Introduction
Bone metastasis is a serious and costly complication
of cancer and is usually incurable (1). Approximately 70% of patients with
advanced breast and prostate cancers and up to 30-40% of patients
with advanced lung, thyroid and kidney cancer develop bone
metastasis (2). Bone metastases may
be characterized as osteolytic or osteoblastic lesions (1). Breast cancer usually forms osteolytic
lesions, and 15-20% of patients with bone metastases develop
osteoblastic lesions (1). By
contrast, patients with prostate cancer more often develop
osteoblastic lesions. Patients with multiple myeloma develop only
osteolytic lesions.
Spinal metastases, which are observed in 60-70% of
patients with systemic cancer, can cause severe pain (usually in
90-95% of patients with metastases), pathologic fractures,
life-threatening hypercalcemia, spinal cord compression and poor
quality of life (3). The goals of
treating spinal metastasis are pain relief and spinal
stabilization. Treatment selection is affected by numerous factors,
including survival prediction, patient health, the number and
localization of involved vertebrae and the degree of expansion of
the spinal metastasis to the surrounding tissue. It is recommended
to start spinal metastasis treatment within 14 days of symptoms
being reported in cases where pain is the only symptom (3). Surgery, chemotherapy and radiotherapy
may be undesirable treatment options, as duration of the required
postoperative hospital stay may last much of the patient's
remaining life expectancy (4).
Percutaneous vertebroplasty (PVP) and percutaneous
kyphoplasty (PKP) are both minimally invasive techniques where
polymethylmethacrylate (PMMA) is injected in the vertebral body
under X-ray or CT guidance. PVP had been demonstrated to be an
economical and effective procedure in controlling pain (usually in
74-100% of patients) and preventing further vertebral collapse in
spinal metastases and also allows for percutaneous biopsy (3,5,6).
Although PVP has been widely used in treatment of osteolytic
metastases, there have been a few reports on the effect of PVP in
painful osteoblastic metastatic spinal lesions (7-10).
In the present study, the clinical data of patients
treated with PVP following painful osteoblastic spinal metastases
were retrospectively analyzed.
Materials and methods
Study design
The present study is a retrospective analysis of
data obtained from 39 consecutive patients with 82 osteoblastic
vertebras who developed painful spinal metastases. These patients
were referred to the Department of Orthopedics of the Cancer
Hospital of the Chinese Academy of Medical Science between August
2017 and February 2019.
Inclusion and exclusion criteria
Patients had to meet the following inclusion
criteria: i) Diagnosed with cancer; ii) aged 18-80 years; iii)
clinical and imaging evidence (MRI or CT) of vertebral metastases
in the cervical, thoracic, lumbar or sacral segments; iv)
osteoblastic appearance of metastases and excruciating pain
corresponding with specific vertebral levels, despite
pharmacological treatment, or adverse effects related to opioids
(constipation, urinary retention and/or confusion), or opioid
tolerance developed in patients with controlled pain; v) patients
treated with spine radiotherapy or waiting to receive radiotherapy
sessions; vi) expected survival time >3 months; and vii)
vertebral fractures without posterior wall disruption, or fractures
with posterior wall disruption but no epidural involvement.
Exclusion criteria included patients with: i)
Clinical signs of spinal cord compression or cauda equina syndrome;
ii) fractures with epidural involvement and contact with spinal
cord or nerve roots; iii) complete vertebral destruction; iv)
posterior arch involvement and v) local infection at the puncture
site or septicemia. Relative contraindications included transient
chemotherapy-induced hematologic anomalies, including leucopenia
(<2.5x103/µl), thrombocytopenia
(<100.0x103/µl) and c) elevated international
normalized ratio >1.5. Only those patients whose abnormalities
had resolved underwent PVP.
Clinical information was obtained through electronic
medical records while imaging was obtained from the hospital
picture archiving and communication system. CT or MR images were
evaluated. Data including primary tumor site, age of spinal
metastases, date of the procedure, modality of associated
chemotherapy and radiotherapy, pain assessment of spinal
metastases, vertebral level treated, technical incidents and
details of complications, such as patients drop out from follow-up
were recorded.
The visual analogue scale (VAS) score was used to
evaluate pain intensity before PVP procedures and at 1 week and 3
months after the procedure. The VAS assesses pain level on a scale
of 0-10, with 0 being no pain and 10 indicating the worst pain
(11). To assess quality of life
the EORTC QLQ-BM22 module was used, which contains 22 items
conceptualized into symptom scales (five painful sites and three
pain characteristics) and functional scales (eight functional
interference) and six psychosocial aspects (12). QLQ-BM22 scores were recorded by the
attending oncologist before PVP and at 1 week and 3 months after
PVP.
The technique of PVP has been described in detail
elsewhere (13,14) and official guidelines have also been
published (15,16). PVP involves a biopsy through a
transpedicle approach and the injection of polymethylmethacrylate
(PMMA) into the vertebral body. All PVP procedures were performed
by senior orthopedists with >10 years of experience of
performing the PVP procedure, always using a digital subtraction
angiography unit with a C-arm (Angiostar, Siemens). The patient was
under conscious sedation in combination with local anaesthesia with
lidocaine 1% at pedicle levels (transpedicular approach)
administered by fluoroscopic guidance. General anaesthesia with
orotracheal intubation was used when the patient was unable to be
in the prone position, as well as when the anesthesiologist
considered it necessary. The bone needle (11-13G; WEGO, Inc.) was
used to slowly puncture the anterior one third of the vertebral
body through a posterior transpedicular approach under fluoroscopic
guidance. The trocar was removed and a biopsy device placed to
obtain a bone core. Then the polymethyl methacrylate (PMMA) cement
(SimplexP; Howmedica Osteonics Corporation) was injected into the
cavity within the vertebral body avoiding the osteoblastic lesion.
The injection process was monitored continuously under fluoroscopy
in the lateral plane. Injection was stopped when substantial
resistance was met or when the PMMA cement reached the posterior
margin of the vertebral body.
At the end of the procedure the presence of cement
leakage to the vertebral disk, anterolateral, lateral, foraminal
and epidural veins, paravertebral plexus, vena cava, intercostal
arteries, soft tissue or spinal canal was recorded. All
complications were recorded in the database, including haematoma,
radicular pain and pulmonary embolism due to cement migration and
settlement in the pulmonary vasculature (confirmed by CT in all
suspicious cases).
Statistical analysis
Descriptive statistical analysis was performed on
all assessed variables. VAS was evaluated preoperatively and at 1
week and 3 months after the surgery. Quality of life (QOL) was
evaluated with QLQ-BM22 score (12)
in the studied population before the surgery and at one week and 3
months after the surgery. All statistical analyses were performed
using SPSS for Windows version 22.0 (SPSS, Inc). Data are presented
as the median ± SD for the VAS and QLQ-BM22 score at different time
points. The Wilcoxon signed rank test was used to compare the
median VAS and QLQ-BM22 scores at the different study time point
after surgery versus pre-operation. P<0.05 was considered
statistically significant.
Results
PVP was performed for 39 consecutive patients with
82 osteoblastic metastatic spinal vertebras, of which 19 vertebras
had pathologic compressive fracture sand the other 63 vertebras had
no compressive fracture with obvious imaging abnormalities. Among
all 82 vertebras, 35 vertebras had been injected bilaterally and
the other 47 vertebras unilaterally. The patients were 19 males and
20 females with a mean age of 58.5 years (age range, 40-77 years;
Fig 1, Fig 2 and Fig
3). The postoperavie pathological diagnoses of spinal
metastases were all malignant: 14 with lung cancer, 10 with breast
cancer, 4 with kidney cancer, 4 with colon cancer, 1 with
esophageal cancer, 1 with gastric cancer, 1 with ovarian cancer, 1
with prostate cancer, 1 with salivary gland carcinoma and 2 with
unknown malignancies. The amount of cement injected per lesion
ranged from 0.5 to 4.5 ml with a mean volume of 1.6±0.8 ml. Cement
deposition in all lesions was uniform.
The patients were followed up from 3 to 15.5 months
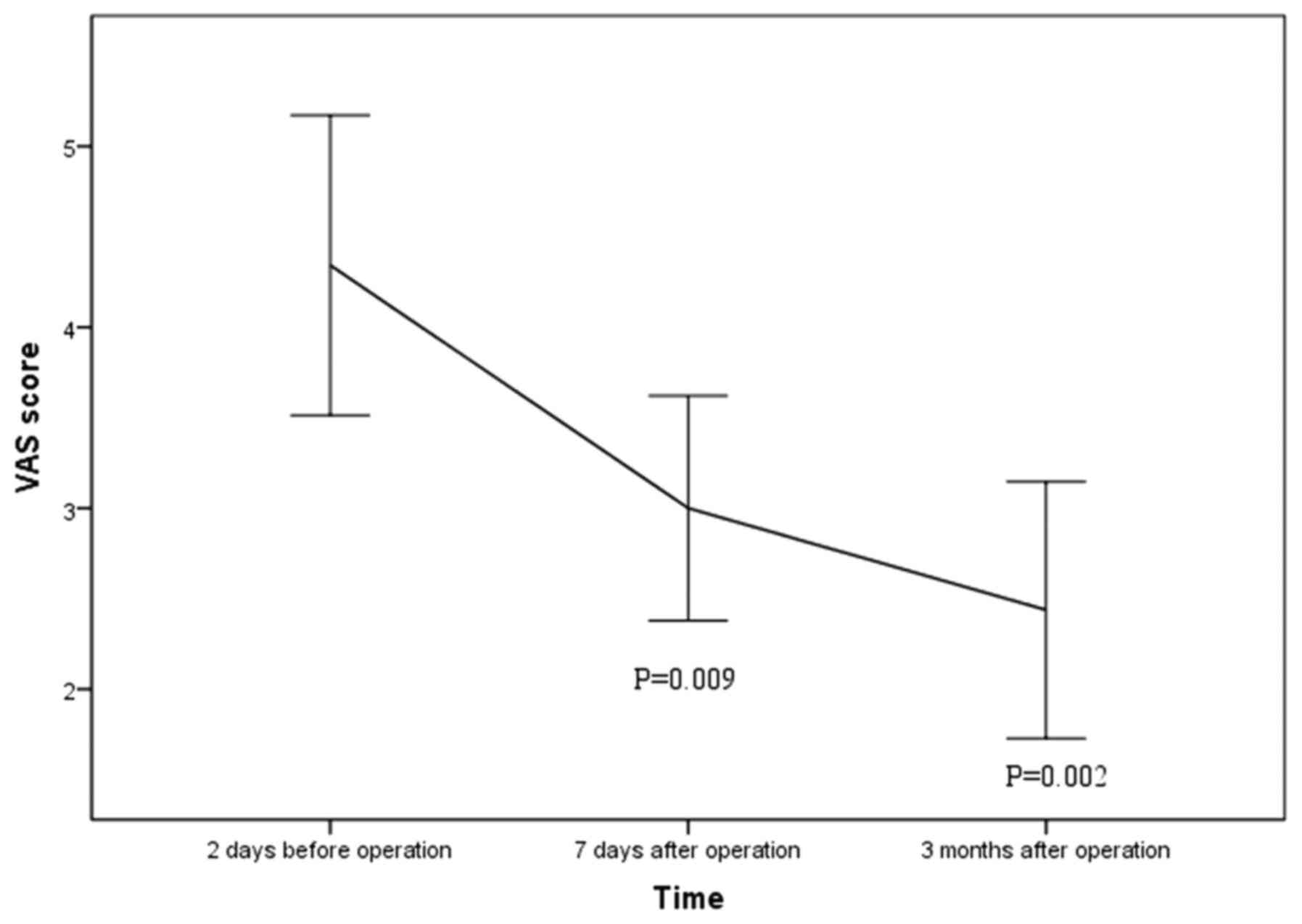
with a mean follow up time of 5.6±3.4 months. Mean VAS score
declined significantly from preoperative 4.3±2.4 to postoperative
3.0±1.7 at 1 week (P=0.009) and 2.4±2.0 at 3 months after the
procedures (P=0.002; Fig. 4). Mean
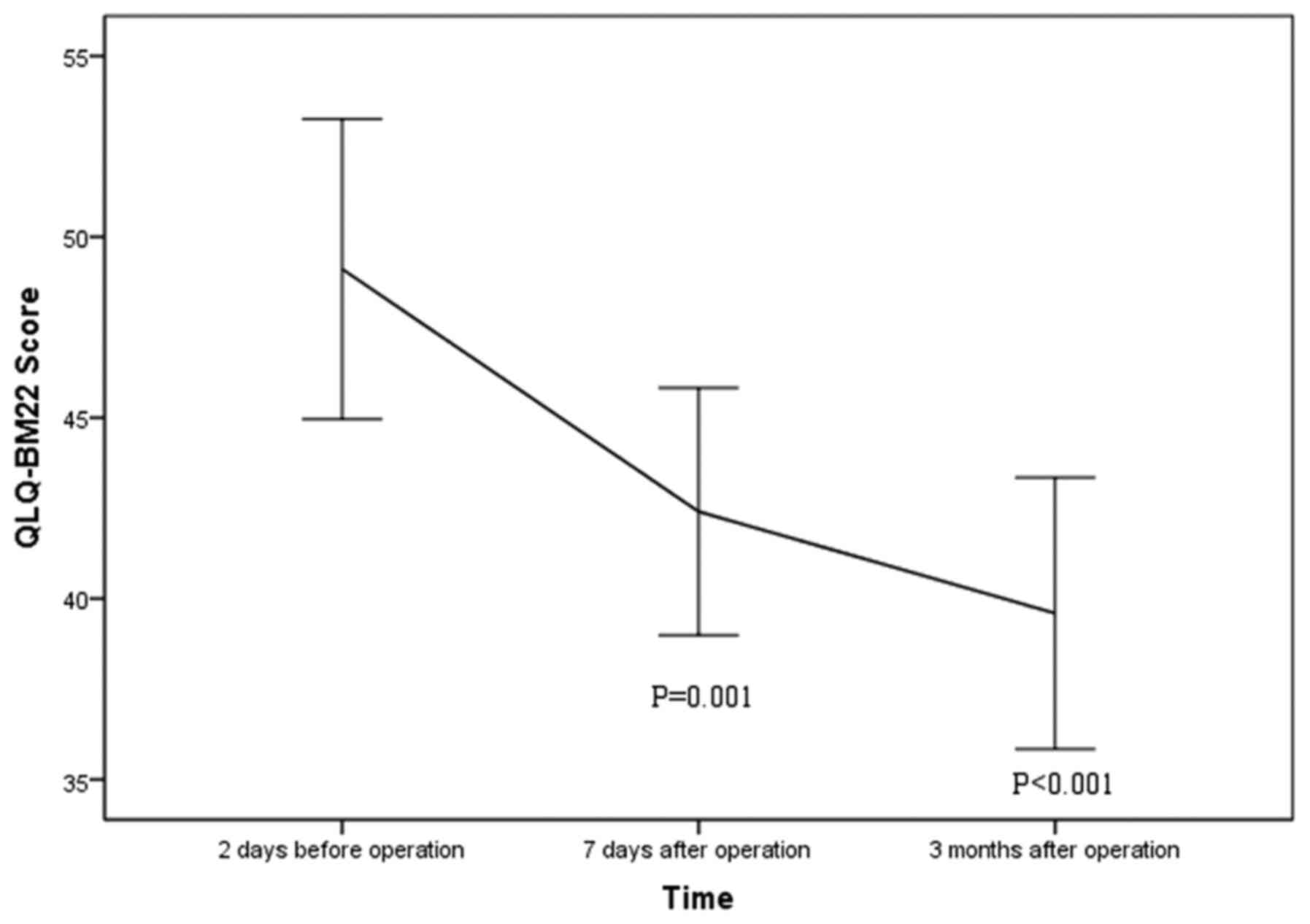
QLQ-BM22 score declined significantly from preoperative 49.1±12.3
to postoperative 42.4±9.5 at 1 week (P=0.001) and 39.6±10.4 at 3
months after the procedure (P<0.001; Fig. 5). Extraosseous cement leakage
occurred in 21 vertebras of 13 patients and in 1 case into the
thoracic vertebra canal without causing any clinical complications.
No further procedures were performed after leakage.
Discussion
Patients with bone metastases are at risk of
suffering due to severe symptoms such as bone pain and
life-threatening hypercalcemia, pathological fracture, neurological
deficit and epidural spinal cord compression (17,18).
Therefore, providing appropriate pain relief by minimally invasive
surgery to improve patient quality of life is of importance and
also a major challenge for these patients.
Bone metastases can be characterized as osteolytic
or osteoblastic lesions (1). These
classifications represent two extremes of a continuum in which
dysregulation of the normal bone remodeling process occurs
(19). In addition, secondary
formation of bone occurs in response to bone destruction. The
mechanisms of osteoblastic metastasis and the factors involved are
unknown. Previous research (11,20,21)
has suggested that blocking osteoblast-stimulating activity by
tumor cells may decrease tumor growth and osteoblast activity,
which suggests that a cycle may be involved in osteoblastic
metastasis in which tumors induce osteoblast activity and thus the
subsequent release of growth factors from these osteoblasts that
increase tumor growth.
The histology of the tumor-bone interface in both
humans and mouse models of tumor bone colonization reveals much
about the cellular content and context of the bone marrow in the
presence of metastatic tumor cells (22). Endothelin-1(20), platelet-derived growth factor
(23), urokinase (24) and prostate-specific antigen (PSA)
(25) have been identified to be
involved in osteoblastic metastasis process. Bone metastases caused
by prostate cancer are commonly osteoblastic, with levels of
bone-resorption markers higher in patients with these metastases
than in patients without bone metastasis. The extent of bone
metastasis in these patients is more accurately measured in these
patients by bone-resorption markers than the PSA level (26). It is still unclear whether bone
resorption precedes bone formation in the development of
osteoblastic metastases. The antiosteolytic action of drugs,
including bisphosphonates (27),
has led to the evaluation of their use in prostate cancer, which
demonstrated their efficacy in patients with hormone-refractory
metastatic prostate cancer (28).
Bone-protection agents, including bisphosphonates and denosumab
(29,30), lead to a reduction in osteoclastic
activity and induction of apoptosis, inhibiting bone resorption as
well as inhibiting tumor cell adhesion to bone (18). This could ultimately control pain
relief, even in osteoblastic metastasis (18,31).
Emerging agents targeting osteoblasts, such as romosozumab, can
activate bone formation (32).
These drugs may improve bone formation and indirectly be involved
in osteoblastic metastatic processes.
Patients with spinal metastases are highly likely to
achieve significant improvements in pain control and reduced
pain-related disability through minimally-invasive surgery
(33-35).
Previous reports published on minimally invasive surgery in spinal
metastasis were largely focused on osteolytic metastasis.
Improvements in preoperative definable vertebral collapse (36) and postoperative pain relief and QOL
were demonstrated (33). Both PVP
and PKP are effective, and no difference could be found in their
relative effectiveness (37,38).
PVP could achieve pain relief and improvement of life quality of
patients with multiple myeloma spinal metastasis (33). Concerning the subsequent cost dure
to the care requirements and serious clinical consequences of
spinal metastasis, the use of PVP could be a cost-effective
strategy at commonly accepted willingness-to-pay thresholds
(35). The mechanism of pain relief
by PVP in vertebral compressive fracture is that PMMA mechanically
stabilizes the vertebral body and its fragments causing an
exothermic reaction during the polymerization of the cement and a
neurotoxic effect to the surrounding micronerves (4). Considering the rarity of local
recurrence of metastatic tumors after PVP, it has been hypothesized
that PVP may have an antitumoral effect by the space occupying
effect and the vascular structure destruction related to PMMA
(4).
Although PVP has been widely used in treatment of
osteolytic metastases, few published reports are focused on
osteoblastic metastasis (7-10,39-41)
(Table I), the findings of which
are summarized here. In 39 consecutive patients with 51
osteoblastic metastases, vertebroplasty could relieve pain, reduce
disability, and improve function (10). Immediate pain relief was achieved in
4 patients with painful osteoblastic spinal metastases (39). A total of 86% of patients with
osteoblastic metastases experienced pain relief up to 92% at 6
months after PVP procedure (40). A
contralateral unipedicular approach was suggested to access the
vertebral body and strengthen the nonblastic side of the metastasic
vertebra body, which might lead to bone pain relief (7). In 6 patients with painful osteoblastic
metastases, pain relief and function improvement was been achieved
after PKP procedure without any complications (41). A combination of PVP and
125I implantation was conducted for 50 patients with
spinal osteoblastic metastases, which showed clinical efficacy with
immediate pain relief, QOL improvement and reduction of paraplegia
occurrence (8). However, the PVP
attempt failed in a patient with a painful lytic vertebral fracture
related to a lung cancer spinal metastasis under medical treatment
with denosumab, which induced a fast and marked sclerotic response
on vertebral bodies that may not be accompanied by a satisfactory
improvement in pain control (42).
These findings raised the question of the optimal treatment order
and the best timeframe for combination of PVP and bone protection
agents in patients with painful spinal metastases.
 | Table IReports forcusing minimally invasive
surgery in osteoblastic spinal metastasis. |
Table I
Reports forcusing minimally invasive
surgery in osteoblastic spinal metastasis.
| | | | Cohort Size | | | | |
|---|
| Author | Year | Minimally invasive
surgery | Case | Control | Lesion site (cement
volume) | Primary tumor
(number) | Follow-up
duration | Assessment |
|---|
| Murphy et al
(7) | 2007 | PVP | 1 | 0 | T10 (Not
mentioned) | Breast cancer
(1) | 3 years | None |
| Chen et al
(39) | 2011 | PVP | 4 | 0 | Thoracic and lumbar
vertebra (2.2-3.5 ml) | Lung (2), Prostate (1) and Pancreatic (1) Cancer | 14-24 weeks | VAS |
| Chen et al
(41) | 2013 | PKP | 6 | 0 | Thoracic and lumbar
vertebra (3.3±1.0 ml) | Lung (2), breast (2), liver (1) and prostate (1) cancer | 16-96 weeks | VAS, ODI |
| Yang et al
(8) | 2013 | PVP and
125I | 50 | 50 | Thoracic (2.8 ml)
and lumbar (3.1 ml) vertebra | Lung (20), breast (19), prostate (10) and colon (1) cancer | 6 months-5
years | VAS ECOG
(QLQ-C30) |
| Chih et al
(9) | 2016 | PVP | 1 | 0 | L2 (5 ml) | Pancreatic Cancer
(1) | 1 year | VAS ECOG |
| Tian et al
(10) | 2016 | PVP | 39 | 0 | Thoracic and lumbar
vertebra (2-5 ml) | Lung (15), prostate (11) breast (9), liver (3), and colon (1) cancer | 3-30 months | KPS |
Pain relief
The treatment choices available for painful
metastases are varied (5). The
Dutch National Guideline noted that surgical techniques range from
minimally invasive options to en bloc resection of the
segments affected by spinal metastases (3). The injection of bone cement may
stabilize the vertebrae and prevent further collapse of the
osteoblastic vertebral body. In osteobalstic metastasis, it also
hypothesized that the asymmetry of vertebral oeteoblastic
compressibility might result in shear stress fractures causing
pain, which could be equalized by vertebroplasty into the
nonblastic side (7). A
meta-analysis with 26 studies involving 1,351 patients with PVP
treatment for spinal tumors demonstrated that PVP was significantly
associated with pain relief and life quality and could improve
outcomes in these metrics in metastatic spinal tumor patients
(34). It is hypothesized that pain
relief in osteoblastic metastasis following bone cement application
is related not only the reinforcement of the vertebra, but also to
chemical and thermal effects of the cement compound, which may
damage sensory nerve endings and kill the tumor cells (4,39,41).
Pain scores are classified as follows: VAS 1-4, mild
pain; 5-8, moderate pain; and 9-10 as severe pain (43). Previous studies of osteoblastic
spinal metastases showed postoperative pain relief. It has been
reported that a VAS score decline could be found after PVP from
preoperative 7.4±1.1 to postoperative 2.5±0.9 at 3 days and 2.1±1.1
at 1 month, 2.0±1.1 at 3 months (10), which suggested pain score was
reduced from moderate to mild at 3 months after the operation. In a
patient with painful osteoblastic pancreatic spinal metastases, a
VAS scores decline could be found after PVP from preoperative 9-10
to postoperative 3-4 at follow-up after <1-year (9), which suggested pain was reduced from
severe to mild. In 4 patient with painful osteoblastic spinal
metastases, a VAS score decline could also be found after PVP from
preoperative 8.5±0.6 to postoperative 1.5±0.6 at on month, which
suggested a pain score reduction from severe and moderate to mild.
In the present study, the VAS score declined significantly from
preoperative 5.0±2.8 to postoperative 3.0±1.7 at 1 week and 2.4±2.0
at 3 months (P<0.001), which suggested a pain reduction from
moderate to mild. Minimally invasive procedurse for the
stabilization of both osteoblastic and osteoclastic spinal
metastases, could achieve statistically significant pain relief,
function improvement, preventing further local kyphotic deformity,
and vertebra body height (40,44).
For patients with advanced cancer who have developed
bone metastases, increased life expectancy has made metastases more
observable, which has resulted in a change in treatment strategy
from curative to palliative (33).
Minimally invasive procedures performed after spinal metastases,
including vertebroplasty and kyphoplasty, could significantly
improve the patient's QOL (33).
Questionnaires for this group of patients should be brief while
still including the most important QOL issues so as not to burden
the patient (45). In 2009, Chow
et al (12) developed a
comprehensive HRQOL measurement tool for patients with bone
metastases. The EORTC QLQ-BM22 module contains 22 items
conceptualized into both symptom scales, with five painful sites
and three pain characteristics, and also functional scales, with
eight functional interference and six psychosocial aspects
(12). Compared with BOMET-QOL,
QLQ-BM22 gives a more in-depth analysis of symptoms and well-being
and includes issues such as mobility, side effects, complications
and dependency for patients with bone metastases (46). The BOMET-QOL is shorter and gives an
overall assessment of pain and mobility. Both questionnaires have
been determined to be valid and reliable. In the present study
QLQ-BM22 was used to measure the QOL of patients with bone
metastases.
A relatively high rate of cement leakage occurred in
21/82 (25.7%) vertebras and 13/39 (33.3%) patients, of which 2/39
(5%) patients experienced leakage into the vertebral canal. The two
patients presented with the immediate complication of radicular
pain, of which one resolved within 3 days, and the other one within
two weeks following treatment with oral medication. No mortality
episodes, such as cement related pulmonary embolism, were recorded
during the present study. These data suggested that no severe
systemic complications occur following PVP in patients with
osteoblastic spinal metastases.
The present study reported technical incidents
related to cement leakages, but otherwise patients were
asymptomatic in the immediate and follow-up post-PVP, similar to
those reported by other authors. In a study with 39 consecutive
patients with 51 osteoblastic metastatic spinal lesions,
extraosseous cement leakage occurred in 15 cases without causing
any clinical complications (10).
In osteoporotic vertebral compression fractures, the most
frequently reported complication of PVP was cement leakage,
occurring in up to 75% of patients (47) and is usually asymptomatic (3). Low viscosity, a larger quantity of
injected PMMA and greater cortical destruction of the vertebra seem
to increase the risk of cement leakage (3).
The high number of cement leakages recorded in the
present study could be explained by the following: a) Careful
monitoring of cement distribution during the PVP procedure; b) use
of CT imaging for post PVP observation; c) treatment of two or more
vertebras per patient, with up to 6 vertebras; d) the treatment of
osteoblastic lesions that are more prone to leakage (40); and e) a relatively high volume of
cement injected per vertebra, up to 4.5 ml.
To improve clinical outcomes, it is advised to
follow these approaches: i) If possible, try to use the high
viscosity cement to avoid leakage, which will shorten the injection
duration; ii) place the puncture needle at the place without
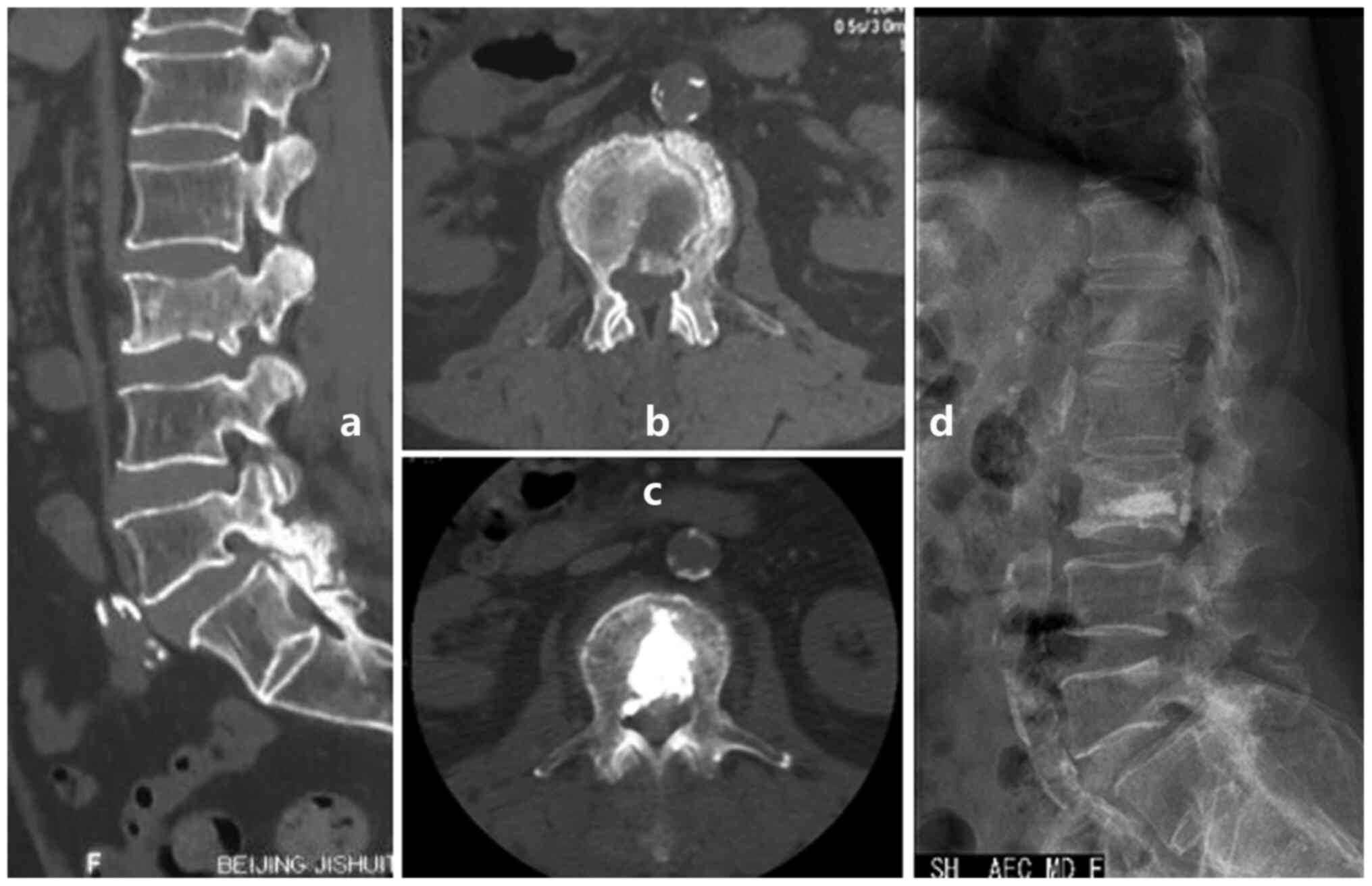
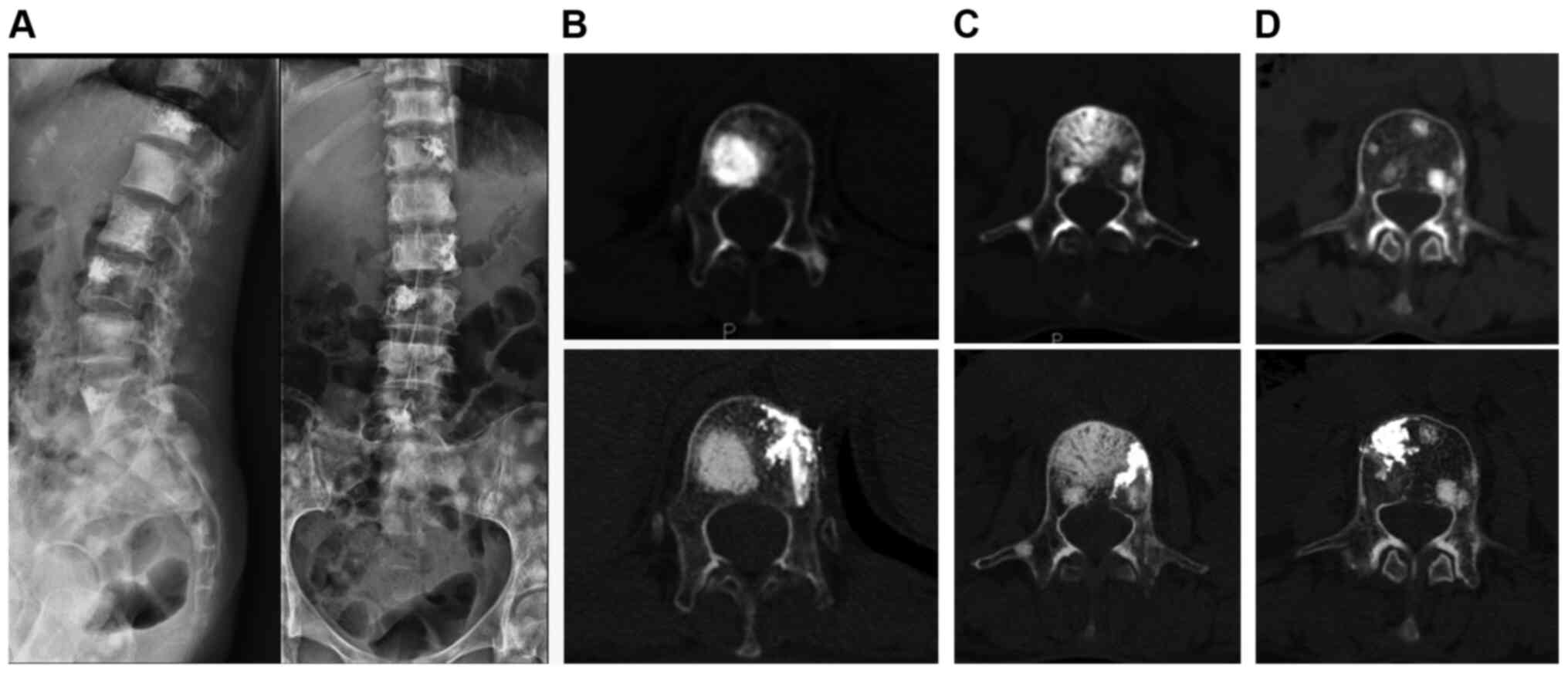
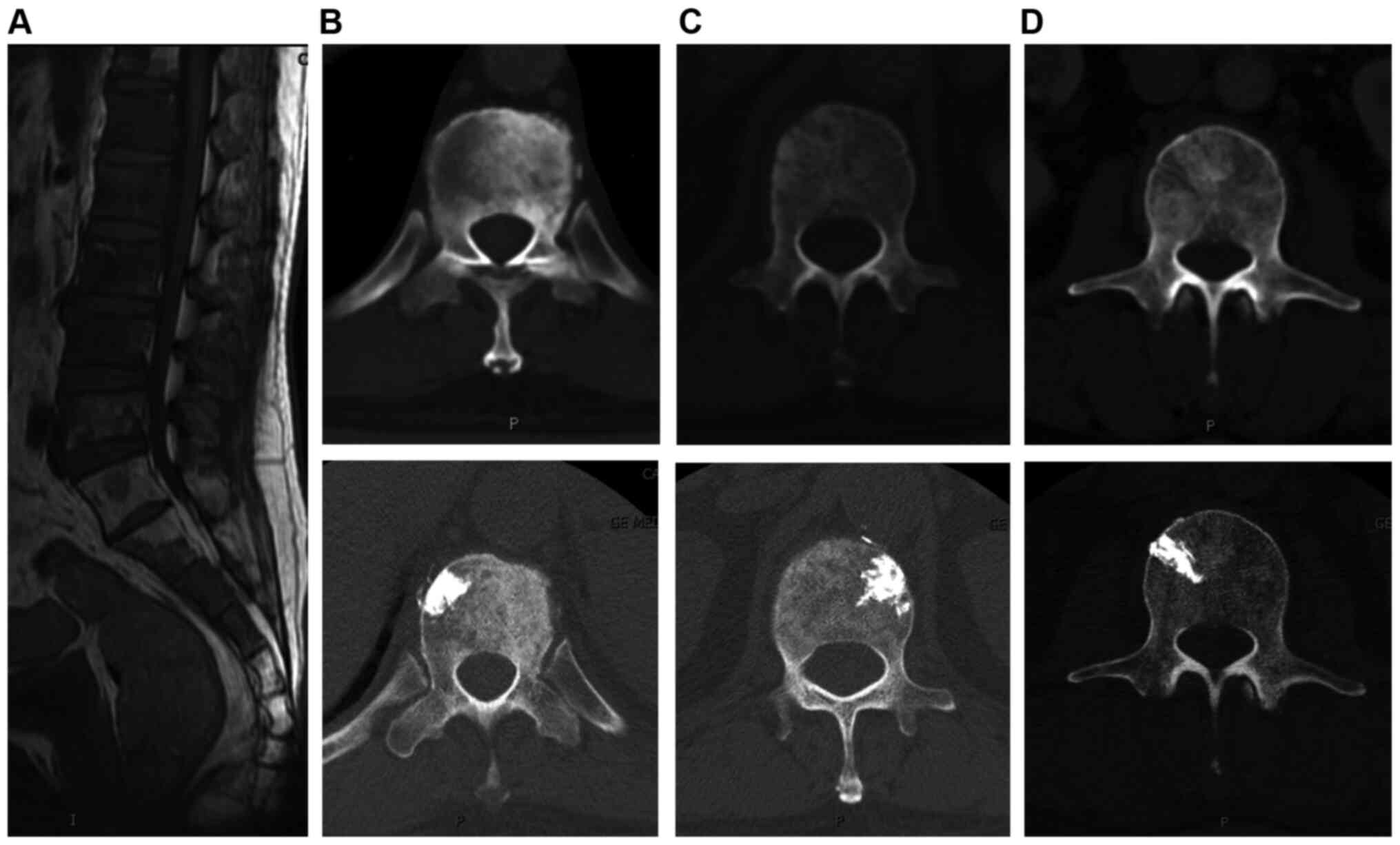
osteoblastic side to equally strengthen the nonblastic side
(Fig. 1); iii) apply unilateral
injection rather than bilateral injections which is usually
difficult to finish due to the osteoblastic strength (Fig. 2); and iv) use a thin trephine and a
surgical hammer (Fig. 3; 10,39) to
aid penetration of the transpedicular due to the hardness of the
osteoblastic bone at the start of the procedure.
The present study had certain limitations. First, a
control group undergoing conservative treatment was not available.
Second, the number of participants was relatively small. Third, the
general status, previous treatment, life expectancy, and tumor type
of the cancer patient may all influence the treatment outcome.
Additional high-quality data are necessary to draw more reliable
conclusions.
PVP is an effective treatment for painful
osteoblastic spinal metastases. It can relieve pain, reduce
disability, and improve function. The main complications are bone
cement leakage and unfavorable pain relief.
Acknowledgements
The authors would like to thank Professor Shao Mng
Wang from the Office of Cancer Registry, National Cancer
Center/National Clinical Research Center for Cancer/Cancer
Hospital, Chinese Academy of Medical Science and Peking Union
Medical College for her assistance with statistical analysis.
Funding
Funding: This study was supported by grants from the Beijing
Municipal Science & Technology Comission (grant no.
Z171100001017210), Beijing Hope Run Special Fund of Cancer
Foundation of China (grant no. LC2016L01) and Beijing Union Medical
College (PUMC) Youth Fund (grant no. 2017320016)
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Author's contributions
SFX and SJY confirm the authenticity of all the raw
data. SFX and SJY conceived and designed the surgical plan. XXZ and
HML carried out the data collection and analysis. TL, XXZ, LBX and
ZGZ contributed surgery and data collection. SFX and TL contributed
to the writing of the article. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Roodman GD: Mechanisms of bone metastasis.
N Engl J Med. 350:1655–1664. 2004.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ferreira AR, Abrunhosa-Branquinho A, Jorge
M, Costa L and Vaz-Luís I: Bone metastases. In: International
Manual of Oncology Practice: (iMOP)-Principles of Medical Oncology.
de Mello RA, Tavares Á and Mountzios G (eds). Springer
International Publishing, Cham, pp867-889, 2015.
|
|
3
|
Bollen L, Dijkstra SPD, Bartels R, de
Graeff A, Poelma DLH, Brouwer T, Algra PR, Kuijlen JMA, Minnema MC,
Nijboer C, et al: Clinical management of spinal metastases-The
Dutch national guideline. Eur J Cancer. 104:81–90. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yang HL, Sun ZY, Wu GZ, Chen KW, Gu Y and
Qian ZL: Do vertebroplasty and kyphoplasty have an antitumoral
effect? Med Hypotheses. 76:145–146. 2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
O'Toole GC and Boland P: Metastatic bone
cancer pain: Etiology and treatment options. Curr Pain Headache
Rep. 10:288–292. 2006.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yang PL, He XJ, Li HP, Zang QJ and Wang
GY: Image-guided minimally invasive percutaneous treatment of
spinal metastasis. Exp Ther Med. 13:705–709. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Murphy KJ, Nwankwo IJ and Gailloud P:
Percutaneous vertebroplasty in the treatment of blastic vertebral
column metastasis from breast cancer. J Vasc Interv Radiol.
18:321–323. 2007.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yang Z, Tan J, Zhao R, Wang J, Sun H, Wang
X, Xu L, Jiang H and Zhang J: Clinical investigations on the spinal
osteoblastic metastasis treated by combination of percutaneous
vertebroplasty and 125I seeds implantation versus radiotherapy.
Cancer Biother Radiopharm. 28:58–64. 2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Chih YP, Wu WT, Lin CL, Jou HJ, Huang YH,
Chen LC and Chou LW: Vertebral compression fracture related to
pancreatic cancer with osteoblastic metastasis: A case report and
literature review. Medicine (Baltimore). 95(e2670)2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Tian QH, Sun XQ, Lu YY, Wang T, Wu CG, Li
MH and Cheng YS: Percutaneous vertebroplasty for palliative
treatment of painful osteoblastic spinal metastases: A
single-center experience. J Vasc Interv Radiol. 27:1420–1424.
2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
McCormack HM, Horne DJ and Sheather S:
Clinical applications of visual analogue scales: A critical review.
Psychol Med. 18:1007–1019. 1988.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chow E, Hird A, Velikova G, Johnson C,
Dewolf L, Bezjak A, Wu J, Shafiq J, Sezer O, Kardamakis D, et al:
The European organisation for research and treatment of cancer
quality of life questionnaire for patients with bone metastases:
The EORTC QLQ-BM22. Eur J Cancer. 45:1146–1152. 2009.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Deramond H, Depriester C, Galibert P and
Le Gars D: Percutaneous vertebroplasty with polymethylmethacrylate.
Technique, indications, and results. Radiol Clin North Am.
36:533–546. 1998.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Mansoorinasab M and Abdolhoseinpour H: A
review and update of vertebral fractures due to metastatic tumors
of various sites to the spine: Percutaneous vertebroplasty. Interv
Med Appl Sci. 10:1–6. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
McGraw JK, Cardella J, Barr JD, Mathis JM,
Sanchez O, Schwartzberg MS, Swan TL and Sacks D: SIR Standards of
Practice Committee. Society of Interventional radiology quality
improvement guidelines for percutaneous vertebroplasty. J Vasc
Interv Radiol. 14:827–831. 2003.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Helmberger T, Bohndorf K, Hierholzer J,
Noldge G and Vorwerk D: German Radiological Society. Guidelines of
the german radiological society for percutaneous vertebroplasty.
Radiologe. 43:703–708. 2003.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
17
|
Quinn RH, Randall RL, Benevenia J, Berven
SH and Raskin KA: Contemporary management of metastatic bone
disease: Tips and tools of the trade for general practitioners. J
Bone Joint Surg Am. 95:1887–1895. 2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Dushyanthen S, Cossigny DA and Quan GM:
The osteoblastic and osteoclastic interactions in spinal metastases
secondary to prostate cancer. Cancer Growth Metastasis. 6:61–80.
2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ortiz A and Lin SH: Osteolytic and
osteoblastic bone metastases: Two extremes of the same spectrum?
Recent Results Cancer Res. 192:225–233. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Guise TA, Yin JJ and Mohammad KS: Role of
endothelin-1 in osteoblastic bone metastases. Cancer. 97 (Suppl
3):S779–S784. 2003.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kasperk CH, Börcsök I, Schairer HU,
Schneider U, Nawroth PP, Niethard FU and Ziegler R: Endothelin-1 is
a potent regulator of human bone cell metabolism in vitro. Calcif
Tissue Int. 60:368–374. 1997.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Johnson RW and Suva LJ: Hallmarks of bone
metastasis. Calcif Tissue Int. 102:141–151. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yi B, Williams PJ, Niewolna M, Wang Y and
Yoneda T: Tumor-derived platelet-derived growth factor-BB plays a
critical role in osteosclerotic bone metastasis in an animal model
of human breast cancer. Cancer Res. 62:917–923. 2002.PubMed/NCBI
|
|
24
|
Achbarou A, Kaiser S, Tremblay G,
Ste-Marie LG, Brodt P, Goltzman D and Rabbani SA: Urokinase
overproduction results in increased skeletal metastasis by prostate
cancer cells in vivo. Cancer Res. 54:2372–2377. 1994.PubMed/NCBI
|
|
25
|
Cramer SD, Chen Z and Peehl DM: Prostate
specific antigen cleaves parathyroid hormone-related protein in the
PTH-like domain: Inactivation of PTHrP-stimulated cAMP accumulation
in mouse osteoblasts. J Urol. 156:526–531. 1996.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Maeda H, Koizumi M, Yoshimura K, Yamauchi
T, Kawai T and Ogata E: Correlation between bone metabolic markers
and bone scan in prostatic cancer. J Urol. 157:539–543.
1997.PubMed/NCBI
|
|
27
|
Diel IJ, Solomayer EF, Costa SD, Gollan C,
Goerner R, Wallwiener D, Kaufmann M and Bastert G: Reduction in new
metastases in breast cancer with adjuvant clodronate treatment. N
Engl J Med. 339:357–363. 1998.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Saad F, Gleason DM, Murray R, Tchekmedyian
S, Venner P, Lacombe L, Chin JL, Vinholes JJ, Goas JA and Chen B:
Zoledronic Acid Prostate Cancer Study Group. A randomized,
placebo-controlled trial of zoledronic acid in patients with
hormone-refractory metastatic prostate carcinoma. J Natl Cancer
Inst. 94:1458–1468. 2002.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Xu SF, Adams B, Yu XC and Xu M: Denosumab
and giant cell tumour of bone-a review and future management
considerations. Curr Oncol. 20:e442–e447. 2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Sousa S and Clézardin P: Bone-targeted
therapies in cancer-induced bone disease. Calcif Tissue Int.
102:227–250. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Dorff TB and Agarwal N: Bone-targeted
therapies to reduce skeletal morbidity in prostate cancer. Asian J
Androl. 20:215–220. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
McClung MR, Brown JP, Diez-Perez A, Resch
H, Caminis J, Meisner P, Bolognese MA, Goemaere S, Bone HG,
Zanchetta JR, et al: Effects of 24 months of treatment with
romosozumab followed by 12 months of denosumab or placebo in
postmenopausal women with low bone mineral density: A randomized,
double-blind, phase 2, parallel group study. J Bone Miner Res.
33:1397–1406. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Tekin SB, Karslı B, Büyükbebeci O, Demir
İH, Gökalp AY and Kılınçoğlu V: How do vertebroplasty and
kyphoplasty affect the quality of life of patients with multiple
myeloma spinal metastasis? Eur J Orthop Surg Traumatol.
30:1447–1451. 2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Qi L, Li C, Wang N, Lian H, Lian M, He B
and Bao G: Efficacy of percutaneous vertebroplasty treatment of
spinal tumors: A meta-analysis. Medicine (Baltimore).
97(e9575)2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Health Quality Ontario. Vertebral
augmentation involving vertebroplasty or kyphoplasty for
cancer-related vertebral compression fractures: An economic
analysis. Ont health Technol Assess Ser. 16:1–34. 2016.PubMed/NCBI
|
|
36
|
Peh WC and Gilula LA: Percutaneous
vertebroplasty: An update. Semin Ultrasound CT MR. 26:52–64.
2005.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Wolman DN and Heit JJ: Recent advances in
vertebral augmentation for the treatment of vertebral body
compression fractures. Curr Phys Med Rehabilitation Rep. 5:1–14.
2017.
|
|
38
|
Wang H, Sribastav SS, Ye F, Yang C, Wang
J, Liu H and Zheng Z: Comparison of percutaneous vertebroplasty and
balloon kyphoplasty for the treatment of single level vertebral
compression fractures: A meta-analysis of the literature. Pain
Physician. 18:209–222. 2015.PubMed/NCBI
|
|
39
|
Chen L, Ni RF, Liu SY, Liu YZ, Jin YH, Zhu
XL, Zou JW and Xiao XS: Percutaneous vertebroplasty as a treatment
for painful osteoblastic metastatic spinal lesions. J Vasc Interv
Radiol. 22:525–528. 2011.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Calmels V, Vallee JN, Rose M and Chiras J:
Osteoblastic and mixed spinal metastases: evaluation of the
analgesic efficacy of percutaneous vertebroplasty. AJNR Am J
Neuroradiol. 28:570–574. 2007.PubMed/NCBI
|
|
41
|
Chen G, Luo ZP, Zhang H, Nalajala B and
Yang H: Percutaneous kyphoplasty in the treatment of painful
osteoblastic metastatic spinal lesions. J Clin Neurosci.
20:948–950. 2013.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Mattei TA, Mendel E and Bourekas EC:
Vertebral compression fractures in patients under treatment with
denosumab: A contraindication for percutaneous vertebroplasty?
Spine J. 14:e29–e35. 2014.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Chow E, Ding K, Parulekar WR, Wong RK, van
der Linden YM, Roos D, Hartsell WF, Hoskin P, Wu JS, Nabid A, et
al: Revisiting classification of pain from bone metastases as mild,
moderate, or severe based on correlation with function and quality
of life. Support Care Cancer. 24:1617–1623. 2016.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Wang Y, Liu H, Pi B, Yang H, Qian Z and
Zhu X: Clinical evaluation of percutaneous kyphoplasty in the
treatment of osteolytic and osteoblastic metastatic vertebral
lesions. Int J Surg. 30:161–165. 2016.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Costa L, Badia X, Chow E, Lipton A and
Wardley A: Impact of skeletal complications on patients' quality of
life, mobility, and functional independence. Support Care Cancer.
16:879–889. 2008.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Bedard G, Zeng L, Poon M, Lam H, Lauzon N
and Chow E: Comparison of the EORTC QLQ-BM22 and the BOMET-QOL
quality of life questionnaires in patients with bone metastases.
Asia Pac J Clin Oncol. 10:118–123. 2014.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Nieuwenhuijse MJ, Van Erkel AR and
Dijkstra PD: Cement leakage in percutaneous vertebroplasty for
osteoporotic vertebral compression fractures: Identification of
risk factors. Spine J. 11:839–848. 2011.PubMed/NCBI View Article : Google Scholar
|