Introduction
Calcaneal fractures constitute approximately 2% of
all fractures presenting to the emergency room. Most of them are
highly comminuted and involve the posterior subtalar joint
(1-4).
The optimal treatment remains controversial, with conservative and
surgical procedures still in use to date (5-7).
The preferred method of the present research group
is surgical reduction with the addition of an autologous bone graft
from the iliac crest. However, the high morbidity of this type of
fracture is well known; most patients returning to work after a
lengthy period, while some are unable to work even after one year.
This fact produces physical and psychological disabilities for the
patient, as well as increased costs to society (8-10).
Bone healing is a biological and physiological
process controlled by biochemical, hormonal and mechanical factors.
The bone is a continuous evolving entity through remodeling. Bone
deposition and resorption is dependent on the blood supply, the
cells that promote healing and the stability of the fixation, in
case of fractures (11).
Autologous bone grafts were the first choice for
numerous years. In theory, they have the characteristics required
for bone healing and integration. However, the resources for bone
grafts are limited, and second interventions increase residual pain
for the patient (12-14).
In addition, due to the fact that bone grafting from
the iliac crest is a painful limited procedure with complications
ranging from nerve injury, infection, persistent pain to cosmetic
deformity, a safe and solid alternative was investigated (15).
In recent years, new materials have been developed
to replace natural bone. The widely used products were composed of
calcium phosphate ceramics and other biosynthetic composites. In
clinical trials, these types of grafts were revealed to be too weak
and with limited osteoinduction (16-20).
The calcaneus fracture occurs in one of the few
places in the human body where the stress shielding is limited at
low levels. Moreover, it requires at least 3 months to heal
properly, thus a slow resorption graft is required. All these
characteristics promote the use of magnesium (Mg) as a grafting
option.
Mg has acceptable mechanical properties and it is
one of the most important elements, stored in bones and essential
in metabolic processes. Daily doses of Mg are provided continuously
in regular food, in a sufficient amount that can stimulate bone
cell growth and can improve the healing time of the bone. Mg is
eliminated from the body in the presence of chlorine throughout the
kidneys (21,22).
Since massive Mg alloy grafts are not commonly used,
the calcaneus is in our opinion a perfect site for a structural
graft in a preclinical study. An anticipated successful result will
allow us to preserve a markedly favorable graft donor site for more
life threatening pathologies, including malignancies, and to reduce
morbidity of the lower foot. In the future, this type of synthetic
graft can be used for an increasing number of different
pathologies.
The data in specialty literature is vast, thus, the
most important Mg alloys are considered and reviewed for their
properties and a direct clinical future approach is focused on by
finding the appropriate design for the need of patients in this
preclinical study.
Materials and methods
From a therapeutic point of view, calcaneal
fractures represent a challenge. Most of the highly comminuted
injuries require bone grafts to restore Bohler's angle. This
variable represents the degree of inclination of the articular
surfaces of the calcaneus. A lateral approach is required to access
the smashed bone. After open reduction, a large ‘missing’ bone is
found. At this level, a graft is generally used to restore and hold
the height of the calcaneus and to correct varus deviation
(23). The need for using a plate
is frequent, fixing it with screws. This is, in our opinion, a
perfect place for the usage of a synthetic graft. There is no major
blood supply and the artificial metal graft does not come in
contact with the steel plate, thus avoiding the risk of metallosis.
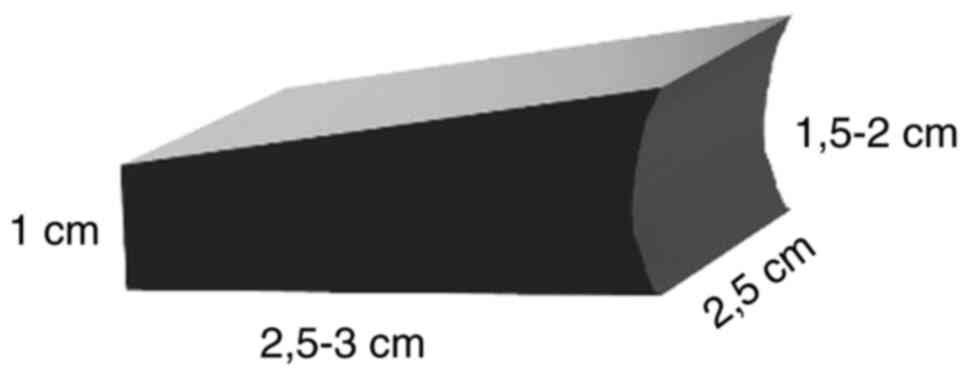
The graft is protected and is held in place by a proposed design
and hidden behind the lateral wall of the calcaneus. This design
for the grafts, presented in the following section, is reproducible
and can be used in this form and dimension to all Sanders 3 and 4
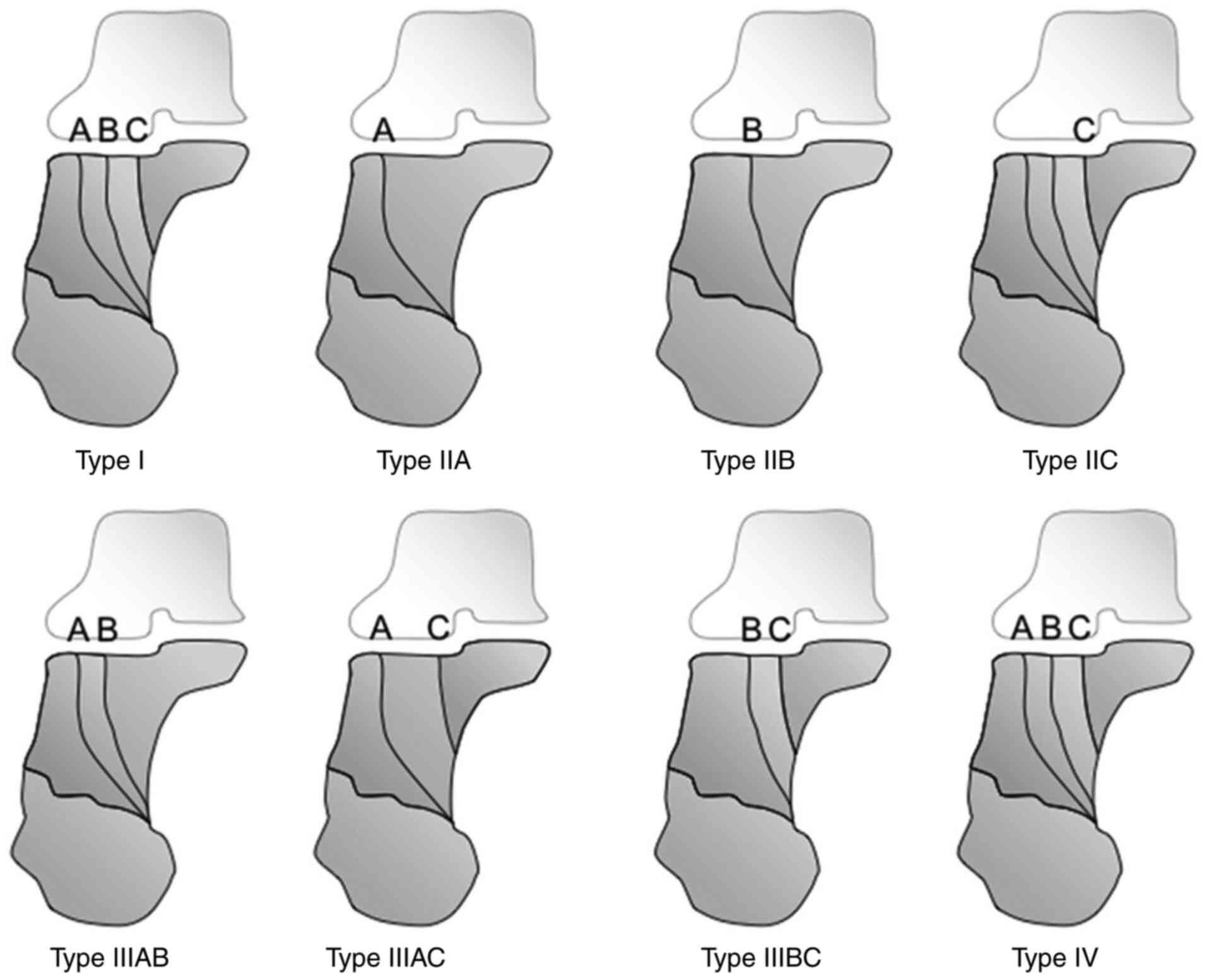
of the calcaneal fractures (Sanders classification) (Fig. 1) (24).
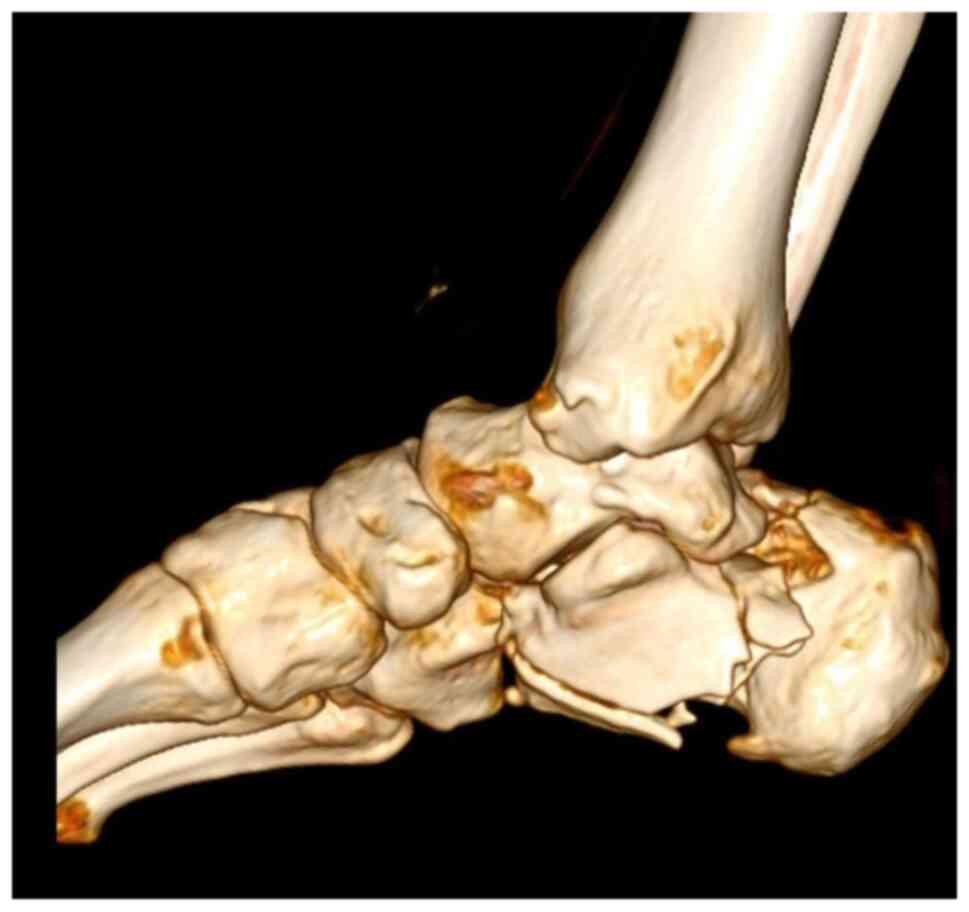
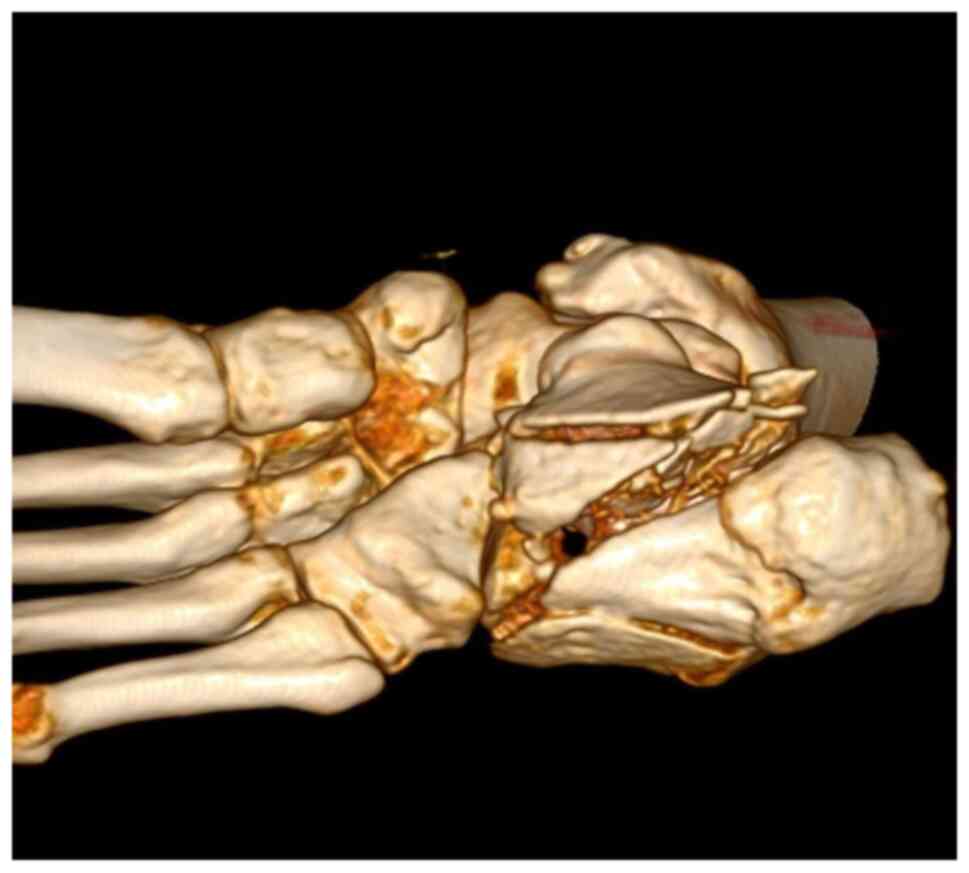
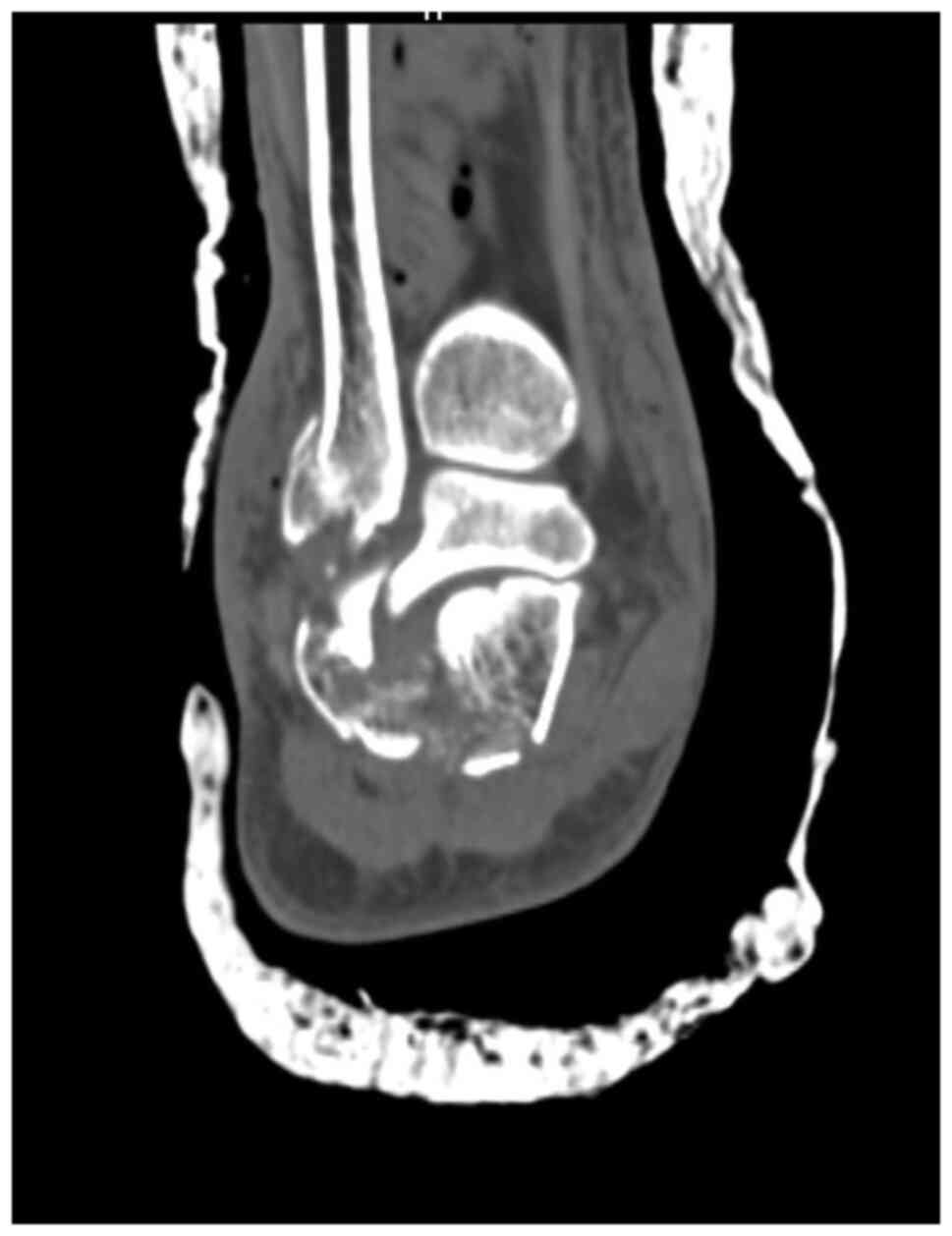
A CT scan with 3D reconstruction is absolutely
necessary in evaluating the degree of comminution and assessing the
decision to use a graft (Fig. 2,
Fig. 3 and Fig. 4). Thus, using these CT scans and
measurements, and taking into consideration that most patients with
comminuted calcaneal fractures are males, men between 30 to 60
years old and non-smokers, should be selected as the perfect
candidates, to prevent skin necrosis, for the next, clinical phase
of this study.
Mg alone has a high corrosion rate (25-30),
thus in theory, it could not be used in orthopedics. Nevertheless,
alloys have markedly improved characteristics including
biodegradation, resistance to fatigue, ductility or strength being
required as to be fully used in vivo.
In addition, Mg structures are potentially
compatible to the human body because they are osteoconductive and
biodegradable. The mechanical properties are similar to that of
bones and the most important aspect is the capacity to stimulate
the formation of new bone. However, the rapid degradation of this
material, faster than bone healing and its formation, can lead to
gas cavities, osteolysis and poor clinical results (25). Therefore, the appropriate type of Mg
alloy had to theoretically be identified for our application and
the optimal surface appearance of the design had to be decided.
Using CT reconstruction, multiple angle x-rays and our surgical
experience, a 3D model similar to the iliac graft was developed in
Windows 3D Viewer (Microsoft Corporation).
Previous research, in vitro, which suggested
the appearance of red blood cell lysis and decreased survival of
mesenchymal stromal cells, when using some Mg alloys, was
considered (26), because of the
tendency for tissue damage.
An attempt was also made to identify the
relationship between Mg alloy products and bacterial load, since
calcaneal fractures are prone to skin necrosis and septic
complications.
Results
In the first stage of finding the appropriate alloy,
the already commercially available products were identified and the
data from literature was used to include them in further tests.
After a close review of international prestigious publications
(21,22,25-45),
the mechanical properties of Mg1Ca and MgYREZr were selected and
compared to normal cortical and cancellous bone. For references,
Ti6Al4V and a markedly close to pure form of Mg were also assessed.
Results are presented in the Table
I.
 | Table IMechanical properties of test
prototypes and bone. |
Table I
Mechanical properties of test
prototypes and bone.
| Tissue | Tensile strength
(MPa) | Young's modulus
(GPa) | Density
(g/cm3) |
|---|
| Cortical Bone | 35-280 | 5-22 | 1.8-2.0 |
| Cancellous Bone | 1-40 | 0.01-1.58 | 1.0-1.4 |
| Pure Mg | 90 | 44 | 1.74 |
| MgYREZr | 250-280 | 44-46 | 1.84 |
| Mg1Ca | 75-240 | - | 1.73 |
| Ti6Al4V | 895-930 | 110-114 | 4.43 |
Clearly, from a mechanical point of view, the
synthetic graft needs to be as strong as the bone, in order to
maintain the correction of the Bohler's angle to a normal range of
20˚ to 40˚.
The fixation of the graft is not necessary because
it will be covered and kept in place by the superior calcaneus
fragments and the lateral wall. A plate is required to stabilize
the fragments. Since the construction of an Mg alloy plate is
beyond our capabilities at the moment, but may be attempted in a
future study, a 3D render with the design is presented in Fig. 5.
Due to the corrosion issue, a selenium-containing
coating using micro-arc oxidation was selected. Data from
literature clearly indicated that improved corrosion resistance was
achieved in this manner (27). In
theory, a minimum of a 3-month period of implant and reduction
stability is required until the external shell of the calcaneus is
healed. After this period, the full strength of the alloy graft is
not required anymore. Tensile strength is the main reason why
MgYREZr was selected, since Mg1Ca has a tensile strength as low as
75 MPa.
In addition, a small Mg alloy graft implanted in a
female mouse (4 months old; weight, 31 g; provided by the
Experimental Medicine Center of the Faculty of Veterinary Medicine
of Bucharest), that was already infected with Pseudomonas
for antibiotic testing, was also assessed. During the experiment
the mouse was maintained in a cage specially designed for this type
of species. The cage was placed in a controlled environment with a
10-h light/14-h dark cycle, a temperature between 18-22˚C and
40-50% humidity. Food and water were available ad libitum.
The use of the animal was approved by the Ethics Committee of the
Faculty of Veterinary Medicine of Bucharest (Bucharest, Romania).
No changes in response to the antibiotics were observed, however
this test was not sufficient in our opinion. Considering data from
literature, caution should be taken when affirming that Mg alloys
do not influence the antibacterial response of the biological
matter (28). Since calcaneal
fractures are prone to infections, the need for extra antibacterial
procedures and antibiotic prophylaxis is highlighted.
Discussion
AZ alloys have been recently tested, especially AZ31
(Mg3Al1Zn) and AZ91 (Mg9Al1Zn), in vitro and in vivo.
Both release large quantities of hydrogen on biodegradation in
organic matter, increasing the pH and Mg concentration (29-32).
In a small study, Witte et al determined that a Ca phosphate
coating could stimulate bone formation around the graft and
decrease absorption (33).
Magnesium alloys containing rare earth elements have
excellent corrosion resistance due to the formation of an oxide
film (rare earth oxide film) in a ‘humid’ environment. WE54 (4.85
Yttrium, 1.58 Neodymium, 0.28 Zirconium, 0.08 Cerium, 0.13
Gadolinium, 0.16 Erbium and Mg balanced) has an improved rate of
degradation in vitro than Mg alone if heat treatment is
applied. WE43 (4.16 Yttrium, 3.80 RE, 0.36 Zirconium, 0.20 Zinc,
and 0.13 Manganese) has good biocompatibility, but it increases
aluminum ions in the brain, increasing the risk of Alzheimer's
disease. Rare-earth elements are associated with severe
hepatotoxicity (34,35).
ZK40 (Mg4Zn0.5Zr) and ZK60 (Mg6Zn0.5Zr) have
excellent biocompatibility and small doses of daily zinc are safe
for humans, thus, Mg-Zinc alloys are better than alloys that
contain aluminum. However, these materials have high rates of
degradation and are not candidates for orthopedic use (36,37).
Medical Mg alloys, used before in orthopedic
surgeries include calcium, strontium, zinc or a combination of
rare-earth elements. Alloys containing Ca as a grain-refining
agent, and strontium (similar proprieties to calcium) are used to
increase the compatibility with the human bone combined with low
toxicity (38). Strontium alloys
are known to be the slowest in degrading. In data published, Zhao
et al (39) concluded that
Mg2Sr has the highest corrosion resistance with high tensile
strength and good compatibility (40).
Zinc is present in the human body, it has almost no
toxicity and it can be used to strengthen the Mg alloy through
solid solution hardening. Therefore, biocompatibility is excellent
(41).
Some elements in the Mg alloy implants form
second-phase particles which can precipitate in grains, conferring
better mechanical properties by strengthening the material
(42-44).
When selecting Mg alloy implants as bone grafts, the
stability and strength of second phases and the Mg matrix in
vivo conditions, can influence the degradation time and
biological response of the body concerning the implant. Yang et
al used the Dmol3 calculation method (44) for determining the stability of four
second-phase Mg-Ca, Mg-Sr, Mg-Zn-Zr, Mg-Al-Zn alloys, these having
higher phase stability than the Mg matrix. In the Mg-Al-Zn alloy,
the Mg17Al12 second phase had good
cytocompatibility in vitro and did not induce hemolysis
(42).
Also, not all alloys form second phase particles.
For example, in high quantities, Yttrium does not form second-phase
particles due to the high solid solubility in Mg, thus presenting
under the form of solid solutions and achieving solid solution
strengthening (41-43).
This solid solution strengthening affects also the degradation of
the Mg alloys improving the corrosion rate because of reducing the
internal galvanic corrosion between the second phase and Mg matrix
(45).
One important aspect in designing and proposing this
Mg alloy for medical use, is the problem of impurities. During
casting, impurities are introduced in the alloy, especially iron,
nickel and copper. They can influence the corrosion characteristics
of the product and can also be toxic for the human body. For
example, nickel is extremely toxic for the human body. Copper has a
high toxicity on the cell membrane. Therefore, when casting, molds
without such elements should be used (21,46,47).
Since a surgical approach to the calcaneus is prone
to skin complications and sometimes sepsis, antibiotic prophylaxis
should always be used. Vancomycin 1-1.5 g, one dose, 2 h before
surgery is recommend. After surgery, the lower limb should be
rested at a higher body level, in a fiber cast. Ice should be
applied daily to prevent hematoma (48).
Data in literature regarding any bond between Mg
implants and hematoma could not be found, but attention should also
be paid to the prevention of infections.
The goal of the present study was to determine a
close to perfect site location for artificial metal grafts. Since
the iliac crest is a markedly favorable graft, being used in
extremely complicated diseases and it exists in limited quantity,
it is considered that the novelty of using large magnesium alloy
implants is beneficial (11).
In conclusion, artificial materials are the future
in medicine replacing the body-limiting grafting capabilities. They
are safe and incur less comorbidities (49). This method could pave the way in
reducing the discomfort and increasing the satisfaction of
patients. Although further testing is required, this research
represents a great starting point (50).
Numerous aspects have to be refined until this
solution can be widely used. Our goal is not only patient
satisfaction, but also the decrease of hospital charges and
markedly faster rehabilitation. Patients can go back to work
faster, in theory, without extra risks associated. Another problem
that is often encountered is that a great number of patients are
not in favor of the bone grafting therapeutic option. A fixation
for this type of injury, without grafting is less stable and more
prone to failure and dissatisfaction (1,3,5). Using
an Mg alloy graft assists this issue and appears to be a safe
‘fix’.
In addition, in the case of failure, there is always
the option of second-stage bone grafting or talo-calcaneal
arthrodesis (51).
It is considered that the future of Mg alloys in
orthopedics and in general medicine is certain, but more studies
are required, for refining and testing and multi-disciplinary teams
should work together to discover and demonstrate the best
options.
The present study represents a starting point, by
finding a favorable biological site for Mg grafts. A suitable Mg
alloy was revealed, appropriate for our strategy. The present study
is not an ending, but a beginning.
Acknowledgements
Professional editing, linguistic and technical
assistance was performed by Irina Radu, Individual Service
Provider, certified translator in Medicine and Pharmacy
(certificate credentials: Series E no. 0048).
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Author's contributions
SD planned the clinical study, contributed to the
conception and design of the study, and the acquisition, analysis
and interpretation of the data. DCC contributed to the analysis and
interpretation of the data and the critical revision for important
intellectual content. CDMD contributed to the conception and design
of the study, analysis of data, the drafting of the manuscript and
its critical revision for important intellectual content. CS
contributed to the conception and design of the study and the
critical revision of the manuscript for important intellectual
content. CIS contributed to the conception and design of the study,
the interpretation of the data and the critical revision of the
manuscript for important intellectual content. All authors read and
approved the final version of the manuscript and agree to be
accountable for all aspects of the study.
Ethics approval and consent to
participate
The study on the one mouse used was approved by the
Ethics Committee from the Faculty of Veterinary Medicine in
Bucharest (Bucharest, Romania) with respect to the current
legislation regarding the protection of laboratory animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Schepers T, van Lieshout EM, van Ginhoven
TM, Heetveld MJ and Patka P: Current concepts in the treatment of
intra-articular calcaneal fractures: Results of a nationwide
survey. Int Orthop. 32:711–715. 2008.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Atkins RM, Allen PE and Livingstone JA:
Demographic features of intra-articular fractures of the calcaneum.
Foot Ankle Surg. 7:77–84. 2001.
|
|
3
|
Soeur R and Remy R: Fractures of the
calcaneus with displacement of the thalamic portion. J Bone Joint
Surg Br. 57:413–421. 1975.PubMed/NCBI
|
|
4
|
Slatis P, Kiviluoto O, Santavirta S and
Laasonen EM: Fractures of the calcaneum. J Trauma. 19:939–943.
1979.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Buckley R, Tough S, McCormack R, Pate G,
Leighton R, Petrie D and Galpin R: Operative compared with
nonoperative treatment of displaced intra-articular calcaneal
fractures: A prospective, randomized, controlled multicenter trial.
J Bone Joint Surg Am. 84:1733–1744. 2002.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Essex-Lopresti P: The mechanism, reduction
technique, and results in fractures of the os calcis. Br J Surg.
39:395–419. 1952.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Rak V, Ira D and Masek M: Operative
treatment of intra-articular calcaneal fractures with calcaneal
plates and its complications. Indian J Orthop. 43:271–280.
2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Bajammal S, Tornetta P III, Sanders D and
Bhandari M: Displaced intra-articular calcaneal fractures. J Orthop
Trauma. 19:360–364. 2005.PubMed/NCBI
|
|
9
|
Brauer CA, Manns BJ, Ko M, Donaldson C and
Buckley R: An economic evaluation of operative compared with
nonoperative management of displaced intra-articular calcaneal
fractures. J Bone Joint Surg Am. 87:2741–2749. 2005.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Pozo JL, Kirwan EO and Jackson AM: The
long-term results of conservative management of severely displaced
fractures of the calcaneus. J Bone Joint Surg Br. 66:386–390.
1984.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Stark JG: Use of selective estrogen
receptor modulator for joint fusion and other healing of connective
tissue. US Patent 8,501,690 B2, Filed April 30, 2010; issued August
6, 2013.
|
|
12
|
Liu C, Wan P, Tan LL, Wang K and Yang K:
Preclinical investigation of an innovative magnesium-based bone
graft substitute for potential orthopaedic applications. J Orthop
Translat. 2:139–148. 2014.
|
|
13
|
Calori GM, Mazza E, Colombo M and
Ripamonti C: The use of bone-graft substitutes in large bone
defects: Any specific needs? Injury. 42 (Suppl 2):S56–S63.
2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Van der Stok J, Van Lieshout EM,
El-Massoudi Y, Van Kralingen GH and Patka P: Bone substitutes in
the Netherlands-a systematic literature review. Acta Biomater.
7:739–750. 2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Almaiman M, Al-Bargi HH and Manson P:
Complication of anterior iliac bone graft harvesting in 372 adult
patients from May 2006 to May 2011 and a literature review.
Craniomaxillofac Trauma Reconstr. 6:257–266. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Böstman O and Pihlajamäki H: Clinical
biocompatibility of biodegradable orthopaedic implants for internal
fixation: A review. Biomaterials. 21:2615–2621. 2000.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Auer JA, Rechenberg BV, Bohner M and
Hofmann-Amtenbrink M: Bone grafts and bone replacements. Equine
Surg. 2012:1081–1096. 2012.
|
|
18
|
Dias AG, Lopes MA, Gibson IR and Santos
JD: In vitro degradation studies of calcium phosphate glass
ceramics prepared by controlled crystallization. J Non Cryst
Solids. 330:81–89. 2003.
|
|
19
|
Kim HM: Ceramic bioactivity and related
biomimetic strategy. Curr Opin Solid State Materials Sci.
7:289–299. 2003.
|
|
20
|
Giannoudis PV, Dinopoulos H and Tsiridis
E: Bone substitutes: An update. Injury. 36 (Suppl 3):S20–S27.
2005.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Waizy H, Seitz JM, Reifenrath J, Weizbauer
A, Bach FW, Meyer-Lindenberg A, Denkena B and Windhagen H:
Biodegradable magnesium implants for orthopedic applications. J
Mater Sci. 48:39–50. 2013.
|
|
22
|
Seal CK, Vince K and Hodgson MA:
Biodegradable surgical implants based on magnesium alloys-A review
of current research. IOP Conf Ser: Mater Sci Eng: 4, 2009.
|
|
23
|
Su Y, Chen W, Zhang T, Wu X, Wu Z and
Zhang Y: Bohler's angle's role in assessing the injury severity and
functional outcome of internal fixation for displaced
intra-articular calcaneal fractures: A retrospective study. BMC
Surg. 13(40)2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Sanders R, Fortin P, DiPasquale T and
Walling A: Operative treatment in 120 displaced in-traarticular
calcaneal fractures. Results using a prognostic computed
Tomogra-phy scan classification. Clin Orthop Relat Res. 290:87–95.
1993.PubMed/NCBI
|
|
25
|
Kuhlmann J, Bartsch I, Willbold E,
Schuchardt S, Holz O, Hort N, Höche D, Heineman WR and Witte F:
Fast escape of hydrogen from gas cavities around corroding
magnesium implants. Acta Biomaterialia. 9:8714–8721.
2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lukyanova E, Yu N, Yu AN, Martynenko AN,
Martynenko N and Estrin Y: Features of in vitro and in vivo
behaviour of magnesium alloy WE43. Materials Lett. 215:308–311.
2018.
|
|
27
|
Xiaoting S, Yuanyuan Z, Shufang Z,
Rongfang Z, Rongfa Z, Chen L and Yijia Z: Characteristics of
selenium-containing coatings on WE43 magnesium alloy by micro-arc
oxidation. Mater Lett. 261(126944)2020.
|
|
28
|
Rahim MI, Ullah S and Mueller PP: Advances
and challenges of biodegradable implant materials with a focus on
magnesium-alloys and bacterial infections. Metals. 8(532)2018.
|
|
29
|
Ding Y, Wen C, Hodgson P and Li Y: Effects
of alloying elements on the corrosion behavior and biocompatibility
of biodegradable magnesium alloys: A review. J Mater Chem B.
2:1912–1933. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wang H and Shi Z: In vitro biodegradation
behavior of magnesium and magnesium alloy. J Biomed Mater Res Part
B Appl Biomater. 98:203–209. 2011.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Song Y, Shan D, Chen R, Zhang F and Han
EH: Biodegradable behaviors of AZ31 magnesium alloy in simulated
body fluid. Mater Sci Eng C. 29:1039–1045. 2009.
|
|
32
|
Yan T, Tan L, Xiong D, Liu X, Zhang B and
Yang K: Fluoride treatment and in vitro corrosion behavior of an
AZ31B magnesium alloy. Mater Sci Eng C. 30:740–748. 2010.
|
|
33
|
Witte F, Fischer J, Nellesen J, Crostack
HA, Kaese V, Pisch A, Beckmann F and Windhagen H: In vitro and in
vivo corrosion measurements of magnesium alloys. Biomaterials.
27:1013–1018. 2006.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Witte F, Kaese V, Haferkamp H, Switzer E,
Meyer-Lindenberg A, Wirth CJ and Windhagen H: In vivo corrosion of
four magnesium alloys and the associated bone response.
Biomaterials. 26:3557–3563. 2005.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Tan L, Yu X, Wan P and Yang K:
Biodegradable materials for bone repairs: A review. J Mater Sci
Technol. 29:503–513. 2013.
|
|
36
|
Hong D, Saha P, Chou DT, Lee B, Collins
BE, Tan Z, Dong Z and Kumta PN: In vitro degradation and
cytotoxicity response of Mg-4% Zn-0.5% Zr (ZK40) alloy as a
potential biodegradable material. Acta Biomater. 9:8534–8547.
2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Gu XN, Li N, Zheng YF and Ruan L: In vitro
degradation performance and biological response of a Mg-Zn-Zr
alloy. Mater Sci Eng B. 176:1778–1784. 2011.
|
|
38
|
Li Z, Gu X, Lou S and Zheng Y: The
development of binary Mg-Ca alloys for use as biodegradable
materials within bone. Biomaterials. 29:1329–1344. 2008.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Zhao C, Pan F, Zhang L, Pan H, Song K and
Tang A: Microstructure, mechanical properties, bio-corrosion
properties and cytotoxicity of as-extruded Mg-Sr alloys. Mater Sci
Eng C Mater Biol Appl. 70:1081–1088. 2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Kannan MB: Influence of microstructure on
the in-vitro degradation behaviour of magnesium alloys. Mater Lett.
64:739–742. 2010.
|
|
41
|
Zhang S, Zhang X, Zhao C, Li J, Song Y,
Xie C, Tao H, Zhang Y, He Y, Jiang Y and Bian Y: Research on an
Mg-Zn alloy as a degradable biomaterial. Acta Biomater. 6:626–640.
2010.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Liu C, He P, Wan P, Li M, Wang K, Tan L,
Zhang Y and Yang K: The in vitro biocompatibility and macrophage
phagocytosis of Mg17Al12 phase in Mg-Al-Zn alloys. J Biomed Mater
Res A. 103:2405–2415. 2015.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Gao L, Chen RS and Han EH: Solid solution
strengthening behaviors in binary Mg-Y single phase alloys. J
Alloys Compd. 472:234–240. 2009.
|
|
44
|
Yang H, Liu C, Wan P, Tan L and Yang K:
Study of second phase in bioabsorbable magnesium alloys: Phase
stability evaluation via Dmol3 calculation. APL
Materials: Nov 8, 2013 (Epub ahead of print) doi:
org/10.1063/1.4828935.
|
|
45
|
Zhang X, Zhang K, Li X, Deng X, LI Y, Ma M
and Shi Y: Effect of solid-solution treatment on corrosion and
electrochemical behaviors of Mg-15Y alloy in 3.5 wt.% NaCl
solution. J Rare Earths. 30:1158–1167. 2012.
|
|
46
|
Südholz AD, Kirkland NT, Buchheit RG and
Birbilis N: Electrochemical properties of intermetallic phases and
common impurity elements in magnesium alloys. Electrochem Solid St
Lett. 14(C57)2011.
|
|
47
|
Hofstetter J, Martinelli E, Pogatscher S,
Schmutz P, Povoden-Karadeniz E, Weinberg AM, Uggowitzer PJ and
Löffler JF: Influence of trace impurities on the in vitro and in
vivo degradation of biodegradable Mg-5Zn-0.3Ca alloys. Acta
Biomater. 23:347–353. 2015.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Crawford T, Rodvold KA and Solomkin JS:
Vancomycin for surgical prophylaxis? Clin Infect Dis. 54:1474–1479.
2012.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Chen L, Peng W, Li LT, Kehong W and Ke Y:
Preclinical investigation of an innovative magnesium-based bone
graft substitute for potential orthopaedic applications. J Orthop
Translat. 2:139–148. 2014.
|
|
50
|
Sommar P, Granberg Y, Halle M, Skogh AC,
Lundgren KT and Jansson KÅ: Effects of a formalized collaboration
between plastic and orthopedic surgeons in severe extremity trauma
patients; a retrospective study. J Trauma Manag Outcomes.
9(3)2015.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Buch BD, Myerson MS and Miller SD: Primary
subtalar arthrodesis for the treatment of comminuted calcaneal
fractures. Foot Ankle Int. 17:61–70. 1996.PubMed/NCBI View Article : Google Scholar
|