Introduction
Leukemia is the most common type of childhood
malignancy worldwide (1). Every
year, >10,000 new cases of acute lymphocytic leukemia (ALL) in
children occur in China, and T-ALL accounts for 15% of them
(2-5).
T-ALL is caused by the diffuse bone marrow infiltration of immature
T lymphoblasts, which clinically manifests as central nervous
system infiltration and mediastinal mass with pleural effusion in
the early stage (6). T-ALL is far
more difficult to treat than B-ALL and has a poor long-term
prognosis (7,8) resulting from poor sensitivity and
resistance to chemotherapeutic drugs, issues with remission and
clearance of bone marrow microresidual lesions, and a high
recurrence rate in the central nervous system (9,10).
With the advancement of chemotherapy regimens, the 5-year
disease-free survival rate of pediatric T-ALL has substantially
improved (11-13).
However, the adverse effects of the high-intensity chemotherapy
regimen are unfavorable, including liver and kidney damage, as well
as severe infections secondary to bone marrow suppression (14,15).
Hematopoietic stem cell transplantation may maximize the treatment
efficiency of refractory ALL, but due to the shortage of donor
sources and low degree of matching, as well as insufficient
economic resources, its benefit is limited in the majority of cases
of pediatric T-ALL (16,17). Therefore, there is an urgent
requirement to discover novel therapeutic targets and develop drugs
for the treatment of T-ALL.
By screening a natural compound library owned by
State Key Laboratory of Quality Research in Chinese Medicine
(Macau, China), it was revealed that hirsutanol A exerted a clear
inhibitory effect on the viability of the T-ALL cell line Jurkat.
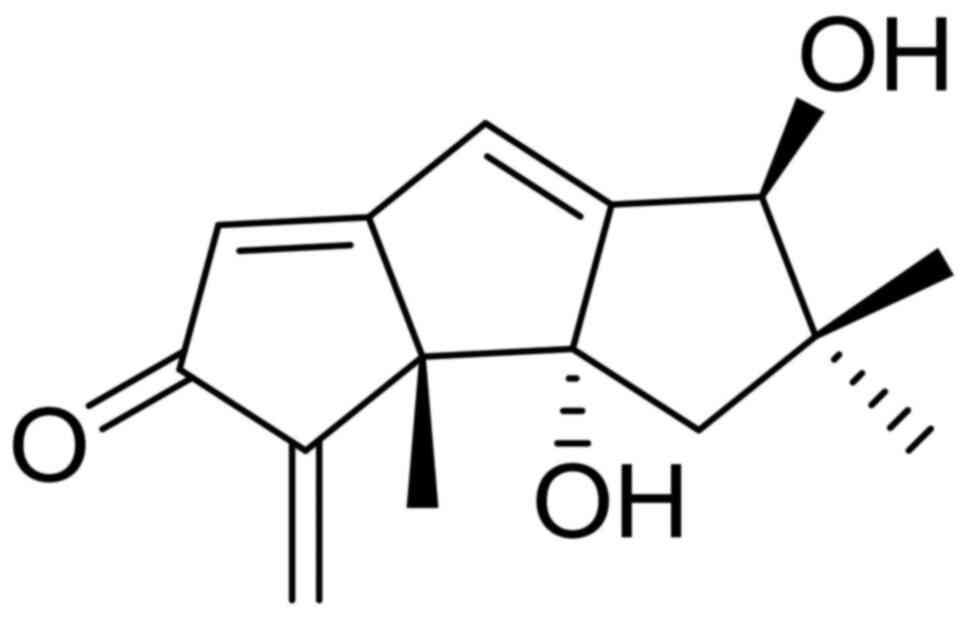
Hirsutanol A is a sesquiterpenoid compound initially isolated from
an unidentified fungus isolated from the Indo-Pacific sponge
Haliclona sp. with antitumor, antibacterial and antioxidant
activities (18). The chemical
structure of hirsutanol A is presented in Fig. 1 (18). Subsequent studies have indicated
that hirsutanol A potently inhibits the proliferation of a variety
of tumor cells by increasing the level of reactive oxygen species,
and inducing apoptosis and autophagy (19,20).
However, these studies were focused on solid tumor cells, including
liver and breast cancer, and to the best of our knowledge, the
antileukemia activities of hirsutanol A have not been previously
assessed.
The aim of the present study was to evaluate the
in vitro anticancer activity of hirsutanol A against T-acute
lymphocytic leukemia Jurkat cells by Cell Counting Kit-8 assay,
western blot analysis and other related biological techniques, and
to explore the mechanism of action. It is hoped to lay the
experimental foundation for finding a new drug to treat T-ALL.
Materials and methods
Cell lines and culture
The human T-ALL cell line Jurkat and the human
T-lymphoid cell line H9 were purchased from the American Type
Culture Collection. Cells were cultured in complete medium
containing 90% RPMI-1640 (HyClone; Cytiva) and 10% FBS (Hangzhou
Sijiqing Biological Engineering Materials Co., Ltd.) with 1%
penicillin-streptomycin mixture (Beijing Solarbio Science &
Technology Co., Ltd.) in an incubator with 5% CO2 and
saturated humidity at 37˚C.
Reagents
Hirsutanol A, a sesquiterpenoid compound isolated
and synthesized in the laboratory of Professor Lan Wenjian, School
of Pharmacy, Sun Yat-sen University, China, was dissolved in DMSO
and stored in a refrigerator at -40˚C until use. p53 inhibitor
pifithrin-α (PFTα) was purchased from APeXBIO Technology LLC. The
Cell Counting Kit-8 (CCK-8) was purchased from Dojindo Molecular
Technologies Inc. Hoechst 33258 was purchased from Beijing Solarbio
Science & Technology Co., Ltd. An apoptosis test kit (Annexin
V-FITC Apoptosis Detection Kit I) was purchased from BD
Biosciences, and the cell cycle detection kit was purchased from
Nanjing KeyGen Biotech Co., Ltd. Rabbit anti-human p53 (1:1,000;
cat. no. 2527), p53 upregulated modulator of apoptosis (Puma;
1:1,000; cat. no. 12450), Bcl-2 (1:1,000; cat. no. 4223), Caspase-3
(1:1,000; cat. no. 9662), poly (ADP-ribose) polymerase (PARP;
1:1,000; cat. no. 9532), cleaved caspase-3 (cCaspase-3; 1:1,000;
cat. no. 9664) and cleaved poly(ADP-ribose) polymerase (cPARP;
1:1,000; cat. no. 9185) monoclonal antibodies were purchased from
Cell Signaling Technology, Inc. The rabbit anti-human GAPDH
(1:1,000; cat. no. 10494-1-AP) primary antibody was purchased from
ProteinTech Group, Inc. The horseradish peroxidase-labeled goat
anti-rabbit secondary antibody (1:1,000; cat. no. A0208) was
purchased from Shanghai Beyotime Biotechnology Co., Ltd.
Cell viability assay
Jurkat cells were collected and resuspended in
medium to adjust the cell density to 2x105 cells/ml.
This cell suspension was added to a 96-well plate at 90 µl/well.
Subsequently, hirsutanol A (10 µl/well) at different concentrations
(0.71, 1.42, 2.84, 5.68 and 11.36 µM) was added for incubation. In
parallel, a blank group and a control group (containing only cells
and culture medium) were used, and each group was set up in 3
duplicate wells. Following incubation for 48 h, 5 µl CCK-8 solution
was added to each well, followed by incubation for another 4 h. The
optical density (OD) value of each well was measured using an
ultraviolet spectrophotometer (Beckman Coulter, Inc.) at a
wavelength of 450 nm. Human T lymphocyte H9 cells were treated with
a series of hirsutanol A concentrations (2, 4, 8, 16, and 32 µM)
for 48 h, and the other experimental procedures were the same as
Jurkat cells. In addition, Jurkat cells were treated with 5 µM
hirsutanol A, and viability was assessed at different time points
(24, 48, and 72 h) after treatment. The cell viability rate was
calculated as follows: Cell viability rate (%) = (OD value of the
experimental group - OD value of the blank group) / (OD value of
the control group - OD value of the blank group). The half-maximal
inhibitory concentration (IC50) of hirsutanol A was
determined as the concentration of the drug leading to 50% cell
inhibition.
Apoptosis assay
Cell apoptosis was assessed using the Annexin V-FITC
Apoptosis Detection Kit I. Jurkat cells were collected and
resuspended to adjust the cell density to 2x105
cells/ml. This cell suspension was added to a 6-well plate at 2
ml/well and different concentrations (0, 3, 5, 6, and 9 µM) of
hirsutanol A and/or PFTα (10 µM) were added. After 48 h of
incubation at 37˚C, the cells were collected, washed twice with
cold PBS and resuspended with 1X binding buffer. Of this
suspension, 100 µl (~1x105 cells/ml) were placed in a
5-ml culture tube and propidium iodide (PI; 5 µl) and Annexin
V-FITC (5 µl) were added. Following incubation for 15 min in the
dark at room temperature, 200 µl 1X binding buffer was added per
tube and apoptotic cells were detected using a BD FACScan™ flow
cytometer (BD Biosciences) within 1 h. The results were analyzed
using BD FACSuite™ (BD Biosciences). Early and late apoptosis were
assessed.
Cell cycle analysis
The cell cycle was assessed using the cell cycle
detection kit. Jurkat cells were collected and resuspended to
adjust the cell density to 2x105 cells/ml. This cell
suspension was added to a 6-well plate at 2 ml/well and different
concentrations (0, 3, 5, 6, and 9 µM) of hirsutanol A and/or PFTα
(10 µM) were added. After 48 h of incubation, the cells were
collected, washed with PBS and fixed with 70% cold ethanol at 4˚C
overnight. Following centrifugation (300 x g at 4˚C and 5 min) and
washing with PBS, 400 µl PI/RNase A staining solution (PI/RNase A
ratio, 1:9; prepared in advance) was added, followed by incubation
at room temperature in the dark for 30 min. Subsequently, the cell
cycle was assessed using a BD FACScan™ flow cytometer. The results
were analyzed using ModFit LT 4.0 (Verity Software House,
Inc.).
Hoechst 33258 staining assay
Jurkat cells were collected and resuspended to
adjust the cell density to 2x105 cells/ml. The
suspension was added to a 6-well plate at 2 ml/well. Following the
addition of different concentrations (0, 3, 6, and 9 µM) of
hirsutanol A, the cells were incubated for 48 h. Subsequently, the
cells were collected, Hoechst 33258 working solution (5 µg/ml) was
added to fully cover the cells and the cells were then placed in an
incubator at 37˚C for 30 min. The staining solution was discarded
via centrifugation (300 x g at room temperature for 5 min) and the
cells were washed with PBS twice, followed by observation via
fluorescence microscopy (maximum excitation wavelength, 352 mm;
maximum emission wavelength, 461 mm). Each sample was viewed from
five fields with roughly the same number of cells.
Mitochondrial membrane potential
detection
JC-1 buffer (1X) was prepared from 5X JC-1 buffer
(Beijing Solarbio Science & Technology Co., Ltd) and distilled
water at a ratio of 1:4 and maintained in an ice bath for later
use. Jurkat cells were collected and resuspended to adjust the cell
density to 2x105 cells/ml. This cell suspension was
transferred to a 6-well plate at 2 ml/well and the cells were
treated with different concentrations (0, 3, 6, and 9 µM) of
hirsutanol A for 48 h. The cells were then collected and
resuspended in medium, JC-1 staining solution was added and then
the suspension was inverted several times to mix. The cells were
cultured in the incubator at 37˚C for 30 min. Following
centrifugation (300 x g at 4˚C and 5 min), the supernatant was
discarded and the cells were washed twice with JC-1 buffer (1X).
The cells were then observed under a fluorescence microscope. Each
sample was viewed from five fields with roughly the same number of
cells.
Western blot analysis
Jurkat cells treated with different concentrations
(0, 3, 5 and 6 µM) of hirsutanol A and/or PFTα (10 µM) were
collected and washed twice with PBS. Then, lysis buffer (80:10:10:1
ratio of RIPA lysate (Beyotime Institute of Biotechnology),
protease inhibitor, phosphatase inhibitor and PMSF) was added, and
cells were lysed on ice for 30 min. Following centrifugation at
4,000 x g for 10 min at 4˚C, the supernatant was obtained and the
protein concentration was measured via the bicinchoninic acid
method. The supernatant was mixed with SDS-PAGE protein loading
buffer (5X) and the mixture was placed in a metal bath at 100˚C for
5 min to denature the protein. Protein samples (20 µg/lane) were
separated via 10% SDS-PAGE (the current and time were appropriately
adjusted according to different proteins), followed by transfer
onto Immobilon® polyvinylidene difluoride membranes
(Sigma-Aldrich; Merck KGaA) and blocking with 5% skimmed milk at
room temperature for 1 h. The membrane was incubated with the
aforementioned primary antibodies at 4˚C overnight and then
incubated with a secondary antibody for 1 h at room temperature.
The bands were visualized using an enhanced chemiluminescence kit
(EMD Millipore) and images were captured on a chemiluminescence
imager (Vilber Lourmat). GAPDH was used as the loading control.
Protein expression was semi-quantified using ImageJ version 2.0
software (National Institutes of Health).
Statistical analysis
The data were analyzed with SPSS 19.0 software
(SPSS, Inc.) and expressed as the mean ± standard deviation of at
least three independent experiments. Differences between two groups
were assessed using the paired Student's t-test. Differences
amongst multiple groups were evaluated using one-way ANOVA followed
by Tukey's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Hirsutanol A inhibits the viability of
Jurkat cells
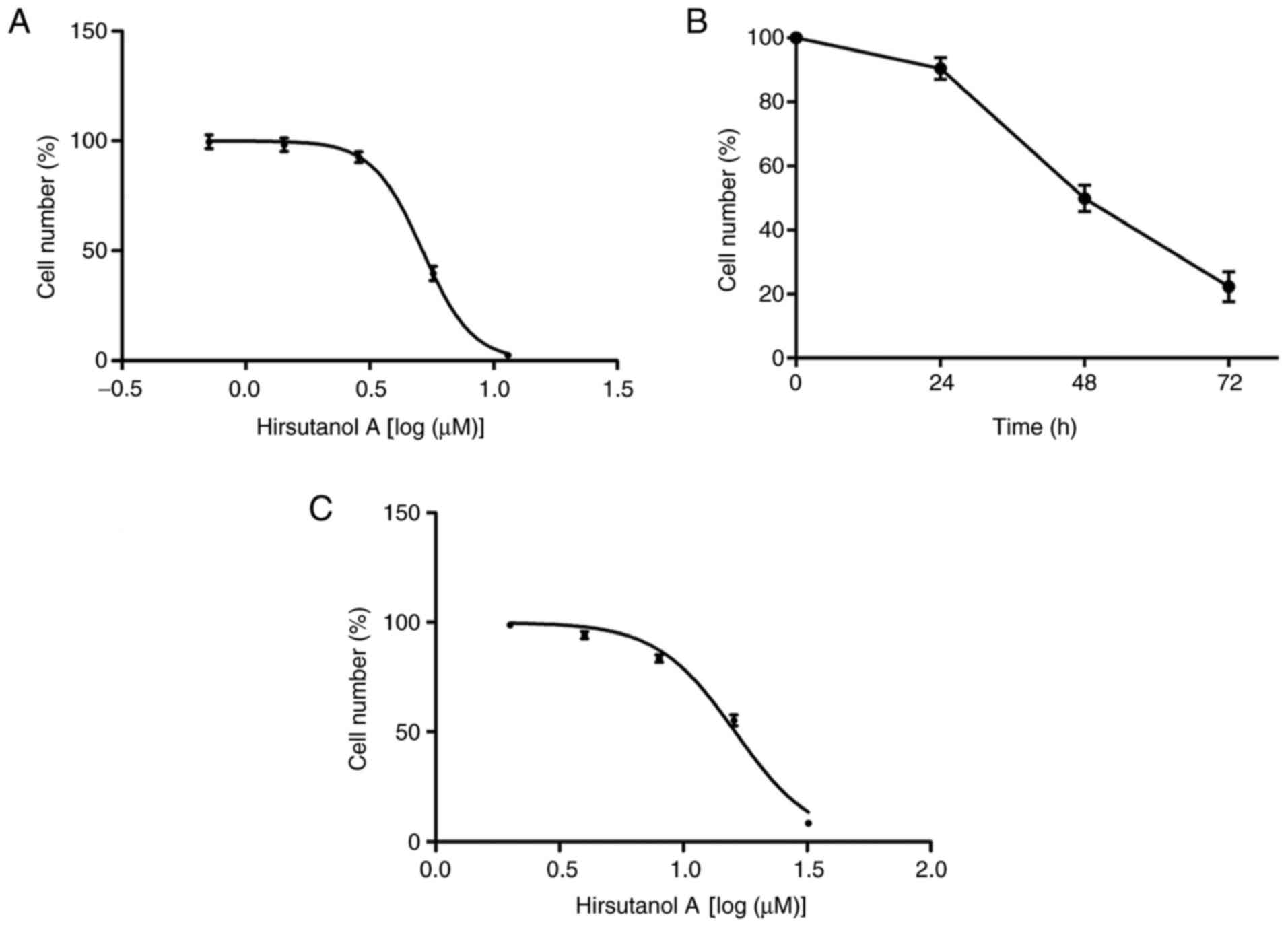
The antiproliferative effect of hirsutanol A on the
T-ALL cell line Jurkat was examined using a CCK-8 assay. Jurkat
cells were treated with a series of hirsutanol A concentrations
from 0.71 to 11.44 µM for 48 h. As presented in Fig. 2A, hirsutanol A markedly reduced the
viability of Jurkat cells in a concentration-dependent manner, with
an IC50 value of 5.16 µM. Therefore, three
concentrations (3, 6 and 9 µM) were selected in the subsequent
experiments. Furthermore, human T lymphocyte H9 cells were treated
with a series of hirsutanol A concentrations from 2 to 32 µM for 48
h, displaying an IC50 value of 16.22 µM (Fig. 2C). These results indicated that
hirsutanol A was less toxic to the normal cell line compared with
the T-ALL cell line. Consistent with the dose effect of hirsutanol
A, the viability of Jurkat cells was also inhibited in a
time-dependent manner. Jurkat cells were then treated with 5 µM
hirsutanol A, and cytotoxicity was assessed at different time
points after treatment. At 24 h, the cell viability rate of Jurkat
cells was 89.24±2.58%, which declined to 21.83±0.61% at 72 h
(Fig. 2B). Thus, these results
indicated that hirsutanol A inhibited the viability of T-ALL Jurkat
cells in a dose- and time-dependent manner.
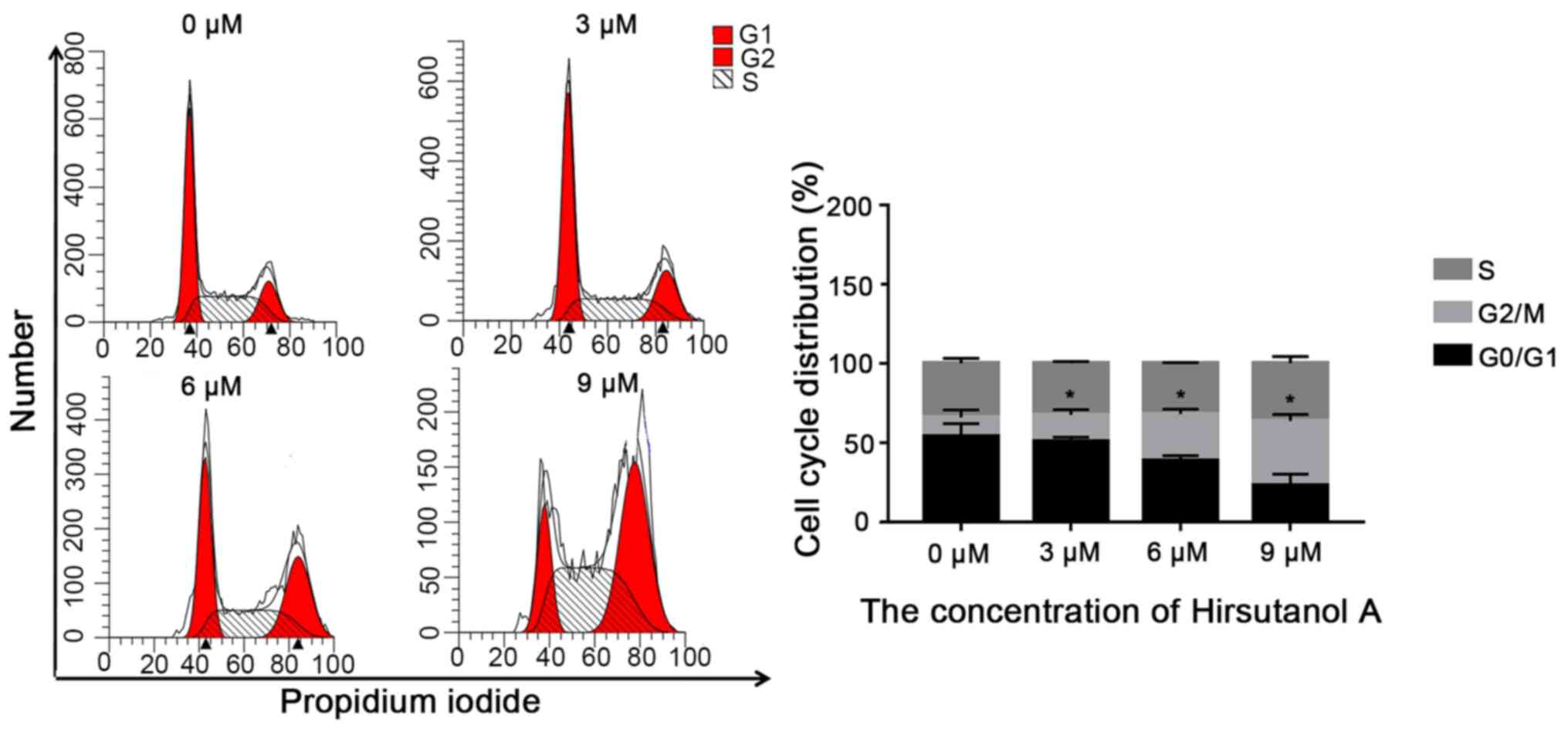
Hirsutanol A inhibits cell cycle
progression in Jurkat cells
It is well known that cell cycle arrest is a key
intracellular event contributing to reduced cell proliferation
(21). It was therefore assessed
whether hirsutanol A altered the cell cycle progression of Jurkat
cells via flow cytometric analysis. As presented in Fig. 3, after 48 h of hirsutanol A
treatment, the population of Jurkat cells in the G2
phase increased significantly, whereas there was no significant
change in the proportion of cells in the S phase compared with the
control group, suggesting that hirsutanol A blocked the cell cycle
of Jurkat cells in the G2 phase.
Hirsutanol A induces apoptosis in
Jurkat cells
To further clarify whether the inhibitory effect of
hirsutanol A on Jurkat cell viability resulted from the induction
of apoptosis, Jurkat cells were treated with a series of
concentrations of the compound for 48 h, stained with Hoechst 33258
and then visualized under a fluorescence microscope. As presented
in Fig. 4A, with increasing
hirsutanol A concentrations, Jurkat cells exhibited typical
morphological characteristics of apoptosis, including nuclear
fragmentation and chromatin condensation. The induction of
apoptosis was further demonstrated via flow cytometric analysis of
cells stained with Annexin V-FITC/PI. The results indicated that,
compared with the control group, hirsutanol A significantly induced
the apoptosis of Jurkat cells in a dose-dependent manner (Fig. 4B and C). The percentages of total apoptotic
cells following hirsutanol A treatment for 48 h were 15.94±0.33% at
3 µM, 47.53±0.74% at 6 µM and 81.25±1.42% at 9 µM, compared with
5.72±0.31% in the control group.
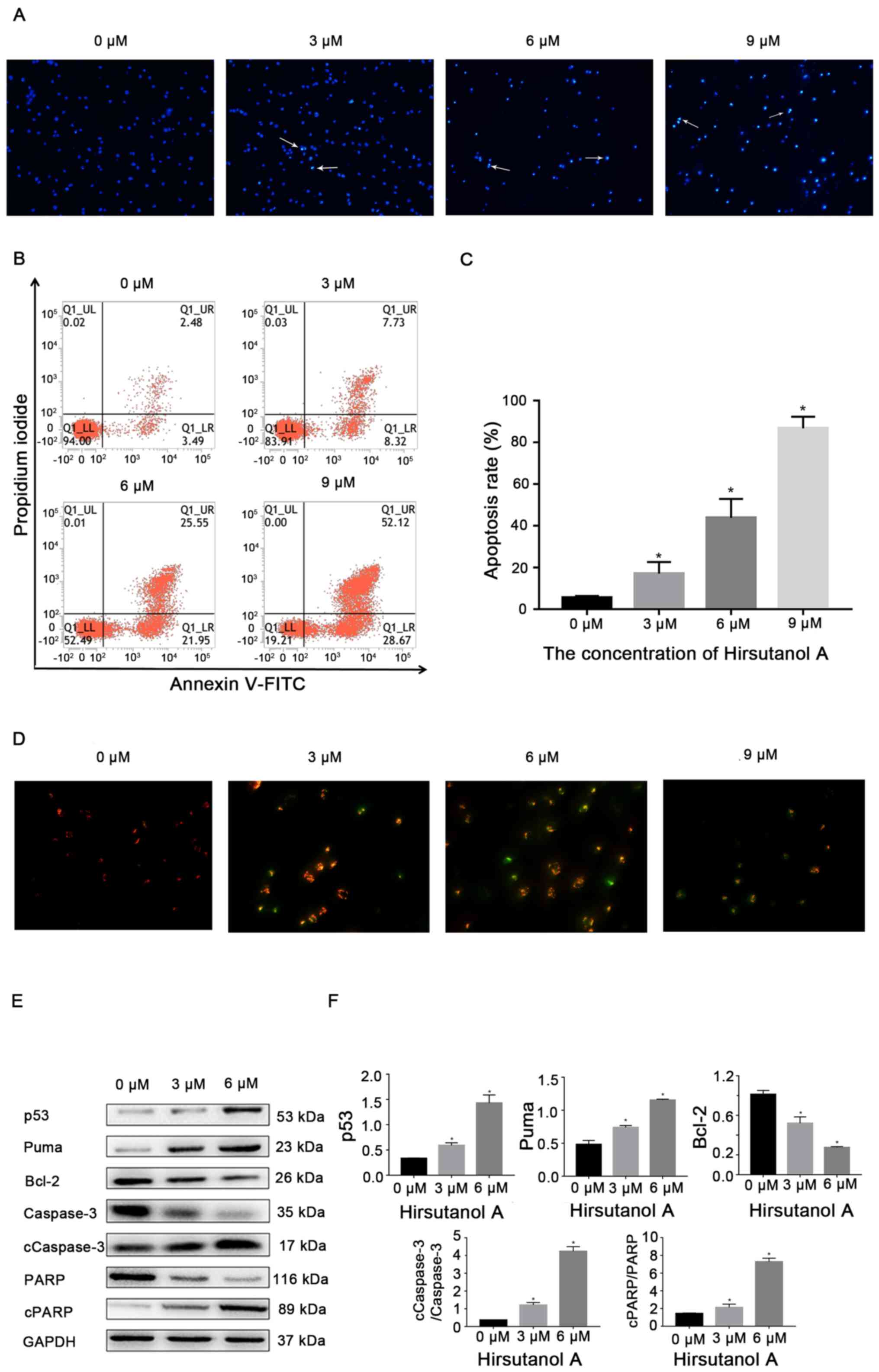 | Figure 4Hirsutanol A induces apoptosis in
Jurkat cells. Jurkat cells were treated with hirsutanol A at
indicated concentrations for 48 h. (A) Visualization of apoptotic
morphological changes using a fluorescent microscope following
Hoechst 33258 staining. Representative images are provided and
apoptotic cells are indicated by arrows (magnification, x200). (B)
Representative dot plots of the flow cytometric analysis of
apoptosis. (C) Fractions of apoptotic cells were quantified. (D)
Changes in mitochondrial membrane potential were detected using
JC-1 (magnification, x400). The decreased red/green fluorescence
ratio with hirsutanol A treatment indicated mitochondrial
depolarization and early stages of apoptosis in Jurkat cells. With
increasing concentrations, the level of green fluorescence,
representing the early stage of apoptosis, increased. (E) Western
blot analysis of apoptosis- and survival-associated proteins. (F)
Semi-quantification of p53, Puma, Bcl-2, cCaspase-3 and cPARP
protein expression levels. *P<0.05 vs. 0 µM. Data are
presented as the mean ± standard deviation of three independent
experiments. c, cleaved; PARP, poly(ADP-ribose) polymerase; Puma,
p53 upregulated modulator of apoptosis. |
The mitochondrial pathway is one of the most
classical pathways of cell apoptosis (22). In the early stage of cell apoptosis,
the mitochondrial structure undergoes certain crucial changes, one
of which is loss of the mitochondrial membrane potential (23,24).
Therefore, in the present study, changes in the mitochondrial
membrane potential of Jurkat cells after 48 h of hirsutanol A
treatment at different concentrations were detected via JC-1
staining. As presented in Fig. 4D,
the green fluorescence was markedly increased in a hirsutanol A
dose-dependent manner, indicating that cells lost their
mitochondrial membrane potential following hirsutanol A
treatment.
Consistent with the aforementioned results of
hirsutanol A inducing apoptosis, compared with the control group,
the expression levels of cCaspase-3 and cPARP, well-known apoptotic
markers (25), increased
significantly following hirsutanol A treatment in a dose-dependent
manner (Fig. 4E and F). Furthermore, the expression levels of
Puma were significantly increased, whereas the expression of Bcl-2
was significantly decreased after hirsutanol A treatment compared
with the control group. As hypothesized, compared with the control
group, the expression of p53 in Jurkat cells increased
significantly after hirsutanol A treatment in a
concentration-dependent manner.
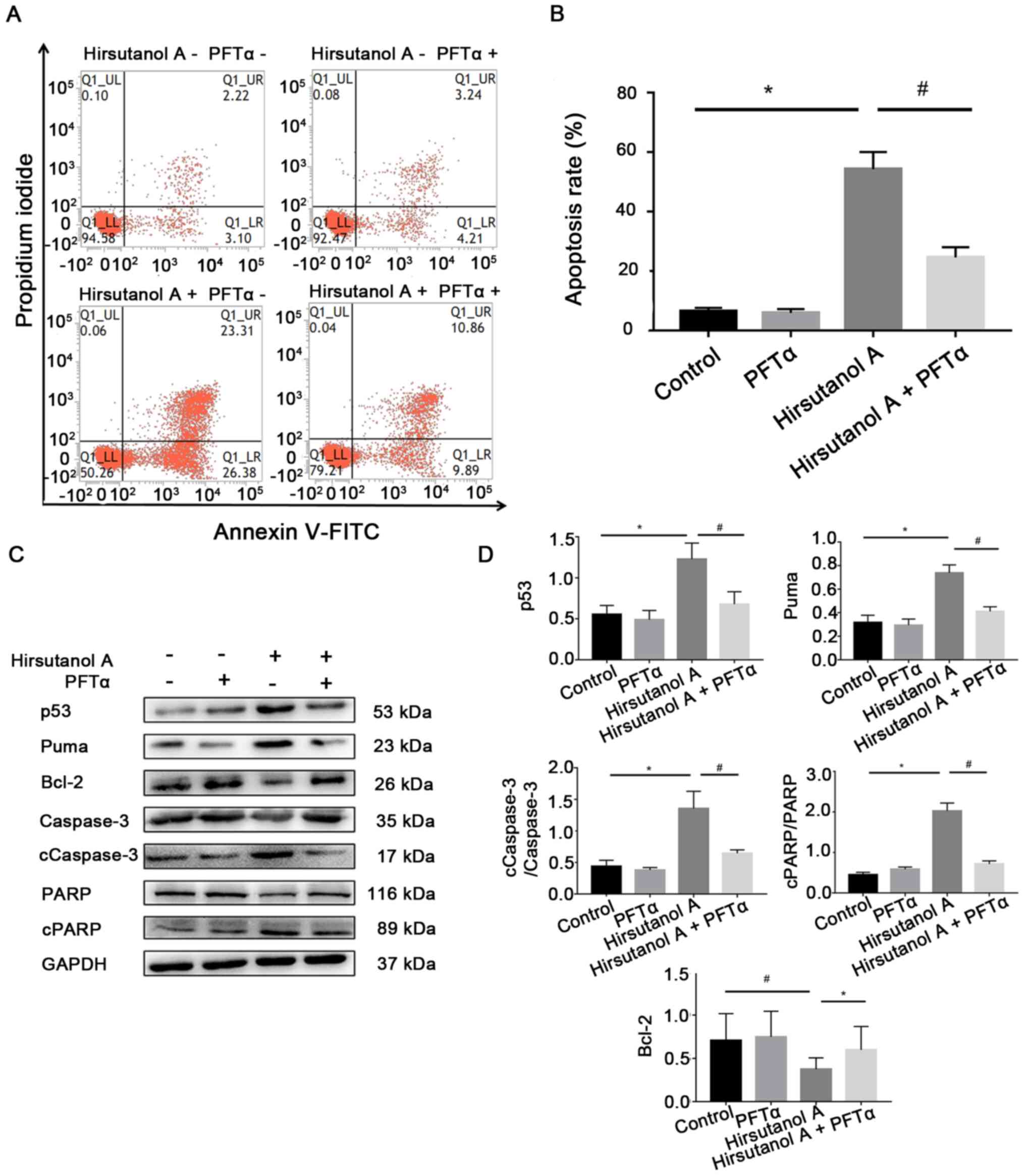
Hirsutanol A induces the apoptosis of
Jurkat cells via the p53 pathway
As p53 is one of the master regulators of cell
proliferation and cell death (26),
whether the induction of apoptosis of Jurkat cells by hirsutanol A
was dependent on p53 was assessed. For this, Jurkat cells were
treated with the p53 inhibitor PFTα in combination with hirsutanol
A. As presented in Fig. 5,
apoptosis induced by hirsutanol A was significantly inhibited from
48.92±0.83 to 19.24±0.46% by PFTα. Consistently, the expression
levels of proapoptotic proteins (p53, Puma, cCaspase-3 and cPARP)
were significantly decreased in the group treated with hirsutanol A
+ PFTα compared with those in the group treated with hirsutanol A
alone, whereas the expression of the antiapoptotic protein Bcl-2
was significantly upregulated (Fig.
5). Collectively, the present results indicated that hirsutanol
A inhibited the proliferation of Jurkat cells by inducing cell
cycle arrest and p53-dependent apoptosis.
Discussion
At present, chemotherapy remains the major treatment
strategy for T-ALL, which has substantially improved the 5-year
survival rate of childhood leukemia (27). However, side effects and cancer cell
resistance limit the benefits of chemotherapeutic drugs in current
clinical use. Marine organisms live in a special environment of low
oxygen, high pressure and high salt, and their metabolites may have
clinically relevant biological activities (28). Therefore, the search for efficient
and low-toxicity natural medicines from the ocean has attracted
increasing interest. Hirsutanol A is a sesquiterpenoid compound
extracted from marine organisms and its antitumor activity is
widely recognized (29,30). At present, studies into the
antitumor activity of hirsutanol A are limited to solid tumors,
including liver and nasopharyngeal cancer (29,31).
To the best of our knowledge, the present study was the first to
explore its inhibitory effect on the proliferation of leukemia
cells, a type of blood cancer cell.
In the present study, to the best of our knowledge,
it was observed for the first time in vitro that hirsutanol
A inhibited the viability of Jurkat cells, displaying an
IC50 value of 5.16 µM at 48 h. The CCK-8 assay of Jurkat
cells exposed to hirsutanol A at different concentrations or
incubation times revealed that the compound markedly inhibited the
viability of Jurkat cells compared with the control group. The
present results indicated that hirsutanol A may serve as a
candidate drug for the treatment of T-ALL. However, prior to its
clinical application, further research is required, including the
assessment of the in vitro activities of hirsutanol A in
more T-ALL cell lines and evaluation in animal models of T-ALL
in vivo, as well as optimization of hirsutanol A to achieve
the strongest possible anticancer activity.
Puma is a powerful proapoptotic protein that is able
to inhibit the expression of the antiapoptotic protein
Bcl-2(32). If the balance between
proapoptotic factors and antiapoptotic factors is altered, the
permeability of the mitochondrial membrane increases and cytochrome
c is released into the cytoplasm, thus activating the caspase
cascade, which results in caspase-mediated breakdown of the
mitochondrial membrane (33-35).
Puma is transcriptionally activated by p53 (36,37).
To investigate the specific mechanism underlying hirsutanol
A-mediated inhibition of Jurkat cell activity, the expression
levels of the apoptosis-associated proteins Puma and Bcl-2, as well
as the intracellular apoptosis indicator protein cCaspase-3 and its
downstream protein cPARP were detected. The results indicated that,
compared with the control group, hirsutanol A significantly reduced
the expression of the antiapoptotic protein Bcl-2 in Jurkat cells,
but significantly increased the expression of the other three
proapoptotic proteins, which indicated that hirsutanol A induced
the apoptosis of Jurkat cells. This was consistent with another
study reporting that hirsutanol A induced apoptosis of human colon
cancer SW620 cells and human breast cancer MDA-MB-231 cells
(30). At the same time, flow
cytometric analysis suggested that hirsutanol A led to cell cycle
arrest of Jurkat cells in the G2 phase. Therefore, it
was hypothesized that hirsutanol A inhibited the proliferation of
Jurkat cells by promoting their apoptosis and blocking the cell
cycle.
The present results indicated that the p53 pathway
may serve a major role in the inhibition of Jurkat cell
proliferation by hirsutanol A. As a transcription factor, p53
regulates a variety of genes involved in apoptosis, the cell cycle,
DNA repair and senescence (38,39).
p53 induces apoptosis through a number of key proteins that
regulate apoptosis signaling and execution, including death
receptors (Fas cell surface death receptor 4 and 5), proapoptotic
proteins (Puma, NADPH oxidase activator and Bax) and apoptotic
executioners (Caspase-6, apoptotic peptidase activating factor 1
and Bcl2-interacting protein 3 like) (40,41).
Therefore, drugs that induce apoptosis and cell cycle arrest by
activating p53 are promising for the treatment of tumors. In the
present study, the expression of p53 protein was significantly
increased in Jurkat cells treated with hirsutanol A compared with
the control group. However, after the addition of p53 inhibitor
PFTα, the apoptosis-inducing effect of hirsutanol A on Jurkat cells
was significantly attenuated. At the same time, following PFTα
treatment of hirsutanol A-treated cells, the expression of p53
protein was significantly decreased and the expression trend of
apoptosis-associated proteins (Puma and Bcl-2) and apoptosis
indicators (cCaspase-3 and cPARP) downstream of p53 was
significantly reversed. These results suggested that hirsutanol A
promoted the apoptosis of Jurkat cells via activation of the p53
pathway, thus inhibiting cell proliferation. However, the specific
mechanisms underlying hirsutanol A-mediated activation of p53
require further investigation.
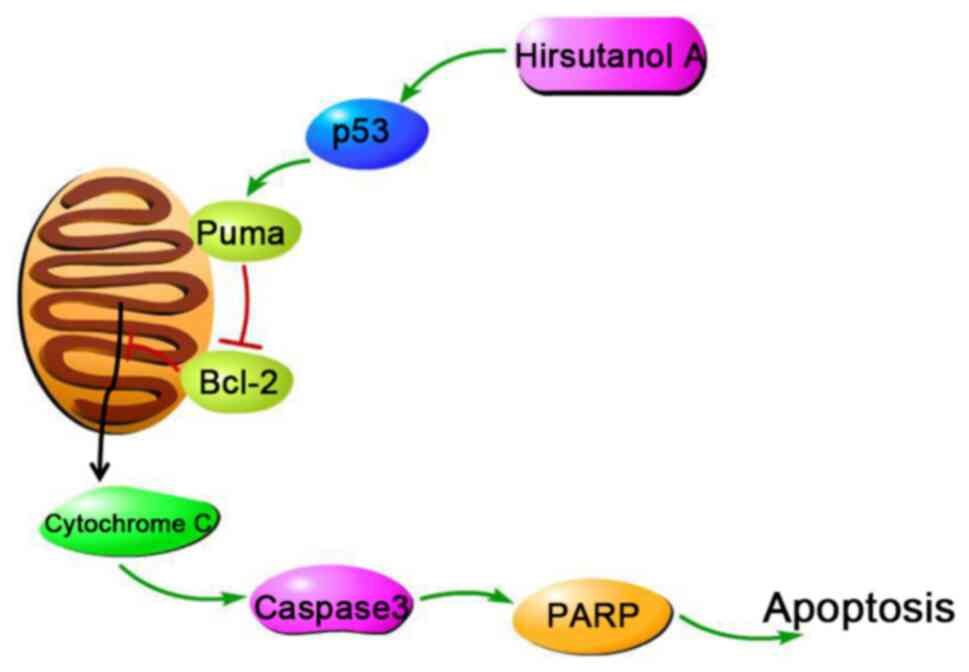
Overall, the present study indicated that hirsutanol
A inhibited the viability of T-ALL Jurkat cells. It was further
demonstrated that the inhibitory effect of hirsutanol A was due to
the induction of apoptosis and cell cycle arrest. It is proposed
that hirsutanol A induced the apoptosis of Jurkat cells through the
p53/Puma/Bcl-2/cCaspase-3 pathway (Fig.
6). However, it is undeniable that there are still some
limitations in the present study. For example, only one type of
T-ALL cells was used and no animal experiments were conducted.
Evaluation of the in vivo anticancer activities,
structure-activity relationship and direct targets of hirsutanol A
will be the focus of further research. In conclusion, the present
study demonstrated that hirsutanol A inhibited Jurkat cell
viability through cell cycle arrest and p53-dependent induction of
apoptosis, rendering it a promising lead compound for the treatment
of T-ALL.
Acknowledgements
Not applicable.
Funding
Funding: This study was funded by the Science and Technology
Project of Health Commission of Sichuan (grant no. 19PJ288), the
Basic Application Research of Sichuan Science and Technology
Department (grant no. 2019YJ0690), the Key R&D projects of
Sichuan Science and Technology Department (grant no. 2019YFS0531)
and the Science and Technology Development Fund, Macau SAR (grant
no. 0013/2019/A1).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WLi and WM designed the study. FZ performed the
CCK-8 assay, Hoechst staining, mitochondrial membrane potential
detection, western blot analysis and manuscript writing. YY and DR
conducted the other functional experiments. FZ and SL collected and
analyzed the data. XQ and WLa performed the statistical analysis.
JL performed western blot analysis and edited the manuscript. YZ
analyzed of data and searched the literature. FZ, WM and WLi
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gao J and Liu WJ: Prognostic value of the
response to prednisone for children with acute lymphoblastic
leukemia: A meta-analysis. Eur Rev Med Pharmacol Sci. 22:7858–7866.
2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Terwilliger T and Abdul-Hay M: Acute
lymphoblastic leukemia: A comprehensive review and 2017 update.
Blood Cancer J. 7(e577)2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Papadantonakis N and Advani AS: Recent
advances and novel treatment paradigms in acute lymphocytic
leukemia. Ther Adv Hematol. 7:252–269. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Matloub Y, Stork L, Asselin B, Hunger SP,
Borowitz M, Jones T, Bostrom B, Gastier-Foster JM, Heerema NA,
Carroll A, et al: Outcome of children with standard-risk T-lineage
acute lymphoblastic leukemia-comparison among different treatment
strategies. Pediatr Blood Cancer. 63:255–261. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Qin X, Zhang MY and Liu WJ: Application of
minimal residual disease monitoring in pediatric patients with
acute lymphoblastic leukemia. Eur Rev Med Pharmacol Sci.
22:6885–6895. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wang M, Wen J, Guo Y, Shen Y, An X, Hu Y
and Xiao J: Clinical characterization and prognosis of T cell acute
lymphoblastic leukemia with high CRLF2 gene expression in Children.
PLoS One. 14(e0224652)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Takahashi H, Kajiwara R, Kato M, Hasegawa
D, Tomizawa D, Noguchi Y, Koike K, Toyama D, Yabe H, Kajiwara M, et
al: Treatment outcome of children with acute lymphoblastic
leukemia: The Tokyo Children's Cancer Study Group (TCCSG) Study
L04-16. Int J Hematol. 108:98–108. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Li Y, Buijs-Gladdines JG, Canté-Barrett K,
Stubbs AP, Vroegindeweij EM, Smits WK, van Marion R, Dinjens WN,
Horstmann M, Kuiper RP, et al: IL-7 receptor mutations and steroid
resistance in pediatric T cell acute lymphoblastic leukemia: A
genome sequencing study. PLoS Med. 13(e1002200)2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Raetz EA and Teachey DT: T-cell acute
lymphoblastic leukemia. Hematology Am Soc Hematol Educ Program.
2016:580–588. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Punzo F, Manzo I, Tortora C, Pota E,
Angelo V, Bellini G, Di Paola A, Verace F, Casale F and Rossi F:
Effects of CB2 and TRPV1 receptors' stimulation in pediatric acute
T-lymphoblastic leukemia. Oncotarget. 9:21244–21258.
2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Tosello V and Ferrando A: The NOTCH
signaling pathway: Role in the pathogenesis of T-cell acute
lymphoblastic leukemia and implication for therapy. Ther Adv
Hematol. 4:199–210. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Juliusson G and Hough R: Leukemia. Prog
Tumor Res. 43:87–100. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Tan SH, Bertulfo FC and Sanda T:
Leukemia-initiating cells in T-cell acute lymphoblastic leukemia.
Front Oncol. 7(218)2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Rose-Inman H and Kuehl D: Acute leukemia.
Hematol Oncol Clin North Am. 31:1011–1028. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kuhlen M, Klusmann JH and Hoell JI:
Molecular approaches to treating pediatric leukemias. Front
Pediatr. 7(368)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wang Y, Chen H and Chen J: The consensus
on the monitoring, treatment, and prevention of leukemia relapse
after allogeneic hematopoietic stem cell transplantation in China.
Cancer Lett. 438:63–75. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Peters C, Cornish JM, Parikh SH and
Kurtzberg J: Stem cell source and outcome after hematopoietic stem
cell transplantation (HSCT) in children and adolescents with acute
leukemia. Pediatr Clin North Am. 57:27–46. 2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wang GYS, Abrell LM, Avelar A, Borgeson BM
and Crews P: New hirsutane based sesquiterpenes from salt water
cultures of a marine sponge-derived fungus and the terrestrial
fungus Coriolus consors. Tetrahedron. 26:7335–7342. 1998.
|
|
19
|
Li HJ, Lan WJ, Lam CK, Yang F and Zhu XF:
Hirsutane sesquiterpenoids from the marine-derived fungus
Chondrostereum sp. Chem Biodivers. 8:317–324.
2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Yang F, Chen WD, Deng R, Li DD, Wu KW,
Feng GK, Li HJ and Zhu XF: Hirsutanol A induces apoptosis and
autophagy via reactive oxygen species accumulation in breast cancer
MCF-7 cells. J Pharmacol Sci. 119:214–220. 2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wenzel ES and Singh ATK: Cell-cycle
checkpoints and aneuploidy on the path to cancer. In Vivo. 32:1–5.
2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wang Y, Zhong J, Bai J, Tong R, An F, Jiao
P, He L, Zeng D, Long E, Yan J, et al: The application of natural
products in cancer therapy by targeting apoptosis pathways. Curr
Drug Metab. 19:739–749. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Green DR and Reed JC: Mitochondria and
apoptosis. Science. 281:1309–1312. 1998.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Sinha K, Das J, Pal PB and Sil PC:
Oxidative stress: The mitochondria-dependent and
mitochondria-independent pathways of apoptosis. Arch Toxicol.
87:1157–1180. 2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Julien O and Wells JA: Caspases and their
substrates. Cell Death Differ. 24:1380–1389. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ranjan A and Iwakuma T: Non-canonical cell
death induced by p53. Int J Mol Sci. 17(2068)2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Abdelmabood S, Fouda AE, Boujettif F and
Mansour A: Treatment outcomes of children with acute lymphoblastic
leukemia in a middle-income developing country: High mortalities,
early relapses, and poor survival. J Pediatr (Rio J). 96:108–116.
2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Khalifa SAM, Elias N, Farag MA, Chen L,
Saeed A, Hegazy MF, Moustafa MS, Abd El-Wahed A, Al-Mousawi SM,
Musharraf SG, et al: Marine natural products: A source of novel
anticancer drugs. Mar Drugs. 17(491)2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yang F, Gao YH, Wu KW, Deng R, Li DD, Wei
ZX, Jiang S, Wu XQ, Feng GK, Li HJ and Zhu XF: A novel
sesquiterpene Hirsutanol A induces autophagical cell death in human
hepatocellular carcinoma cells by increasing reactive oxygen
species. Chin J Cancer. 29:655–660. 2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yang F, Chen WD, Deng R, Zhang H, Tang J,
Wu KW, Li DD, Feng GK, Lan WJ, Li HJ and Zhu XF: Hirsutanol A, a
novel sesquiterpene compound from fungus Chondrostereum sp.,
induces apoptosis and inhibits tumor growth through
mitochondrial-independent ROS production: Hirsutanol A inhibits
tumor growth through ROS production. J Transl Med.
11(32)2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Li HJ, Jiang WH, Liang WL, Huang JX, Mo
YF, Ding YQ, Lam CK, Qian XJ, Zhu XF and Lan WJ: Induced marine
fungus Chondrostereum sp. as a means of producing new
sesquiterpenoids chondrosterins I and J by using glycerol as the
carbon source. Mar Drugs. 12:167–175. 2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Correia C, Lee SH, Meng XW, Vincelette ND,
Knorr KL, Ding H, Nowakowski GS, Dai H and Kaufmann SH: Emerging
understanding of Bcl-2 biology: Implications for neoplastic
progression and treatment. Biochim Biophys Acta. 1853:1658–1671.
2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Porter AG and Jänicke RU: Emerging roles
of caspase-3 in apoptosis. Cell Death Differ. 6:99–104.
1999.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wang Y, Liu C, Wang J, Zhang Y and Chen L:
Iodine-131 induces apoptosis in human cardiac muscle cells through
the p53/Bax/caspase-3 and PIDD/caspase-2/t-BID/cytochrome
c/caspase-3 signaling pathway. Oncol Rep. 38:1579–1586.
2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Burke PJ: Mitochondria, bioenergetics and
apoptosis in cancer. Trends Cancer. 3:857–870. 2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Tichy A, Marek J, Havelek R, Pejchal J,
Seifrtova M, Zarybnicka L, Filipova A, Rezacova M and Sinkorova Z:
New light on an old friend: Targeting PUMA in radioprotection and
therapy of cardiovascular and neurodegenerative diseases. Curr Drug
Targets. 19:1943–1957. 2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Vávrová J and Rezáčová M: Importance of
proapoptotic protein PUMA in cell radioresistance. Folia Biol
(Praha). 60:53–56. 2014.PubMed/NCBI
|
|
38
|
Hickman ES, Moroni MC and Helin K: The
role of p53 and pRB in apoptosis and cancer. Curr Opin Genet Dev.
12:60–66. 2002.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Brázda V and Fojta M: The rich world of
p53 DNA binding targets: The role of DNA structure. Int J Mol Sci.
20(5605)2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Riley T, Sontag E, Chen P and Levine A:
Transcriptional control of human p53-regulated genes. Nat Rev Mol
Cell Biol. 9:402–412. 2008.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Matt S and Hofmann TG: The DNA
damage-induced cell death response: A roadmap to kill cancer cells.
Cell Mol Life Sci. 73:2829–2850. 2016.PubMed/NCBI View Article : Google Scholar
|