Introduction
Schizophrenia (SZ) belongs to a group of serious
mental diseases whose etiology is not completely known. The key
clinical characteristics of this disease include perceptual,
thinking, emotional and behavioral disorders (1,2). The
lifetime prevalence rate is approximately 1%. The onset age of SZ
is early and the disease has a chronic progression (3). Progressive aggravation or
deterioration, accompanied by social dysfunction along with high
disability rate and death rate can create a great burden to the
society (4). At present, the
pathogenesis of SZ is unclear, however results obtained from prior
studies have revealed that genetic factors play an important role
in the pathogenesis of SZ (5,6).
With the development of molecular genetics, numerous
genes have been revealed to be closely related to the pathogenesis
of SZ (7). It has been revealed
that the zinc finger protein 804a (ZNF804a) gene polymorphism was
closely related to the susceptibility of schizophrenia and the
efficacy of antipsychotics (8,9).
ZNF804a gene can control the formation of synapses, the
proliferation of neurites and the migration of neurons. It plays an
important role in the development of central nervous system
(10). ZNF804a gene can regulate
the expression of schizophrenia-related genes, such as dopamine D2
receptor, catecholamine oxidative methyl transferase, and
phosphodiesterase 4B gene (11).
ZNF804a which is located on chromosome 8p12-21, acts on the
epidermal growth factor receptor (EGFR) family. It spans over 1.4
trillion bases and encodes 15 types of proteins. ZNF804a has been
also revealed to play a role in white matter development and SZ
susceptibility (12). Other studies
revealed that the G-A allele mutation may increase the risk of SZ
and affect the cognitive function of SZ patients (13,14).
It has also been revealed that the genetic defects and
neurodevelopmental abnormalities of SZ were related to the
dysfunction of glutamatergic neurons (15). The mutation of the ZNF804a gene in
some SZ patients leads to the dysregulation of the glutamatergic
synapse and nerve fiber, which leads to the abnormal development
and function of the nerve fiber, and affects the development of
brain white matter (16). In
addition, it has been revealed that ZNF804a can regulate GABA
energy transmission by pre-synaptic ErbB4 receptor (17). Any changes to the ZNF804a gene
sequence may lead to abnormal development of GABAergic
interneurons, and the abnormal signal transmission of these neurons
may be a step in the pathogenesis of SZ (13,18).
These studies indicated that ZNF804a gene abnormality may affect
the development of white matter in the brain. This may be related
to the pathogenesis of SZ. These previous studies also revealed
that any abnormality in white matter plays an important role in the
pathogenesis of SZ. At present, diffusion tensor imaging (DTI) is
the only non-invasive method used for observing and tracking the
white matter fiber bundle (19).
The fractional anisotropy (FA) of water molecules is the proportion
of anisotropic components of water molecules in the total diffusion
tensor, and its variation range is from 0 to 1(20). DTI reflects the integrity of white
matter and the abnormality of white matter fiber bundle by
measuring the FA value (21). It
has been revealed that the FA value is decreased in patients
suffering from disorder in myelination of the central nervous
system (22). This observation
demonstrated that the FA value is an abnormal sensitive factor in
the measurement of myelination. A decrease in the FA value reflects
the abnormality of white matter fiber bundle, such as the decrease
of axon density and number, the abnormality of oligodendrocyte and
myelin lecithin (23). According to
these findings, the application of DTI technology can better
clarify the changes of brain white matter.
Several researchers have revealed a wide range of
brain areas with abnormal white matter in SZ patients. These areas
include the corpus callosum, the cingulate tract, the upper and
lower longitudinal tracts, the lower fronto-occipital tract and the
radial crown (24-26).
A previous study demonstrated that compared with HC, the FA value
of white matter in SZ patients is substantially decreased. These
areas include bilateral posterior radial crowns, bilateral
posterior optic nerve internal capsule, bilateral posterior
thalamic radiation, left anterior radial crowns, left upper
longitudinal bundle, left sagittal plane, right cerebral foot and
knee of corpus callosum (27).
Previous studies on ZNF804a polymorphism revealed
that the white matter density of the anterior limb of the inner
capsule and the connectivity of the DTI structure in the same
region of the TT homozygote, the risk gene of rs1344706, was
decreased in healthy subjects (28,29).
These results revealed that ZNF804a not only affected the migration
of neurons, the growth of axons and myelin sheath, but also was
related to the decrease of white matter density and integrity in
human subjects (30). The decrease
in the FA value of the anterior cingulate in SZ patients with
rs1344706 allele indicates that ZNF804a mutation may negatively
affect the anterior cingulate in SZ patients (31). However, for the ZNF804a site
rs1344706, it has been revealed that the GG homozygote of the
ZNF804a site rs1344706 was related to the increased risk of early
onset of SZ. It has also been suggested that the mutation of
rs1344706 in ZNF804a gene may affect the cognitive function of SZ
patients (32). There is also
research revealing that ZNF804a site rs1344706 has no significant
effects on the cognitive ability of SZ patients. It has been
reported that genetic variation related to different pathways may
affect the brain structure and function of SZ patients (33). However, the exact association
between rs1344706 polymorphism of the ZNF804a gene locus and the
whole brain white matter integrity of SZ patients is not clearly
understood. In addition, the internal mechanism of rs1344706 and
the whole brain white matter integrity of SZ patients is not clear.
There have been few studies on the interaction between rs1344706
single nucleotide polymorphism of the ZNF804a gene and brain white
matter integrity of SZ patients, as well as the physiological roles
in cell differentiation, proliferation, and inflammatory processes,
which may be part of MS/SZ common pathophysiological processes that
participate in the pathophysiological mechanism of SZ (34).
Materials and methods
Research subjects
In total, 160 subjects, including 76 males and 84
females, aged 14 to 50 years (average age, 42.1±4.3 years) were
enrolled in the present study, diagnosed and treated at the
Department of MRI of the Third Affiliated Hospital of Qiqihar
Medical University (Qiqihar, China) from May 2018 to February 2019.
There were 60 SZ patients and 100 healthy controls (HC). Inclusion
criteria for the SZ group were as follows: The diagnosis of mental
illness was confirmed by structured clinical interview using the
DSM IV classification system (35).
All patients with SZ were assessed with the Brief Psychiatric
Rating Scale (BPRS) (36). In the
HC group, there was no disease history diagnosed by DSM-IV axial
workers and no disease history diagnosed by first-degree relatives.
No psychiatric drugs were taken in the previous month. The
exclusion criteria were as follows: History of major physical
diseases, unstable physical diseases, IQ less than 70; autism or
extensive developmental disorders; or claustrophobia. The study was
approved by the Ethics Committee of The Third Affiliated Hospital
of Qiqihar Medical University. Signed written informed consents
were obtained from the patients and/or guardians.
Genotyping
Peripheral venous blood (2 ml) was collected on an
empty stomach at 8:00 am on the day of collection. Plasma was
removed using low-speed centrifugation (500 x g for 10 min) at
37˚C. Red blood cell lysate containing 150 mm/l NH4Cl, 10 mmol/l
KHC03 and 0.1 mmol/l EDTA was used to separate white blood cells.
After 6 h of treatment with SDS lysate + proteinase K, proteins
were extracted using phenol/chloroform. After centrifugation at
2,000 x g for 5 min at 37˚C, the supernatant was collected, and the
genomic DNA was precipitated with 75% ethanol. The extracted DNA
was stored at -80˚C for later use. Venous blood samples (2 ml) were
collected, and the single nucleotide polymorphism (SNP) typing kit
(product no. HNSV-01; Sigma-Aldrich; Merck KGaA) was used to detect
160 samples for 22 SNP genotyping. In the present study, only the
G/A genotype at rs1344706 of ZNF804a gene was used. The PCR primers
were as follows: rs1344706 forward, 5'-AAACCTTTGGAATGGTGCCTTGA-3'
and rs1344706 reverse, 3'-CTTATTGCTGGGGGCAGTCTCTAC-5'.
The PCR conditions were as follows: First
denaturation at 95˚C for 2 min, then denaturation at 94˚C for 20
sec, annealing at 65˚C for 40 sec, extension for 1.5 min at 72˚C
for 11 cycles; then the annealing temperature of each cycle was
decreased to 59˚C, and finally denaturation at 94˚C for 20 sec,
annealing at 59˚C for 30 sec, and extension for 1.5 min at 72˚C for
24 cycles.
Interpretation of results: The alleles determined by
the analysis were G and A alleles, which were divided into AA, AG
and GG genotypes. The genotype and diagnosis were divided into four
groups, including the SZ AA group (A allele control group of SZ
patients, 24 people), SZ-GG/AG group (G-risk allele carrier group
of SZ patients, 36 people), HC-AA group (A allele control group of
HC, 58 people) and HC-GG/AG group (G-risk allele carrier group of
HC, 42 people).
Brain MRI examination methods
All subjects were scanned with MRI when they were
enrolled in the study. Using the 65 layers scanned by the GE
Discovery MR750w 3.0T MRI scanner. The thickness of the layer was 2
mm3, the layer spacing was 1 mm, the voxel size was 2
mm3, and the diffusion gradient was applied in 25
directions, B=1000 smm.
Processing of experimental data and
statistical analysis
The standardized FA images of each subject were
obtained, and registered on a 1x1x1 mm3 standard spatial
image mni-152. We used Pipeline for Analyzing Brain Diffusion
Images DTI (PANDA software) (http://www.nitrc.org/projects/panda) in order to
preprocess the data. The steps included head movement and eddy
current correction, removal of motion artifacts caused by head
movement, removing excess scalp and brain tissue, and fitting a
tensor model by least squares method to calculate and obtain the FA
value. Subsequently, the FA brain map was calculated and smoothed
by 6 mm3 half height and full width Gaussian kernel for
the next step of analysis and statistics. SPSS 13.0 (SPSS, Inc.)
was used for statistical analysis. Chi square test, two-sample
unpaired t-test and two-factor factorial design analysis of
variance (ANOVA) were used for data statistical analysis. Scheffe's
post hoc test was used following ANOVA. Diagnosis (SZ and HC) and
genotype (AA, GG/AG) were used as intergroup factors. Two-factor
ANOVA (GRF correction, voxel level P<0.05, regional level
P<0.05) was carried out on four groups of FA images with SPM8
software (https://www.fil.ion.ucl.ac.uk/spm/software/spm8/),
with diagnosis (SZ and HC) and genotype (AA and GG/AG) as
intergroup factors, and age and years of education as covariates.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Statistical results of the clinical
data in the present study
In total, 160 subjects were divided into four
groups: SZ-AA group (A allele control group of SZ patients),
SZ-GG/AG group (G risk allele carrier group of SZ patients), HC-AA
group (A allele control group of HC) and HC-GG/AG group (G risk
allele carrier group of HC). Statistical analysis of demographic
and clinical data revealed that there was no significant difference
between the four groups with regard to sex and the course of
disease between the two genotypes of SZ patients (P>0.05). There
were significant differences in age, education years and BPRS
scores (P<0.05). Detailed demographic data and statistical
results are presented in Table I.
The distribution of the rs1344706 genotype of ZNF804a gene in all
samples was in accordance with the Hardy-Weinberg genetic
equilibrium law (16) (P>0.05),
and the selected samples were representative of the population. The
secondary allele frequency was 26.7%. The genotype frequency of SZ:
χ2=0.238 and P=0.626 (Table
II).
 | Table IDemographic and clinical data. |
Table I
Demographic and clinical data.
| | SZ (n=60) | HC (n=100) | |
|---|
| Index | AA (n=24) | GG/AG (n=36) | AA (n=58) | GG/AG (n=42) | P-value |
|---|
| Sex, M/F | 11/24 | 4/36 | 25/58 | 21/42 |
χ2=4.451 | 0.220 |
| Age (years) |
24.1±6.0a |
26.5±8.2a |
34.7±8.9a |
31.8±10.0a | F=16.566 | <0.05 |
| Education
years |
12.0±4.4a |
13.1±4.2a |
14.5±4.6a |
15.4±5.0a | F=3.428 | 0.019 |
| Course of disease
(mouth) | 10.5±6.3 | 11.0±6.0 | | | t=0.361 | 0.719 |
| BPRS score |
57.8±23.2a |
51.8±18.1a |
25.3±5.8a |
25.9±6.4a | F=59.312 | <0.05 |
 | Table IIHardy-Weinberg equilibrium
constant. |
Table II
Hardy-Weinberg equilibrium
constant.
| | Allele frequency
(%) | Genotype frequency
(%) | |
|---|
| Groups | G | A | GG | AG | AA | χ2 | P-value |
|---|
| SZ | 26.7 | 73.3 | 7.1 | 32.1 | 60.7 | 0.238 | 0.626 |
| HC | 23.2 | 76.8 | | | | | |
DTI analysis of multivariate
ANOVA
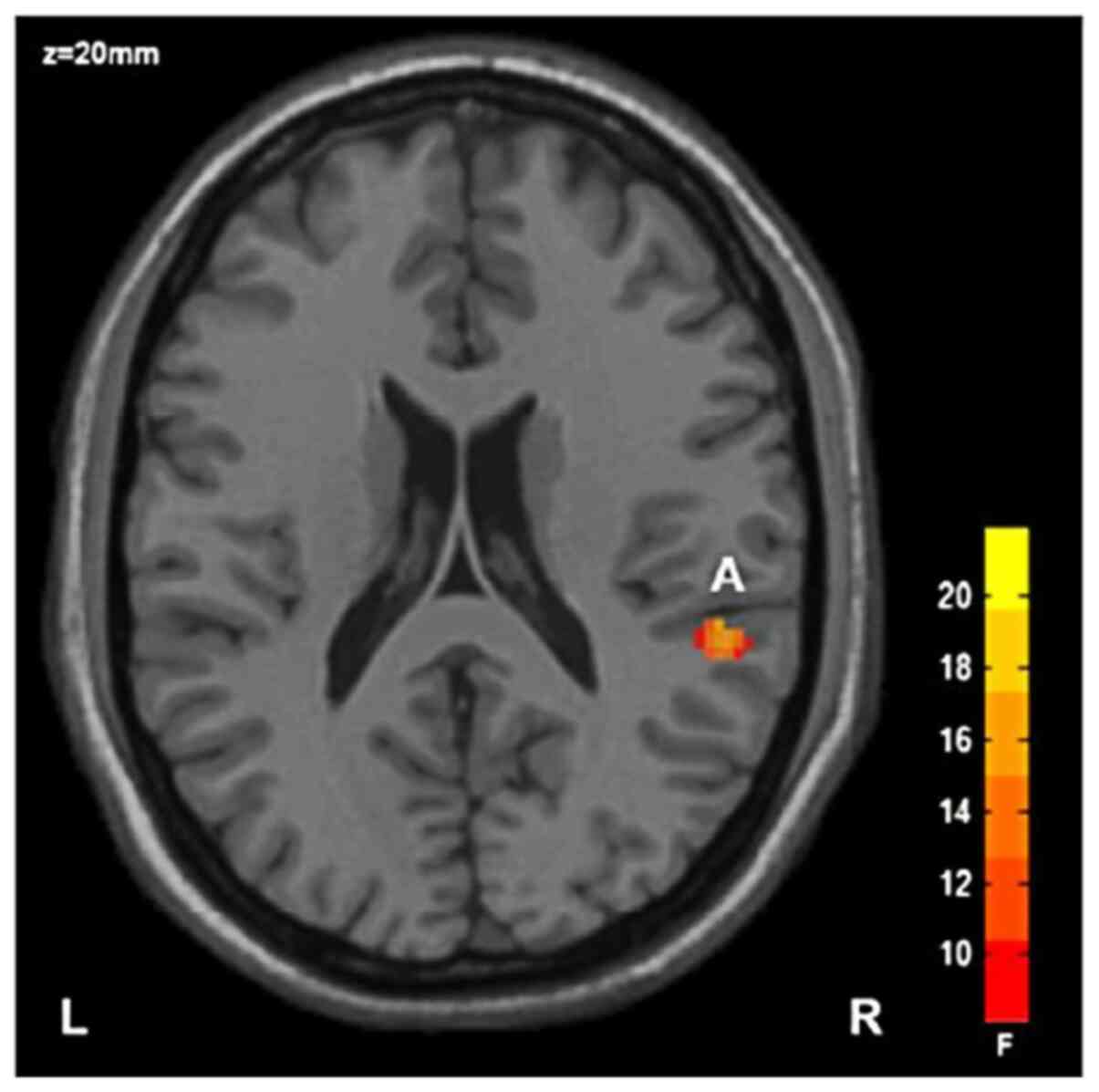
i) Main effect of diagnosis: The FA value of the
right posterior radiocrown in the SZ group was lower than that in
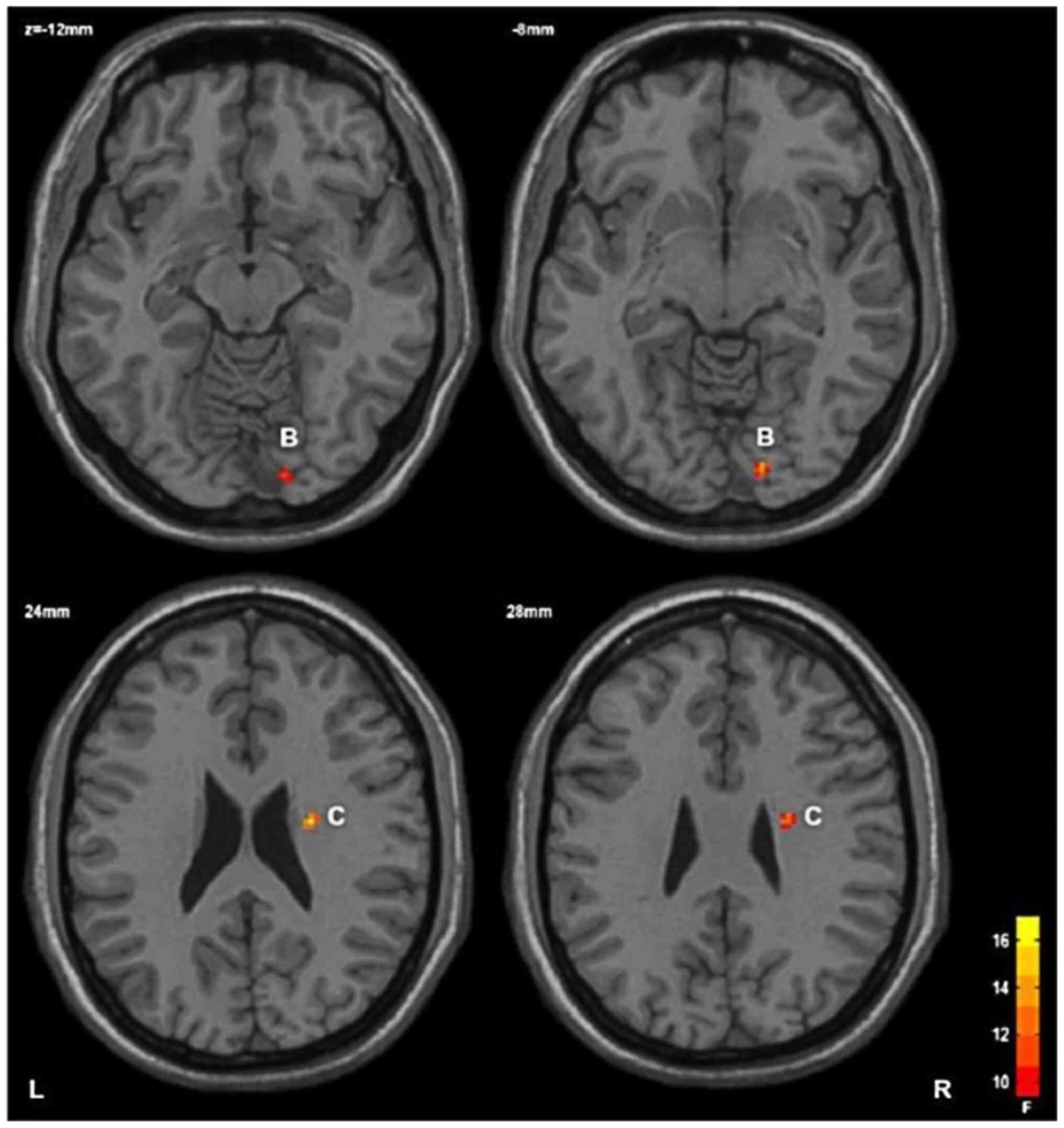
HC group, with statistical significance (Table III; Fig. 1); and ii) main effect of the gene:
The FA value of the right lower frontal occipital tract and right
upper radiocrown in the GG/AG group was lower than that in the AA
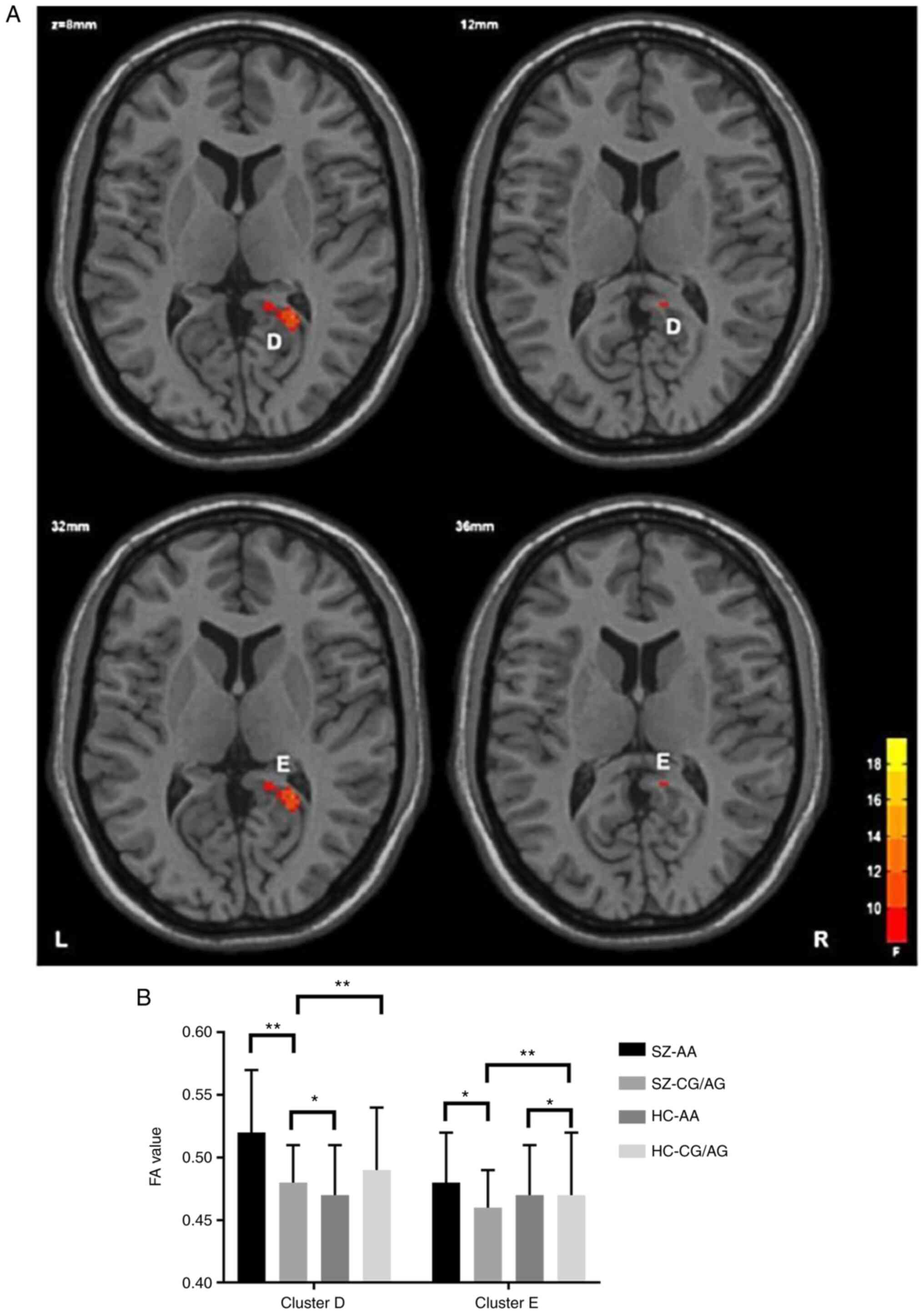
group, with statistical significance (Table III; Figs. 2 and 3).
 | Table IIIResults of two-factor ANOVA. |
Table III
Results of two-factor ANOVA.
| | Maximum difference
pointa MNI
coordinateb | |
|---|
| Brain area | Voxel no. | x | y | z | FA
valuec |
|---|
| Main effect of
diagnosis | | | | | |
|
Right rear
radial crown | 42 | 52 | -34 | 20 | 18.04 |
|
Right lower
frontal screening bundle | 26 | 14 | -86 | -8 | 14.17 |
|
Right upper
radial crown | 29 | 24 | -8 | 26 | 17.67 |
| Diagnosis x gene
interaction | | | | | |
|
Splenium of
the corpus callosum | 55 | 22 | -48 | 6 | 16.18 |
|
Corpus
callosum/right cingulate gyrus | 49 | 12 | -10 | 36 | 12.69 |
Discussion
In the present study, the rs1344706 polymorphism of
ZNF804a gene was compared between a patient group (SZ) and a
control group (HC). The results indicated that: i) the main effect
of disease factors revealed that the FA value of the right
posterior radiocrown in the SZ group was significantly lower than
that in the HC group; ii) the main effect of rs1344706 polymorphism
in the ZNF804a gene revealed that the FA value of the right lower
occipital east and right upper radiocrown were significantly lower;
and iii) the interaction of the same gene polymorphism in the SZ
group revealed that the FA value of the patient group was
significantly lower than that in the control group, Compared with
the AA control group, the FA value in the splenium of the corpus
callosum, the body part of the corpus callosum part and the right
cingulate tract in the gene carrier group was significantly
decreased. The findings of Cui et al (16) suggest that ZNF804A affects the
resting-state functional activation by interacting with COMT and
may improve our understanding of the neurobiological effects of
ZNF804A and its association with schizophrenia (16,37).
The FA value of the SZ group was significantly lower
than that of HC group. The corona radiata is a radial fiber from
the inner capsule to the cerebral cortex (38). It is the main fiber bundle
connecting the frontal lobe and other brain areas and regulates
sensation and perception. It is mainly related to the cognitive
functions of language and memory processing and may be related to
positive mental symptoms such as auditory hallucinations (7). Luhrmann et al (38) reported that the FA value of the
white matter radiation crown in SZ patients was decreased. Zhao
et al also discovered a decrease in the FA value of the
radiation crown in SZ patients (39). These findings which were related to
the facial expression recognition function of patients, were
consistent with our results presented in the present study. These
findings suggest that the local white matter fiber bundle and other
micro structure abnormalities of the radiation crown may be related
to the pathological mechanism of SZ, however there was no gene
correlation. Research by Meller et al which focused on the
relationship between ZNF804 gene polymorphism and Alzheimer's
disease did not evaluate the clinical indications of patients, but
our study focused more on the correlation between ZNF804 gene and
clinical manifestations of schizophrenia disease, such as MRI
examination and analysis of the white matter of the head of
patients (37). Other studies
(5,11,12)
revealed that the FA value of the right anterior radiocrown was
positively correlated to the age of onset, although no decrease in
the FA value of the SZ group compared with the HC group was
reported. These results were inconsistent with the results of the
present study. This inconsistence may be related to the age
difference and our small sample size. Consistent with a previous
study suggesting that functional connectivities within the
frontoparietal central executive network are prone to the effect of
working memory training (22), the
present study provided direct RCT evidence for the effect
ofrs1344706 polymorphism of ZNF804a gene related to the integrity
of white matter fiber bundle in schizophrenics (39).
In the area where the FA value of the GG/AG genotype
in the SZ group demonstrated a significant decrease, we revealed
for the first time, to the best of our knowledge, that the FA value
of thesplenium of the corpus callosum, the body part of the corpus
callosum and right cingulate band in the patient risk gene carrier
group was lower than that in the control group. It has been
revealed that the dysfunctionality of the glutamatergic system is
related to genetic polymorphism (40-42).
In the present study, it was theorized that rs1344706 of ZNF804a
gene may affect the white matter function in SZ patients through
the hypothesis of ‘glutamatergic dysfunction’. The corpus callosum
is an important nerve fiber bundle connecting the left and right
cerebral hemispheres, which is divided into four parts: The
splenium part, the body part, the genu part and the rostrum part.
It is responsible for the integration of the perception and
cognitive functions between the two hemispheres, as well as the
transmission of information such as learning, memory and will. It
is an important part of human spiritual activities. Among them, the
corpus callosum has been revealed to exhibit abnormalities in SZ
patients (10). It was revealed in
the present study, that the damage of white matter fiber integrity
in the corpus callosum and corpus may be related to glutamatergic
dysfunction caused by rs1344706 mutation at the ZNF804a site in SZ
patients. The cingulate tract is an important part of the limbic
green system. It is the white matter fiber connecting the cingulate
with the frontal lobe, parietal lobe, parietal region and thalamus.
It is important for the functional integrity of SZ patients. The
cingulate band is mainly related to emotion regulation, learning
ability, attention, memory and other cognitive functions (7). Knöchel et al (27) and revealed that the FA value of
white matter was decreased in SZ patients. Seitz et al
(43) revealed that the FA value of
the right cingulate tract was decreased in SZ patients. They
speculated that the abnormality of the right cingulate tract may be
related to the memory decline and attention deficit in SZ patients,
and the white matter change of the right cingulate tract may be
caused by the destruction of the myelin sheath. It was suggested
that structural abnormalities interact with cognitive function and
were related to clinical symptoms. In the present study, it was
revealed that the FA value of the right cingulate tract in the risk
gene carrier group was lower than that in the control group,
indicating that rs1344706 at ZNF804a site may affect the
myelination of the central nervous system through the hypothesis of
‘glutamatergic dysfunction’. If this process is damaged, it may
affect the development of white matter in the brain, thus
increasing the possibility of SZ. It was concluded that the
rs1344706 single nucleotide polymorphism of ZNF804a gene may affect
the integrity of brain white matter in SZ patients.
A previous study revealed that the pathogenesis of
SZ is related to the destruction of white matter integrity and the
rs1344706 mutation at ZNF804a site (14). This study further indicated that the
rs1344706 SNP of ZNF804a gene was related to the integrity of white
matter in schizophrenics, and may be related to the pathogenesis of
schizophrenia. However, there are still some limitations in the
present study. The age and education years of the four groups were
not similar. This study used SNP as a covariate, but could not
completely exclude (SNP) influence on the results. In the present
study, we only addressed the rs1344706 locus and the effect on the
structure of white matter. Future studies are required to further
explore the interaction between brain function and gene
polymorphism. In these future studies, the sample size will be
further expanded, more comprehensive analysis of multiple sites
that may be related to SZ will be identified, which will aid in
further discovering the role of ZNF804a in brain function. In
addition, these studies may explore the response of different
genotypes to the treatment for schizophrenics, and provide
practical basis for precision medicine.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by Clinical Research
Foundation of Qiqihar Academy of Medical Sciences (QMSI2019L-22);
The General Scientific Research Project of Heilongjiang Education
Department (grant no. 2016-KYYWF-0875).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JZ, QB, SL, HG, XM and GZ conceived and designed the
study, and drafted the manuscript. JZ, QB, SL, HG, XM and PL
collected, analyzed and interpreted the experimental data. JZ and
XM revised the manuscript for important intellectual content. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
The Third Affiliated Hospital of Qiqihar Medical University. Signed
written informed consents were obtained from the patients and/or
guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Guloksuz S and van Os J: The slow death of
the concept of schizophrenia and the painful birth of the psychosis
spectrum. Psychol Med. 48:229–244. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ozaki N: Right treatment for the right
schizophrenic patients based on carbonyl stress pathophysiology.
Psychiatry Clin Neurosci. 72(2)2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lehman AF, Lieberman JA, Dixon LB,
McGlashan TH, Miller AL, Perkins DO and Kreyenbuhl J: American
Psychiatric Association; Steering Committee on Practice Guidelines.
Practice guideline for the treatment of patients with
schizophrenia, second edition. Am J Psychiatry. 161 (Suppl 2):1–56.
2004.PubMed/NCBI
|
|
4
|
Bi XJ, Lv XM, Ai XY, Sun MM, Cui KY, Yang
LM, Wang LN, Yin AH and Liu LF: Childhood trauma interacted with
BDNF Val66Met influence schizophrenic symptoms. Medicine
(Baltimore). 97(e0160)2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Thyme SB, Pieper LM, Li EH, Pandey S, Wang
Y, Morris NS, Sha C, Choi JW, Herrera KJ, Soucy ER, et al:
Phenotypic landscape of schizophrenia-associated genes defines
candidates and their shared functions. Cell. 177:478–491.e20.
2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Oh SB, Kim MS, Park S, Son H, Kim SY, Kim
MS, Jo DG, Tak E and Lee JY: Clusterin contributes to early stage
of Alzheimer's disease pathogenesis. Brain Pathol. 29:217–231.
2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Bocci V, Zanardia I, Valacchi G, Borrelli
E and Travagli V: Validity of oxygen-ozone therapy as integrated
medication form in chronic inflammatory diseases. Cardiovasc
Hematol Disord Drug Targets. 15:127–138. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Voineskos AN, Lerch JP, Felsky D, Tiwari
A, Rajji TK, Miranda D, Lobaugh NJ, Pollock BG, Mulsant BH and
Kennedy JL: The ZNF804A gene: Characterization of a novel neural
risk mechanism for the major psychoses. Neuropsychopharmacology.
36:1871–1878. 2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhang F, Chen Q, Ye T, Lipska BK, Straub
RE, Vakkalanka R, Rujescu D, St Clair D, Hyde TM, Bigelow L, et al:
Evidence of sex-modulated association of ZNF804A with
schizophrenia. Biol Psychiatry. 69:914–917. 2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhou Y, Dong F, Lanz TA, Reinhart V, Li M,
Liu L, Zou J, Xi HS and Mao Y: Interactome analysis reveals
ZNF804A, a schizophrenia risk gene, as a novel component of protein
translational machinery critical for embryonic neurodevelopment.
Mol Psychiatry. 23:952–962. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lencz T, Szeszko PR, DeRosse P, Burdick
KE, Bromet EJ, Bilder RM and Malhotra AK: A schizophrenia risk
gene, ZNF804A, influences neuroanatomical and neurocognitive
phenotypes. Neuropsychopharmacology. 35:2284–2291. 2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Steinberg S, Mors O, Børglum AD,
Gustafsson O, Werge T, Mortensen PB, Andreassen OA, Sigurdsson E,
Thorgeirsson TE, Böttcher Y, et al: Expanding the range of ZNF804A
variants conferring risk of psychosis. Mol Psychiatry. 16:59–66.
2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Girgenti MJ, LoTurco JJ and Maher BJ:
ZNF804a regulates expression of the schizophrenia-associated genes
PRSS16, COMT, PDE4B, and DRD2. PLoS One. 7(e32404)2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wu S, Wang P, Tao R, Yang P, Yu X, Li Y,
Shao Q, Nie F, Ha J, Zhang R, et al: Schizophrenia-associated
microRNA-148b-3p regulates COMT and PRSS16 expression by targeting
the ZNF804A gene in human neuroblastoma cells. Mol Med Rep.
22:1429–1439. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wang Z, Zhang T, Liu J, Wang H, Lu T, Jia
M, Zhang D, Wang L and Li J: Family-based association study of
ZNF804A polymorphisms and autism in a Han Chinese population. BMC
Psychiatry. 19(159)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Cui L, Wang F, Chang M, Yin Z, Fan G, Song
Y, Wei Y, Xu Y, Zhang Y, Tang Y, et al: Spontaneous regional brain
activity in healthy individuals is nonlinearly modulated by the
interaction of ZNF804A rs1344706 and COMT rs4680 polymorphisms.
Neurosci Bull. 35:735–742. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Gambarotta G, Garzotto D, Destro E,
Mautino B, Giampietro C, Cutrupi S, Dati C, Cattaneo E, Fasolo A
and Perroteau I: ErbB4 expression in neural progenitor cells
(ST14A) is necessary to mediate neuregulin-1beta1-induced
migration. J Biol Chem. 279:48808–48816. 2004.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhang R, Lu SM, Qiu C, Liu XG, Gao CG, Guo
TW, Valenzuela RK, Deng HW and Ma J: Population-based and
family-based association studies of ZNF804A locus and
schizophrenia. Mol Psychiatry. 16:360–361. 2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Roberts RM, Mathias JL and Rose SE:
Relationship between diffusion tensor imaging (DTI) findings and
cognition following pediatric TBI: A meta-analytic review. Dev
Neuropsychol. 41:176–200. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Commowick O, Fillard P, Clatz O and
Warfield SK: Detection of DTI white matter abnormalities in
multiple sclerosis patients. Med Image Comput Comput Assist Interv.
11:975–982. 2008.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Mazal AT, Ashikyan O, Cheng J, Le LQ and
Chhabra A: Diffusion-weighted imaging and diffusion tensor imaging
as adjuncts to conventional MRI for the diagnosis and management of
peripheral nerve sheath tumors: Current perspectives and future
directions. Eur Radiol. 29:4123–4132. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Grazzini I, Redi F, Sammartano K and Cuneo
GL: Diffusion tensor imaging in idiopathic normal pressure
hydrocephalus: Clinical and CSF flowmetry correlations. Neuroradiol
J. 33:66–74. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Visser MM, Yassi N, Campbell BC, Desmond
PM, Davis SM, Spratt N, Parsons M and Bivard A: White matter
degeneration after ischemic stroke: A longitudinal diffusion tensor
imaging study. J Neuroimaging. 29:111–118. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Du J, Ma G, Li S, Carl M, Szeverenyi NM,
VandenBerg S, Corey-Bloom J and Bydder GM: Ultrashort echo time
(UTE) magnetic resonance imaging of the short T2 components in
white matter of the brain using a clinical 3T scanner. Neuroimage.
87:32–41. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Minkner K, Lovblad KO, Yilmaz H, Alimenti
A, Sekoranja L, Delavelle J, Sztajzel R and Rüfenacht DA: White
matter lesions in watershed territories studied with MRI and
parenchymography: A comparative study. Neuroradiology. 47:425–430.
2005.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wu MK, Lu YT, Huang CW, Lin PH, Chen NC,
Lui CC, Chang WN, Lee CC, Chang YT, Chen SF and Chang CC: Clinical
significance of cerebrovascular biomarkers and white matter tract
integrity in Alzheimer disease: Clinical correlations With
Neurobehavioral Data in Cross-Sectional and After 18 Months
Follow-ups. Medicine (Baltimore). 94(e1192)2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Knöchel C, Schmied C, Linden DE, Stäblein
M, Prvulovic D, de A, de Carvalho L, Harrison O, Barros Junior PO,
Carvalho AF, Reif A, et al: White matter abnormalities in the
fornix are linked to cognitive performance in SZ but not in BD
disorder: An exploratory analysis with DTI deterministic
tractography. J Affect Disord. 201:64–78. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Mallas E, Carletti F, Chaddock CA,
Shergill S, Woolley J, Picchioni MM, McDonald C, Toulopoulou T,
Kravariti E, Kalidindi S, et al: The impact of CACNA1C gene, and
its epistasis with ZNF804A, on white matter microstructure in
health, schizophrenia and bipolar disorder. Genes Brain Behav.
16:479–488. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhang Z, Chen X, Yu P, Zhang Q, Sun X, Gu
H, Zhang H, Zhai J, Chen M, Du B, et al: Effect of rs1344706 in the
ZNF804A gene on the connectivity between the hippocampal formation
and posterior cingulate cortex. Schizophr Res. 170:48–54.
2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Gurung R and Prata DP: What is the impact
of genome-wide supported risk variants for schizophrenia and
bipolar disorder on brain structure and function?A systematic
review. Psychol Med. 45:2461–2480. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Sprooten E, McIntosh AM, Lawrie SM, Hall
J, Sussmann JE, Dahmen N, Konrad A, Bastin ME and Winterer G: An
investigation of a genomewide supported psychosis variant in
ZNF804A and white matter integrity in the human brain. Magn Reson
Imaging. 30:1373–1380. 2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Calabrò M, Mandelli L, Crisafulli C,
Nicola MD, Colombo R, Janiri L, Lee SJ, Jun TY, Wang SM, Masand PS,
et al: ZNF804A gene variants have a cross-diagnostic influence on
psychosis and treatment improvement in mood disorders. Clin
Psychopharmacol Neurosci. 18:231–240. 2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Chen X, Zhang Z, Zhang Q, Zhao W, Zhai J,
Chen M, Du B, Deng X, Ji F, Wang C, et al: Effect of rs1344706 in
the ZNF804A gene on the brain network. Neuroimage Clin.
17:1000–1005. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
de Castro-Catala M, Mora-Solano A, Kwapil
TR, Cristóbal-Narváez P, Sheinbaum T, Racioppi A, Barrantes-Vidal N
and Rosa A: The genome-wide associated candidate gene ZNF804A and
psychosis-proneness: Evidence of sex-modulated association. PLoS
One. 12(e0185072)2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Uher R, Payne JL, Pavlova B and Perlis RH:
Major depressive disorder in DSM-5: Implications for clinical
practice and research of changes from DSM-IV. Depress Anxiety.
31:459–471. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
36
|
Schooler NR: Precursors to the PANSS: The
BPRS and its progenitors. Innov Clin Neurosci. 14:10–11.
2017.PubMed/NCBI
|
|
37
|
Meller T, Schmitt S, Stein F, Brosch K,
Mosebach J, Yüksel D, Zaremba D, Grotegerd D, Dohm K, Meinert S, et
al: Associations of schizophrenia risk genes ZNF804A and CACNA1C
with schizotypy and modulation of attention in healthy subjects.
Schizophr Res. 208:67–75. 2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Luhrmann TM, Alderson-Day B, Bell V, Bless
JJ, Corlett P, Hugdahl K, Jones N, Larøi F, Moseley P, Padmavati R,
et al: Beyond trauma: A multiple pathways approach to auditory
hallucinations in clinical and nonclinical populations. Schizophr
Bull. 45 (Suppl 1):S24–S31. 2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Zhao W, Chen X, Zhang Q, Du B, Deng X, Ji
F, Xiang YT, Wang C, Dong Q, Chen C and Li J: Effect of ZNF804A
gene polymorphism (rs1344706) on the plasticity of the functional
coupling between the right dorsolateral prefrontal cortex and the
contralateral hippocampal formation. Neuroimage Clin.
27(102279)2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Zarate CA, Quiroz J, Payne J and Manji HK:
Modulators of the glutamatergic system: Implications for the
development of improved therapeutics in mood disorders.
Psychopharmacol Bull. 36:35–83. 2002.PubMed/NCBI
|
|
41
|
Sanacora G, Zarate CA, Krystal JH and
Manji HK: Targeting the glutamatergic system to develop novel,
improved therapeutics for mood disorders. Nat Rev Drug Discov.
7:426–437. 2008.PubMed/NCBI View
Article : Google Scholar
|
|
42
|
Spanagel R, Pendyala G, Abarca C, Zghoul
T, Sanchis-Segura C, Magnone MC, Lascorz J, Depner M, Holzberg D,
Soyka M, et al: The clock gene Per2 influences the glutamatergic
system and modulates alcohol consumption. Nat Med. 11:35–42.
2005.PubMed/NCBI View
Article : Google Scholar
|
|
43
|
Seitz J, Lyall AE, Kanayama G, Makris N,
Hudson JI, Kubicki M, Pope HG Jr and Kaufman MJ: White matter
abnormalities in long-term anabolic-androgenic steroid users: A
pilot study. Psychiatry Res Neuroimaging. 260:1–5. 2017.PubMed/NCBI View Article : Google Scholar
|