Introduction
Ectopic pregnancy (EP) is the main cause of maternal
morbidity and mortality in the first trimester (1,2). With
the occurrence of embryo transfer and in vitro
fertilization, the incidence of EP has increased sharply, leading
to mass maternal and sporadic deaths (3,4), which
account for approximately 10% of all pregnancy-related deaths
(5). Complications caused by EP
remain a major cause of morbidity and mortality in early pregnancy
(6). In addition, little is known
about the treatment and predictive factors of this complication
(7), and its early symptoms are not
obvious, and easily confused with threatened abortion (8). Therefore, determining the development,
occurrence, prognosis and potential mechanism of EP is conducive
for clinicians to explore a more feasible treatment plan for this
condition.
First found in the plasma of pregnant women, PAPP-A
is a metalloproteinase that plays a key part in regulating the
activity of insulin-like growth factors (9), and is subsequently acknowledged to be
a multifunctional regulator in various pathological processes
(10). In addition, it is a major
physiological regulator of insulin-like growth factor binding
protein-4 (IGFBP-4), which cleaves the IGFBP4/IGF1 complex to
release insulin-like growth factor 1 (IGF-1), and then regulates
its bioavailability (11). However,
there are other studies indicating that the aberrant expression of
PAPP-A disrupts the regulation of the availability of IGF-1 and
thus affects the biology of tumors (12-14).
Previous studies (15,16) have demonstrated that, the prevention
and prognosis of an adverse pregnancy is of great clinical
significance from a medical point of view, and is particularly
challenging for the scientific community, family and society, and
that ultrasounds combined with PAPP-A can better diagnose and
predict an abnormal pregnancy. However, the relationship between
serum PAPP-A and the diagnosis, prognosis, related factors, as well
as the possible molecular mechanism of EP remains poorly
understood.
Therefore, by examining the expression of PAPP-A in
EP, its clinical value in EP, the related factors inducing the
disease and the possible molecular mechanism were explored, with
the aim to identify reliable diagnostic and prognostic markers and
potential drug targets for EP.
Patients and methods
General information
From January 2018 to February 2019, 75 patients with
EP admitted to the Affiliated Hospital of Jining Medical University
were included in the research group, and another 59 healthy
pregnant women of the corresponding age, gravidity and gestational
week were enrolled in the control group (17). Patients in the research group were
21-35 years old, with an average age of 25.73±7.23 years, while
those in the control group were 20-35 years old, with an average
age of 26.64±7.35 years.
Inclusion and exclusion criteria
The inclusion criteria were as follows: Aged 20-35
years and naturally conceived; patients diagnosed with EP
laparoscopically (18); with
complete clinical general data, amenorrhea between 27-88 days; and
no history of fetus protection during pregnancy. Ultrasound
examination confirmed that there was no pregnancy sac in the
uterine cavity of the patient, and there were heterogeneous
abnormal echogenic masses outside the uterine cavity revealing a
trend of gradual increase, or there were fetal buds and fetal heart
beats. All of the enrolled patients were informed of this study and
signed the written informed consent. The experimental process was
approved by the Medical Ethics Committee of the Affiliated Hospital
of Jining Medical University and was in accordance with the 2013
version of the Declaration of Helsinki. The exclusion criteria were
as follows: Patients with communication barriers or severe mental
illness, those combined with malignant tumor or serious heart,
lung, liver, kidney and other functional disorders, or those who
were in urgent need of surgery for intraperitoneal hemorrhage with
unstable vital signs were excluded.
Methods
Elbow venous blood (5 ml) was extracted from an
empty stomach in the morning into vacuum blood collection tubes
without anticoagulant, centrifuged at 1,500 x g and 4˚C for 10 min,
and then stored in a low-temperature refrigerator at -75˚C for
later use. The serum was then removed from the freezer and
dissolved in a 4˚C refrigerator before placing it at room
temperature for complete dissolution. The serum expression levels
of PAPP-A (cat. no. ab174314; Kemin Biotechnology Co., Ltd.), IL-8
[cat. no. Ant-111 (0.5 mg); Jingke Chemical Technology Co., Ltd.]
and TNF-α (cat. no. BL-E1290h; Bdlisa Technology Co., Ltd.) were
detected by enzyme-linked immunosorbent assay (ELISA) (19). The present study was carried out in
strict accordance with the manufacturer's instructions. Firstly,
sample, standard and blank wells were set up. Then, 50 µl of the
sample to be tested was added to the sample wells, 50 µl of the
standard was added to the standard wells, and blank wells contained
no reagents. Subsequently, 100 µl of horseradish peroxidase-labeled
detection antibody (cat. no. P39810-100 mg; Acmec Biochemical Co.,
Ltd.) was added to the sample wells and standard wells, and then
the plates were sealed and incubated at 37˚C for 60 min. Next, the
liquid was discarded, the plates were patted dry and washed
repeatedly 5 times and the substrates A and B (1:1) (included in
the kit) were thoroughly mixed before their addition (100 µl) to
all the wells. The plates were then sealed and incubated at 37˚C
for 15 min. Finally, 50 µl termination solution was added to each
well, and the absorbance [optical density (OD)] of each well at 450
nm was read by a fully-automatic enzyme label analyzer (M15;
Chenlian Biotechnology Development Co., Ltd.) to calculate the
expression levels of PAPP-A, IL-8 and TNF-α.
Statistical analysis
Statistical analysis was performed using SPSS 19.0
(IBM Corp.), and the data was visualized by GraphPad Prism 6
(GraphPad Software, Inc.). The counting data were expressed as
case/percentage [n (%)] and the chi-square test was adopted for
inter-group comparisons. The measurement data were expressed in the
form of the mean ± SD, and the comparison of measurement data
between the two groups was conducted by independent sample t-test.
The area under the receiver operating characteristic (ROC) curve
(AUC) was applied to evaluate the diagnostic value of peripheral
blood PAPP-A in patients with EP. The correlation between PAPP-A
and inflammatory factors IL-8 and TNF-α was assessed by Pearson
correlation coefficient, and the independent risk factors affecting
the incidence of EP were analyzed using Cox regression analysis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
General information
Seventy-five patients with EP admitted to our
hospital were enrolled as the research group, and another 59
healthy pregnant women were enrolled as the control group. The
participants in the research group were 21-35 years old, with an
average age of 25.73±7.23 years, while those in the control group
were 20-35 years old, with an average age of 26.64±7.35 years. No
significant difference was observed in terms of age, gravidity,
ethnicity, allergic reaction, smoking history, drinking history,
diet, height, gestational age, abdominal circumference, systolic
blood pressure, or diastolic blood pressure of patients in the two
groups, while other baseline data represented by pre-pregnancy BMI,
weight gain during pregnancy, and level of PAPP-A revealed
statistically significant differences (P<0.05; Table I).
 | Table IComparison of general data between two
groups [n (%)] (mean ± SD). |
Table I
Comparison of general data between two
groups [n (%)] (mean ± SD).
| Categories | Research group
(n=75) | Control group
(n=59) | t/χ2
value | P-value |
|---|
| Age (years) | 25.73±7.23 | 26.64±7.35 | 0.718 | 0.474 |
| Gravidity
(times) | 1.40±0.52 | 1.50±0.62 | 1.015 | 0.312 |
| Ethnicity | | | 0.273 | 0.602 |
|
Han | 39 (52.00) | 28 (47.46) | | |
|
Ethnic
minorities | 36 (48.00) | 31 (52.54) | | |
| Allergic
reaction | | | 0.970 | 0.325 |
|
Yes | 42 (56.00) | 38 (64.41) | | |
|
No | 33 (44.00) | 21 (35.59) | | |
| Smoking | | | 0.158 | 0.691 |
|
Yes | 23 (30.67) | 20 (33.90) | | |
|
No | 52 (69.33) | 39 (66.10) | | |
| Drinking | | | 0.001 | 0.980 |
|
Yes | 24 (32.00) | 19 (32.20) | | |
|
No | 51 (68.00) | 40 (67.80) | | |
| Diet | | | 0.970 | 0.325 |
| Dietary
restriction | 33 (44.00) | 21 (35.59) | | |
| None dietary
restriction | 42 (56.00) | 38 (64.41) | | |
| Height (cm) | 161.54±5.23 | 162.01±5.12 | 0.521 | 0.603 |
| Pre-pregnancy BMI
(kg/m2) | | | 9.104 | 0.002 |
|
≥23 | 11 (14.67) | 22 (37.29) | | |
|
<23 | 64 (85.33) | 37 (62.71) | | |
| Weight gain during
pregnancy (kg) | 13.52±4.26 | 15.61±4.53 | 2.742 | 0.007 |
| Gestational age
(weeks) | 23.95±1.85 | 24.45±1.55 | 1.666 | 0.098 |
| Abdominal
circumference (cm) | 99.98±6.36 | 101.87±6.65 | 1.674 | 0.097 |
| Systolic blood
pressure (mmHg) | 114.12±9.06 | 115.59±8.99 | 0.936 | 0.351 |
| Diastolic blood
pressure (mmHg) | 72.98±7.16 | 75.04±6.88 | 1.682 | 0.095 |
| PAPP-A (pg/ml) | 4.94±1.36 | 5.68±1.59 | 2.902 | 0.004 |
Expression and diagnostic value of
PAPP-A in the two groups
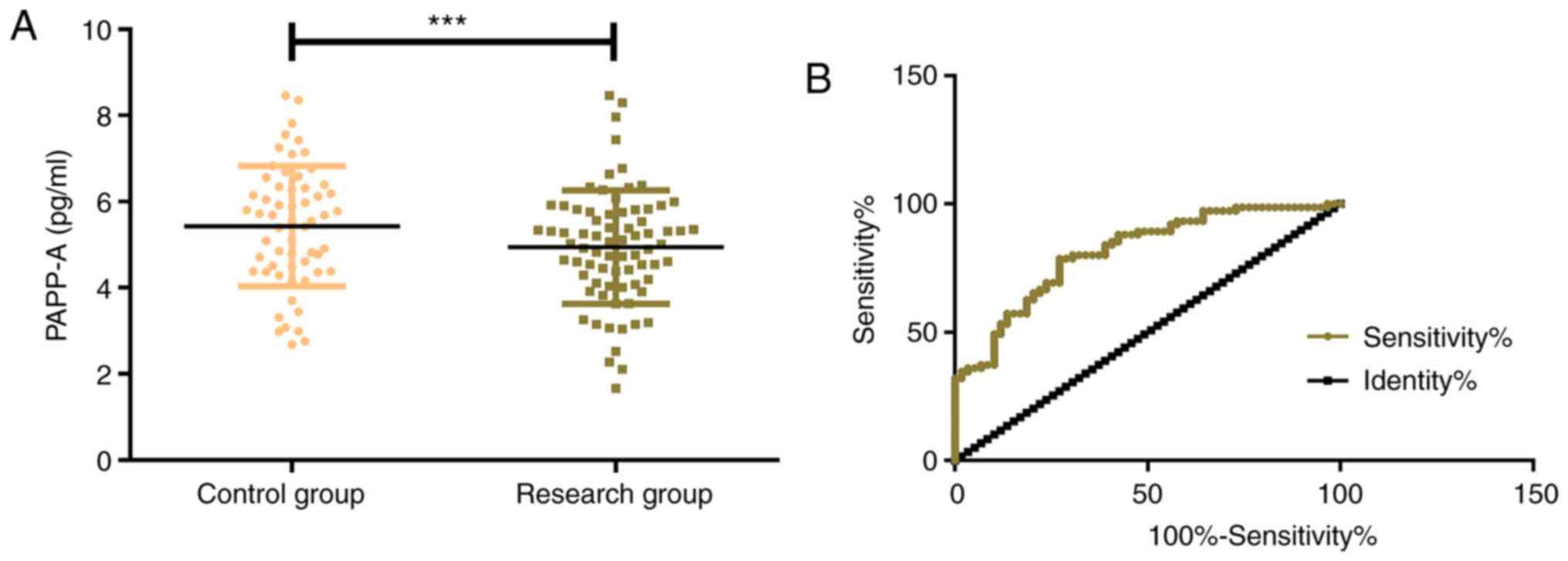
The expression levels of PAPP-A in the control group
and the research group were 5.68±1.59 pg/ml and 4.94±1.36 pg/ml,
respectively, which indicated that PAPP-A in the control group was
significantly higher than that in the research group (P<0.001).
By further drawing the ROC curve, it was determined that the serum
PAPP-A in the diagnosis of EP was 0.812 (95% CI, 0.741-0.884), with
an optimal cut-off value of 5.648, a sensitivity of 92.13, and a
specificity of 78.33 (Fig. 1).
Changes of serum PAPP-A expression at
different gestational weeks in the two groups
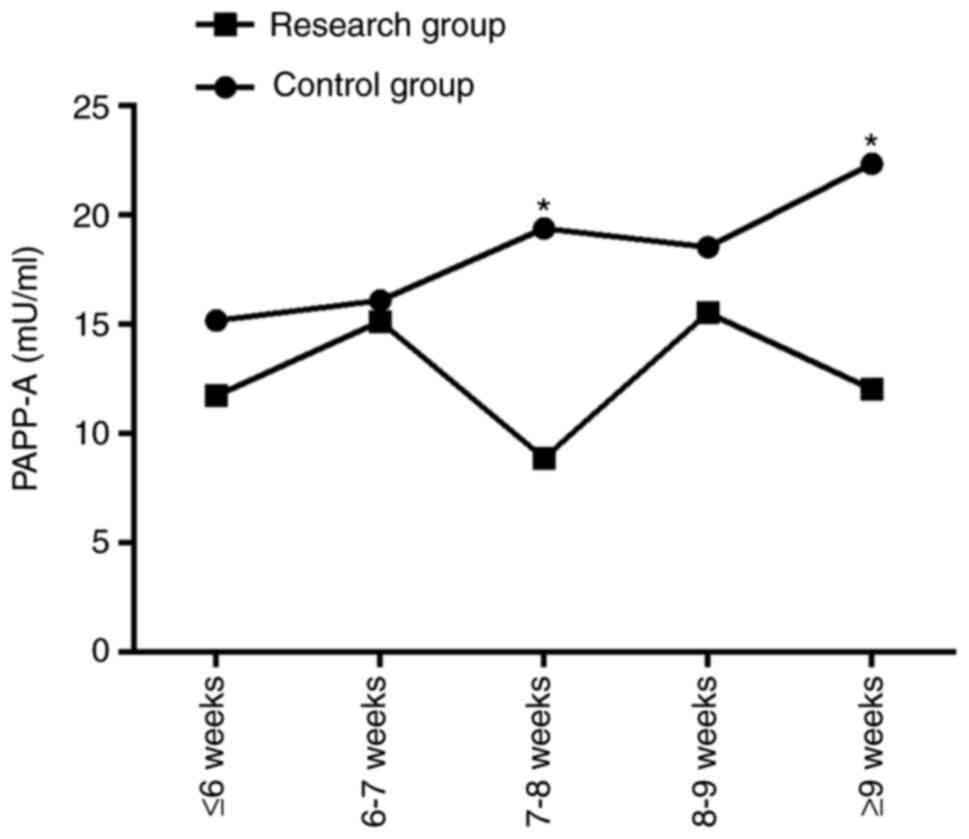
The corresponding serum PAPP-A expression levels at
different gestational weeks in the control group and the research
group were as follows: ≤6 weeks: 15.16±15.27 vs. 11.71±10.97 mU/ml;
6-7 weeks: 16.08±7.60 vs. 15.12±9.30 mU/ml; 7-8 weeks: 19.37±10.23
vs. 8.86±7.62 mU/ml; 8-9 weeks: 18.52±16.92 vs. 15.52±5.15 mU/ml;
≥9 weeks: 22.33±14.64 vs. 12.02±10.11 mU/ml. From the
aforementioned data, no significant difference in serum PAPP-A
expression was observed between the two groups at weeks ≤6, week
6-7, and week 8-9 (P>0.001), while the PAPP-A value of the
control group at week 7-8 and ≥9 weeks was significantly higher
than that of the research group (P<0.001; Table II and Fig. 2).
 | Table IIChanges in the serum PAPP-A level at
different gestational weeks in two groups [(mean ± sd), mU/ml]. |
Table II
Changes in the serum PAPP-A level at
different gestational weeks in two groups [(mean ± sd), mU/ml].
| Groups | ≤6 weeks | 6-7 weeks | 7-8 weeks | 8-9 weeks | ≥9 weeks |
|---|
| Control group | 15.16±15.27 | 16.08±7.60 | 19.37±10.23 | 18.52±16.92 | 22.33±14.64 |
| Research group | 11.71±10.97 | 15.12±9.30 | 8.86±7.62 | 15.52±5.15 | 12.02±10.11 |
| t | 1.521 | 0.742 | 6.815 | 1.454 | 4.814 |
| P-value | 0.131 | 0.459 | <0.001 | 0.148 | <0.001 |
Expression of inflammatory factors
IL-8 and TNF-α in the two groups
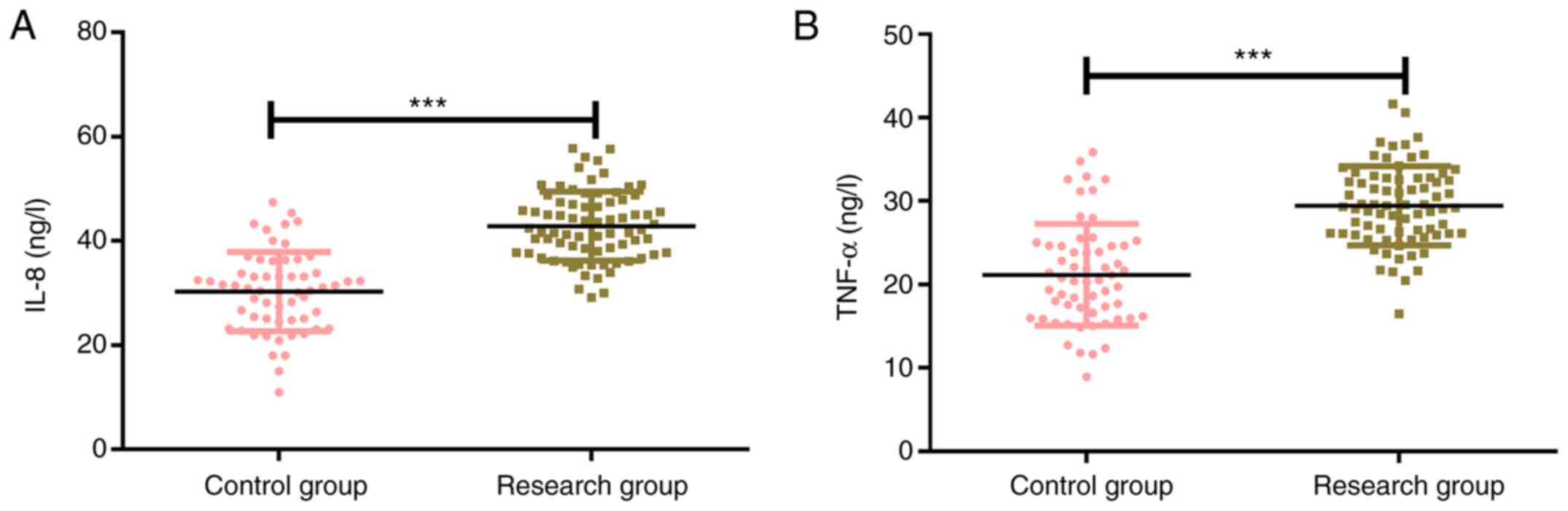
The expression levels of IL-8 in the control group
and the research group were 27.73±4.79 ng/l and 42.93±6.28 ng/l,
respectively, and the corresponding expression levels of TNF-α in
the control group and the research group were 20.83±4.37 ng/l and
29.37±4.38 ng/l. The results revealed that the expression levels of
inflammatory factors IL-8 and TNF-α in the control group were
significantly lower than those in the research group (P<0.001;
Fig. 3).
Correlation analysis between
inflammatory factors IL-8, TNF-α and PAPP-A
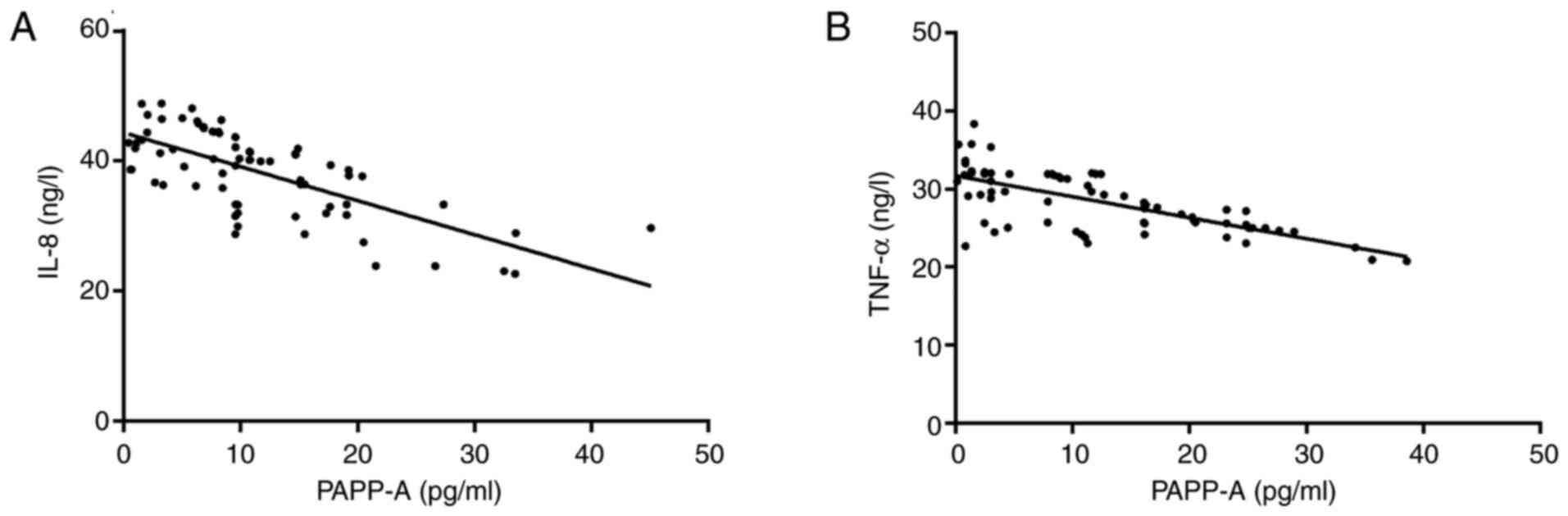
Pearson correlation coefficients were applied to
analyze the correlation of PAPP-A with IL-8 and TNF-α. The results
revealed that serum PAPP-A and IL-8 were negatively correlated
(r=-0.691; P<0.001), and in addition, serum PAPP-A was
negatively correlated with TNF-α (r=-0.692; P<0.001; Fig. 4).
Cox regression analysis of factors
affecting the incidence of EP
Multivariate logistic regression analysis was
carried out for the factors with differences. The results indicated
that a history of genital surgery (P=0.022), salpingotomy
(P=0.005), pelvic infection (P=0.041), EP (P=0.013) and PAPP-A
(P=0.003) were independent risk factors affecting the incidence of
EP. Among the risk factors aforementioned above, history of
salpingotomy, EP and low PAPP-A expression increased the risk of EP
(Tables III and IV).
 | Table IIICox regression analysis assignment
table. |
Table III
Cox regression analysis assignment
table.
| Factors | Variable | Assignment |
|---|
| Age | X1 | No=0, yes=1 |
| Allergic
reaction | X2 | No=0, yes=1 |
| Smoking
history | X3 | No=0, yes=1 |
| Drinking
history | X4 | No=0, yes=1 |
| Diet | X5 | No=0, yes=1 |
| History of genital
surgery | X6 | No=0, yes=1 |
| History of
salpingotomy | X7 | No=0, yes=1 |
| History of pelvic
infection | X8 | No=0, yes=1 |
| History of lower
abdominal surgery | X9 | No=0, yes=1 |
| History of ectopic
pregnancy | X10 | No=0, yes=1 |
| PAPP-A | X11 | No=0, yes=1 |
 | Table IVUnivariate and multivariate Cox
regression analysis of ectopic pregnancy. |
Table IV
Univariate and multivariate Cox
regression analysis of ectopic pregnancy.
| | Univariate | Multivariate |
|---|
| Factors | HR (95 CI%) | P-value | HR (95 CI%) | P-value |
|---|
| Age | 1.549
(0.519-4.628) | 0.438 | | |
| Allergic
reaction | 0.553
(0.157-2.042) | 0.372 | | |
| Smoking
history | 1.008
(0.991-1.022) | 0.368 | | |
| Drinking
history | 0.564
(0.201-1.580) | 0.276 | | |
| Diet | 2.748
(0.705-10.701) | 0.143 | | |
| History of genital
surgery | 1.406
(1.092-1.812) | 0.043 | 1.138
(0.857-1.514) | 0.022 |
| History of
salpingotomy | 6.459
(1.408-29.575) | 0.017 | 12.852
(2.297-71.238) | 0.005 |
| History of pelvic
infection | 6.108
(1.385-24.954) | 0.018 | 2.601
(1.032-6.538) | 0.041 |
| History of lower
abdominal surgery | 2.270
(0.901-1.792) | 0.175 | - | - |
| History of ectopic
pregnancy | 0.292
(0.124-0.689) | 0.005 | 0.357
(0.158-0.735) | 0.013 |
| PAPP-A | 8.356
(2.128-3.846) | 0.002 | 5.817
(2.157-15.756) | 0.003 |
Discussion
Worldwide, gestational placenta related-diseases are
one of the leading causes of maternal and neonatal morbidity and
mortality (20). Of these, EP is a
pregnancy implanted outside the endometrium, which hazards the
health of patients and is a major cause of sudden death in women of
childbearing age (21). In
addition, these women often suffer from complications, such as
organ rupture with massive bleeding, treatment-related risks,
recurrent EP and infertility risk (22). Therefore, the diagnosis of EP is of
vital significance for reducing the morbidity and mortality
associated with this disease (23).
PAPP-A, a placental-derived glycoprotein produced by
trophoblast cells that gradually increases during the first few
weeks of a viable pregnancy, has been revealed to be diagnostic in
a variety of abnormal obstetric conditions (24). The present study firstly detected
the expression of PAPP-A in EP. It was observed that the serum
PAPP-A expression was significantly lower in EP patients in the
research group than that of patients in the control group, and the
AUC of patients diagnosed with EP by PAPP-A was 0.812, with a
sensitivity of 92.13 and a specificity of 78.33, indicating that
PAPP-A may be a potential diagnostic and therapeutic target for EP.
PAPP-A expression was further analyzed in the two groups at
different gestational weeks, and different PAPP-A levels were
observed, that is, the control group presented a stable increasing
trend of PAPP-A at each gestational week, while the research group
exhibited an irregular but insignificant increase. At 7-8 weeks and
≥9 weeks, the PAPP-A value in the control group was significantly
higher than that in the research group, which was probably due to
the fact that the PAPP-A value between the two groups gradually
increased with the prolonging of amenorrhea. Therefore, according
to the detection of low expression of PAPP-A in the serum of EP
patients, it was theorized that the determination of PAPP-A in
serum could be used as a routine measurement for pregnant patients.
In a study of Kaijomaa et al on adverse pregnancy (25), PAPP-A was underexpressed in patients
with adverse pregnancy, indicating that PAPP-A was of certain
diagnostic value and was an important risk factor for EP, which was
consistent with the results of the present study.
IL-8 is a chemokine that has been clinically
demonstrated to play a major role in tumor immune escape by
promoting an immunosuppressive tumor microenvironment, and it has
been revealed that high levels of IL-8 are associated with poor
prognosis in a variety of tumors (26). According to previous studies, serum
IL-8 was revealed to be significantly higher in EP women than in
women with normal pregnancy in utero, and the diagnosis and
identification of EP women with chlamydia trachomatis infection was
related to the level of IL-8 (27,28).
TNF-α is an inflammatory cytokine secreted by macrophages and
monocytes, which exerts a marked effect on inflammatory response,
cell apoptosis and proliferation (29). There is also research showing that
TNF-α has attracted the attention of clinicians and scholars due to
its involvement in the development of inflammatory response,
autoimmunity, neoplastic disease and the endocrine system (30). For example, TNF-α induces the
apoptosis of cytotrophoblast cells, which also suggests that
abnormal expression of TNF-α may adversely affect the development
and function of the placenta (31).
Growing evidence reveals that TNF-α mediates pregnancy
complications and increases the sensitivity of infertility, while
increased TNF-α in the placenta increases the abortion rate
(32). Soriano et al
(33) revealed that the expression
of serum inflammatory factors IL-6, IL-8 and TNF-α in EP women was
significantly higher than that in normal pregnant women, suggesting
that the overexpression of these three inflammatory mediators
stimulated the inflammatory cascade in patients and aggravated the
progression of EP. Furthermore, other researchers have reported
that the pro-inflammatory factors TNF-α, IL-1β and IL-6 stimulate
the expression of PAPP-A in cultured cells (34). In the present study, the detection
of inflammatory factors demonstrated that the expression levels of
inflammatory factors IL-8 and TNF-α in the research group were
significantly higher than those in the control group, while PAPP-A
was negatively correlated with the two, suggesting that the low
expression of PAPP-A in an inflammatory environment may be related
to the occurrence and development of EP. Concerning the risk
factors of EP, Zhang et al (35) revealed that a history of EP,
infertility, and salpingotomy were all risk factors. In the present
study, Cox regression analysis revealed that a history of genital
surgery, salpingotomy, pelvic infection, EP and low expression of
PAPP-A were the risk factors of EP, among which a history of
salpingotomy, EP and low expression of PAPP-A increased the risks.
Therefore, PAPP-A is anticipated to be a biomarker for the
diagnosis and prognosis of EP.
The present study strictly screened the participants
according to the inclusion and exclusion criteria and ensured the
rigor and reliability of the research. Although in the present
study it was confirmed that PAPP-A is lowly expressed in EP
patients and is negatively correlated with inflammatory factors
IL-8 and TNF-α, there are still some certain limitations in this
study. We will perform more basic experiments in the future,
investigating the specific regulatory mechanism of PAPP-A on EP,
thus further verifying that PAPP-A can be a potential therapeutic
target for EP.
In conclusion, PAPP-A was downregulated in patients
with EP and may be a useful marker for the diagnosis and prognosis
assessment of this condition.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XZ and CW conceived and designed the study,
collected, analyzed and interpreted the experimental data, drafted
this paper, and revised the manuscript critically for important
intellectual content. Both authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the Affiliated Hospital of Jining Medical University (Jining,
China). Signed written informed consents were obtained from the
patients and/or guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Perkins KM, Boulet SL, Kissin DM and
Jamieson DJ: National ART Surveillance (NASS) Group. Risk of
ectopic pregnancy associated with assisted reproductive technology
in the United States, 2001-2011. Obstet Gynecol. 125:70–78.
2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bronson R: Ectopic pregnancy-still a
challenge. Fertil Steril. 110:1265–1266. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Refaat B, Dalton E and Ledger WL: Ectopic
pregnancy secondary to in vitro fertilisation-embryo transfer:
Pathogenic mechanisms and management strategies. Reprod Biol
Endocrinol. 13(30)2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ashshi AM, Batwa SA, Kutbi SY, Malibary
FA, Batwa M and Refaat B: Prevalence of 7 sexually transmitted
organisms by multiplex real-time PCR in Fallopian tube specimens
collected from Saudi women with and without ectopic pregnancy. BMC
Infect Dis. 15(569)2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Li C, Zhao WH, Zhu Q, Cao SJ, Ping H, Xi
X, Qin GJ, Yan MX, Zhang D, Qiu J and Zhang J: Risk factors for
ectopic pregnancy: A multi-center case-control study. BMC Pregnancy
Childbirth. 15(187)2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lagana AS, Vitale SG, De Dominici R,
Padula F, Rapisarda AM, Biondi A, Cianci S, Valenti G, Capriglione
S, Frangez HB and Sturlese E: Fertility outcome after laparoscopic
salpingostomy or salpingectomy for tubal ectopic pregnancy A
12-years retrospective cohort study. Ann Ital Chir. 87:461–465.
2016.PubMed/NCBI
|
|
7
|
Hsu JY, Chen L, Gumer AR, Tergas AI, Hou
JY, Burke WM, Ananth CV, Hershman DL and Wright JD: Disparities in
the management of ectopic pregnancy. Am J Obstet Gynecol.
217:49.e1–49.e10. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Chanana C, Gupta N, Bansal I, Hooda K,
Sharma P, Gupta M, Gandhi D and Kumar Y: Different sonographic
faces of ectopic pregnancy. J Clin Imaging Sci. 7(6)2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Bøtkjær JA, Noer PR, Oxvig C and Yding
Andersen C: A common variant of the pregnancy-associated plasma
protein-A (PAPPA) gene encodes a protein with reduced proteolytic
activity towards IGF-binding proteins. Sci Rep.
9(13231)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Bale LK, Chakraborty S and Conover CA:
Inducible reduction in pregnancy-associated plasma protein-A gene
expression inhibits established atherosclerotic plaque progression
in mice. Endocrinology. 155:1184–1187. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Smith YE, Toomey S, Napoletano S, Kirwan
G, Schadow C, Chubb AJ, Mikkelsen JH, Oxvig C and Harmey JH:
Recombinant PAPP-A resistant insulin-like growth factor binding
protein 4 (dBP4) inhibits angiogenesis and metastasis in a murine
model of breast cancer. BMC Cancer. 18(1016)2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Prithviraj P, Anaka M, McKeown SJ,
Permezel M, Walkiewicz M, Cebon J, Behren A and Jayachandran A:
Pregnancy associated plasma protein-A links pregnancy and melanoma
progression by promoting cellular migration and invasion.
Oncotarget. 6:15953–15965. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sert A, Leung K, Waring ME,
Rojas-Rodriguez R, Corvera S and Moore Simas TA: Association
between first trimester pregnancy associated plasma protein-A
(PAPP-A) and gestational diabetes mellitus development. 2016.
|
|
14
|
Peeva G, Oakley L, Von Rège I, Nicolaides
K and Oteng-Ntim E: Does first trimester serum pregnancy-associated
plasma protein A differ in pregnant women with sickle cell disease?
Prenat Diagn. 39:921–924. 2019.PubMed/NCBI View
Article : Google Scholar
|
|
15
|
Antsaklis P, Fasoulakis Z, Theodora M,
Diakosavvas M and Kontomanolis EN: Association of low maternal
pregnancy-associated plasma protein A with adverse perinatal
outcome. Cureus. 11(e4912)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Papastefanou I, Wright D, Syngelaki A,
Lolos M, Anampousi K and Nicolaides KH: Competing-risks model for
prediction of small-for-gestational-age neonate from maternal
characteristics and serum pregnancy-associated plasma protein-A at
11-13 weeks' gestation. Ultrasound Obstet Gynecol. 56:541–548.
2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Xin H, Liu W and Li P: Diagnostic value of
detection of serum β-HCG and CT-IgG combined with transvaginal
ultrasonography in early tubal pregnancy. Exp Ther Med. 16:277–281.
2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hoffmann S, Abele H and Bachmann C:
Spontaneous bilateral tubal ectopic pregnancy: Incidental finding
during laparoscopy-brief report and review of literature.
Geburtshilfe Frauenheilkd. 76:413–416. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hornbeck PV: Enzyme-linked immunosorbent
assays. Curr Protoc Immunol. 110:2.1.1–2.1.23. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Browne JL, Klipstein-Grobusch K, Koster
MP, Ramamoorthy D, Antwi E, Belmouden I, Franx A, Grobbee DE and
Schielen PC: Pregnancy associated plasma protein-a and placental
growth factor in a sub-Saharan African population: A nested
cross-sectional study. PLoS One. 11(e0159592)2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sowter M: Finding an ectopic pregnancy.
Imaging. 17:2015.
|
|
22
|
Perlman BE, Guerrero K, Karsalia R and
Heller DS: Reproductive outcomes following a ruptured ectopic
pregnancy. Eur J Contracept Reprod Health Care. 25:206–208.
2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Murano T, Shaker L and Marco CA:
Evaluation and management of ectopic pregnancy in the emergency
department. Emergency Med Rep. 40:2019.
|
|
24
|
Batson RJ, Mills BB, Nagy Z and Roudebush
W: Pregnancy-associated plasma protein A (PAPP-A): A biomarker for
the aid in risk stratification of nonviable pregnancy. Fertil
Steril. 104(e351)2015.
|
|
25
|
Kaijomaa M, Rahkonen L, Ulander VM,
Hämäläinen E, Alfthan H, Markkanen H, Heinonen S and Stefanovic V:
Low maternal pregnancy-associated plasma protein A during the first
trimester of pregnancy and pregnancy outcomes. Int J Gynaecol
Obstet. 136:76–82. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Melero Bermejo I, Jaffee EM, Davar D,
Cardarelli J, Williams D, Phillips P, Phillips P, Carleton M, Zhou
M, De Henau O, et al: Phase 1b/2 study of nivolumab in combination
with an anti-IL-8 monoclonal antibody, BMS-986253, in a
biomarker-enriched population of patients with advanced cancer. J
Clin Oncol. 36 (Suppl 15)(TPS3109)2018.
|
|
27
|
Shao R, Feng Y, Zou S, Li X, Cui P and
Billig H: Quantitative analysis of hormones and inflammatory
cytokines in Chlamydia trachomatis-infected women with tubal
ectopic pregnancy and early intrauterine pregnancy. Data Brief.
6:135–142. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ma L, Li Z, Xi S, Guo Q, Zhao P, Li W, Ai
J and Chen X: Tubal ectopic pregnancy occurrence is associated with
high expressions of prokineticin receptors and aberrant secretion
of inflammatory cytokines. Am J Transl Res. 12:5741–5751.
2020.PubMed/NCBI
|
|
29
|
Jiang PR, Cao Z, Qiu ZL, Pan JW, Zhang N
and Wu YF: Plasma levels of TNF-α and MMP-9 in patients with
silicosis. Eur Rev Med Pharmacol Sci. 19:1716–1720. 2015.PubMed/NCBI
|
|
30
|
Zaka M, Abbasi BH and Durdagi S: Novel
tumor necrosis factor-α (TNF-α) inhibitors from small molecule
library screening for their therapeutic activity profiles against
rheumatoid arthritis using target-driven approaches and binary QSAR
models. J Biomol Struct Dyn. 37:2464–2476. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Cha HH, Hwang JR, Kim HY, Choi SJ, Oh SY
and Roh CR: Autophagy induced by tumor necrosis factor α mediates
intrinsic apoptosis in trophoblastic cells. Reprod Sci. 21:612–622.
2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Li HH, Xu XH, Tong J, Zhang KY, Zhang C
and Chen ZJ: Association of TNF-α genetic polymorphisms with
recurrent pregnancy loss risk: A systematic review and
meta-analysis. Reprod Biol Endocrinol. 14(6)2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Soriano D, Hugol D, Quang NT and Darai E:
Serum concentrations of interleukin-2R (IL-2R), IL-6, IL-8, and
tumor necrosis factor alpha in patients with ectopic pregnancy.
Fertil Steril. 79:975–980. 2003.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Tang SL, Zhao ZW, Liu SM, Wang G, Yu XH,
Zou J, Wang SQ, Dai XY, Fu MG, Zheng XL, et al:
Pregnancy-associated plasma protein-A accelerates atherosclerosis
by regulating reverse cholesterol transport and inflammation. Circ
J. 83:515–523. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Zhang D, Shi W, Li C, Yuan JJ, Xia W, Xue
RH, Sun J and Zhang J: Risk factors for recurrent ectopic
pregnancy: A case-control study. BJOG. 123 (Suppl 3):S82–S89.
2016.PubMed/NCBI View Article : Google Scholar
|