Introduction
One of the most common malignant tumor types of the
biliary system is gallbladder carcinoma. Its major clinical
manifestations include right upper abdominal pain, gallbladder
enlargement, jaundice, nausea and vomiting, upper gastrointestinal
bleeding, and cachexia signs such as low fever and wasting
(1). With an annual incidence of
~528 per 100,000 individuals, China is a region with a high
incidence of this cancer type (2).
Surgical resection remains the preferred treatment for gallbladder
carcinoma and postoperative adjuvant therapy has an important role
in prolonging the survival time of patients. However, it is
difficult to detect gallbladder carcinoma at an early stage because
of its high malignancy and lack of specific symptoms. Diagnosis is
typically associated with lymph node metastasis and liver
metastasis. This leads to a low resection rate and poor prognosis,
causing serious harm to human health (3). Signal transducer and activator of
transcription 3 (STAT3) is one of the most important members of the
STAT family and is the junction point of numerous carcinogenic
signaling pathways. It has been well established that all
successful tumors must undergo neovascularization (4). Vascular endothelial growth factor
(VEGF) is one of the most important of all known inducers of
angiogenesis. Previous evidence indicated that constitutive STAT3
activation directly promotes VEGF expression and stimulates tumor
angiogenesis, and STAT3 is a direct factor in VEGF expression
(5). Multiple studies have
confirmed that STAT3 is continuously activated in numerous tumor
cell types (6-8).
It is closely related to the proliferation, invasion, immune escape
and microvascularization of tumor cells (9-12).
Upregulation of STAT3 expression may promote the early abnormal
division of gallbladder epithelial cells by improving the
activation degree of the tumor signaling pathway in tumor cells and
transcriptional activation degree of tumor-associated genes
(13).
Vasculogenic mimicry (VM), which is a vascular
structure formed by tumor cells that mimics the function of
endothelial cells, is a novel tumor vascular model (14). Angiogenesis, mosaic vessels and VM
are considered as the three major factors promoting intracranial
vascular formation in tumors. These factors are common in melanoma,
inflammatory breast cancer, osteosarcoma, liver cancer and other
highly invasive malignant tumor types. VM involves tumor cells that
have been generated internally and gradually integrated with
endothelial-dependent vessels (EDV) of host origin. The
intermediate states of these two vessels are known as mosaic
vessels, which are transitional vessels surrounded by tumor and
host endothelial cells (15).
Increasing evidence has indicated that inflammatory
signals in the diseased tissue environment are closely related to
the occurrence, maintenance and development of tumors (16). Gallbladder carcinoma is frequently
associated with gallstones. Stimulation of calculi and the
concurrent inflammation of gallbladder mucosa may lead to the
occurrence of gallbladder carcinoma. STAT3 is a key molecule
mediating inflammation in tumors (17). Its status in the tumor
microenvironment determines the immune response, including whether
the tumor is promoted or inhibited. Its continued activation in
tumor cells promotes cell proliferation and survival (18). Therefore, STAT3, as a control
target, may inhibit the proliferation and invasiveness of tumor
cells, similar to the effects of inhibiting the development of VM
and EDV. STAT3 may be a useful target for antitumor angiogenesis
therapy. Therefore, in the present study, the gallbladder tissue
expression and correlation of STAT3 and VM were analyzed in
patients with gallbladder carcinoma. The relationship between the
occurrence and development of gallbladder carcinoma and its
potential for targeted gene therapy were examined. Targeted gene
therapy may help to improve treatment strategies and long-term
curative effects in patients with gallbladder carcinoma.
Materials and methods
Patients, tissue samples and clinical
data collection
The experimental study of clinical tissue samples
was reviewed and approved by the Ethics Committee of the Fifth
Affiliated Hospital of the Medical School of Nantong University
(Taizhou, China). All patients participating in the study or their
family members signed an informed consent form for the clinical
study. Paraffin tissue samples were collected from 72 patients with
gallbladder carcinoma and 10 cases of chronic cholecystitis at the
Fifth Affiliated Hospital of the Medical School of Nantong
University between January 2013 and December 2016. The study cohort
for gallbladder carcinoma included 40 female patients and 32 male
patients (male-to-female ratio, 1:1.4) with an age range of 42-82
years. The male-to-female ratio of patients with chronic
cholecystitis was 1:1.5, with an age range of 45-76 years. Only
subjects with complete medical records were enrolled. None of the
patients included in the study had undergone any preoperative
chemotherapy, radiation therapy or other treatment. A total of 72
wax blocks of gallbladder cancer and 10 wax blocks of chronic
cholecystitis tissue were selected, fixed on a slicer and sliced to
4-µm sections. The manual slicer was used with uniform rotation,
the selected slice was brushed and immersed in water. After the
sections were fully flattened on the surface of warm water at 45˚C,
they were picked up with anti-slip slides, and then placed in an
electrically heated constant temperature air drying oven at 65˚C
for 30-60 min until the sections were completely dried. All tissue
samples were pathologically diagnosed. There were 18 cases of
highly differentiated carcinoma, 25 cases of moderately
differentiated carcinoma, and 29 cases of poorly differentiated
carcinoma according to the TNM staging criteria published by the
International Union Against Cancer (19). Follow-up of the patients was mainly
performed via telephone and outpatient reexamination. The
postoperative recurrence time was calculated by month, and the
truncation time was from the first operation to the recurrence
time. The cut-off date for follow-up was December 2019.
Main reagents
Rabbit anti-human STAT3 monoclonal antibody (1:100;
cat. no. BSM-52235R; BIOSS); Diaminobenzidine (DAB) color reagent
(Beijing Zhongshan Jinqiao Biotechnology Co., Ltd.); poly-l-lysine
coated slides (cat. no. AR1065; Wuhan Boster Biological Technology,
Ltd.); HRP-conjugated AffiniPure goat anti-rabbit IgG testing kit
(cat. no. BA1054; Wuhan Boster Biological Technology, Ltd.);
Periodic Acid-Schiff Stain kit, (cat. no. DG0005; Beijing Leigen
Biotechnology Co., Ltd.); 5% BSA (cat. no. AR0004; Wuhan Boster
Biological Technology, Ltd.) and normal goat serum (cat. no.
AR0009; Wuhan Boster Biological Technology, Ltd.)
Immunohistochemical staining
Gallbladder lesion tissues were taken from patients
with gallbladder carcinoma and chronic cholecystitis. After baking,
tissue specimens were dewaxed and hydrated, washed with PBS for 20
min at room temperature, incubated in EDTA antigen retrieval
solution (1 mM) at 95-100˚C for ~15 min and blocked with 5% BSA.
Rabbit anti-human STAT3 monoclonal antibody was used as a primary
antibody, incubated at 4˚C overnight, followed by washing with PBS.
Subsequently, the sample was incubated with goat anti-rabbit
antibody labeled with horseradish peroxidase (HRP) (diluted
1:1,000) at room temperature for 2 h and then washed with PBS three
times for 5 min. DAB solution was added, color development was
checked under the microscope and the solution was washed with
distilled water after satisfactory color development. After
counterstaining for 1 min with hematoxylin, the slides were
dehydrated in sequential ethanol gradients (75, 80 and 100%) of 1
min each. The slides were incubated in xylene until transparent and
they were sealed with neutral gum. As the negative control, normal
goat serum was used instead of primary antibody, while the other
steps remained unchanged, and the result was negative. As the blank
control, PBS was used instead of primary antibody; the other steps
remained unchanged, and the results were negative.
Periodic acid Schiff (PAS)/CD34 double
staining
For double staining, CD34 (1:100; cat. no. ab8536;
Abcam) staining was performed first and the specific labeling steps
were the same as those described above. CD34 staining was completed
by color development with DAB and subsequently, the PAS staining
procedure was performed. For this, 1% PAS was oxidized under the
exclusion of light for 10 min. Slices were rinsed with double
distilled water after running tap water. At room temperature, drops
of PAS stain were added to the slices, followed by incubation in
the dark for 20 min. PAS solution was rinsed off thoroughly with
running water. The nucleus was stained with hematoxylin at room
temperature for 5 min, and hematoxylin was rinsed off with running
water. The sections underwent dehydration with an ethanol gradient
(70, 80, 90, 95 and 100%) and made transparent with xylene,
followed by sealing with neutral gum.
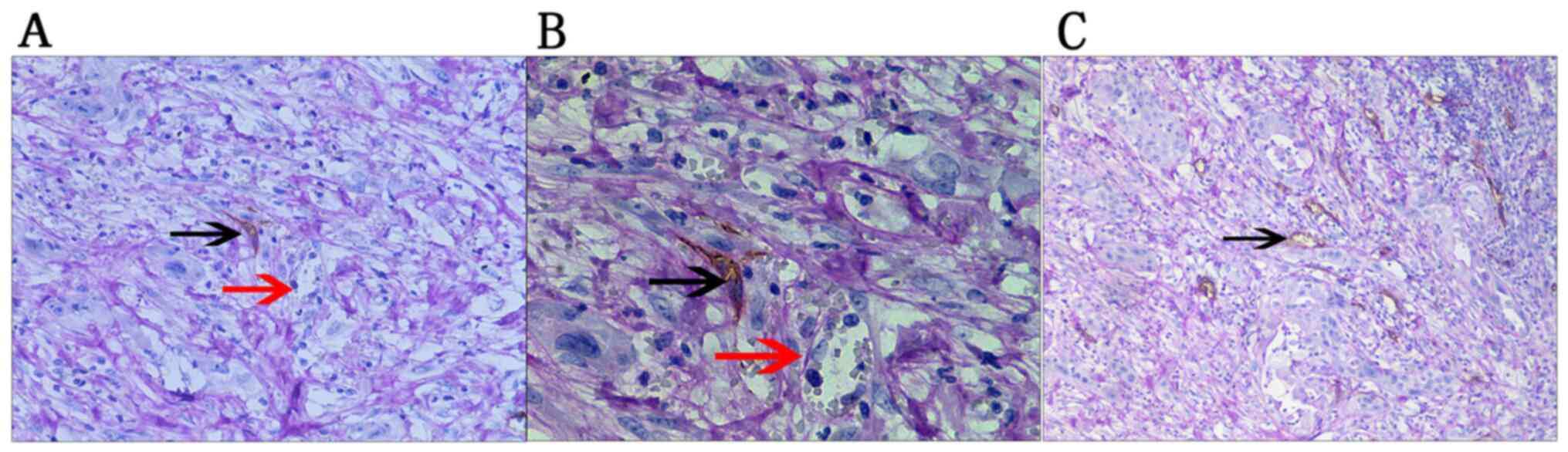
VM is surrounded by tumor cells and its wall is rich
in extracellular matrix (20). VM
structures may be stained purplish red by PAS. CD34 immunostaining
is negative due to lack of endothelial involvement. Therefore, in
PAS/CD34 double staining, the lumen structure with PAS-positive and
CD34-negative staining may be used to identify VM-positive
structures with red blood cells in them.
Immunohistochemical staining result
scoring
For each tissue slice, staining results were
determined and the pathological diagnosis was made by two senior
physicians independently, who were blinded to the
clinicopathological data and research content. STAT3 protein is
mainly located in the cytoplasm and was determined from 10 randomly
selected views at high magnification (x100), counting 1,000 cells.
The results were obtained using a semi-quantitative integral method
(21), according to the
immunohistochemical score for the intensity of each section and the
proportion of positive cells. Color intensity was scored as
follows: No color, 0 points; light yellow, 1 point; brownish
yellow, 2 points with; Tan, 3 points. The percentage of stained
cells was scored as follows: <5%, 0; 5-24%, 1; 25-49%, 2;
50-74%, 3; 75-100%, 4. Multiplication of the two values was used to
obtain the semi-quantitative results. A final score of 0-2 was
defined as negative expression, while a score of >2 was
considered to indicate positive expression.
Statistical analysis
SPSS version 21.0 statistical software (IBM Corp.)
was used for data analysis and the results were expressed as the
mean ± standard deviation. Wilcoxon rank sum test and
independent-sample Student's t-test were used to analyze
differences in age and sex distribution between the two groups of
patients. Pearson's χ2 test and Fisher's exact test were
used to statistically analyze the expression levels of STAT3 in the
tissues from the two groups of patients. Spearman rank correlation
analysis method was also used to analyze the correlation between
STAT3 and VM expression levels in gallbladder carcinoma tissues.
The association between the two indicators and clinicopathological
data was also statistically analyzed with Pearson's χ2
test and Fisher's exact test methods. The cut-off value for STAT3
was calculated using a receiver operating characteristic curve.
Survival curves were plotted using Kaplan-Meier analysis and the
log-rank test was used to compare differences between the survival
curves. P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression of STAT3 in gallbladder
carcinoma and cholecystitis tissues
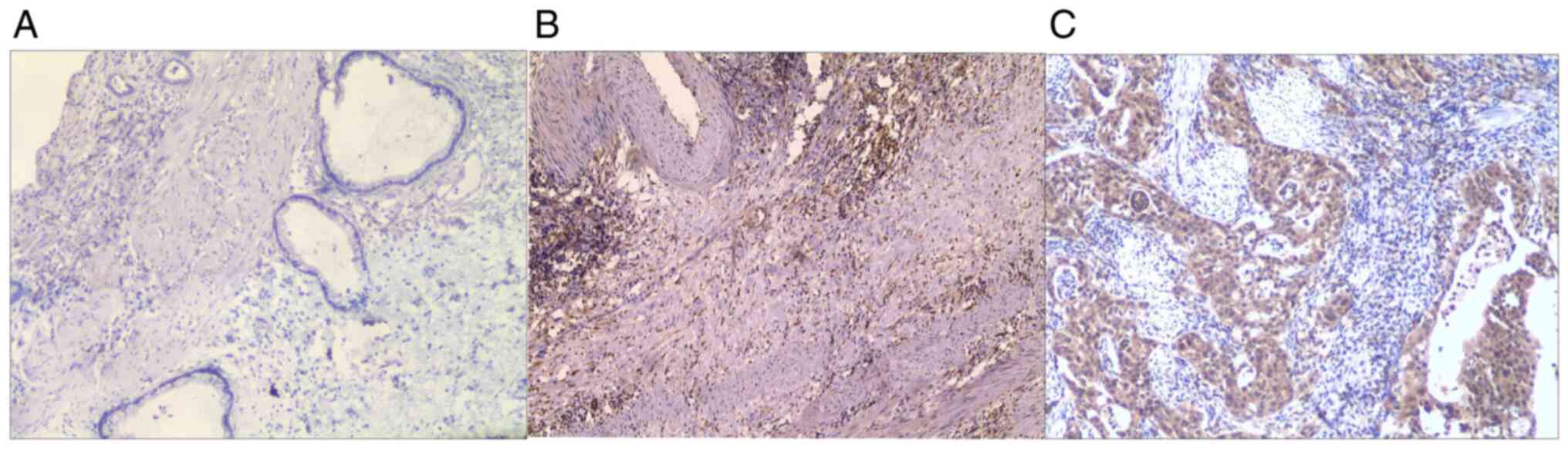
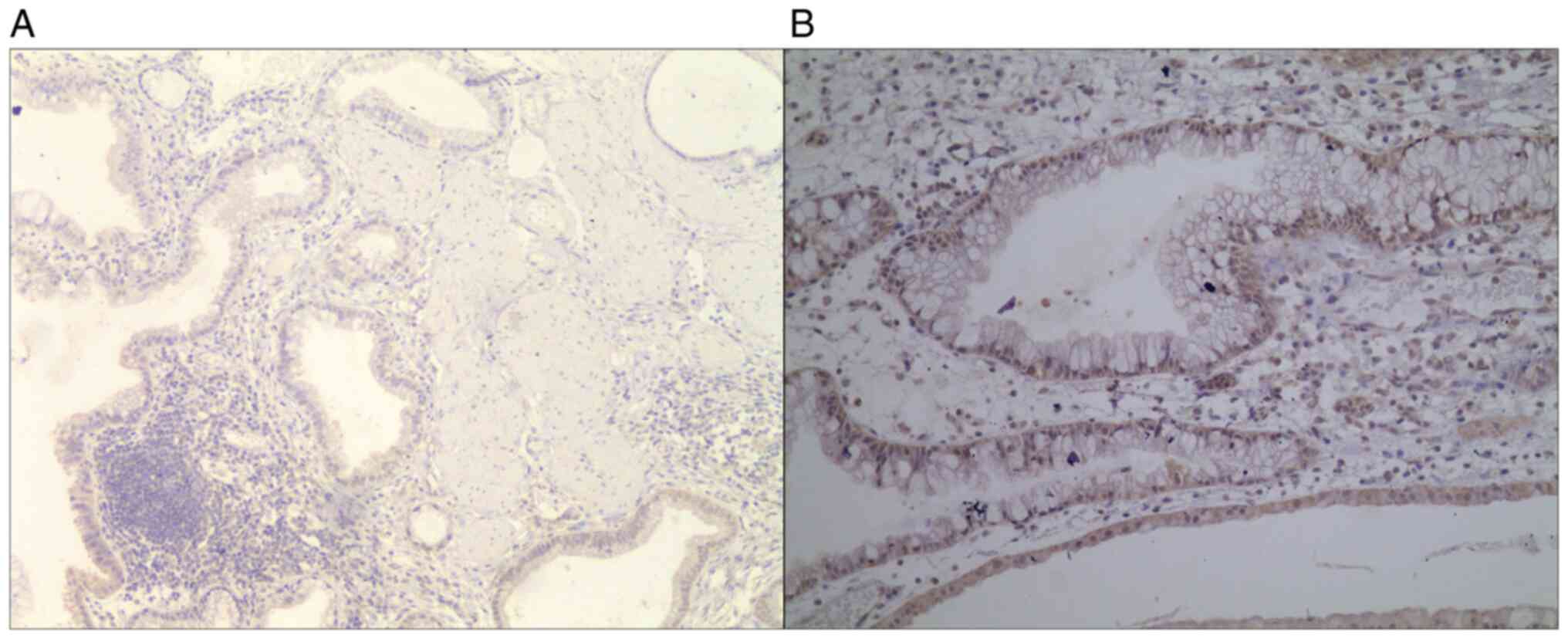
In the present study, STAT3 expression was assessed
in 72 cases of primary gallbladder carcinoma and 10 cases of
chronic cholecystitis using immunohistochemistry. The
male-to-female ratio of patients with gallbladder carcinoma was
1:1.4, with an age range of 42-82 years. The male-to-female ratio
of patients with chronic cholecystitis was 1:1.5, with an age range
of 45-76 years (Table I). The
median age of the patients with gallbladder carcinoma was 59.53
years and the median age of the patients with chronic cholecystitis
was 57.76 years (Table I). There
was no significant difference in age (F=0.004, P=0.950; Table I) or sex between the two groups (sum
of ranks: 2,982 in the gallbladder carcinoma group and 421 in the
chronic cholecystitis group; P=0.921; Table I). The positive expression rate of
STAT3 in gallbladder carcinoma and chronic cholecystitis was 83.3
and 10.0%, respectively. Fisher's exact test indicated that the
expression rate of STAT3 in gallbladder carcinoma tissues was
significantly higher than that in chronic cholecystitis tissues
(P<0.001; Table II and Figs. 1 and 2).
 | Table IAge and sex distribution of patients
in chronic cholecystitis and gallbladder carcinoma. |
Table I
Age and sex distribution of patients
in chronic cholecystitis and gallbladder carcinoma.
| Factor | Chronic
cholecystitis | Gallbladder
carcinoma | P-value |
|---|
| Sex, n (%) | | | 0.921 |
|
Male | 4 (40%) | 30 (41.7%) | |
|
Female | 6 (60%) | 42 (58.3%) | |
| Male-to-female
ratio | 1:1.5 | 1:1.4 | |
| Age, years (%) | | | 0.950 |
|
<60 | 6 (60%) | 32 (44.4) | |
|
≥60 | 4(40) | 40 (55.6) | |
| Median age,
years | 57.76 | 59.53 | |
 | Table IIExpression of STAT3 in gallbladder
carcinoma and chronic cholecystitis tissues. |
Table II
Expression of STAT3 in gallbladder
carcinoma and chronic cholecystitis tissues.
| | STAT3
expression | |
|---|
| Group | n | Positive | Negative | Positive rate
(%) | P-value |
|---|
| Gallbladder
carcinoma | 72 | 60 | 12 | 83.3 | <0.001 |
| Chronic
cholecystitis | 10 | 1 | 9 | 10.0 | |
Association between STAT3 expression
in gallbladder carcinoma and clinicopathological factors
Clinical stage analysis indicated that the STAT3
expression rate in gallbladder carcinoma of stages I-III was 73.7%
and that of stages IV/V was 94.1%. This suggested that a higher
clinical stage was significantly positively associated with STAT3
expression (Fisher's exact test F=5.867, P=0.027; Table III). The rate of STAT3 expression
in well, moderately and poorly differentiated gallbladder carcinoma
tissues was 66.7, 80.0 and 96.6%, respectively, and accordingly,
the rate of STAT3 expression significantly increased with
decreasing histological grade (Fisher's exact test P=0.017;
Table III). There was no
significant difference in STAT3 expression in gallbladder carcinoma
tissues between different subgroups according to sex, age and lymph
node metastasis (sex, χ2=0.411, P=0.521; age,
χ2=0.180, P=0.671; lymph node metastasis, Fisher's exact
test F=4.292, P=0.061; Table
III).
 | Table IIIAssociation between STAT3 and
clinicopathological factors of patients with gallbladder
carcinoma. |
Table III
Association between STAT3 and
clinicopathological factors of patients with gallbladder
carcinoma.
| | STAT3
expression | |
|---|
| Factor | n | Positive | Negative | Positive rate
(%) |
χ2/Fisher's exact test
value | P-value |
|---|
| Sex | | | | | 0.411 | 0.521 |
|
Male | 30 | 24 | 6 | 80.0 | | |
|
Female | 42 | 36 | 6 | 85.7 | | |
| Age (years) | | | | | 0.180 | 0.671 |
|
<60 | 32 | 26 | 6 | 81.3 | | |
|
≥60 | 40 | 34 | 6 | 85.0 | | |
| Histological
grade | | | | | 7.645 | 0.017 |
|
Well | 18 | 12 | 6 | 66.7 | | |
|
Moderate | 25 | 20 | 5 | 80.0 | | |
|
Poor | 29 | 28 | 1 | 96.6 | | |
| Lymph node
metastasis | | | | | 4.292 | 0.061 |
|
N1/N2 | 54 | 48 | 6 | 88.9 | | |
|
N0 | 18 | 12 | 6 | 66.7 | | |
| Clinical stage | | | | | 5.867 | 0.027 |
|
I-III | 38 | 28 | 10 | 73.7 | | |
|
IV/V | 34 | 32 | 2 | 94.1 | | |
Association between VM in gallbladder
carcinoma and clinicopathological factors
In the present study, 58 cases of gallbladder
carcinoma presented with typical VM structures as follows: i) Tumor
cells connected with each other through a cell bridge to form the
vascular lumen, in which red blood cells or free endothelial cells
were occasionally observed; and ii) the tumor cells were stained
using PAS into a purplish red net-like structure (Fig. 3). Statistical analysis revealed no
significant difference in VM in gallbladder carcinoma tissues
between different sexes, ages and lymph node metastasis status
(sex, χ2=0.010, P=0.920; age, χ2=0.217,
P=0.641; lymph node metastasis, Fisher's exact test F=1.004,
P=0.318; Table IV).
 | Table IVAssociation between VM and
clinicopathological factors in patients with gallbladder
carcinoma. |
Table IV
Association between VM and
clinicopathological factors in patients with gallbladder
carcinoma.
| | VM | |
|---|
| Factor | n | Positive | Negative | Positive rate
(%) |
χ2/Fisher's exact test
value | P-value |
|---|
| Sex | | | | | 0.010 | 0.920 |
|
Male | 30 | 24 | 6 | 80.0 | | |
|
Female | 42 | 34 | 8 | 81.0 | | |
| Age (years) | | | | | 0.217 | 0.641 |
|
<60 | 32 | 25 | 7 | 78.1 | | |
|
≥60 | 40 | 33 | 7 | 82.5 | | |
| Histological
grade | | | | | 9.202 | 0.002 |
|
Well | 18 | 11 | 7 | 61.1 | | |
|
Moderate | 25 | 19 | 6 | 76.0 | | |
|
Poor | 29 | 28 | 1 | 96.6 | | |
| Lymph node
metastasis | | | | | 1.004 | 0.318 |
|
N1+2 | 54 | 45 | 9 | 83.3 | | |
|
N0 | 18 | 13 | 5 | 72.2 | | |
| Clinical stage | | | | | 8.324 | 0.007 |
|
I-III | 38 | 26 | 12 | 68.4 | | |
|
IV-V | 34 | 32 | 2 | 94.1 | | |
The rate of VM in the poor histological grade group
was 96.6%, which was significantly higher than that in the
well-differentiated histological grade group (61.1%;
χ2=9.202, P=0.002). The VM-positive rate in patients
with stage I-III gallbladder carcinoma was 68.4% and that in
patients with stage IV/V was 94.1%, with a significant difference
(Fisher's exact test F=8.324, P=0.007; Table IV).
Correlation between VM and STAT3 in
gallbladder carcinoma
In the present study, VM was present in 52 of the 60
cases of gallbladder carcinoma with positive STAT3 expression
(86.7%). In addition, among the 12 patients with negative STAT3
expression in gallbladder carcinoma tissues, 6 exhibited VM, with a
rate of 50.0%. Spearman's rank correlation analysis indicated a
close and positive correlation between STAT3 expression and VM in
gallbladder carcinoma (R=0.345, P<0.05; Table V).
 | Table VCorrelation between VM and STAT3 in
gallbladder carcinoma. |
Table V
Correlation between VM and STAT3 in
gallbladder carcinoma.
| | VM | |
|---|
| STAT3
expression | Total | Positive | Negative | Positive rate
(%) | r | P-value |
|---|
| Positive | 60 | 52 | 8 | 86.7 | 0.345 | 0.003 |
| Negative | 12 | 6 | 6 | 50.0 | | |
| Total | 72 | 58 | 14 | 80.6 | | |
STAT3 expression is associated with
early postoperative recurrence of gallbladder carcinoma
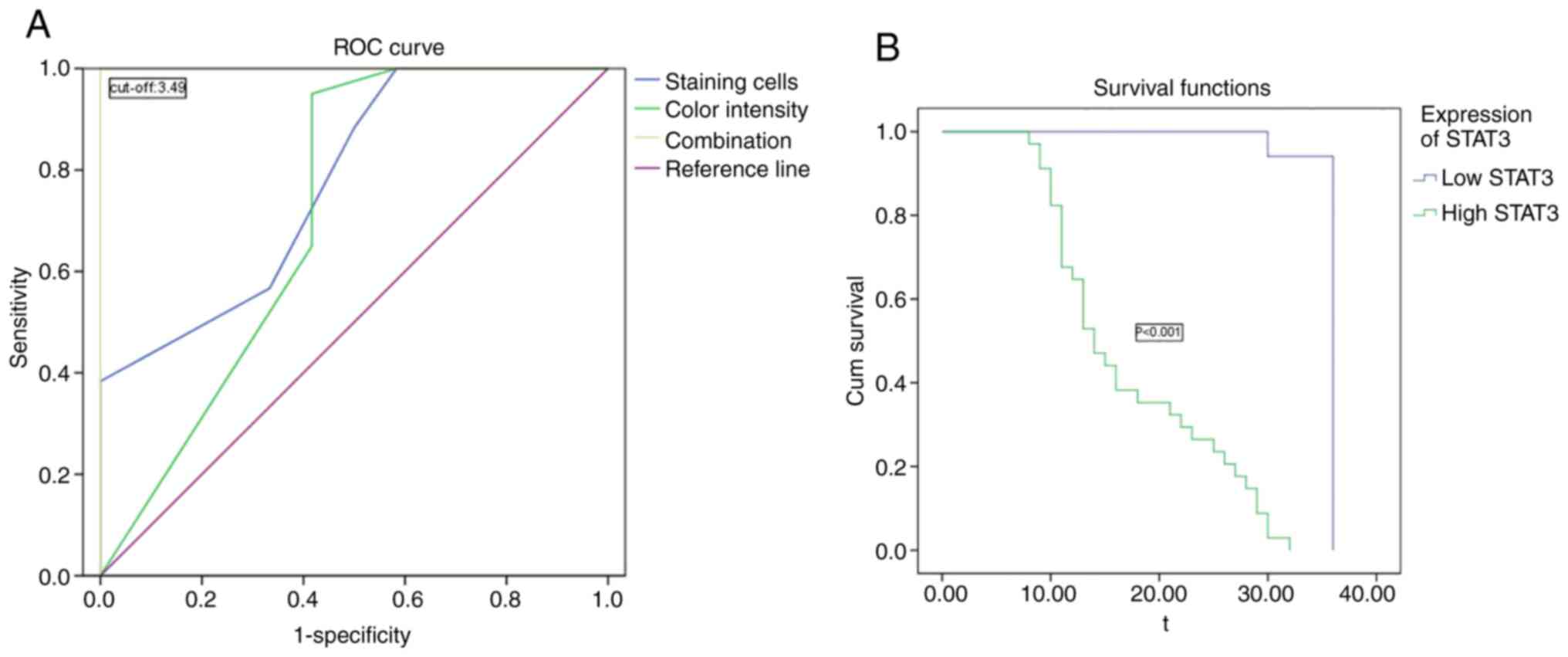
The relationship between the expression level of
STAT3 and time to postoperative recurrence (TTR) of gallbladder
carcinoma was then analyzed. As staining for STAT3 was mild to
moderate in chronic cholecystitis tissues and its expression was
significantly increased in carcinoma tissues, the cut-off value for
STAT3 was calculated using a receiver operating characteristic
curve to evaluate the expression level of STAT3 in liver cancer
tissues. The semi-quantitative cut-off value of the combined factor
score was generated by combining two immunohistochemical diagnostic
methods for STAT3, which were the coloration intensity diagnostic
score and the number of stained cells diagnostic score. STAT3
diagnostic score >3.49 was regarded as high expression. Among
them, 12 cases had low expression and 39 cases had high expression.
The Youden index, which is a method to evaluate the authenticity of
screening tests, was used (22).
The Youden index of the semi-quantitative integration method for
coloring intensity diagnosis =0.971, the Youden index of the number
of stained cells diagnostic =0.774 and Joint Youden index =1
(Fig. 4A). Among the 51 patients
with gallbladder carcinoma who completed a 3-year follow-up, the
mean TTR of patients in the high-STAT3 group was 17.35 months and
that in the low-STAT3 group was 35.65 months, with a significant
difference (P<0.001; Fig. 4B). A
total of 8 patients were followed up for 36 months without
postoperative recurrence, but the statistical data were analyzed
according to the time of postoperative recurrence for 36 months.
Thus, increased expression of STAT3 in gallbladder carcinoma tissue
indicated early postoperative recurrence of gallbladder
carcinoma.
VM is associated with early
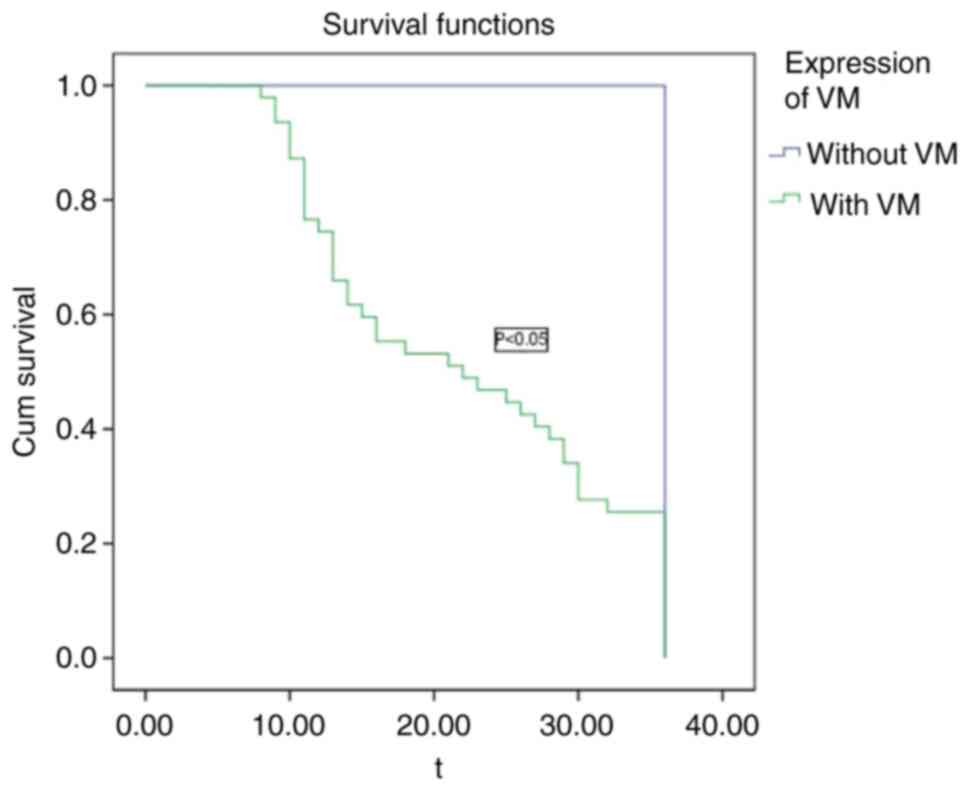
postoperative recurrence of gallbladder carcinoma
The VM structure was detected in 47 (92.15%, 47/51)
of 51 cases of gallbladder carcinoma with 3 years of follow-up,
with an average TTR of 22.38 months. The average TTR of the four
cases (7.84%, 4/51) with no VM structure was 36 months, because 4
patients with no VM structure were followed up for 36 months and
exhibited no recurrence, but the statistical data were analyzed
according to the time of postoperative recurrence for 36 months
(Fig. 5). VM-positive gallbladder
carcinoma cases were more likely to have early postoperative
recurrence and there was a significant difference in TTR between
the two groups (P<0.05).
Discussion
To date, surgical resection remains the major
treatment for gallbladder carcinoma, supplemented by adjuvant
treatments such as chemotherapy or radiotherapy. The surgical mode
for gallbladder carcinoma treatment should be determined according
to its clinical stage. For patients with lesions limited to the
gallbladder mucosa, conventional cholecystectomy may achieve an
optimum therapeutic effect (23).
Gemcitabine and platinum-based chemotherapy combined with molecular
targeted therapy has become a promising avenue of research for
treating gallbladder carcinoma. Targeted therapy utilizes a
specific signaling molecule overexpressed on the surfaces of tumor
cells as a target, and through the selection of specific blockers
to interfere with the signal transduction pathway regulated by this
molecule, it may inhibit the growth and invasion of tumors.
Currently, the major targets for biliary system malignancies
include epidermal growth factor receptor, vascular endothelial
factor (VEGF) and mitogen-activated protein kinase (24,25).
Identifying targets for gallbladder carcinoma treatment is the
basis of targeted therapy.
STAT3 was purified as an acute-phase response factor
in interleukin-6 (IL-6) signaling and is a member of the tyrosine
phosphorylation-activated family (26). IL-6 promotes tumor development by
various mechanisms, for example, regulating the cell cycle, local
inflammation, angiogenesis and tumor stem cell self-renewal
(27). Numerous studies have
indicated that STAT3 has a crucial role in the proliferation,
invasion, metastasis, angiogenesis and immune escape of numerous
types of tumor cell (28). Zhang
et al (29) reported that
Tanshinone IIA (Tan IIA) exerted potent anticancer activity in
gastric cancer cells, mediated by downregulation of STAT3
activation. Overexpression of STAT3 significantly ameliorated the
Tan IIA-induced suppression of cell growth and apoptosis. In
addition, STAT3 is able to promote the proliferation of biliary
tumors. It was reported that upregulation of STAT3 expression
significantly enhanced the proliferation activity of RBE and 9810
cell lines of bile duct cancer, whereas inhibition of STAT3
expression reduced the proliferation activity of the two cell lines
(30). These results indicated that
activation of STAT3 may promote the growth of tumor cells. Thus,
inhibiting the expression and activation of STAT3 may be an
effective approach for preventing the proliferation of tumor
cells.
Invasion and metastasis are the major
characteristics that distinguish malignant tumors from benign
lesions (31). Tumor invasion and
metastasis are the result of multiple factors. Neovascularization
is a key step in this process and is regulated by various cytokines
and growth factors, among which VEGF is the most important
(32). VEGF produced by tumor cells
is able to bind to tyrosine kinase receptors and promote the
formation of new blood vessels, while STAT3 is a direct factor in
activating VEGF expression (33).
As early as in 2002, Niu et al (5) reported that activated STAT3
upregulated the expression of VEGF and promoted the formation of
blood vessels in melanoma. STAT3 also regulated the migration of
lymphatic endothelial cells and formation of lymphatic vascular
lumen by upregulating the expression of VEGF and assisted tumor
cells in lymphatic metastasis (34). Accumulating evidence has indicated
that targeted inhibition of STAT3 is able to enhance the anticancer
immune response and preserve the suppressed immune microenvironment
in tumors, making it a promising target in cancer immunotherapy
(35).
STAT3 is highly expressed in various cancer types,
including endometrial cancer (36),
glioma of the brain (37), breast
cancer (38) and lung cancer
(39). However, studies focusing on
the relationship between STAT3 and gallbladder carcinoma are
currently limited. Enyu et al (40) detected the expression of STAT3 in
gallbladder carcinoma tissues and paraneoplastic tissues and
determined that STAT3 expression was abnormally high in gallbladder
carcinoma tissues. In the present study, the expression of STAT3 in
gallbladder carcinoma tissues was also significantly higher than
that in chronic cholecystitis tissues, with a positive expression
rate of 83.3%. These results are consistent with those of the study
by Enyu et al (40), who
suggested that STAT3 is related to the occurrence and development
of gallbladder carcinoma. Furthermore, in the present study, the
positive expression rate of STAT3 in chronic cholecystitis was
10.0%, while the small sample size of the control group is a
limitation of the present study. In addition, the STAT3 expression
rate in stage Ⅰ-Ⅲ gallbladder carcinoma was 73.7% and that in stage
IV/V, it was 94.1%. In highly, moderately and poorly differentiated
gallbladder carcinoma, the expression rates of STAT3 were 66.7,
80.0 and 96.6%, respectively; the expression rate of STAT3
increased with decreased differentiation and these differences were
significant (P<0.05). High expression of STAT3 was correlated
with the clinical stage and histological grade of gallbladder
carcinoma. However, no significant differences in STAT3 expression
in gallbladder carcinoma tissues were obtained between different
sexes, nor between different age and lymph node metastasis groups
(P>0.05). In the present study, 51 patients with gallbladder
carcinoma who completed a 3-year follow-up had an average TTR of
17.353 months for high STAT3 expression and 35.647 months for low
STAT3 expression. The results indicated that increased expression
of STAT3 in gallbladder carcinoma tissues was positively associated
with early postoperative recurrence of the malignancy. Due to the
small sample size and follow-up time in the present study, the
study results may be affected. In the future, the sample size
should be increased, and the follow-up time should be extended to
obtain more accurate research results.
Angiogenesis has been widely recognized as an
important factor in the growth of solid tumors and angiogenesis has
been widely studied in tumor therapy. VM, as a tumor vascular model
that differs from angiogenesis, has been detected in melanoma,
inflammatory breast cancer, liver cancer and other malignant tumor
types with high invasive characteristics (41-43).
VM has a completely different blood supply mode from classical
tumor angiogenesis. Tumor cells imitate the blood vessels in the
body to form a ‘pipe-like’ structure. There is no endothelial cell
covering in the tube, but blood is able to flow through the ‘pipe’.
Since VM morphology in chromatoma was first described, numerous
basic studies have focused on the formation mechanism of VM
(44).
Hypoxia is one of the key conditions inducing VM
formation and the hypoxia-inducible factor is highly localized in
the VM region of tumors (45).
Hypoxia is able to directly regulate the expression of genes such
as those encoding VEGF-A, VEGF receptor 1, EPH receptor A2, Twist,
Nodal, osteopontin and cyclooxygenase-2. It may also directly
promote VM formation (46) and
tumor metastasis (47). The present
study indicated that the VM rate in the low-differentiation group
was 96.6%, which was significantly higher than that in the
high-differentiation group (61.1%). Therefore, the incidence of VM
in highly malignant gallbladder carcinoma is higher than that in
low malignant gallbladder carcinoma, which may be related to
differences in gene expression and the cell phenotype. In addition,
the VM-positive rate in stage I-III gallbladder carcinoma was
68.4%, while it was 94.1% in stage IV/V, with a significant
difference (P<0.05). This indicates that VM was correlated with
the clinical stage of gallbladder carcinoma, with later clinical
stages exhibiting higher VM positivity.
In clinical practice, patients with early
gallbladder carcinoma may have liver metastasis and lymph node
metastasis, and early recurrence after surgery may be associated
with poor prognosis. This is consistent with the present results,
according to which the VM structure was detected in 47 (92.15%) of
51 cases of gallbladder carcinoma when followed up for 36 months
after surgery and the mean TTR was 22.38 months. A total of 4
patients with no VM structure were followed up for 36 months and
had no immediate recurrence after surgery, which may limit the
results of the present study. Thus, patients with VM-positive
gallbladder carcinoma were more likely to have early postoperative
recurrence. These results suggested that VM formation has an
important role in the progression of gallbladder carcinoma. In
addition, there were no significant differences in VM expression in
gallbladder carcinoma tissues between patients with different
sexes, nor between different age and lymph node metastasis groups
(P>0.05). According to the currently known results of clinical
applications, neutralizing monoclonal antibodies and small-molecule
drugs targeting angiogenesis-promoting factors such as VEGF,
platelet-derived growth factor and fibroblast growth factor are not
able to significantly reduce the postoperative recurrence rate and
prolong the survival time (48). By
contrast, inhibition of EDV leads to increased hypoxia in the
tumor, aggravating the phenotypic deterioration of tumor cells and
promoting the formation of VM (49-51).
Therefore, studies to evaluate therapeutic strategies for
inhibiting VM in gallbladder carcinoma and identify drugs targeting
key molecules in the process of VM formation are required.
In the present study, the expression of STAT3 and VM
in gallbladder carcinoma tissues was significantly increased and
positively correlated. In gallbladder carcinoma tissues, a lower
degree of differentiation, higher malignancy degree and higher
clinical stage of malignancy were associated with higher expression
rates of STAT3 and VM. This may indicate that STAT3 directly or
indirectly promotes the structure formation of VM, thus having an
important role in the process of tumor occurrence, development and
metastasis.
It can be concluded from the present study that VM
was associated with poor prognosis of gallbladder carcinoma. As
activation of STAT3 increased the expression of VEGF to promote the
formation of tumor blood vessels, increases in the expression of
STAT3 may promote the formation of gallbladder carcinoma. Future
studies by our group will examine the roles of STAT3 in gallbladder
carcinoma and VM, as well as their possible mechanisms of action.
By regulating the expression levels of STAT3 in gallbladder
carcinoma cells, changes in the expression of STAT3 in gallbladder
carcinoma and the influence of VM may be determined. STAT3, as a
regulatory target, may inhibit the proliferation and invasion of
tumor cells and block the development of VM and EDV, thereby
representing a suitable target for antitumor angiogenesis
therapy.
Acknowledgements
The authors thank Dr Qian Haixin from the Department
of General Surgery (The First Affiliated Hospital of Soochow
University, Suzhou, China) for guidance with the study design and
technical support.
Funding
Funding: This work was supported by a grant from The Fifth
Affiliated Hospital of the Medical School of Nantong University
(Taizhou, China; grant no. ZL201931).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
HZ and HQ designed experiments and wrote the
manuscript. HZ and YY performed experiments and provided technical
support. YY and HQ confirmed the authenticity of the raw data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was reviewed and approved by the ethics
committee of The Fifth Affiliated Hospital of the Medical School of
Nantong University (Taizhou, China). All patients participating in
the present study or their family members signed an informed
consent form to participate in the clinical study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hundal R and Shaffer EA: Galltesticles
Cancer: Epidemiology and outcome. Clin Epidemiol. 6:99–109.
2014.
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Liu S, Wang X and Liu Y: Advances in
comprehensive treatment of gallbladder cancer. Surgery Concepts
& Practice. 21:365–368. 2016.
|
|
4
|
Folkman J: Tumor angiogenesis: Therapeutic
implications. N Engl J Med. 285:1182–1186. 1971.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Niu G, Wright KL, Huang M, Song L, Haura
E, Turkson J, Zhang S, Wang T, Sinibaldi D, Coppola D, et al:
Constitutive Stat3 activity up-regulates VEGF expression and tumor
angiogenesis. Oncogene. 21:2000–2008. 2002.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lee SE, Jang JY, Lim CS, Kang MJ and Kim
SW: Systematic review on the surgical treatment for T1 gallbladder
cancer. World J Gastroenterol. 17:174–180. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Isambert M, Leux C, Métairie S and Paineau
J: Incidentally-discovered gallbladder cancer: When, why and which
reoperation. J Visc Surg. 148:e77–e84. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Cavallaro A, Piccolo G, Panebianco V, Lo
Menzo E, Berretta M, Zanghì A, Di Vita M and Cappellani A:
Incidental gallbladder cancer during laparoscopic cholecystectomy:
Managing an unexpected finding. World J Gastroenterol.
18:4019–4027. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Horiguchi A, Miyakawa S, Ishihara S,
Miyazaki M, Ohtsuka M, Shimizu H, Sano K, Miura F, Ohta T, Kayahara
M, et al: Gallbladder bed resection or hepatectomy of segments 4a
and 5 for pT2 gallbladder carcinoma: Analysis of Japanese
registration cases by the study group for biliary surgery of the
Japanese Society of Hepato-Biliary-Pancreatic Surgery. J
Hepatobiliary Pancreat Sci. 20:518–524. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Fathi N, Rashidi G, Khodadadi A, Shahi S
and Sharifi S: STAT3 and apoptosis challenges in cancer. Int J Biol
Macromol. 117:993–1001. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kitamura H, Ohno Y, Toyoshima Y, Ohtake J,
Homma S, Kawamura H, Takahashi N and Taketomi A:
Interleukin-6/STAT3 signaling as a promising target to improve the
efficacy of cancer immunotherapy. Cancer Sci. 108:1947–1952.
2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Nagakura S, Shirai Y, Yokoyama N and
Hatakeyama K: Clinical significance of lymph node micrometastasis
in gallbladder carcinoma. Surgery. 129:704–713. 2001.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wang H, Zhan M, Liu Q and Wang J:
Glycochenodeoxycholate promotes the metastasis of gallbladder
cancer cells by inducing epithelial to mesenchymal transition via
activation of SOCS3/JAK2/STAT3 signaling pathway. J Cell Physiol.
235:1615–1623. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Fernandez-Cortes M, Delgado-Bellido D and
Oliver FJ: Vasculogenic mimicry: Become an endothelial cell ‘but
not so much’. Front Oncol. 9:803–809. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kim SH, Lee HS, Kang BJ, Song BJ, Kim HB,
Lee H, Jin MS and Lee A: Dynamic contrast-enhanced MRI perfusion
parameters as imaging biomarkers of angiogenesis. PLoS One.
11(E0168632)2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Dutta S, Going JJ, Crumley AB, Mohammed Z,
Orange C, Edwards J, Fullarton GM, Horgan PG and McMillan DC: The
relationship between tumour necrosis, tumour proliferation, local
and systemic inflammation, microvessel density and survival in
patients undergoing potentially curative resection of oesophageal
adenocarcinoma. Brit J Cancer. 106:702–710. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lazcano-Ponce EC, Miquel JF, Muñoz N,
Herrero R, Ferrecio C, Wistuba II, Alonso de Ruiz P, Aristi Urista
G and Nervi F: Epidemiology and molecular pathology of gallbladder
cancer. CA Cancer J Clin. 51:349–364. 2001.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lesina M, Kurkowski MU, Ludes K, Rose-John
S, Treiber M, Klöppel G, Yoshimura A, Reindl W, Sipos B, Akira S,
et al: Stat3/Socs3 activation by IL-6 transsignaling promotes
progression of pancreatic intraepithelial neoplasia and development
of pancreatic cancer. Cancer Cell. 19:456–69. 201l.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Edge S, Byrd DR, Compton CC, Fritz AG,
Greene F and Trotti A (eds): AJCC Cancer Staging Manual. 7th
edition, Springer, New York, 2010. https://www.springer.com/us/book/9780387884424.
|
|
20
|
Maniotis AJ, Folberg R, Hess A, Seftor EA,
Gardner LM, Pe'er J, Trent JM, Meltzer PS and Hendrix MJ: Vascular
channel formation by human melanoma cells in vivo and in vitro:
Vasculogenic mimicry. Am J Pathol. 155:739–752. 1999.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Okayasu I and Hara A: Cyclooxygenase-2 and
inducible nitric oxide synthase expression in human astrocytic
gliomas: Correlation with angiogenesis and prognostic significance.
Acta Neuropathol. 108:43–48. 2004.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Li C, Chen J and Qin G: Partial Youden
index and its inferences. J Biopharm Stat. 29:385–399.
2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lee SE, Jang JY, Lim CS, Kang MJ and Kim
SW: Systematic review on the surgical treatment for T1 gallbladder
cancer. World J Gastroenterol. 17:174–180. 2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Fratto ME, Santini D, Vincenzi B,
Silvestris N, Azzariti A, Tommasi S, Zoccoli A, Galluzzo S, Maiello
E, Colucci G and Tonini G: Targeting EGFR in bilio-pancreatic and
liver carcinoma. Front Biosci (Schol Ed). 3:16–22. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
25
|
Chen Y, Jiang L, She F, Tang N, Wang X, Li
X, Han S and Zhu J: Vascular endothelial growth factor-C, promotes
the growth and invasion of gallbladder cancer via an autocrine
mechanism. Mol Cell Biochem. 345:77–89. 2010.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lirdprapamongkol K, Sakurai H, Abdelhamed
S, Yokoyama S, Athikomkulchai S, Viriyaroj A, Awale S, Ruchirawat
S, Svasti J and Saiki I: Chrysin overcomes TRAIL resistance of
cancer cells through Mcl-1 downregulation by inhibiting STAT3
phosphorylation. Int J Oncol. 43:329–337. 2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wu YS, Chung I, Wong WF, Masamune A, Sim
MS and Looi CY: Paracrine IL-6 signaling mediates the effects of
pancreatic stellate cells on epithelial-mesenchymal transition via
Stat3/Nrf2 pathway in pancreatic cancer cells. Biochim Biophys Acta
Gen Subj. 1861:296–306. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
O'Sullivan T, Saddawi-Konefka R, Vermi W,
Koebel CM, Arthur C, White JM, Uppaluri R, Andrews DM, Ngiow SF,
Teng MW, et al: Cancer immunoediting by the innate immune system in
the absence of adaptive immunity. J Exp Med. 209:1869–1882.
2012.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhang Y, Guo S, Fang J, Peng B, Zhang Y
and Cao T: Tanshinone IIA inhibits cell proliferation and tumor
growth by downregulating STAT3 in human gastric cancer. Exp Ther
Med. 16:2931–2937. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ke F, Wang Z, Song X, Ma Q, Hu Y, Jiang L,
Zhang Y, Liu Y, Zhang Y and Gong W: Cryptotanshinone induces cell
cycle arrest and apoptosis through the JAK2/STAT3 and PI3K/Akt/NFκB
pathways in cholangiocarcinoma cells. Drug Des Devel Ther.
11:1753–1766. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ruiz P and Günthert U: The cellular basis
of metastasis. World J Urol. 14:141–150. 1996.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Sohn EJ, Jung DB, Lee H, Han I, Lee J, Lee
H and Kim SH: CNOT2 promotes proliferation and angiogenesis via
VEGF signaling in MDA-MB-231 breast cancer cells. Cancer Lett.
412:88–98. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Chen Z and Han ZC: STAT3: A critical
transcription activator in angiogenesis. Med Res Rev. 28:185–200.
2008.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Okazaki H, Tokumaru S, Hanakawa Y,
Shiraishi K, Shirakata Y, Dai X, Yang L, Tohyama M, Hashimoto K and
Sayama K: Nuclear translocation of phosphorylated STAT3 regulates
VEGF-A-induced lymphatic endothelial cell migration and tube
formation. Biochem Biophys Res Commun. 412:441–445. 2011.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wang Y, Shen Y, Wang S, Shen Q and Zhou X:
The role of STAT3 in leading the crosstalk between human cancers
and the immune system. Cancer Lett. 415:117–128. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Bao W, Wang HH, Tian FJ, He XY, Qiu MT,
Wang JY, Zhang HJ, Wang LH and Wan XP: A TrkB-STAT3-miR-204-5p
regulatory circuitry controls proliferation and invasion of
endometrial carcinoma cells. Mol Cancer. 12(155)2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Kruczyk M, Przanowski P, Dabrowski M,
Swiatek-Machado K, Mieczkowski J, Wallerman O, Ronowicz A,
Piotrowski A, Wadelius C, Kaminska B and Komorowski J: Integration
of genome-wide of Stat3 binding and epigenetic modification mapping
with transcriptome reveals novel Stat3 target genes in glioma
cells. Biochim Biophys Acta. 1839:1341–1350. 2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Cheng GZ, Zhang WZ, Sun M, Wang Q, Coppola
D, Mansour M, Xu LM, Costanzo C, Cheng JQ and Wang LH: Twist is
transcriptionally induced by activation of STAT3 and mediates STAT3
oncogenic function. J Biol Chem. 283:14665–14673. 2008.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Cao C, Zhao G, Yu W, Xie X, Wang W, Yang
R, Lv X and Liu D: Activation of STAT3 stimulates AHSP expression
in K562 cells. Sci China Life Sci. 57:488–494. 2014.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Enyu L, Na W, Chuanzong Z, Ben W, Xiaojuan
W, Yan W, Zequn L, Jianguo H, Jiayong W, Benjia L, et al: The
clinical significance and underlying correlation of pStat-3 and
integrin αvβ6 expression in gallbladder cancer. Oncotarget.
8:19467–19477. 2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Sun B, Zhang D, Zhao N and Zhao X:
Epithelial-to-endothelial transition and cancer stem cells: Two
cornerstones of vasculogenic mimicry in malignant tumors.
Oncotarget. 8:30502–30510. 2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Vartanian A, Karshieva S, Dombrovsky V and
Belyavsky A: Melanoma educates mesenchymal stromal cells towards
vasculogenic mimicry. Oncol Lett. 11:4264–4268. 2016.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Pereira JA, Bilhim T, Rio Tinto H,
Fernandes L, Martins Pisco J and Goyri-O'Neill J: Radiologic
anatomy of arteriogenic erectile dysfunction: A systematized
approach. Acta Med Port. 26:219–225. 2013.PubMed/NCBI
|
|
44
|
Bissell MJ: Tumor plasticity allows
vasculogenic mimicry, a novel form of angiogenic switch. A rose by
anyother name? Am J Pathol. 155:675–679. 1999.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Wang HF, Wang SS, Zheng M, Dai LL, Wang K,
Gao XL, Cao MX, Yu XH, Pang X, Zhang M, et al: Hypoxia promotes
vasculogenic mimicry formation by vascular endothelial growth
factor A mediating epithelial-mesenchymal transition in salivary
adenoid cystic carcinoma. Cell Prolif. 52(e12600)2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Li S, Meng W, Guan Z, Guo Y and Han X: The
hypoxia-related signaling pathways of vasculogenic mimicryin tumor
treatment. Biomed Pharmacother. 80:127–135. 2016.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Liu K, Sun B, Zhao X, Wang X, Li Y, Qiu Z,
Liu T, Gu Q, Dong X, Zhang Y, et al: Hypoxia promotes vasculogenic
mimicry formation by the Twist1-Bmi1 connection in hepatocellular
carcinoma. Int J Mol Med. 36:783–791. 2015.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Sun H, Zhang D, Yao Z, Lin X, Liu J, Gu Q,
Dong X, Liu F, Wang Y, Yao N, et al: Anti-angiogenic treatment
promotes triple-negative breast cancer invasion via vasculogenic
mimicry. Cancer Biol Ther. 18:205–213. 2017.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Hendrix MJ, Seflor EA, Seflor RE, Chao JT,
Chien DS and Chu YW: Tumor cell vascular mimicry: Novel targeting
opportunity in melanoma. Pharmacol Ther. 159:83–92. 2016.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Huang B, Xiao E and Huang M: MEK/ERK
pathway is positively involved in hypoxia-induced vasculogenic
mimicry formation in hepatocellular carcinoma which is regulated
negatively by protein kinase A. Med Oncol. 32(408)2015.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Vacca A, Ria R, Reale A and Ribatti D:
Angiogenesis in multiple myeloma. Chem Immunol Allergy. 99:180–196.
2014.PubMed/NCBI View Article : Google Scholar
|