Introduction
Gastric cancer (GC) is a common malignant tumor type
of the digestive system. Its incidence is the fifth-highest among
all malignant tumors and GC is the third leading cause of
cancer-associated mortality worldwide (1). A survey conducted by the National
Central Cancer Registry of China reported ~410,000 novel cases of
GC in China annually and ~290,000 GC-associated deaths (2). Despite the downward trend in the
overall incidence and mortality related to GC due to the gradual
improvement in diagnosis and treatment strategies (3), reduction of Helicobacter pylori
infection due to better hygiene and antibiotics use and a deeper
understanding of its molecular mechanisms, it still poses enormous
challenges (4,5).
Early GC (EGC) refers to gastric lesions confined to
the gastric mucosa or submucosa (6). Studies have indicated that radical
surgery, primarily endoscopic submucosal dissection (ESD) and
endoscopic mucosal resection (EMR), are the optimal surgical
approach for patients with GC (7,8). ESD
has advantages of minimal invasiveness and high treatment
efficiency. Furthermore, ESD has a good therapeutic effect on a
relatively wide range of tissues wherein pathological examination
may determine the residual cancer cell status (9,10).
Golgi protein 73 (GP73), initially isolated from a
complementary DNA liver library of patients with
Cytomegalovirus hepatitis, is a protein unique to epithelial
cells. It is abnormally expressed in hepatocarcinoma, bile duct
carcinoma and lung cancer (11-13).
Trypsinogen-2 (TAT-2) is a serine protease encoded by T-8 amino
acid, which is closely associated with the growth and metastasis of
cancer cells. It is able to activate proteases and receptors.
Receptors are present on different tumor cells, as well as on cells
that form the tumor microenvironment, including vascular
endothelial cells, smooth muscle cells and macrophages that promote
tumor growth and development (14,15).
Ichikawa et al (16)
reported that serum TAT-2 expression levels were higher in patients
with Borrmann type IV GC/leucogastric cancer than in those with
other GC types; furthermore, they reported that it may be
associated with lymph node metastasis, liver metastasis and poorly
differentiated adenocarcinoma.
The treatment of patients with EGC using ESD has
been extensively studied (17,18),
whereas limited progress has been made regarding the specific
therapeutic effect of treatment for EGC using ESD and its effect on
serum TAT-2 and GP73 expression levels. Therefore, the present
study aimed to observe the early treatment effectiveness of ESD and
the effect on TAT-2 and GP73 expression levels in patients with EGC
to provide a reference for clinical treatment.
Materials and methods
General patient information
A total of 161 patients with EGC treated at the
Eighth Affiliated Hospital of Guangxi Medical University (Guigang,
China) from April 2013 to February 2014 were selected as the study
subjects. Patients who underwent ESD were assigned to group A (86
cases), and those who underwent EMR were assigned to group B (75
cases). There were 56 males and 30 females in group A (age, 46-61
years; mean age, 57.36±2.87 years). There were 17 cases of lymph
node metastasis, 69 cases without lymph node metastasis, 36 cases
with tumor infiltration of the mucosal layer, 19 cases with tumor
infiltration of the superficial muscle layer and 31 cases with
tumor infiltration of the submucosal layer. In group B, there were
48 males and 37 females (age, 43-60 years; mean age, 58.06±2.84
years). There were 19 cases of lymph node metastasis, 5 cases
without lymph node metastasis, 33 cases with tumor infiltration of
the mucosal layer, 15 cases with tumor infiltration of the
superficial muscle layer and 27 cases with tumor infiltration of
the submucosal layer. The present study was approved by the Ethics
Committee of the Eighth Affiliated Hospital of Guangxi Medical
University (Guigang, China). All study participants provided
written informed consent prior to participating in the study.
Inclusion and exclusion criteria
The inclusion criteria were as follows: Patients
aged 40-65 years; first diagnosed with EGC using gastroscopy and
histopathological examination without prior treatment prior to
admission; no lesion size limits were applied in the patient
selection process; diagnosed with EGC with the GC differentiation
degree, clinical stage and lymph node metastasis being in
accordance with the criteria of the Union for International Cancer
Control for EGC (19). The family
members of all patients were informed and patients or family
members provided written informed consent. Patients with congenital
immune dysfunction, severe birth defects, other heart, liver or
kidney diseases or with coagulation disorders were excluded.
Treatment
An electronic gastroscope (Olympus Corp.) was used
with the Cv-260 endoscope host. The ultrasonic microprobe (model
no. ERBEICC-200) was set to 20 MHz. A high-frequency electrotome,
injection needle, hemostatic clamp, trap, three-claw forceps and
hot biopsy forceps were also used in the surgical procedures. After
admission of all study subjects, group B was treated using EMR as
follows: High-frequency electroacupuncture was performed to
electrocauterize around the lesion and mark the edge. Next, a
submucosal injection of 1:10,000 epinephrine/normal saline was
administered (1 ml). Next, a trap was placed around the lesion
after confirming lesion swelling. The lesion tissue was lifted with
three-claw forceps and the trap was retrieved. Finally, the lesion
was excised, the endoscope was removed and relevant tissues were
examined pathologically for residual GC cells (2,4). Group
A received ESD as follows: A 1:10,000 mixture of adrenaline/normal
saline was injected under the lesion mucosa (1 ml) and the lesion
location was aspirated by a transparent cap after the lesion
swelling was confirmed. Next, dissection was performed along the
lesion edge, the tumor was removed and bleeding was stopped by
electrocoagulation after using an electrical trap. Finally, the
tissue was examined pathologically for residual GC tissue (5,6). All
patients were post-operatively administered routine hemostatic and
anti-infection treatments. The patients fasted for 1 day
post-operatively and proton pump inhibitors were routinely
administered for 2 weeks. The patients were also treated with
gastric mucosal protective agents.
Treatment effectiveness
After 2 weeks, symptoms and treatment effectiveness
were evaluated on a descriptive scale (20): i) Markedly effective - symptoms,
signs and lesions disappeared and imaging and endoscopic findings
were normal; ii) effective - symptoms and signs improved, imaging
and endoscopic findings improved and lesions were smaller than
those prior to treatment; and iii) ineffective - symptoms and signs
did not improve, the fistula exhibited no change or increased
according to imaging and endoscopic examination and the condition
had a progressive trend toward deterioration. The incidence of
post-operative complications was also recorded (21).
Main reagents
A TAT-2 diagnostic kit (cat. no. YS01266B; Shanghai
Yaji Biotechnology Co., Ltd.) a GP73 diagnostic kit (cat. no.
1532405515; Shanghai Jianglai Biotechnology Co., Ltd.) and an
MR-96A enzyme-linked immunoassay (cat. no. 1012; Mindray) were
used.
Detection method
ELISA was performed for analyzing serum TAT-2 and
GP73 expression levels in the two groups prior to and after
treatment. First, the corresponding microplate wells were numbered
sequentially with two negative control wells, two positive control
wells and one blank control well in each plate. Next, the samples
were diluted with sample dilution solution (1:1) and 50 µl was
added to the reaction wells. Next, 50 µl of diluted standard or
sample was added to the wells, immediately followed by the addition
of 50 µl of biotinylated antibody. The plate was covered, shaken
gently to mix and incubated at 37˚C for 1 h. The solution in each
well was discarded, each well was filled with detergent, shaken for
30 sec and the detergent was discarded. Subsequently, the plate was
patted with absorbent paper to absorb residual detergent and the
process was repeated thrice. Termination solution (50 µl) was added
immediately after removing the plate from the dark. The optical
density value of each well was measured within 15 min after the
addition of the termination solution at a wavelength of 450 nm.
Statistical analysis
SPSS 22.0 (IBM Corp.) was employed for statistical
analyses. Count data are expressed as numbers and percentages and
were compared using a Chi-squared test. An unpaired Student's
t-test was used for comparisons between the two groups. Intergroup
comparisons between the pre-treatment and the post-treatment data
were analyzed by one-way ANOVA, whereas the pairwise comparisons
were analyzed by the Bonferroni post hoc test. Kaplan-Meier curves
were drawn to establish survival curves for the two groups and a
log-rank test was performed to evaluate differences between the
survival curves.
Results
General information
Comparison of clinical data between the two groups
indicated no significant differences in terms of parameters such as
sex, age, body mass index, education level, smoking history,
drinking history, residence, body temperature, erythrocyte count,
leukocyte count, pathological classification, infiltration depth
and lymph node metastasis or tumor site between the two groups
(P>0.05; Table I).
 | Table IDemographic and clinical data of the
patients. |
Table I
Demographic and clinical data of the
patients.
| Factors | Group A (n=86) | Group B (n=75) | t/χ2
value | P-value |
|---|
| Sex | | | 0.022 | 0.883 |
|
Male | 56 (65.12) | 48 (64.00) | | |
|
Female | 30 (34.88) | 27 (36.00) | | |
| Age (years) | 57.36±2.87 | 58.06±2.84 | 1.551 | 0.123 |
| Body mass index
(kg/m2) | 22.65±2.52 | 22.34±2.37 | 0.800 | 0.425 |
| Educational
level | | | 0.088 | 0.766 |
|
High school
and below | 41 (47.67) | 34 (45.33) | | |
|
Above high
school | 45 (52.33) | 41 (54.67) | | |
| Smoking history | | | 0.179 | 0.672 |
|
Yes | 51 (59.30) | 42 (56.00) | | |
|
No | 35 (40.70) | 33 (44.00) | | |
| Drinking
history | | | 0.515 | 0.473 |
|
Yes | 22 (25.58) | 23 (30.67) | | |
|
No | 64 (74.42) | 52 (69.33) | | |
| Residence | | | 0.159 | 0.690 |
|
Urban | 63 (73.26) | 57 (76.00) | | |
|
Rural | 23 (26.74) | 18 (24.00) | | |
| Body temperature
(˚C) | 36.62±0.30 | 36.69±0.28 | 1.523 | 0.130 |
| Erythrocytes
(x1012/l) | 6.58±0.49 | 6.61±0.51 | 0.380 | 0.704 |
| Leukocytes
(x109/l) | 12.26±3.53 | 12.32±3.61 | 0.107 | 0.915 |
| Pathological
type | | | 0.396 | 0.821 |
|
Uplift
type | 30 (34.88) | 26 (34.67) | | |
|
Superficial
type | 32 (37.21) | 25 (33.33) | | |
|
Depressed
type | 24 (27.91) | 24 (32.00) | | |
| Infiltration
depth | | | 0.126 | 0.939 |
|
Mucosal
layer | 36 (41.86) | 33 (44.00) | | |
|
Mucosal
muscular layer | 19 (22.09) | 15 (20.00) | | |
|
Submucosal
layer | 31 (36.05) | 27 (36.00) | | |
| Lymph node
metastasis | | | 0.715 | 0.398 |
|
Yes | 17 (19.77) | 19 (25.33) | | |
|
No | 69 (80.23) | 56 (74.67) | | |
| Site | | | 0.770 | 0.681 |
|
Upper
1/3 | 7 (8.14) | 9 (12.00) | | |
|
Medium
1/3 | 44 (51.16) | 35 (46.67) | | |
|
Bottom
1/3 | 35 (40.70) | 31 (41.33) | | |
Treatment effectiveness
The results regarding treatment effectiveness were
as follows: In group A, the treatment was markedly effective in 69
cases (80.23%), effective in 14 (16.28%) and ineffective in 3
patients (3.49%), with a total treatment effectiveness of 96.51%.
In group B, the treatment was markedly effective in 48 cases
(64.00%), effective in 12 (16.00%) and ineffective in 15 patients
(20.00%), with a total effectiveness of 80.00%. The total treatment
effectiveness was significantly higher in group A than in group B
(P<0.05; Table II).
 | Table IIComparison of clinical efficacy
between groups A and B. |
Table II
Comparison of clinical efficacy
between groups A and B.
| Category | Group A (n=86) | Group B (n=75) | χ2
value | P-value |
|---|
| Marked clinical
efficacya | 69 (80.23) | 48 (64.00) | 5.315 | 0.021 |
| Effective | 14 (16.28) | 12 (16.00) | 0.002 | 0.962 |
| Ineffective | 3 (3.49) | 15 (20.00) | 11.000 | 0.001 |
| Total
effectiveness | 83 (96.51) | 60 (80.00) | 11.000 | 0.001 |
Comparison of complications
There were 6 cases (8.00%) of nausea and vomiting, 4
(5.33%) of bleeding due to perforation occurring intraoperatively
and 4 (5.33%) of abdominal distension and abdominal pain after
treatment in group B (total adverse reaction/complication rate,
18.66%). The corresponding post-treatment adverse
reactions/complications in group A were 8 (9.30%), 6 (6.98%) and 7
(8.14%), respectively (total adverse reaction/complication rate,
24.42%; Table III).
 | Table IIIComparison of adverse events and
complications between groups A and B. |
Table III
Comparison of adverse events and
complications between groups A and B.
| Item | Group A (n=86) | Group B (n=75) | χ2
value | P-value |
|---|
| Nausea and
vomiting | 8 (9.30) | 6 (8.00) | 0.083 | 0.773 |
| Bleeding due to
perforation in the perioperative phase | 6 (6.98) | 4 (5.33) | 0.186 | 0.667 |
| Abdominal
distension and abdominal pain | 7 (8.14) | 4 (5.33) | 0.496 | 0.481 |
| Total
incidence | 21 (24.42) | 14 (18.66) | 0.779 | 0.377 |
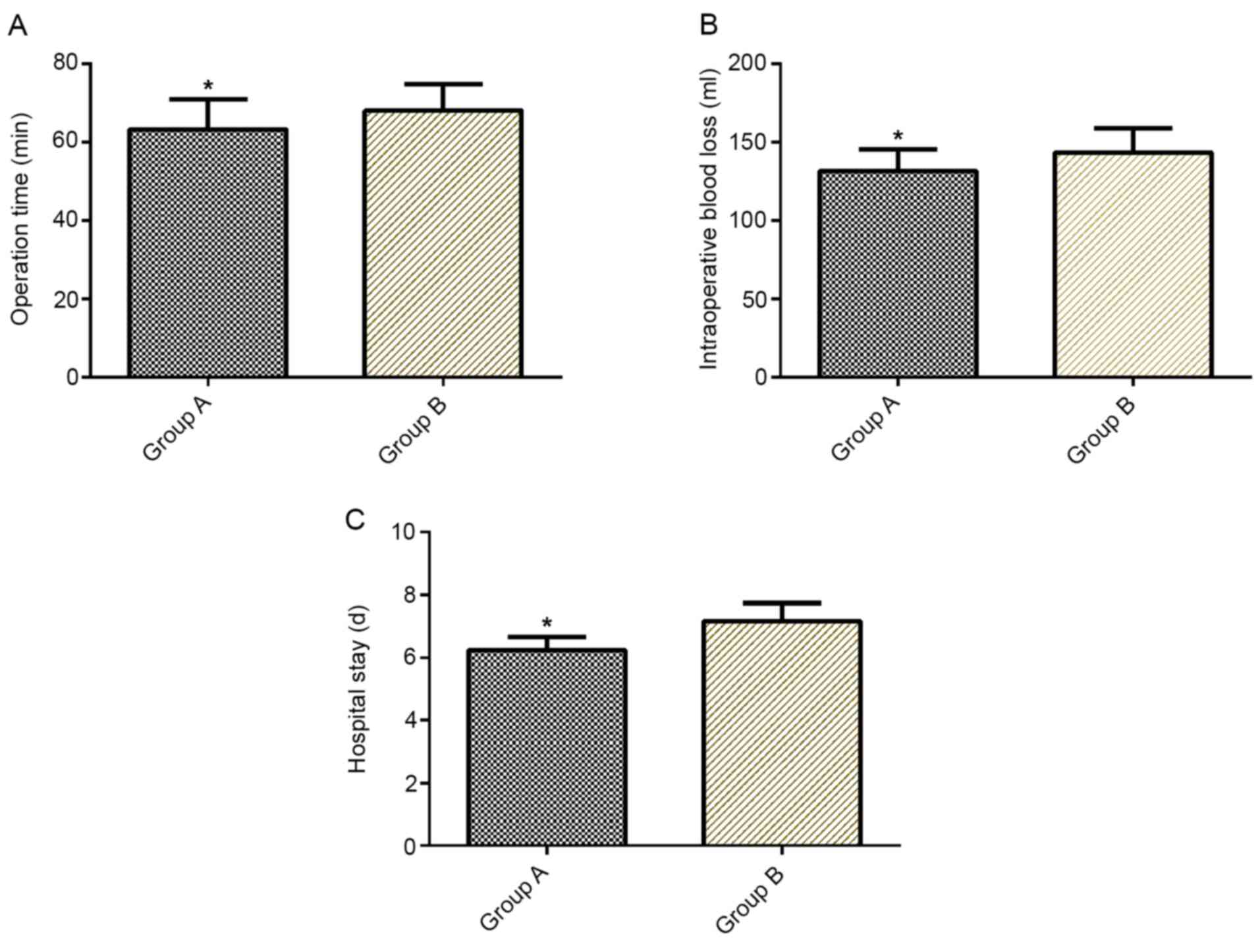
Comparison of operation time,
intraoperative blood loss and length of hospital stay
Operation time, intraoperative blood loss and length
of hospital stay were significantly lower in group A than in group
B (P<0.05; Fig. 1).
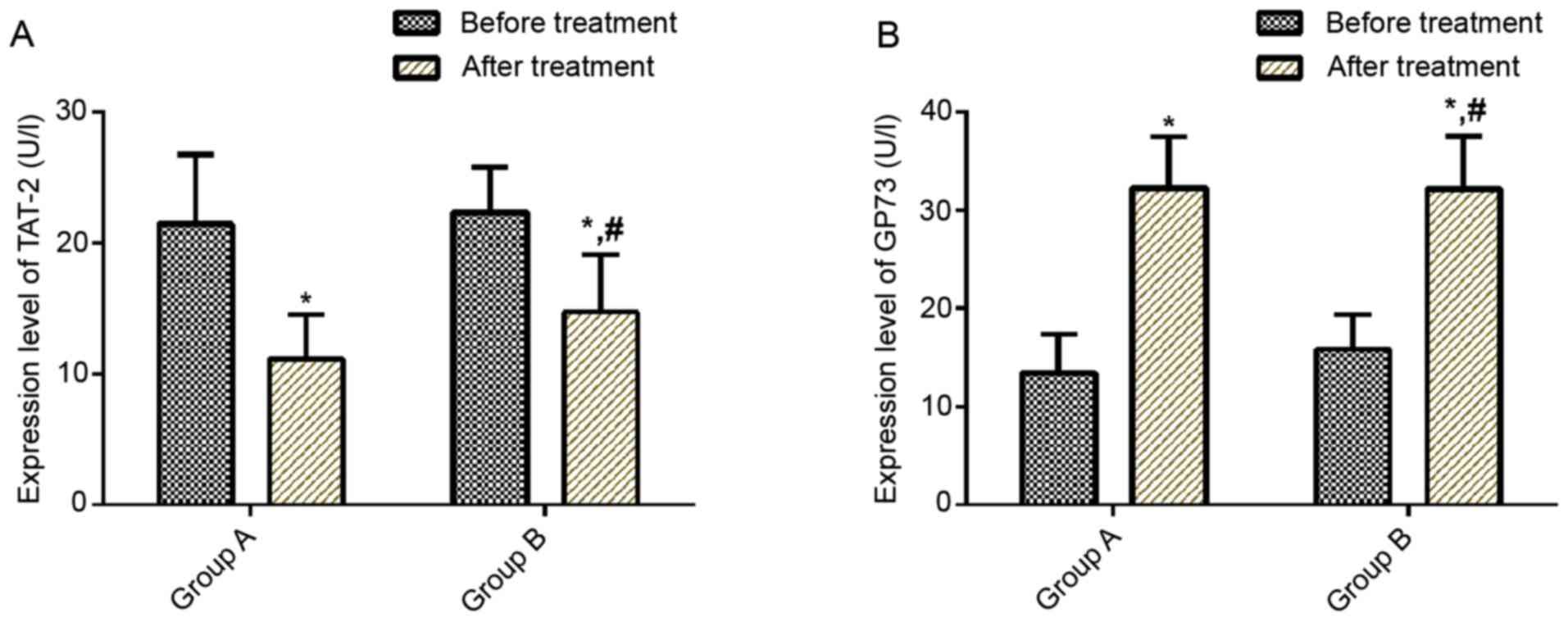
Comparison of serum TAT-2 and GP73
expression levels prior to and after treatment
No significant difference was observed in serum
TAT-2 and GP73 expression levels between the two groups prior to
treatment (P>0.05). After treatment, serum TAT-2 expression
levels decreased in both groups (P<0.05) and serum TAT-2
expression levels were lower in group A than in group B
(P<0.05). After treatment, serum GP73 expression levels
increased in both groups (P<0.05; Fig. 2).
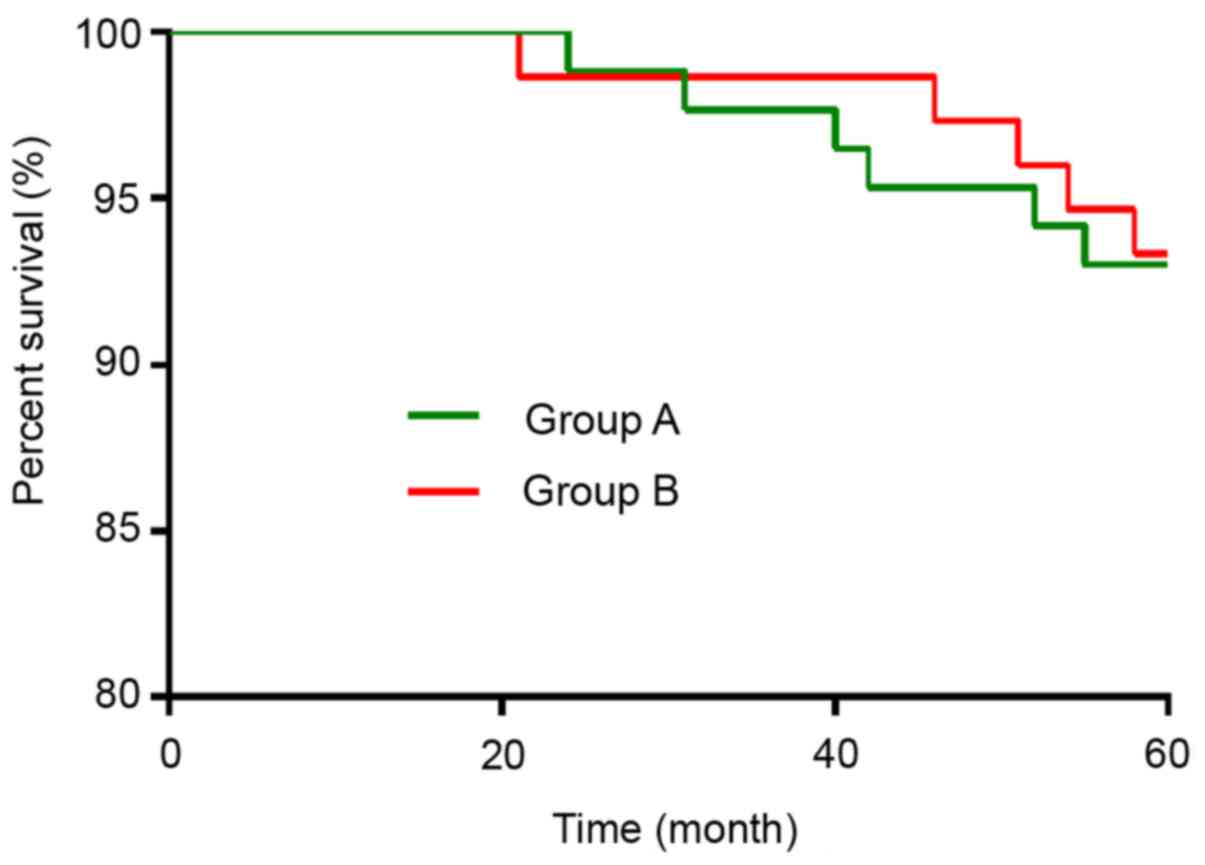
Five-year survival rate after
treatment
All 161 patients were followed up for 5 years and 11
died during the follow-up period (5-year survival rate, 93.17%).
There were 6 deaths in group A (5-year survival rate, 93.02%) and 5
in group B (5-year survival rate, 93.33%). Kaplan-Meier survival
curves revealed no significant differences in 5-year survival rate
between the two groups (P>0.05; Fig.
3).
Discussion
The pathogenesis of GC is complex with possible
causes including Helicobacter pylori infection,
environmental factors and genetic factors. EGC cells are confined
to the gastric mucosa and submucosa and the 5-year survival rate of
patients with EGC may reach >90%. However, if GC cells continue
to develop and invade the muscular layer, the post-operative 5-year
survival rate decreases to 30-50% (22,23).
Therefore, timely and effective treatment of early-onset GC and EGC
are of great importance for favorable patient outcomes.
ESD is a minimally invasive technique that evolved
from endoscopic mucosectomy, which has the advantages of limited
trauma, rapid recovery, high removal rate, fewer complications and
lower surgical costs. It improves treatment effects and
post-operative recovery and it has become one of the most common
microsurgical procedures for the treatment of digestive tract
cancer (24-26).
Meng et al (27)
retrospectively reviewed 126 cases of gastrointestinal stromal
tumor with lesion diameters of <2 cm and indicated that ESD was
superior to laparoscopic surgery for submucosal tumors with smaller
diameters. In addition, Bang et al (28) conducted a meta-analysis of 9 studies
and concluded that ESD is a technically feasible method for the
treatment of subepithelial tumors. The present study suggested that
total treatment effectiveness was higher in group A than in group
B, whereas no significant difference was observed in terms of
incidence of complications between the two groups. Furthermore, the
operation time, intraoperative blood loss and length of hospital
stay were lower in group A than in group B. Certain clinical
studies have suggested that ESD has an ideal efficacy in the
treatment for EGC, with relatively few and controllable
complications and relatively high safety and effectiveness. By
studying long-term patient prognoses after ESD, Park et al
(29) indicated that considering
the resection rates of large lesions, the outcomes for patients
treated with ESD were good and the recurrence rate was low. Ryu
et al (30) reviewed ESD and
cancer resection and their results suggested that the ESD group had
a shorter operation time and fasting period than the surgical
resection group. They suggested that ESD is an acceptable and
effective treatment for EGC compared with surgical resection, which
is consistent with the results of the present study.
Previous studies have demonstrated that TAT-2 and
GP73 were abnormally expressed in GC. Song et al (31) reported that serum TAT2 expression
levels were significantly increased in patients with GC compared
with those in healthy subjects (P<0.05). In addition, its
sensitivity was higher than that of the tumor markers CA242, CA50
and CEA. Furthermore, the specificity of serum TAT2 expression
levels was 95%, suggesting that it may be used as a novel tumor
marker. Chen et al (32)
reported that GP73 mRNA and protein expression in GC tissues were
lower than those of adjacent normal tissues, which was associated
with the differentiation degree of GC and the sex of patients. In
the present study, serum TAT-2 expression levels decreased in both
groups, whereas serum GP73 expression levels increased after
treatment. Serum TAT-2 expression levels were lower but serum GP73
expression levels were higher in group A than in group B,
indicating that ESD improved EGC, reduced serum TAT-2 expression
levels and increased serum GP73 expression levels. Serum TAT-2 and
GP73 expression levels may be associated with the severity of EGC,
but their relationship has not been thoroughly discussed in the
present study. Regarding survival, the 5-year survival rate of
group A and group B was 93.02 and 93.33%, respectively, which were
not significantly different, indicating that the effects of the two
treatments on survival were not different. These results are
consistent with those reported by Rong et al (33) who reported no significant difference
in 5-year morbidity and mortality between ESD and surgical
resection.
The study subjects were selected in strict
accordance with the inclusion and exclusion criteria in the present
study. No significant differences were noted in clinical baseline
data including sex, age, body mass index, education level, smoking
history or drinking history between both groups, which ensured
rigor and reliability of the study. However, there are certain
limitations to the study. First, due to the retrospective
collection of patient data, the obtained data may have been
influenced by subjective factors. Furthermore, the regulatory
mechanisms of ESD in the treatment of EGC and its effects on TAT-2
and GP73 expression levels remain unclear, warranting further
investigation in follow-up experiments.
In conclusion, compared with EMR, ESD was more
efficient in the treatment of EGC, shortening the operation time
and length of hospital stay, reducing intraoperative blood loss,
decreasing serum TAT-2 expression levels and increasing serum GP73
expression levels. However, no significant differences were noted
in adverse reactions or survival between the two groups.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XH, FL and QJ conceived and designed the study and
interpreted the results of the experiments. HG, ZJ, PW and YL
performed the experiments. HG and ZJ authenticated the raw data in
this paper. XH, FL, HG, ZJ, PW, YL and QJ analyzed data. PW and YL
prepared figures. QJ drafted the manuscript and XH and QJ edited
and revised the manuscript. All authors approved the final version
of the manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the Eighth Affiliated Hospital of Guangxi Medical University
(Guigang, China). All study participants provided written informed
consent prior to participating in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wu HQ, Wang HY, Xie WM, Wu SL, Li ZF,
Zhang XM and Li H: Scanning photoacoustic imaging of submucosal
gastric tumor based on a long focused transducer in phantom and in
vitro experiments. J Innov Opt Health Sci. 12(1950011)2019.
|
|
3
|
Rahman R, Asombang AW and Ibdah JA:
Characteristics of gastric cancer in Asia. World J Gastroenterol.
20:4483–4490. 2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Shen L, Li J, Xu J, Pan H, Dai G, Qin S,
Wang L, Wang J, Yang Z, Shu Y, et al: Bevacizumab plus capecitabine
and cisplatin in Chinese patients with inoperable locally advanced
or metastatic gastric or gastroesophageal junction cancer:
Randomized, double-blind, phase III study (AVATAR study). Gastric
Cancer. 18:168–176. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zeng H, Zheng R, Guo Y, Zhang S, Zou X,
Wang N, Zhang L, Tang J, Chen J, Wei K, et al: Cancer survival in
China, 2003-2005: A population-based study. Int J Cancer.
136:1921–1930. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yu L, Wu D, Gao H, Balic JJ, Tsykin A, Han
TS, Liu YD, Kennedy CL, Li JK, Mao JQ, et al: Clinical utility of a
STAT3-regulated miRNA-200 family signature with prognostic
potential in early gastric cancer. Clin Cancer Res. 24:1459–1472.
2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hondo FY, Kishi H, Safatle-Ribeiro AV,
Pessorrusso FC, Ribeiro U Jr and Maluf-Filho F: Characterization of
the mucin phenotype can predict gastric cancer recurrence after
endoscopic mucosal resection. Arq Gastroenterol. 54:308–314.
2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kim SG, Park CM, Lee NR, Kim J, Lyu DH,
Park SH, Choi IJ, Lee WS, Park SJ, Kim JJ, et al: Long-term
clinical outcomes of endoscopic submucosal dissection in patients
with early gastric cancer: A prospective multicenter cohort study.
Gut Liver. 12:402–410. 2018.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Pan J, Zhang X, Shi Y and Pei Q:
Endoscopic mucosal resection with suction vs. endoscopic submucosal
dissection for small rectal neuroendocrine tumors: A meta-analysis.
Scand J Gastroenterol. 53:1139–1145. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Seewald S, Ang TL, Pouw RE, Bannwart F and
Bergman JJ: Management of early-stage adenocarcinoma of the
esophagus: Endoscopic mucosal resection and endoscopic submucosal
dissection. Dig Dis Sci. 63:2146–2154. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hu JS, Wu DW, Liang S and Miao XY: GP73, a
resident Golgi glycoprotein, is sensibility and specificity for
hepatocellular carcinoma of diagnosis in a hepatitis B-endemic
Asian population. Med Oncol. 27:339–345. 2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Riener MO, Stenner F, Liewen H, Soll C,
Breitenstein S, Pestalozzi BC, Samaras P, Probst-Hensch N,
Hellerbrand C, Müllhaupt B, et al: Golgi phosphoprotein 2 (GOLPH2)
expression in liver tumors and its value as a serum marker in
hepatocellular carcinomas. Hepatology. 49:1602–1609.
2009.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sun Y, Yang H, Mao Y, Xu H, Zhang J, Li G,
Lu X, Sang X, Zhao H, Zhong S, et al: Increased Golgi protein 73
expression in hepatocellular carcinoma tissue correlates with tumor
aggression but not survival. J Gastroenterol Hepatol. 26:1207–1212.
2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Fujimura T, Ohta T, Kitagawa H, Fushida S,
Nishimura GI, Yonemura Y, Elnemr A, Miwa K and Nakanuma Y:
Trypsinogen expression and early detection for peritoneal
dissemination in gastric cancer. J Surg Oncol. 69:71–75.
1998.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Itkonen O: Human trypsinogens in the
pancreas and in cancer. Scand J Clin Lab Invest. 70:136–143.
2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ichikawa Y, Koshikawa N, Hasegawa S,
Ishikawa T, Momiyama N, Kunizaki C, Takahashi M, Moriwaki Y,
Akiyama H, Yamaoka H, et al: Marked increase of trypsin(ogen) in
serum of linitis plastica (gastric cancer, borrmann 4) patients.
Clin Cancer Res. 6:1385–1388. 2000.PubMed/NCBI
|
|
17
|
Esaki M, Suzuki S, Hayashi Y, Yokoyama A,
Abe S, Hosokawa T, Tsuruta S, Minoda Y, Hata Y, Ogino H, et al:
Propensity score-matching analysis to compare clinical outcomes of
endoscopic submucosal dissection for early gastric cancer in the
postoperative and non-operative stomachs. BMC Gastroenterol.
18(125)2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hahn KY, Park CH, Lee YK, Chung H, Park
JC, Shin SK, Lee YC, Kim HI, Cheong JH, Hyung WJ, et al:
Comparative study between endoscopic submucosal dissection and
surgery in patients with early gastric cancer. Surg Endosc.
32:73–86. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yuan Y: A survey and evaluation of
population-based screening for gastric cancer. Cancer Biol Med.
10:72–80. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lamb CA, Kennedy NA, Raine T, Hendy PA,
Smith PJ, Limdi JK, Hayee B, Lomer MCE, Parkes GC, Selinger C, et
al: IBD guidelines eDelphi consensus group: British Society of
Gastroenterology consensus guidelines on the management of
inflammatory bowel disease in adults. Gut. 68 (Suppl 3):s1–s106.
2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Askar H, Di Gianfilippo R, Ravida A,
Tattan M, Majzoub J and Wang HL: Incidence and severity of
postoperative complications following oral, periodontal, and
implant surgeries: A retrospective study. J Periodontol.
90:1270–1278. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Buckland G, Travier N, Huerta JM,
Bueno-de-Mesquita HB, Siersema PD, Skeie G, Weiderpass E, Engeset
D, Ericson U, Ohlsson B, et al: Healthy lifestyle index and risk of
gastric adenocarcinoma in the EPIC cohort study. Int J Cancer.
137:598–606. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ghoshal UC, Kumar S, Krishnani N, Kumari
N, Chourasia D and Tripathi S: Serological assessment of gastric
intestinal metaplasia and atrophy using pepsinogen-I, pepsinogen-II
and gastrin-17 levels in a low incidence area of gastric cancer
endemic for H. pylori infection. Trop Gastroenterol.
32:292–298. 2011.PubMed/NCBI
|
|
24
|
Ono S, Fujishiro M, Niimi K, Goto O,
Kodashima S, Yamamichi N and Omata M: Long-term outcomes of
endoscopic submucosal dissection for superficial esophageal
squamous cell neoplasms. Gastrointest Endosc. 70:860–866.
2009.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Onozato Y, Kakizaki S, Ishihara H, Iizuka
H, Sohara N, Okamura S, Mori M and Itoh H: Endoscopic submucosal
dissection for rectal tumors. Endoscopy. 39:423–427.
2007.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Saito Y, Uraoka T, Matsuda T, Emura F,
Ikehara H, Mashimo Y, Kikuchi T, Fu KI, Sano Y and Saito D:
Endoscopic treatment of large superficial colorectal tumors: A case
series of 200 endoscopic submucosal dissections (with video).
Gastrointest Endosc. 66:966–973. 2007.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Meng Y, Li W, Han L, Zhang Q, Gong W, Cai
J, Li A, Yan Q, Lai Q, Yu J, et al: Long-term outcomes of
endoscopic submucosal dissection versus laparoscopic resection for
gastric stromal tumors less than 2 cm. J Gastroenterol Hepatol.
32:1693–1697. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Bang CS, Baik GH, Shin IS, Suk KT, Yoon JH
and Kim DJ: Endoscopic submucosal dissection of gastric
subepithelial tumors: A systematic review and meta-analysis. Korean
J Intern Med (Korean Assoc Intern Med). 31:860–871. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Park JC, Lee SK, Seo JH, Kim YJ, Chung H,
Shin SK and Lee YC: Predictive factors for local recurrence after
endoscopic resection for early gastric cancer: Long-term clinical
outcome in a single-center experience. Surg Endosc. 24:2842–2849.
2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ryu SJ, Kim BW, Kim BG, Kim JH, Kim JS,
Kim JI, Park JM, Oh JH, Kim TH, Kim JJ, et al: Endoscopic
submucosal dissection versus surgical resection for early gastric
cancer: A retrospective multicenter study on immediate and
long-term outcome over 5 years. Surg Endosc. 30:5283–5289.
2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Song WC, Qiao XL and Gao XZ: A comparison
of endoscopic submucosal dissection (ESD) and radical surgery for
early gastric cancer: A retrospective study. World J Surg Oncol.
13(309)2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Chen LG, Wang HJ, Yao HB, Guan TP, Wu F,
He XJ, Ma YY, Tao HQ and Ye ZY: GP73 is down-regulated in gastric
cancer and associated with tumor differentiation. World J Surg
Oncol. 11(132)2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Rong L, Cai Y, Nian W, Wang X, Liang J, He
Y and Zhang J: Efficacy comparison between surgical resection and
endoscopic submucosal dissection of early gastric cancer in a
domestic single center. Zhonghua Wei Chang Wai Ke Za Zhi.
21:190–195. 2018.PubMed/NCBI(In Chinese).
|