
(AFM) analyses indicated that gastroesophageal reflux had a
deleterious effect on the morphology and roughness of enamel and
dentin surfaces. A prolonged exposure of 5-10 years caused
important dento-maxillary functional alterations that severely
affected the dental system.
The period of time of the contact between the
erosive agent and the tooth is more important than the pH acidity
(38).
A profound and detailed knowledge of dental erosion
mechanisms requires ample studies performed on a large number of
samples over a long period of time of up to 30 years. Often DE is
associated with other dental wear mechanisms, which favors rapid
decay of dental structures and fostering difficulties of
etiological diagnosis (25,40-43).
Abrasion and attrition will dislodge more easily the eroded dental
structures; this is obvious in many cases of bruxism or bad habits
in patients with GERD, unhealthy diets, or heavy consumption of
acidic foods or beverages (44,45).
This is how Lussi et al explains the appearance of cupules
at the level of the cusps, which are typic for dental erosion and
in which the acid is retain for long times because it is not washed
away by saliva (39-41).
Composite materials present a porous structure that
determines an increased adherence of the bacterial plaque, even if
their surface is well polished. Ceramic materials being glazed do
not present porosity, which minimizes the adherence of bacterial
plaque (46,47) (Figs.
7 and 8).
We consider that documenting the progressive erosion
in time of dentin and enamel represent a novel study (Figs. 7 and 8). We also wanted to compare the
resistance in time of ceramic materials and composites exposed to
the same acid aggression, in order to obtain conclusive information
on the changes undergoing at surfaces exposed to acid and thus
orient the optimal materials to be used for treatment.
The ceramic and composite material investigated in
the present study proved to have good resistance to the erosion
caused by gastroesophageal reflux disease (GERD), with preserved
form and dimensions, and even roughness after 10 years. The
composite was found more stable regarding roughness than ceramic.
However, we should keep in mind that there are many other
mechanical forces of wear at work on composites as compared to
ceramic masses.
The behavior of the prosthetic materials examined in
our study indicates the choice of composites for crown restorations
of eroded teeth. In a previous study on the behavior of cements in
acid environment, we found that glass ionomers are less resistant
to acid compared to resin cements, therefore contraindicated in
patients with dental erosions (42).
In addition to the health issues consequent to
dental erosion (DE), dental structure loss has important
psychosocial and aesthetic consequences (48,49).
In order to study and establish an aesthetic dental treatment plan,
extraoral and intraoral professional photography is needed. Photos
obtained are used for aesthetic guidelines and can be coordinated
with digital smile design, a special computer program or phone
application that allows the doctor to create and the patient to
previsualize the final result of the treatment, collaborating
together. Indeed, beauty is also enhanced in association with a
healthy oral cavity with a healthy smile. Minimal invasive
treatment for dental erosions that use composites and ceramic
veneers is considered a correct approach among experienced
dentists. The art of creating a smile is motivating doctors to find
advances in technology and to identify more aesthetic correlations
between teeth and facial anatomy. Giving the power of creating
dental morphology in the hands of the dentist, facial harmony is
obtained, and together with functionality, long term results that
satisfy patient needs are reached (50-52).
Special attention to DE must be given by the gastroenterologist in
collaboration with the dentist, as GERD is a disease with a genetic
predisposition, and prevention is required (53).
Acknowledgements
Not applicable.
Funding
Funding: Personal funding was used for the present study.
Availability of data and materials
Data are available at request and are stored in our
personal database.
Authors' contributions
AMP realized the research conception, and was
responsible for the design of the article and acquisition of
materials. AMP also revised the work, examined the patients, edited
the tables and figures, analyzed and interpreted the data. IP
analyzed the dentin, enamel, composite and ceramics with the atomic
force microscope. AP realized the conception of the article,
handled the dentin, enamel, ceramic and composites immersion in
gastric juice mixed with saliva, prepared the substance for the
immersion, edited the tables and figures, analyzed and interpreted
the data. ADP revised the article and edited the data and research
study. ALR and AMT examined the patient and collected and tested pH
level and buffer capacity of saliva. NMP performed the examination
and diagnosis of GERD patients and collected the gastric juice. CP
prepared the restorative materials, ceramics and composites in the
dental laboratory. MEB supervised and revised the work, structure,
steps, information and corrected the article. IDM documented the
work and analyzed and edited the photographs. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was conducted according to the Declaration
of Helsinki on Human and Animal Studies. Written informed consent
was obtained from the patient.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Picos A, Badea ME and Dumitrascu DL:
Dental erosion in gastro-esophageal reflux disease. A systematic
review. Clujul Med. 91:387–390. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Muñoz JV, Herreros B, Sanchis V, Amoros C,
Hernandez V, Pascual I, Mora F, Minguez M, Bagan JV and Benages A:
Dental and periodontal lesions in patients with gastro-oesophageal
reflux disease. Dig Liv Dis. 35:461–467. 2003.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Schroeder PL, Filler SJ, Ramirez B,
Lazarchik DA, Vaezi MF and Richter JE: Dental erosion and acid
reflux disease. Ann Int Med. 122:809–815. 1995.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Meurmann JH, Toskala J, Nuutinien P and
Klemetti E: Oral and dental manifestations in gastrooesophageal
reflux disease. Oral Surg Oral Med Oral Pathol. 78:583–589.
1994.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Gregory-Head B and Curtis DA: Erosion
caused by gastrooesophageal reflux: Diagnostic considerations. J
Prosthodont. 6:278–285. 1997.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bartlett DW, Evans DF and Smith BG: The
relationship between gastro-oesphageal reflux disease and dental
erosion. J Oral Rehabil. 23:289–297. 1996.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Bartlett DW, Evans DF, Anggiansah A and
Smith BG: A study of the association between gastro-oesophageal
reflux and palatal dental erosion. Brit Dent J. 181:125–131.
1996.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Pontefract HA: Erosive toothwear in the
elderly population. Gerodontology. 19:5–16. 2002.PubMed/NCBI View Article : Google Scholar
|
|
9
|
O'Sullivan EA, Curzon ME, Roberts GJ,
Milla PJ and Stringer MD: Gastroesophageal reflux in children and
its relationship to erosion of primary and permanent teeth. Eur J
Oral Sci. 106:765–769. 1998.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Moazzez R, Bartlett D and Anggiansah A:
Dental erosion, gastro-oesophageal reflux disease and saliva: How
are they related? J Dent. 32:489–494. 2004.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Loffeld RJ: Incisor teeth status in
patients with reflux oesophagitis. Digestion. 57:388–390.
1996.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Jarvinen V, Meurman JH, Hyvarinen H,
Rytömaa I and Murtomaa H: Dental erosion and upper
gastrointestinal disorders. Oral Surg Oral Med Oral Pathol.
65:298–303. 1988.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Aine L, Baer M and Mäki M: Dental erosions
caused by gastroesophageal reflux disease in children. ASDC J Dent
Child. 60:210–214. 1993.PubMed/NCBI
|
|
14
|
Böhmer CJ, Klinkenberg-Knol EC, Niezen-de
Boer MC, Meuwissen PR and Meuwissen SG: Dental erosions and
gastro-oesophageal reflux disease in institutionalized
intellectually disabled individuals. Oral Dis. 3:272–275.
1997.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Myklebust S, Espelid I, Svalestad S and
Tveit AB: Dental health behavior, gastroesophageal disorders and
dietary habits among Norwegian recruits in 1990 and 1999. Acta
Odontol Scand. 61:100–104. 2003.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Linnett V, Seow WK, Connor F and Shepherd
R: Oral health of children with gastro-esophageal reflux disease: A
controlled study. Aust Dent J. 47:156–162. 2002.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Dahshan A, Patel H, Delaney J, Wuerth A,
Thomas R and Tolia V: Gastroesophageal reflux disease and dental
erosion in children. J Pediatr. 140:474–478. 2002.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bartlett DW, Lussi A, West NX, Bouchard P,
Sanz M and Bourgeois D: Prevalence of tooth wear on buccal and
lingual surfaces and possible risk factors in young European
adults. J Dent. 41:1007–1013. 2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Pace F, Pallotta S, Tonini M, Vakil N and
Bianchi Porro G: Systematic review: Gastro-oesophageal reflux
disease and dental lesions. Aliment Pharmacol Ther. 27:1179–1186.
2008.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Jaspersen D, Kulig M, Labenz J, Leodolter
A, Lind T, Meyer-Sabellek W, Vieth M, Willich SN, Lindner D, Stolte
M and Malfertheiner P: Prevalence of extra-oesophageal
manifestations in gastro-oesophageal reflux disease: An analysis
based on the ProGERD Study. Aliment Pharmacol Ther. 17:1515–1520.
2003.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hirano I and Richter JE: Practice
Parameters Committee of the American College of Gastroenterology.
ACG practice guidelines: Oesophageal reflux testing. Am J
Gastroenterol. 102:668–685. 2007.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zerbib F, desVarannes SB, Roman S,
Pouderoux P, Artigue F, Chaput U, Mion F, Caillol F, Verin E,
Bommelaer G, et al: Normal values and day-to-day variability of
24-h ambulatory oesophageal impedance-pH monitoring in a
Belgian-French cohort of healthy subjects. Aliment Pharmacol Ther.
22:1011–1021. 2005.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ganss C, Lussi A and Schlueter N: Dental
erosion as oral disease. Insights in etiological factors and
pathomechanisms, and current strategies for prevention and therapy.
Am J Dent. 25:351–364. 2012.PubMed/NCBI
|
|
24
|
Magalhães AC, Wiegand A, Rios D, Honório
HM and Buzalaf MA: Insights into preventive measures for dental
erosion. J Appl Oral Sci. 17:75–86. 2009.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Bartlett D, Ganss C and Lussi A: Basic
erosive wear examination (BEWE): A new scoring system for
scientific and clinical needs. Clin Oral Investig. 12 (Suppl
1):S65–S68. 2008.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lussi A, Schaffner M, Hotz P and Suter P:
Dental erosion in a population of Swiss adults. Community Dent Oral
Epidemiol. 19:286–290. 1991.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Aanen MC, Numans ME, Weusten BL and Smout
AJ: Diagnostic value of the reflux disease questionnaire in general
practice. Digestion. 74:162–168. 2006.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wilder-Smith CH, Materna A, Martig L and
Lussi A: Gastro-oesophageal reflux is common in oligosymptomatic
patients with dental erosion: A pH-impedance and endoscopic study.
United European Gastroenterol J. 3:174–181. 2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Stål P, Lindberg G, Ost A, Iwarzon M and
Seensalu R: Gastroesophageal reflux in healthy subjects.
Significance of endoscopic findings, histology, age, and sex. Scand
J Gastroenterol. 34:121–128. 1999.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Dua KS, Surapaneni SN, Hafeezullah M,
Reddy N, Tatro L and Shaker R: Prevalence of abnormal upper GI
findings in apparently healthy volunteers enrolled for research
studies. Gastrointest Endosc. 69:AB350–AB351. 2009.
|
|
31
|
Marrese M, Guarino V and Ambrosio L:
Atomic force microscopy: A powerful tool to address scaffold design
in tissue engineering. J Funct Biomater. 8(7)2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zheng X, Hu J, Chen Y, Zhu Y and Chen H:
AFM study of the effects of collagenase and its inhibitors on
dentine collagen fibrils. J Dent. 40:163–171. 2012.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lussi A and Jaeggi T (eds): Dental
Erosion: Diagnostic, Risk Assessment, Prevention, Treatment.
Quintessence International, pp55-67, 2012.
|
|
34
|
Goldberg M, Kulkarni AB, Young M and
Boskey A: Dentin: Structure, composition and mineralization. Front
Biosci (Elite Ed). 3:711–735. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
35
|
Ren YF, Zhao Q, Malmstrom H, Barnes V and
Xu T: Assessing fluoride treatment and resistance of dental enamel
to soft drink erosion in vitro: Applications of focus variation 3D
scanning microscopy and stylus profilometry. J Dent. 37:167–176.
2009.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ren YF: Dental erosion: Etiology,
diagnosis and prevention. A peer reviewed publication. Registered
Dental Hygienist Magazine, pp32-38, 2011.
|
|
37
|
Lussi A and Hellwig E: Risk assessment and
preventive measures. From diagnosis to therapy: In: Dental Erosion.
Lussi A (ed). Vol 20. Monogr Oral Sci, Basel, pp190-199, 2006.
|
|
38
|
Picos AM: Eroziunea Dentara in Noala de
Reflux Gastroesofagian. Dental erosion in gastroesophageal reflux
disease. Edit Med Univ ‘Iuliu Haţieganu’, Cluj-Napoca, pp11-19,
2014 (In Romanian).
|
|
39
|
Lussi A, Portmann P and Burhop B: Erosion
on abraded dental hard tissues by acid lozenges: An in situ study.
Clin Oral Invest. 1:191–194. 1997.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Lussi A, Jäggi T and Schärer S: The
influence of different factors on in vitro enamel erosion. Carries
Res. 27:387–393. 1993.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Lussi A and Jaeggi T: Erosion-diagnosis
and risk factors. Clin Oral Investig. 12 (Suppl 1):S5–S13.
2008.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Picoş A, Răchişan AL and Dădârlat A:
Minimally invasive dental treatment using composites and ceramics
in GERD diagnoses patients. Mater Plast. 55:252–254. 2018.
|
|
43
|
Vakil N, van Zanten S, Kahrilas P, Dent J
and Jones R: Global Consensus Group. The Montreal definition and
classification of gastroesophageal reflux disease: A global
evidence-based consensus. Am J Gastroenterol. 101:1900–1920, 1943.
2006.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Picos AM, Poenar S, Opris A, Chira A, Bud
M, Berar A, Picos A and Dumitrascu DL: Prevalence of dental
erosions in BRGE: A pilot study. Clujul Med. 86:344–346.
2013.PubMed/NCBI
|
|
45
|
Picos AM, Chisnoiu AM, Lasserre JF, Spinei
A, Chisnoiu MR and Picos A: Dental erosion-literature update. HVM
Bioflux. 5:135–141. 2013.
|
|
46
|
Picoş AM, D'Incau E, Bonafos C, Berar A,
Chira A and Dumitrascu D: Intrinsic etiology of dental erosion. J
Odonto Stom. 43:56–67. 2014.
|
|
47
|
Picos A, Lasserre JF, Chisnoiu A, Berar A,
D'Incau E, Picos AM, Chira A, des Varannes SB and Dumitrascu DL:
Factors associated with dental erosions in gastroesophageal reflux
disease: A cross-sectional study in patients with heartburn. Med
Pharm Rep. 93:23–29. 2020.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Tsichlaki A, O'Brien K, Benson PE,
Marshman Z, Johal A, Colonio-Salazar FB, Harman NL and Fleming PS:
Development of a core outcome set for use in routine orthodontic
clinical trials. Am J Orthod Dntofacial Orthop. 158:650–660.
2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Nairn W (ed): Principles and Practice of
Esthetic Dentistry. 1st edition. Elsevier, pp165-191, 2014.
|
|
50
|
Alhajj MN, Ariffin Z, Celebić A, Alkheraif
AA, Amran AG and Ismail IA: Perception of orofacial appearance
among laypersons with diverse social and demographic status. PLoS
One. 15(e0239232)2020.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Dong JK, Jin TH, Cho HW and Oh SC: The
esthetics of the smile: A review of some recent studies. Int J
Prosthodont. 12:9–19. 1999.PubMed/NCBI
|
|
52
|
Romano R, Bichacho N and Touati B (eds):
The Art of the Smile: Integrating Prosthodontics, Orthodontics,
Periodontics, Dental Technology, and Plastic Surgery in Esthetic
Dental Treatment. Quintessence Publishing, pp11791, 2005.
|
|
53
|
Picos A, Vulturar R, Picos A, Chis A,
Chiorean I, Piciu A, Petrachescu N and Dumitrascu DL:
Interleukin-1A and interleukin-1B gene polymorphisms in
gastroesophageal reflux disease. Exp Ther Med. 20:3394–3398.
2020.PubMed/NCBI View Article : Google Scholar
|