Introduction
Liver cancer is the sixth most common type of cancer
and the fourth leading cause of cancer-associated mortality
worldwide, accounting for ~841,000 newly diagnosed cases and
782,000 deaths annually (1).
Notably, China accounts for ~1/2 of all cases and deaths (2). In the United States, the morbidity and
mortality of liver cancer is growing at a faster rate compared with
any other type of cancer (3,4).
Surgical resection remains the first-choice treatment option for
early-stage patients (5); however,
a 5-year recurrence rate as high as 70% has been reported following
liver cancer resection (6,7), and there is currently no adjuvant
therapy available for patients to prevent or reduce postoperative
metastases and the formation of de novo tumors (8). Thus, the prognosis and survival
outcomes of patients following curative liver cancer resection
remain unsatisfactory. It has been reported that cellular and
molecular events involved in tumor metastasis may be notably
affected during or immediately following surgery (9). However, it has been suggested that
during curative resection, the surgery-induced stress response and
immunosuppression, in addition to the weakened immune function
following surgery, may activate a series of events involved in the
metastasis of tumors (10-12).
In addition, operative manipulation itself has also been suggested
to promote the dissemination of cancer cells (12). Therefore, the perioperative period
has been suggested as a window of opportunity to effectively reduce
cancer metastasis and recurrence.
Propofol is an anesthetic that has been widely used
in the clinic. Recently, the non-anesthetic properties of propofol
(13,14), such as its immunomodulatory,
neuroprotective, antioxidant and anticancer effects, have been
investigated in more detail. Previous studies in multiple cancer
types, including liver (15,16),
lung (17), breast (18), colorectal (19), gastric (20), prostate (21) and pancreatic cancer (22), have reported that propofol exerts
direct anticancer effects by interfering with the biological
functions of cancer cells through a variety of mechanisms to
inhibit tumor proliferation, invasion and metastasis and promote
apoptosis. In addition, propofol may also induce indirect effects
by affecting the functions of immune cells (23). However, the studies investigating
the role of propofol in cancer have reported conflicting results.
For example, Zhang et al (24) reported that propofol exerts
oncogenic activity by activating NF-E2-related factor 2 (Nrf2),
which leads to an increase in the proliferation and invasion of
gallbladder cancer cells. In another study by Ecimovic et al
(25), the results revealed that
propofol had no effect on the proliferation of breast cancer cells.
Thus, the association between propofol and cancer progression
requires further investigation.
Solid tumor cells usually exist within a hypoxic
microenvironment, which is caused by the imbalance of oxygen supply
and demand (26). Tumor cells in
the hypoxic environment have been observed to undergo a series of
adaptive changes, in which the activation of the hypoxia inducible
factor-1α (HIF-1α) pathway plays an important role. HIF-1α is
rapidly degraded under normoxia, but it can accumulate and be
activated under hypoxic conditions (27). The cobalt ion in cobalt chloride
(CoCl2) can replace Fe2+ on the active site
of prolyl hydroxylase (PHD), which is a key enzyme required for the
degradation of HIF-1α under normoxic conditions. This blocks the
activity of PHD and stabilizes HIF-1α, which subsequently
upregulates the protein levels of HIF-1α and thereby induces its
transcriptional activity (28).
Therefore, CoCl2 was used as a chemical hypoxia simulant
in the present study.
The present study aimed to determine the effects of
propofol on the viability, proliferation and migration of HepG2 and
HCCLM3 cells under normoxia and CoCl2-induced hypoxia
in vitro.
Materials and methods
Cell culture and treatment
HCCLM3 and HepG2 cells (Kunming Cell Bank of Type
Culture Collection) were cultured in DMEM (cat. no. C11995500BT;
Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS
(cat. no. 10099141; Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin-streptomycin (cat. no. P1400; Beijing Solarbio Science
& Technology, Co., Ltd.). Both cells were maintained in an
incubator with 5% CO2 at 37˚C, and routinely digested
with 0.25% Trypsin-EDTA (cat. no. 25200072; Gibco; Thermo Fisher
Scientific, Inc.) for subculture upon reaching 80-90%
confluence.
The plasma concentration of propofol required for
sedation in the intensive care unit is ~2 µg/ml (11.2 µM) (29) and 3-8 µg/ml (16.8-44.8 µM) for
general anesthesia (30).
Therefore, the present study used a range of concentrations of
propofol (10, 25, 50 and 100 µM). Under normoxia, HepG2 and HCCLM3
cells were randomly divided into six groups: i) Control group
(untreated cells); ii) 10 µM propofol (cat. no. Y0000016;
Sigma-Aldrich; Merck KGaA) group; iii) 25 µM propofol group; iv) 50
µM propofol group; v) 100 µM propofol group; and vi) DMSO (cat. no.
D2650; Sigma-Aldrich; Merck KGaA) group. To simulate hypoxia, HepG2
cells were treated with 200 µM CoCl2 (cat. no. C8661;
Sigma-Aldrich; Merck KGaA) or different concentrations of propofol
(10, 25, 50 or 100 µM) in the presence of 200 µM CoCl2
for 12 h. Propofol was dissolved in DMSO, and the final
concentration of DMSO in both the DMSO and propofol groups was
0.05%.
Cell Counting Kit-8 (CCK-8) assay
The viability of HepG2 and HCCLM3 cells was analyzed
using a CCK-8 assay (cat. no. CK04; Dojindo Molecular Laboratories,
Inc.). Briefly, cells were seeded into 96-well plates at a density
of 6x103 cells/well (for 24 h propofol stimulation) or
4x103 cells/well (for 48 h propofol stimulation). Under
normoxia, the cells were treated with different concentrations of
propofol or 0.05% DMSO for 24 or 48 h. For hypoxia simulation,
cells were exposed to propofol in the presence of 200 µM
CoCl2 for 12 h. Following the incubation, 10 µl CCK-8
solution was added into each well and incubated for a further 1 h.
The absorbance of each well was measured at a wavelength of 450 nm
using a microplate reader (Epoch2NS; BioTek Instruments, Inc.).
5-ethynyl-2'-deoxyuridine (EdU)
assay
Cell proliferation was analyzed using a Click-iT™
EdU Imaging kit (cat. no. C10337; Invitrogen; Thermo Fisher
Scientific, Inc.). Briefly, cells were cultured at a density of
5x104 cells/well (for 24 h propofol stimulation) or
3x104 cells/well (for 48 h propofol stimulation) in
24-well plates for 24 h and then treated with different
concentrations of propofol or 0.05% DMSO for 24 or 48 h. Following
the incubation, the cells were labeled for 2 h with 10 µM EdU
solution and then fixed with 4% paraformaldehyde (Shanghai Lingfeng
Chemical Reagent Co., Ltd.) for 15 min at room temperature. Cells
were permeabilized with 0.5% Triton X-100 (cat. no. 0694; Beijing
Solarbio Science & Technology Co., Ltd.) for 20 min.
EdU-incorporated cells and all cells were stained with Alexa Fluor
fluorescent dye and Hoechst 33342 solution, respectively, for 30
min at room temperature, away from light. Subsequently, stained
cells were visualized in five randomly selected fields of view
using a fluorescence microscope (cat. no. DM5500B; Leica
Microsystems GmbH), and the percentage of EdU-positive cells was
calculated. Analysis was performed using ImageJ version 1.8.0
software (National Institutes of Health).
Wound healing assay
A total of 1x106 cells were seeded into
each well of six-well plates and cultured for 24 h. Upon cells
reaching 100% confluence, an artificial scratch was made in the
middle of the cell monolayer using a 200-µl pipette tip. The cell
medium was subsequently removed and replaced with fresh DMEM
containing 10, 25, 50 or 100 µM propofol or 0.05% DMSO under
normoxia. Cells under hypoxia were treated with propofol combined
with 200 µM CoCl2. The wound closure was observed and
images were captured using an inverted microscope (TS100-F; Nikon
Corporation) at 0 and 24 h. Analysis was performed using ImageJ
version 1.8.0 software.
Transwell migration assay
Analysis of cell migration was conducted using a
24-well Transwell chamber (pore size, 8-µm; cat. no. 3422; Corning,
Inc.). Serum-starved HCCLM3 cells (5x104 cells)
suspended in 200 µl serum-free DMEM containing 10, 25, 50 or 100 µM
propofol or 0.05% DMSO were plated into the upper chamber. The
lower chamber was filled with 600 µl complete medium supplemented
with 20% FBS (cat. no. 10099141; Gibco; Thermo Fisher Scientific,
Inc.). Following 48 h of incubation at 37˚C, the migratory cells in
the lower chamber were fixed with 4% paraformaldehyde for 30 min at
room temperature and stained with crystal violet solution (cat. no.
C0121; Beyotime Institute of Biotechnology) for 30 min at room
temperature. The non-migratory cells remaining in the upper chamber
were gently removed with a cotton swab. The number of migratory
cells was counted in five randomly selected fields of view using an
inverted microscope (TS100-F; Nikon Corporation) at a high
magnification (x400). Analysis was performed using ImageJ version
1.8.0 software.
Western blotting
HepG2 cells treated with 100, 200 or 300 µM
CoCl2 for 4, 8, 12 or 24 h were lysed on ice with RIPA
lysis buffer (cat. no. P0013B; Beyotime Institute of Biotechnology)
supplemented with 100 mM phenylmethanesulfonyl fluoride (1%; cat.
no. ST506; Beyotime Institute of Biotechnology) and 1% protein
phosphatase inhibitor (cat. no. P1260; Applygen Technologies,
Inc.). The samples were centrifuged at 12,000 x g for 20 min in a
centrifuge (cat. no. 5417R; Eppendorf) at 4˚C and the supernatants
were collected. The protein concentration was quantified using an
enhanced BCA protein assay kit (cat. no. P0010; Beyotime Institute
of Biotechnology), according to the manufacturer's protocol, and 30
µg protein/lane was separated via 10% SDS-PAGE. The separated
proteins were subsequently transferred onto polyvinylidene fluoride
membranes (cat. no. IPVH00010; EMD Millipore) and blocked with 10%
non-fat milk for 2 h at room temperature. The membranes were then
incubated overnight with the following primary antibodies on a
shaker at 4˚C: Anti-HIF-1α (rabbit; 1:1,000; cat. no. ab51608;
Abcam) and anti-β-tubulin (rabbit; 1:1,000; cat. no. 10068-1-AP;
ProteinTech Group, Inc.). Following the primary antibody
incubation, the membranes were incubated with a horseradish
peroxidase-conjugated goat anti-rabbit IgG secondary antibody
(1:3,000; cat. no. SA00001-2; ProteinTech Group, Inc.) for 1 h at
room temperature. Protein bands were visualized using enhanced
chemiluminescence western blotting substrates (cat. no.
K-12045-D20; Advansta, Inc.). Densitometric analysis was performed
using ImageJ version 1.8.0 software.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism version 5.0 software (GraphPad Software, Inc.). Data of three
of more experimental repeats are presented as the mean ± SD. Data
that were normally distributed and had an equal variance were
analyzed using a one-way ANOVA, after which a Tukey's test was
performed for multiple comparisons. Otherwise, Kruskal-Wallis test
was used, followed by Dunn's multiple comparison test. P<0.05
was considered to indicate a statistically significant
difference.
Results
Propofol exerts no effect on the
viability of HepG2 and HCCLM3 cells under normoxia
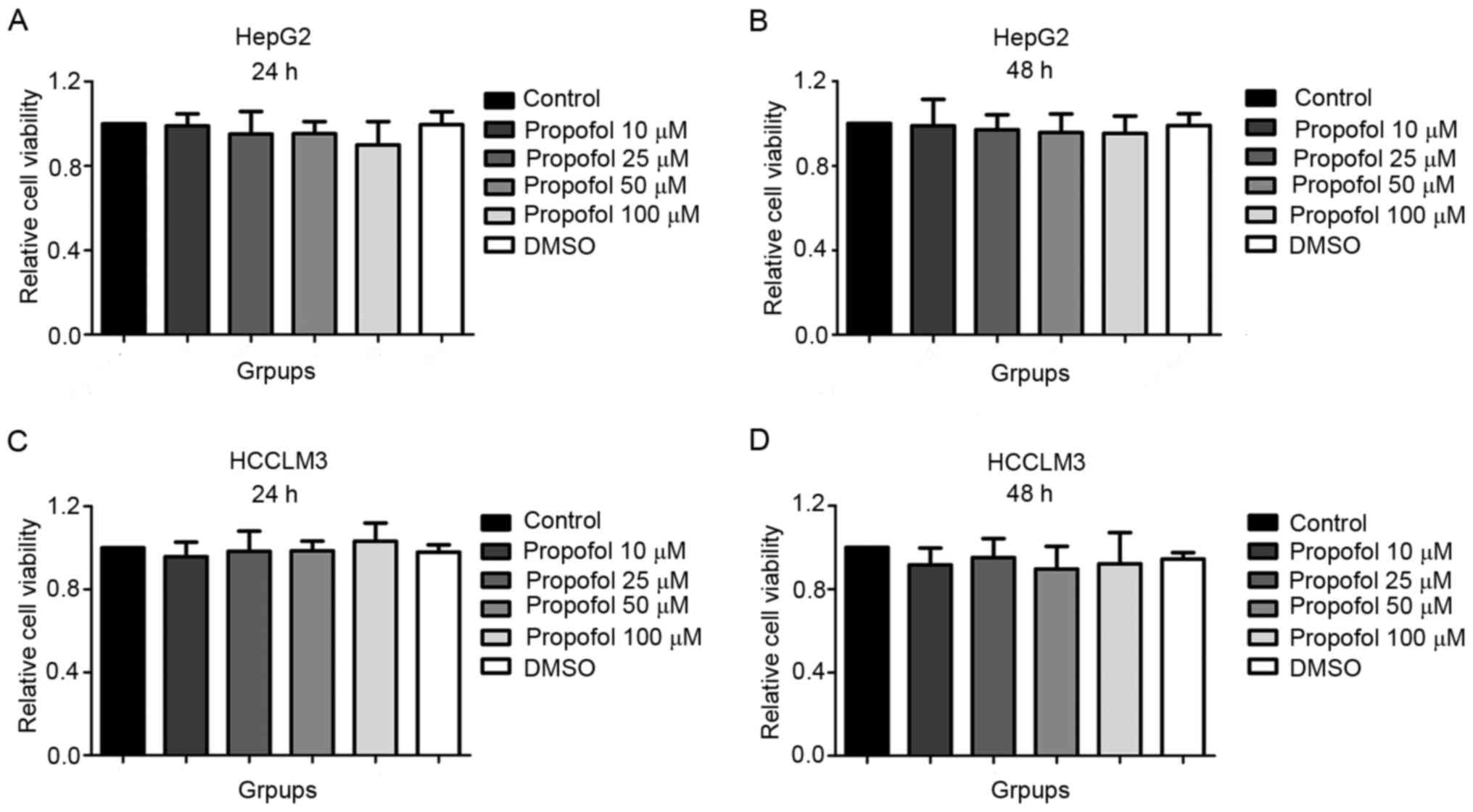
The results of the CCK-8 assay revealed that
compared with the control (1.00±0.00) and DMSO (0.99±0.06) groups,
the viability of HepG2 cells stimulated with 10 (0.99±0.06), 25
(0.95±0.11), 50 (0.95±0.16) or 100 µM (0.90±0.11) propofol for 24 h
was not significantly different (Fig.
1A). Similar findings were observed in HepG2 cells following 48
h of propofol stimulation (control, 1.00±0.00; DMSO, 0.99±0.06; 10
µM propofol, 0.99±0.12; 25 µM propofol, 0.97±0.07; 50 µM propofol,
0.96±0.09; 100 µM propofol, 0.95±0.08; Fig. 1B), in HCCLM3 cells following 24 h of
propofol stimulation (control, 1.00±0.00; DMSO, 0.98±0.04; 10 µM
propofol, 0.96±0.07; 25 µM propofol, 0.98±0.10; 50 µM propofol,
0.98±0.05; 100 µM propofol, 1.03±0.09; Fig. 1C) and in HCCLM3 cells following 48 h
of propofol stimulation (control, 1.00±0.00; DMSO, 0.94±0.03; 10 µM
propofol, 0.92±0.08; 25 µM propofol, 0.95±0.09; 50 µM propofol,
0.90±0.11; 100 µM propofol, 0.92±0.15; Fig. 1D). In addition, no statistically
significant differences in cell viability were observed between the
DMSO and control groups. The above results suggested that propofol
exerted no effect on the viability of HepG2 and HCCLM3 cells under
normoxic conditions.
Propofol exerts no effect on the
proliferation of HepG2 and HCCLM3 cells under normoxia
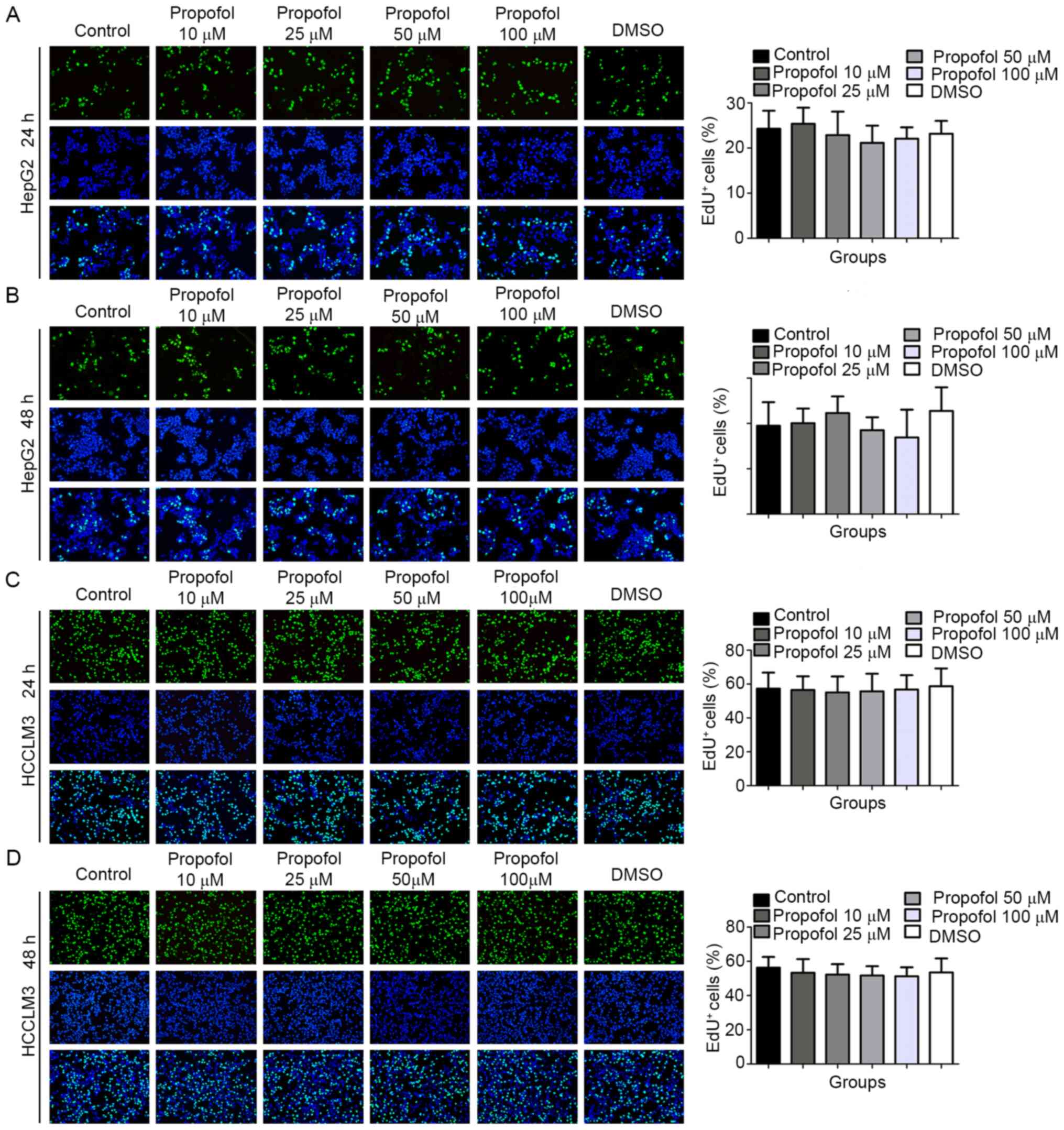
The results of the EdU assay demonstrated that,
compared with the control (24.26±4.01) and DMSO (23.17±2.84)
groups, the percentage of EdU+ HepG2 cells following 10
(25.37±3.56), 25 (22.87±5.19), 50 (21.15±3.82) or 100 µM
(22.05±2.54) propofol stimulation for 24 h was not statistically
significantly different (Fig. 2A).
Similar trends were observed in HepG2 cells following 48 h of
propofol stimulation (control, 19.48±5.21; DMSO, 22.75±5.22; 10 µM
propofol, 20.07±3.23; 25 µM propofol, 22.29±3.67; 50 µM propofol
18.52±2.85; 100 µM propofol, 16.91±6.13; Fig. 2B), in HCCLM3 cells following 24 h of
propofol stimulation (control, 57.31±9.45; DMSO, 58.69±10.54; 10 µM
propofol, 56.47±8.12; 25 µM propofol, 55.05±9.42; 50 µM propofol,
55.72±10.38; 100 µM propofol, 56.77±8.49; Fig. 2C) and in HCCLM3 cells following 48 h
of propofol stimulation (control, 56.30±6.29; DMSO, 53.51±8.21; 10
µM propofol, 53.31±7.98; 25 µM propofol, 52.26±6.11; 50 µM
propofol, 51.69±5.45; 100 µM propofol, 51.25±5.25; Fig. 2D). The above results indicated that
propofol exerted no effect on the proliferation of HepG2 and HCCLM3
cells under normoxic conditions.
Propofol exerts no effect on the
migration of HCCLM3 cells under normoxia
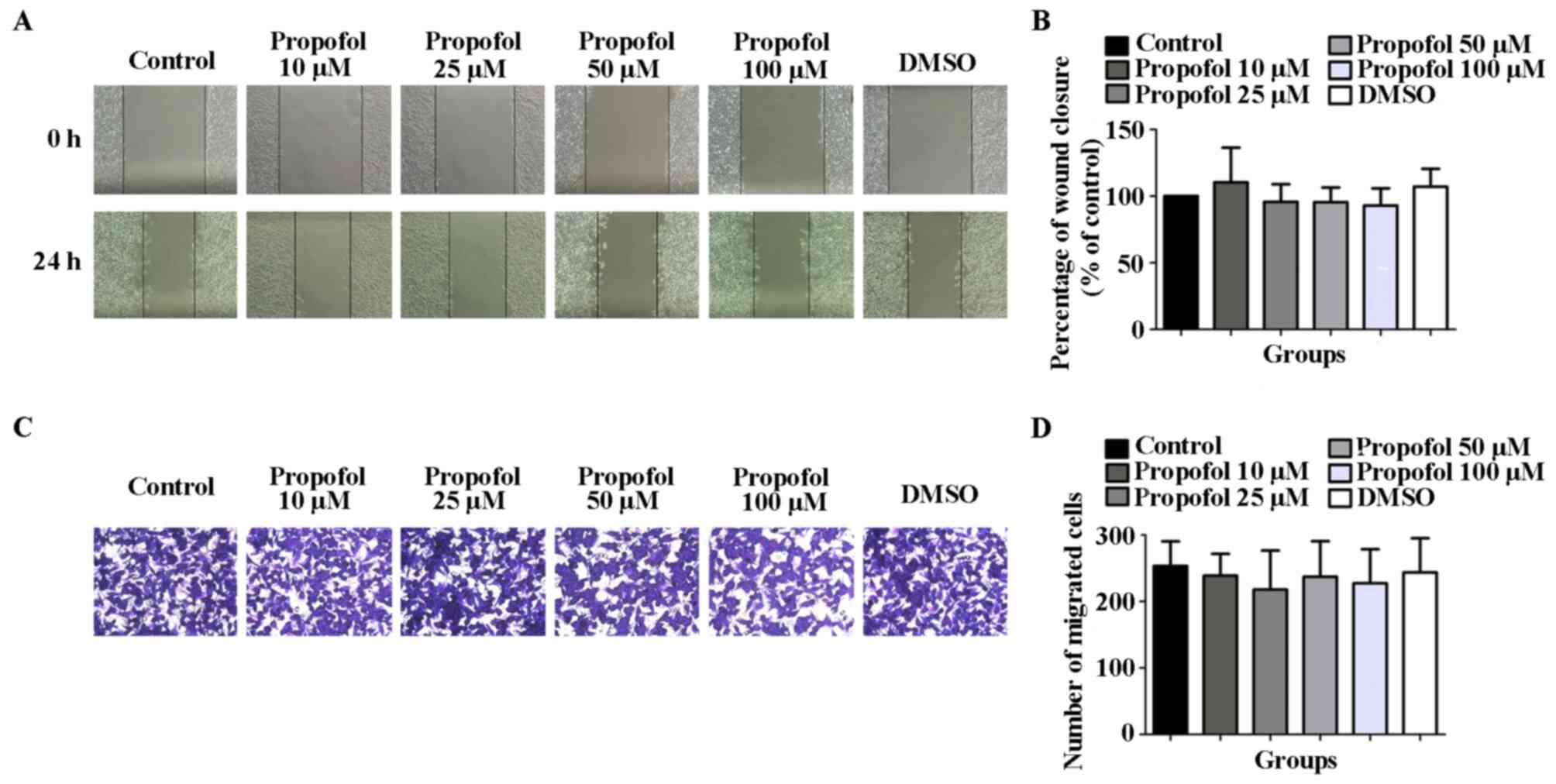
The results of the wound healing and Transwell
migration assays revealed that, compared with the control
(100.0±0.0) and DMSO (107.1±13.37) groups, the wound closure
percentage of HCCLM3 cells stimulated with 10 (110.4±25.97), 25
(95.68±13.30), 50 (95.49±10.91) or 100 (92.92±12.83) µM propofol
for 24 h was not statistically significantly different (Fig. 3A and B). Similarly, the number of migratory
HCCLM3 cells in the propofol groups [10 (239.0±32.72), 25
(217.8±58.77), 50 (237.2±53.40) or 100 (227.5±51.11) µM] following
48 h of stimulation was also not statistically significantly
different compared with the control (253.3±36.97) and DMSO
(243.6±51.54) groups (Fig. 3C and
D). The results suggested that
propofol exerted no effect on the migration of HCCLM3 cells under
normoxic conditions.
Propofol exerts no effect on the
viability and migration of HepG2 cells under
CoCl2-induced hypoxia
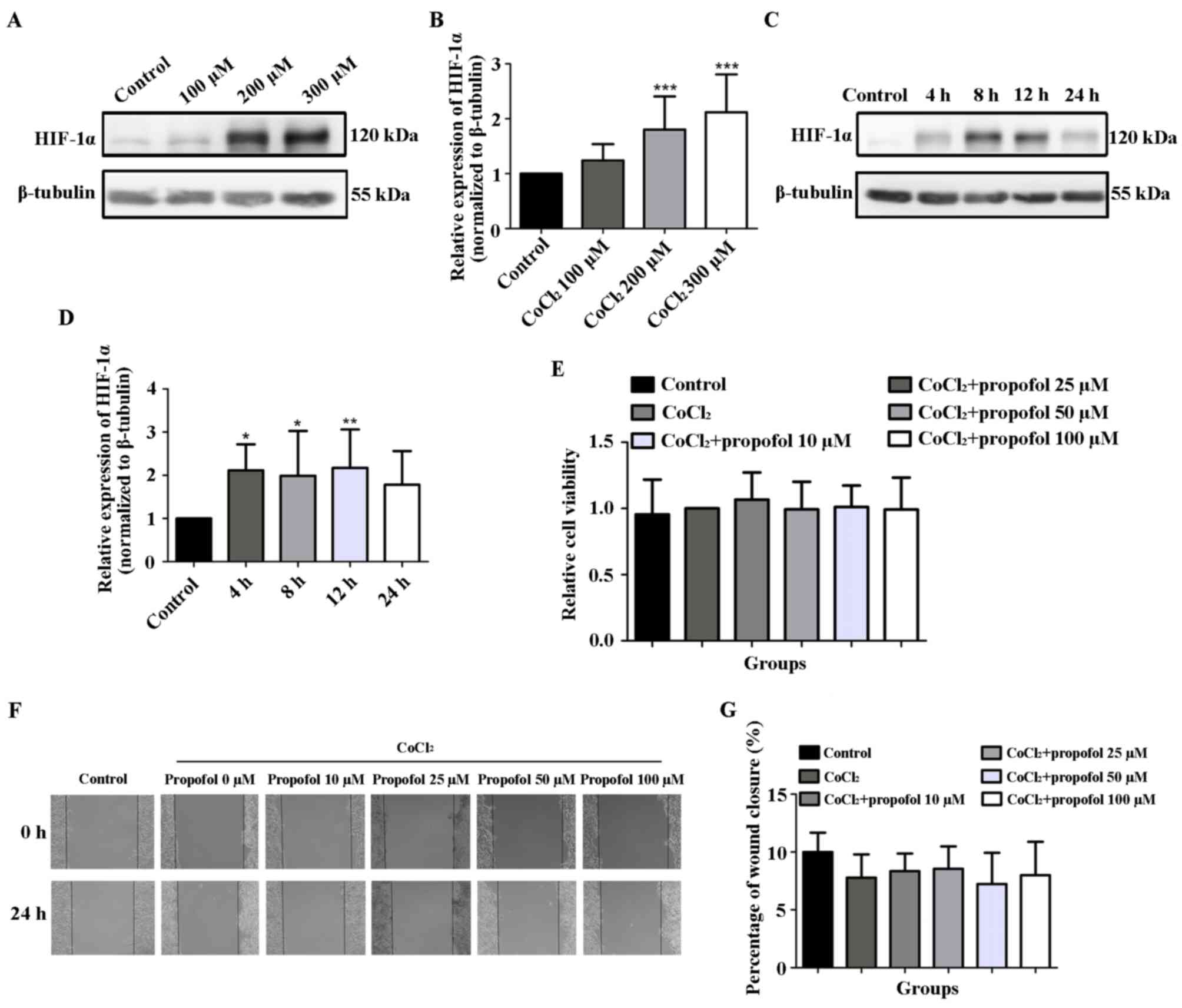
The results of the western blotting analysis
indicated that, compared with the control group, the protein levels
of HIF-1α in HepG2 cells treated with 200 and 300 µM
CoCl2 for 24 h were significantly upregulated (1.00±0.00
vs. 1.80±0.60 and 2.12±0.69, respectively; P<0.001; Fig. 4A and B). In addition, following the treatment of
HepG2 cells with 200 µM CoCl2, the protein levels of
HIF-1α were upregulated at 4 h and reached a peak at 12 h
(P<0.05 and P<0.01, respectively; Fig. 4C and D), and were subsequently downregulated by
24 h. Therefore, the 12-h time point was selected for subsequent
experiments. The effects of propofol on the viability and migration
of HepG2 cells under CoCl2-induced hypoxia were further
investigated. The results demonstrated that compared with the 200
µM CoCl2 group (1.00±0.00), the viability of HepG2 cells
following 12 h of stimulation with 10 (1.07±0.21), 25 (0.99±0.21),
50 (1.01±0.16) or 100 µM (0.99±0.24) propofol in the presence of
CoCl2 was not significantly different (Fig. 4E). Similarly, the wound closure
percentage in HepG2 cells following stimulation with 10
(8.35±1.52), 25 (8.55±1.94), 50 (7.24±2.70) or 100 (8.00±2.89) µM
propofol for 24 h in the presence of CoCl2 was also not
statistically significantly different compared with the 200 µM
CoCl2 group (7.79±2.01) (Fig. 4F and G). In HCCLM3 cells, pre-experimental
results revealed that low concentrations of CoCl2 (≤150
µmol/l) failed to induce an increase in HIF-1α protein levels. An
increase in CoCl2 concentration (>150 µmol/l)
resulted in morphological changes and the death of HCCLM3 cells
(data not shown). The results suggested that the hypoxia model of
HCCLM3 cells was not successfully induced by CoCl2 in
this experiment. These above results indicated that propofol
exerted no effect on the viability and migration of HepG2 cells
under hypoxia induced by CoCl2.
Discussion
The main finding of the present study was that
different concentrations of propofol (10, 25, 50 and 100 µM) had no
effect on the viability, proliferation and migration of HepG2 and
HCCLM3 cells in vitro under normoxic or
CoCl2-induced hypoxic conditions. It has been
demonstrated that anesthetics may affect the long-term prognosis of
patients with cancer who have a compromised immune response during
major surgery with increased risk of regrowth and metastasis of
their malignant tumors (31).
Therefore, evaluating the effects of anesthetics on cancer cells
could provide a basis for the appropriate use of anesthetics in
patients with cancer in order to improve their prognosis. Propofol
is one of the most common anesthetics used for both the induction
and maintenance of anesthesia in cancer surgery (32), and, undoubtedly, numerous such cases
are liver cancer surgery. To date, the effects of propofol on
different types of cancer cell cultured in vitro remain
contradictory (16-18,24,25),
and the mechanisms of action of propofol have not been clarified.
Therefore, the present study aimed to investigate the effects of
propofol on the proliferation and migration of liver cancer
cells.
A previous study demonstrated that cancer cells are
usually exposed to a hypoxic microenvironment in the body as a
result of their rapid proliferation (26). Hypoxia can prompt tumors to
metastasize to distant tissues rich in oxygen, thereby facilitating
the development of tumor cells with highly aggressive phenotypes
(33). One of the most important
adaptive responses to hypoxia is the activation of HIF-1α, which
can upregulate the expression levels of genes involved in cell
proliferation, migration and angiogenesis, and promote the glucose
metabolism of tumor cells to shift from oxidative phosphorylation
to aerobic glycolysis to provide sufficient energy to meet the
needs of rapid tumor proliferation (34). Hypoxia was previously reported to be
associated with a poor prognosis in liver cancer (35). Therefore, the present study aimed to
investigate the effects of propofol on liver cancer cells under
CoCl2-induced hypoxic conditions. The results of the
present study on the CoCl2-induced hypoxia model of
HepG2 cells revealed that propofol stimulation had no effect on
cell viability and migration.
A previous study found that in prostate cancer PC3
cells, propofol stimulation alone does not alter cell proliferation
and migration (21), which is
consistent with the findings of the current study. However,
contrary to the present findings, numerous studies have
demonstrated that propofol exerts significant advantages against
liver cancer by decreasing cell proliferation and migration
(36-38),
while other studies showed that propofol stimulation promotes
cancer cell proliferation and migration (24,39).
Several other studies have reported that propofol suppresses cell
migration without affecting the levels of proliferation (25,40).
In contrast to these, Deegan et al (41) demonstrated that anesthesia with
propofol reduces cell proliferation, but not migration. These
aforementioned findings indicated that propofol may not serve an
anticancer role in all types of neoplasms. The cell type, propofol
concentration and duration of stimulation in the present study were
consistent with those implemented in previous studies (37,38).
There are several possible explanations for the observed
differences between the current results and previous findings.
First, owing to the inter- and intratumor heterogeneity and the
diversity of liver cancer cells (42), the sensitivity to propofol may vary
in different liver cancer cells. Second, it is now widely accepted
that tumor proliferation and metastasis are not independent events
in tumor cells, as tumors are required to interact with their
microenvironment (43). Therefore,
changes in the microenvironment of liver cancer cells cultured
in vitro may affect cell proliferation and migration. Third,
propofol may act through a variety of factors such as microRNA
(18), transforming growth factor-β
(44) and Nrf2(39). Thus, changes in the expression
levels of these factors may also account for the diverse responses
of cancer cells to propofol.
There were also several limitations to the present
study. For example, the effect of propofol on HIF-1α protein levels
in the CoCl2-induced hypoxia model were not
investigated. In addition, the effects of propofol on the
expression levels of proliferation- and migration-related genes
were not determined. The study was also limited because it only
included in vitro cell experiments, while investigating the
effects of propofol in liver cancer model mice in vivo or
using primary cell cultures from tissue specimens obtained from
liver cancer model mice would produce more reliable results and
help validate the present findings.
In conclusion, the results of the present study
indicated that the anticancer effects of propofol may be
questionable and controversial, and further investigations are
required to determine the potential of propofol as a targeted
anticancer drug. Although several studies have suggested that
propofol-based anesthesia may have potential benefits for the
survival of cancer patients (45,46),
to the best of our knowledge, there is currently no clear evidence
to support the selection of propofol as the optimum choice of
anesthesia for liver cancer surgery.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by The Zhejiang Provincial
Public Welfare Technology Application Research Foundation of China
(grant. no. 2018ZD033).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WS and CL contributed to the design of the study and
project administration. CL, QF and JC performed the experiments and
analyzed the data. HM performed the statistical analysis. CL
interpreted the data. WS, CL and QF confirmed the authenticity of
all raw data. CL and WS drafted, reviewed and edited the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Islami F, Miller KD, Siegel RL, Fedewa SA,
Ward EM and Jemal A: Disparities in liver cancer occurrence in the
United States by race/ethnicity and state. CA Cancer J Clin.
67:273–289. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2020. CA Cancer J Clin. 70:7–30. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bruix J and Sherman M: Management of
hepatocellular carcinoma: An update. Hepatology. 53:1020–1022.
2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Xu XF, Xing H, Han J, Li ZL, Lau WY, Zhou
YH, Gu WM, Wang H, Chen TH, Zeng YY, et al: Risk factors, patterns,
and outcomes of late recurrence after liver resection for
hepatocellular carcinoma: A multicenter study from China. JAMA
Surg. 154:209–217. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhang H, Han J, Xing H, Li Z, Schwartz ME,
Zhou Y, Chen T, Wang H, Gu W, Lau WY, et al: Sex difference in
recurrence and survival after liver resection for hepatocellular
carcinoma: A multicenter study. Surgery. 165:516–524.
2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Bruix J, Takayama T, Mazzaferro V, Chau G,
Yang J, Kudo M, Cai J, Poon RT, Han K, Tak WY, et al: Adjuvant
sorafenib for hepatocellular carcinoma after resection or ablation
(STORM): A phase 3, randomised, double-blind, placebo-controlled
trial. Lancet Oncol. 16:1344–1354. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Yeager MP and Rosenkranz KM: Cancer
recurrence after surgery. Region Anesth Pain Med. 35:483–484.
2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lee JW, Shahzad MMK, Lin YG, Armaiz-Pena
G, Mangala LS, Han H, Kim H, Nam EJ, Jennings NB, Halder J, et al:
Surgical stress promotes tumor growth in ovarian carcinoma. Clin
Cancer Res. 15:2695–2702. 2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wall T, Sherwin A, Ma D and Buggy DJ:
Influence of perioperative anaesthetic and analgesic interventions
on oncological outcomes: A narrative review. Br J Anaesth.
123:135–150. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kim R: Effects of surgery and anesthetic
choice on immunosuppression and cancer recurrence. J Transl Med.
16:8–13. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Vasileiou I, Xanthos T, Koudouna E, Perrea
D, Klonaris C, Katsargyris A and Papadimitriou L: Propofol: A
review of its non-anaesthetic effects. Eur J Pharmacol. 605:1–8.
2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Irwin MG, Chung CKE, Ip KY and Wiles MD:
Influence of propofol-based total intravenous anaesthesia on
peri-operative outcome measures: A narrative review. Anaesthesia.
75:e90–e100. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhang J, Shan WF, Jin TT, Wu GQ, Xiong XX,
Jin HY and Zhu SM: Propofol exerts anti-hepatocellular carcinoma by
microvesicle-mediated transfer of miR-142-3p from macrophage to
cancer cells. J Transl Med. 12(279)2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zheng H, Fu Y and Yang T: Propofol
inhibits proliferation, migration, and invasion of hepatocellular
carcinoma cells by downregulating Twist. J Cell Biochem.
120:12803–12809. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Liu W and Liu N: Propofol inhibits lung
cancer A549 cell growth and epithelial-mesenchymal transition
process by upregulation of microRNA-1284. Oncol Res. 27:1–8.
2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Du Q, Zhang X, Zhang X, Wei M, Xu H and
Wang S: Propofol inhibits proliferation and epithelial-mesenchymal
transition of MCF-7 cells by suppressing miR-21 expression. Artif
Cells Nanomed Biotechnol. 47:1265–1271. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhang YF, Li CS, Zhou Y and Lu XH: Effects
of propofol on colon cancer metastasis through STAT3/HOTAIR axis by
activating WIF-1 and suppressing Wnt pathway. Cancer Med.
9:1842–1854. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhu F, Li Q, Yang Y, Wang L and Wang J:
Propofol suppresses proliferation, migration, invasion and promotes
apoptosis by upregulating microRNA-140-5p in gastric cancer cells.
Onco Targets Ther. 12:10129–10138. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Huang H, Benzonana LL, Zhao H, Watts HR,
Perry NJ, Bevan C, Brown R and Ma D: Prostate cancer cell
malignancy via modulation of HIF-1α pathway with isoflurane and
propofol alone and in combination. Br J Cancer. 111:1338–1349.
2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Gao Y, Yu X, Zhang F and Dai J: Propofol
inhibits pancreatic cancer progress under hypoxia via ADAM8. J
Hepatobiliary Pancreat Sci. 26:219–226. 2019.PubMed/NCBI View
Article : Google Scholar
|
|
23
|
Kushida A, Inada T and Shingu K:
Enhancement of antitumor immunity after propofol treatment in mice.
Immunopharm Immunot. 29:477–486. 2008.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhang L, Wang N, Zhou S, Ye W, Jing G and
Zhang M: Propofol induces proliferation and invasion of gallbladder
cancer cells through activation of Nrf2. J Exp Clin Cancer Res.
31(66)2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ecimovic P, Murray D, Doran P and Buggy
DJ: Propofol and bupivacaine in breast cancer cell function in
vitro-role of the NET1 gene. Anticancer Res. 34:1321–1331.
2014.PubMed/NCBI
|
|
26
|
Masoud GN and Li W: HIF-1α pathway: Role,
regulation and intervention for cancer therapy. Acta Pharm Sin B.
5:378–389. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Tang B, Zhao F, Qu Y and Mu D:
Hypoxia-inducible factor-1alpha: A promising target for tumor
therapy. Ai Zheng. 28:775–782. 2009.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Muñoz Sánchez J and Chánez Cárdenas ME:
The use of cobalt chloride as a chemical hypoxia model. J Appl
Toxicol. 39:556–570. 2018.PubMed/NCBI View
Article : Google Scholar
|
|
29
|
Albanese J, Martin C, Lacarelle B, Saux P,
Durand A and Gouin F: Pharmacokinetics of long-term propofol
infusion used for sedation in ICU patients. Anesthesiology.
73:214–217. 1990.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Coetzee JF, Glen JB, Wium CA and Boshoff
L: Pharmacokinetic model selection for target-controlled infusions
of propofol. Assessment of three parameter sets. Anesthesiology.
82:1328–1345. 1995.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kim R: Anesthetic technique and cancer
recurrence in oncologic surgery: Unraveling the puzzle. Cancer
Metastasis Rev. 36:159–177. 2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Yang C, Gao J, Yan N, Wu B, Ren Y, Li H
and Liang J: Propofol inhibits the growth and survival of gastric
cancer cells in vitro through the upregulation of ING3. Oncol Rep.
37:587–593. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Brahimi-Horn MC, Bellot G and Pouysségur
J: Hypoxia and energetic tumour metabolism. Curr Opin Genet Dev.
21:67–72. 2011.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wilson GK, Tennant DA and McKeating JA:
Hypoxia inducible factors in liver disease and hepatocellular
carcinoma: Current understanding and future directions. J Hepatol.
61:1397–1406. 2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Liu Y, Yan W, Tohme S, Chen M, Fu Y, Tian
D, Lotze M, Tang D and Tsung A: Hypoxia induced HMGB1 and
mitochondrial DNA interactions mediate tumor growth in
hepatocellular carcinoma through Toll-like receptor 9. J Hepatol.
63:114–121. 2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ou W, Lv J, Zou X, Yao Y, Wu J, Yang J,
Wang Z and Ma Y: Propofol inhibits hepatocellular carcinoma growth
and invasion through the HMGA2-mediated Wnt/β-catenin pathway. Exp
Ther Med. 13:2501–2506. 2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Gong T, Ning X, Deng Z, Liu M, Zhou B,
Chen X, Huang S, Xu Y, Chen Z and Luo R: Propofol-induced
miR-219-5p inhibits growth and invasion of hepatocellular carcinoma
through suppression of GPC3-mediated Wnt/β-catenin signalling
activation. J Cell Biochem. 120:16934–16945. 2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Sun Y and Sun H: Propofol exerts
anticancer activity on hepatocellular carcinoma cells by raising
lncRNA DGCR5. J Cell Physiol. 235:2963–2972. 2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Meng C, Song L, Wang J, Li D, Liu Y and
Cui X: Propofol induces proliferation partially via downregulation
of p53 protein and promotes migration via activation of the Nrf2
pathway in human breast cancer cell line MDA-MB-231. Oncol Rep.
37:841–848. 2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Li R, Huang Y and Lin J: Distinct effects
of general anesthetics on lung metastasis mediated by
IL-6/JAK/STAT3 pathway in mouse models. Nat Commun.
11(642)2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Deegan CA, Murray D, Doran P, Ecimovic P,
Moriarty DC and Buggy DJ: Effect of anaesthetic technique on
oestrogen receptor-negative breast cancer cell function in vitro.
Brit J Anaesth. 103:685–690. 2009.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Da Silva-Diz V, Lorenzo-Sanz L,
Bernat-Peguera A, Lopez-Cerda M and Muñoz P: Cancer cell
plasticity: Impact on tumor progression and therapy response. Semin
Cancer Biol. 53:48–58. 2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Guan X: Cancer metastases: Challenges and
opportunities. Acta Pharm Sin B. 5:402–418. 2015.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Xu YB, Jiang W, Zhao FR, Li G, Du QH,
Zhang MY and Guo XG: Propofol suppresses invasion and induces
apoptosis of osteosarcoma cell in vitro via downregulation of
TGF-β1 expression. Eur Rev Med Pharmacol Sci. 20:1430–1435.
2016.PubMed/NCBI
|
|
45
|
Wigmore TJ, Mohammed K and Jhanji S:
Long-term survival for patients undergoing volatile versus IV
anesthesia for cancer surgery: A retrospective analysis.
Anesthesiology. 124:69–79. 2016.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Wu ZF, Lee MS, Wong CS, Lu CH, Huang YS,
Lin KT, Lou YS, Lin C, Chang YC and Lai HC: Propofol-based total
intravenous anesthesia is associated with better survival than
desflurane anesthesia in colon cancer surgery. Anesthesiology.
129:932–941. 2018.PubMed/NCBI View Article : Google Scholar
|