Introduction
Diabetes mellitus (DM) includes a group of metabolic
conditions that are caused by disrupted carbohydrate metabolism due
to islet dysfunction, which manifests as hyperglycemia (1). Chronic hyperglycemia results in the
dysfunction of various tissues and organs, including the eyes,
kidney, heart, vessels and nerves, with visual damage appearing in
the early stages of DM (2).
Diabetic retinopathy (DR) is a major classical microvascular
complication of DM, resulting in retinal ischemia, altered retinal
permeability, neovascularization and macular edema, eventually
leading to visual impairment and blindness (3,4). DR is
classified into two stages: Non-proliferative diabetic retinopathy
and proliferative diabetic retinopathy (PDR). PDR is the most
advanced stage of the disease, and new vessels grow in a chaotic
manner, causing vitreous hemorrhage and retinal detachment
(5). Epidemiological data have
revealed that 93 million individuals (35% of diabetic adults aged
20-76 years) suffered from DR in 2010 worldwide, of whom 17 million
exhibited PDR and 21 million displayed diabetic macular edema
(3). It has been estimated that the
number of DR cases will continue to increase by 2050(6).
Vascular dysfunction, including endothelial cell
damage, pericyte death, retinal capillary basement membrane
thickening and tight junction alterations has been a major focus of
DR research; however, diabetic vascular dysfunction alone cannot
explain the loss of retinal function (7). Accumulating evidence has pointed to
the key role of inflammation in the progression of DR (8-10).
Previous studies have reported that the levels of various
inflammatory cytokines, including IL-1β, IL-6, IL-8, TNF-α and
monocyte chemoattractant protein-1, were increased in the vitreous
humor of patients with DR compared with healthy subjects (11-13).
Of note, a recent study demonstrated that the serum and vitreous
concentrations of nesfatin-1 were negatively correlated with DR
(14).
Nesfatin-1 is a secretory peptide distributed in the
hypothalamus and brainstem and is produced by the hydrolysis of
nucleobindin-2 at its N-terminal (15). A previous study has suggested that
nesfatin-1 may ameliorate the high glucose-induced toxicity in PC12
cells via the regulation of oxidative stress, autophagy and
apoptosis (16). Furthermore, it
has been demonstrated that nesfatin-1 suppressed NF-κB-dependent
inflammatory responses and attenuated caspase-3-mediated neuronal
cell apoptosis following traumatic brain injury in rats (17), indicating the anti-inflammatory and
anti-apoptotic effects of nesfatin-1. However, the detailed
function of nesfatin-1 in DR remains elusive.
High expression of NF-κB and NACHT, LRR and PYD
domains-containing protein 3 (NLRP3) has been indicated to be
closely associated with inflammation in DR (18-20).
Enhanced expression level of NLRP3, caspase-1 and IL-1β has been
observed in proliferative membranes of patients with DR compared
with healthy subjects and in high glucose-treated human retinal
endothelial cells compared with untreated cells, whereas inhibition
of NLRP3 significantly alleviated inflammatory responses (21). In addition to NLRP3, high-mobility
group protein B1 (HMGB1) has also been indicated to promote
inflammation in DR, and inhibition of HMGB1 attenuated the NF-κB
activity in human retinal epithelial cells cultured with high
glucose (22). Moreover, HMGB1 has
been indicated to bind with the receptor for advanced glycation end
products to accelerate rat retinal cell apoptosis (23). Previous studies have demonstrated
that HMGB1 promoted the activation of the NLRP3 inflammasome,
upregulated the expression level of NLRP3, apoptosis-associated
speck-like protein containing a CARD (ASC) and caspase-1 (24,25),
whereas nesfatin-1 reduced the activation of the NF-κB pathway via
downregulating the expression of HMGB1(26). Therefore, the present study was
performed to investigate the role of nesfatin-1 in DR and explore
the potential association among HMGB1, NF-κB, NLRP3 and
nesfatin-1.
Materials and methods
Cell culture and treatment
The human retinal epithelial cell line ARPE-19 was
purchased from The Cell Bank of Type Culture Collection of Chinese
Academy of Sciences and cultured in DMEM/F12 (HyClone; Cytiva)
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.)
at 37˚C in an incubator with 95% air/5% CO2. In order to
examine the effects of hyperglycemia, ARPE-19 cells were cultured
with high concentration of glucose (33 mM; Sigma-Aldrich; Merck
KGaA), normal concentration of glucose (5.5 mM) or 27.5 mM mannitol
+ 5.5 mM glucose as an osmotic control for 48 h. ARPE-19 cells were
pretreated with nesfatin-1 (2.5 or 5 ng/ml; Sigma-Aldrich; Merck
KGaA) for 1 h, followed by exposure to 33 mM glucose for 48 h
(16).
Cell transfection
ARPE-19 cells (2x105 cells/well) were
seeded into 6-well plates. Subsequently, cells were transfected
with HMGB1 plasmid (pcDNA3.1-HMGB1; 50 nM) or the empty vector
plasmid (pcDNA3.1; 50 nM) using Lipofectamine® 3000
(Invitrogen; Thermo Fisher Scientific, Inc.) at 37˚C, according to
the manufacturer's instructions. These plasmids were provided by
Shanghai GenePharma Co., Ltd. After 48 h, cells were treated with
glucose/nesfatin as aforementioned. The transfected cells were
harvested and protein expression was examined with western blot
analysis.
Cell Counting Kit-8 (CCK-8) assay
Cell viability was determined using the CCK-8 assay.
Briefly, ARPE-19 cells were seeded into a 96-well plate at a
density of 5,000 cells/well. Following incubation with glucose
and/or nesfatin-1 as aforementioned, 10 µl CCK-8 solution (Nanjing
KeyGen Biotech Co., Ltd.) was added into each well for 1 h at 37˚C.
The absorbance of each well was measured using a microplate reader
at 450 nm.
Colony formation assay
ARPE-19 cells were seeded into 6-well plates (500
cells/well) and incubated for 24 h at 37˚C to allow for adherence.
The medium was changed every 3 days. After 2 weeks of culture, the
cells were fixed with methanol for 30 min at room temperature and
stained with 0.2% crystal violet at room temperature for 30 min.
Images were captured using a light microscope (Olympus Corporation)
at x10 magnification.
ELISA
ARPE-19 cell culture medium was collected into a
centrifuge tube and centrifuged at 1,000 x g for 10 min at 4˚C.
Subsequently, the concentration of TNF-α (cat. no. SEKH-0047;
Beijing Solarbio Science & Technology Co., Ltd.), IL-1β (cat.
no. F01220) and IL-6 (cat. no. F01310; both from Shanghai Xitang
Biotechnology Co., Ltd.) was measured by ELISA kits according to
the manufacturer's instructions. The absorbance of each well was
measured using a microplate reader at 450 nm. The concentration of
nesfatin-1 was determined with a commercially available ELISA kit
(cat. no. JL19919-96T; Shanghai Jianglai Biological Technology Co.,
Ltd.) in accordance with the manufacturer's instructions.
Measurement of oxidative
stress-related malondialdehyde (MDA)
ARPE-19 cells were collected into a centrifuge tube
and centrifuged at 8,000 x g for 10 min at 4˚C. Subsequently, the
reagents of the MDA assay kit (cat. no. BC0025; Beijing Solarbio
Science & Technology Co., Ltd.) were added into the centrifuge
tube according to the manufacturer's protocol. The mixture was
heated at 100˚C for 60 min, cooled on ice and centrifuged at 10,000
x g for 10 min at room temperature. Subsequently, 200 µl
supernatant was added into a 96-well plate. The absorbance of each
well was recorded using a microplate reader at 532 nm.
Measurement of reactive oxygen species
(ROS)
The production of intracellular ROS was determined
with a ROS assay kit (cat. no. D6470; Beijing Solarbio Science
& Technology Co., Ltd.) using 2',7'-dichlorodihydrofluorescein
diacetate (DCFH-DA) as a fluorescence probe. ARPE-19 cells were
seeded into a 6-well plate, and DCFH-DA (10 µM) was added into each
well according to the manufacturer's protocol for 20 min at 37˚C.
Subsequently, the cells were washed with DMEM/F12 three times and
the absorbance of each well was measured using a fluorescence
microplate reader at 488 and 525 nm.
Flow cytometry analysis
To determine apoptosis, 2x105 ARPE-19
cells were prepared and washed with PBS twice. Subsequently, the
cells were suspended in 500 µl binding buffer (Nanjing KeyGen
Biotech Co., Ltd.), followed by the addition of 5 µl Annexin V-FITC
and 5 µl PI (Nanjing KeyGen Biotech Co., Ltd.) for 10 min at room
temperature in the dark. Subsequently, cell apoptosis was analyzed
using a flow cytometer (FACSAria III; BD Biosciences). The data
were analyzed using BD Accuri C6 software (version 1.0; Becton,
Dickinson and Company).
Western blot analysis
Total protein from ARPE-19 cells was extracted using
a RIPA lysis buffer (Beyotime Institute of Biotechnology). Then,
ARPE-19 cells were collected into a centrifuge tube and centrifuged
at 10,000 x g for 10 min at 4˚C. The cell lysate was collected into
another centrifuge tube and the protein concentration of the lysate
was determined using a BCA assay kit (Sigma-Aldrich; Merck KGaA). A
total of 20 µg protein lysate was loaded to 10% SDS-PAGE gels and
then transferred to PVDF membranes (EMD Millipore). The membranes
were incubated with primary antibodies at 4˚C overnight after
blocking with 5% non-fat milk for 1 h at room temperature. Primary
antibodies against NF-κB (cat. no. ab32536; 1:1,000), NLRP3 (cat.
no. ab263899; 1:1,000), caspase-1 (cat. no. ab179515; 1:1,000),
pro-caspase-1 (cat. no. ab207802; 1:1,000), ASC (cat. no. ab151700;
1:1,000), HMGB1 (cat. no. ab18256; 1:1,000), Bcl-2 (cat. no.
ab32124; 1:1,000), Bax (cat. no. ab32503; 1:1,000) and GAPDH (cat.
no. ab8245; 1:1,000) were all obtained from Abcam. Following
incubation with HRP-conjugated goat anti-rabbit IgG (cat. no.
ab205718; Abcam; 1:5,000) or goat anti-mouse IgG (cat. no. ab6789;
Abcam; 1:5,000) secondary antibodies at 37˚C for 1 h, the protein
bands of the membranes were detected using Immobilon Western
Chemiluminescent HRP Substrate (EMD Millipore). The relative
intensity of target bands was semi-quantified using ImageJ software
(version 1.52r; National Institutes of Health) and normalized to
the intensity of GAPDH.
Statistical analysis
Data are presented as mean ± SD. All experiments
were performed in triplicate. Statistical analyses were carried out
using GraphPad Prism v6 software (GraphPad Software, Inc.). One-way
ANOVA followed by Tukey's post hoc test was used to compare the
differences among groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
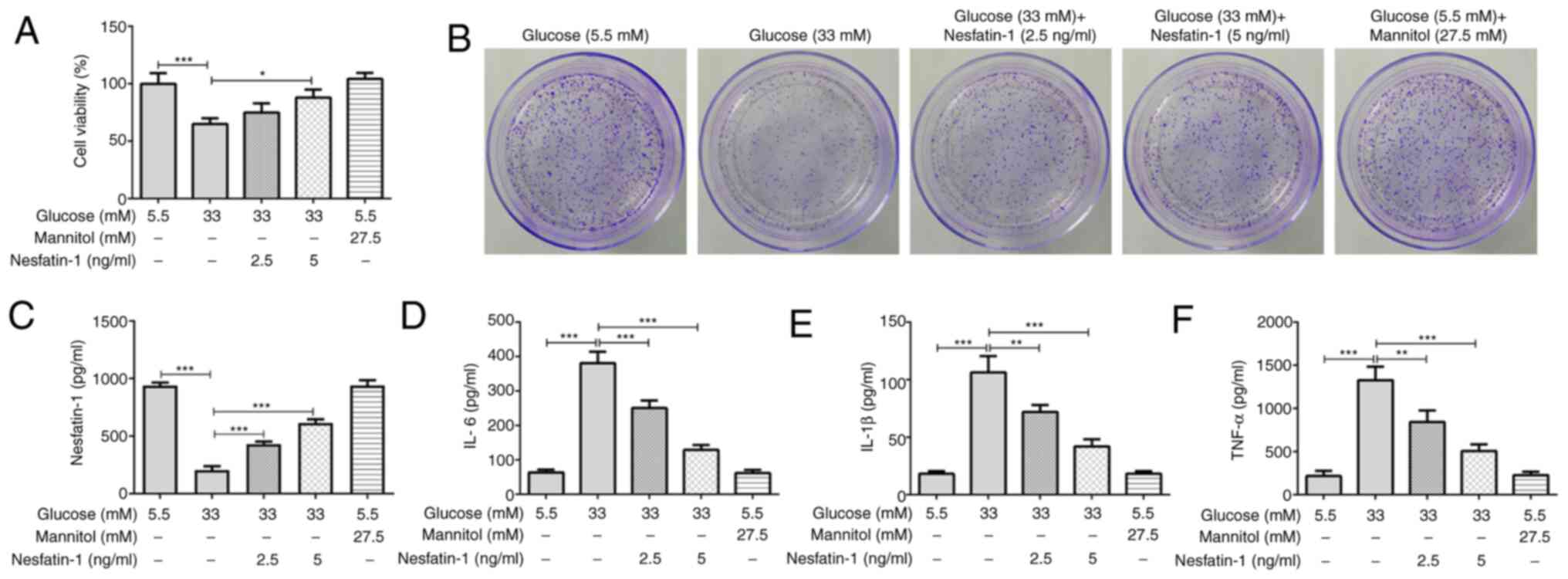
Nesfatin-1 enhances cell viability and
decreases inflammatory responses in high glucose-treated ARPE-19
cells
ARPE-19 cells exposed to high glucose exhibited
lower viability compared with that of the normal glucose group.
Nesfatin-1 treatment alleviated the decrease in cell viability
induced by high glucose, which was dose-dependent. The osmotic
group revealed that osmotic pressure exerted no significant effects
on cell viability, excluding the effect of osmotic pressure on the
high glucose group (Fig. 1A). The
results of the colony formation assay indicated that high glucose
exposure inhibited the proliferation of ARPE-19 cells compared with
the normal glucose group, while nesfatin-1 treatment remarkably
increased the cell proliferation relatively to the high glucose
group (Fig. 1B). Additionally, a
notably reduced nesfatin-1 level was observed following high
glucose stimulation, whereas nesfatin-1 treatment significantly
enhanced the nesfatin-1 level (Fig.
1C). Moreover, osmotic pressure presented no effect on the
levels of IL-6, IL-1β and TNF-α compared with the normal glucose
group, but high glucose significantly induced the secretion of
these inflammatory cytokines, whereas nesfatin-1 treatment reduced
the levels of inflammatory cytokines compared with the high glucose
group (Fig. 1D-F).
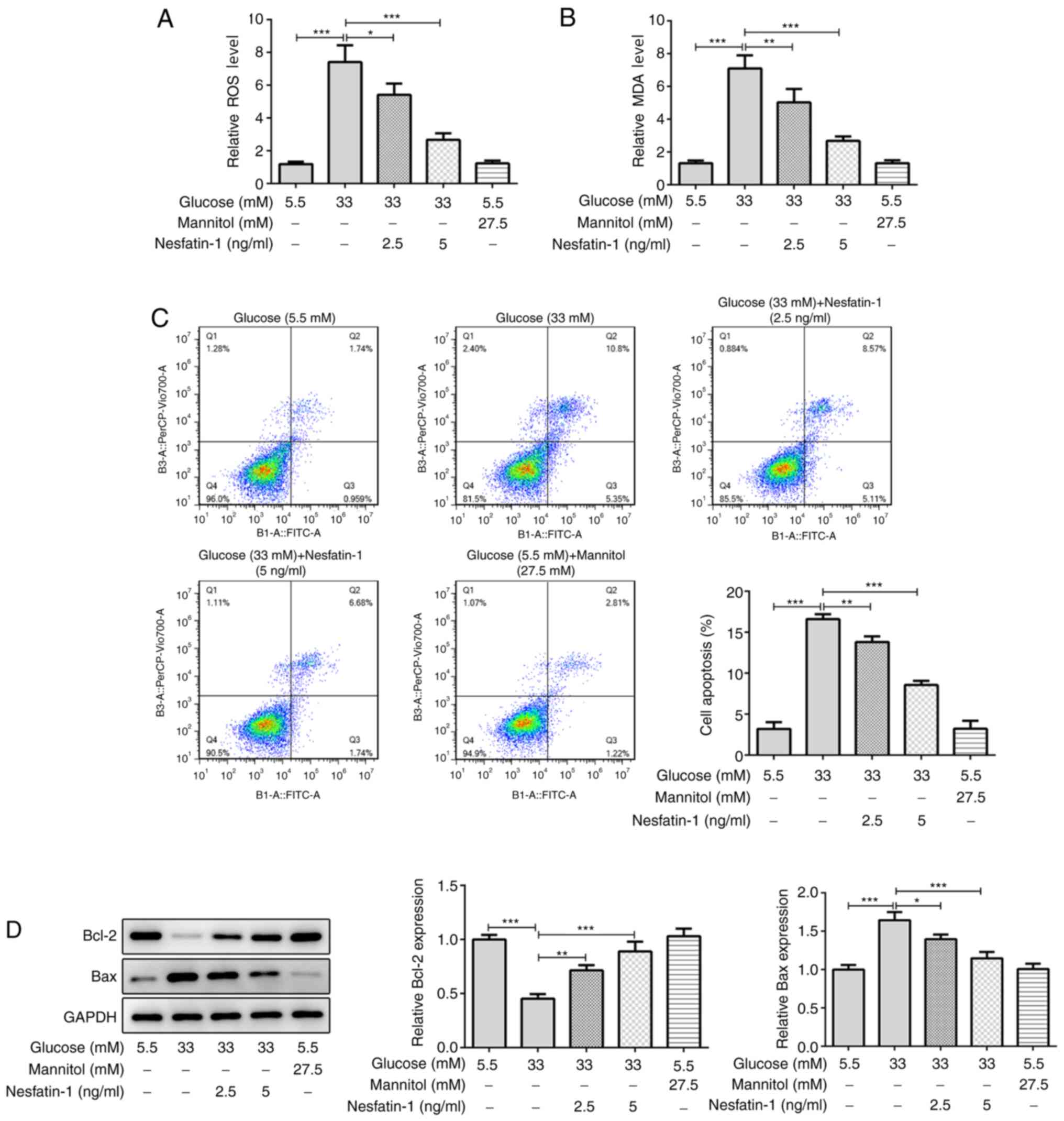
Nesfatin-1 alleviates high
glucose-induced oxidative stress and apoptosis in ARPE-19
cells
A number of studies have demonstrated that high
glucose concentration can induce cellular oxidative stress
(27,28). It was observed that osmotic pressure
exhibited no effect on the levels of ROS and MDA and high glucose
markedly increased the levels of ROS and MDA compared with the
normal glucose group, whereas nesfatin-1 reduced the cellular ROS
and MDA content. Furthermore, the high dose of nesfatin-1 exerted a
more potent antioxidant effect compared with the low dose
nesfatin-1 (Fig. 2A and B). Consistently, there was no significant
difference in cell apoptosis between the mannitol group and the
normal glucose group, but nesfatin-1 reduced high glucose-induced
cell apoptosis (Fig. 2C). In
addition, osmotic pressure exhibited no effect on the expression of
Bcl-2 and Bax compared with the normal glucose group. However, the
decreased Bcl-2 and increased Bax level in high glucose-treated
ARPE-19 cells also demonstrated that high glucose concentration may
increase the cell apoptosis rate, whereas nesfatin-1 treatment
reversed the effect of high glucose on the protein level of Bcl-2
and Bax (Fig. 2D). These data
provided evidence that nesfatin-1 attenuated high glucose-induced
oxidative stress and apoptosis in ARPE-19 cells.
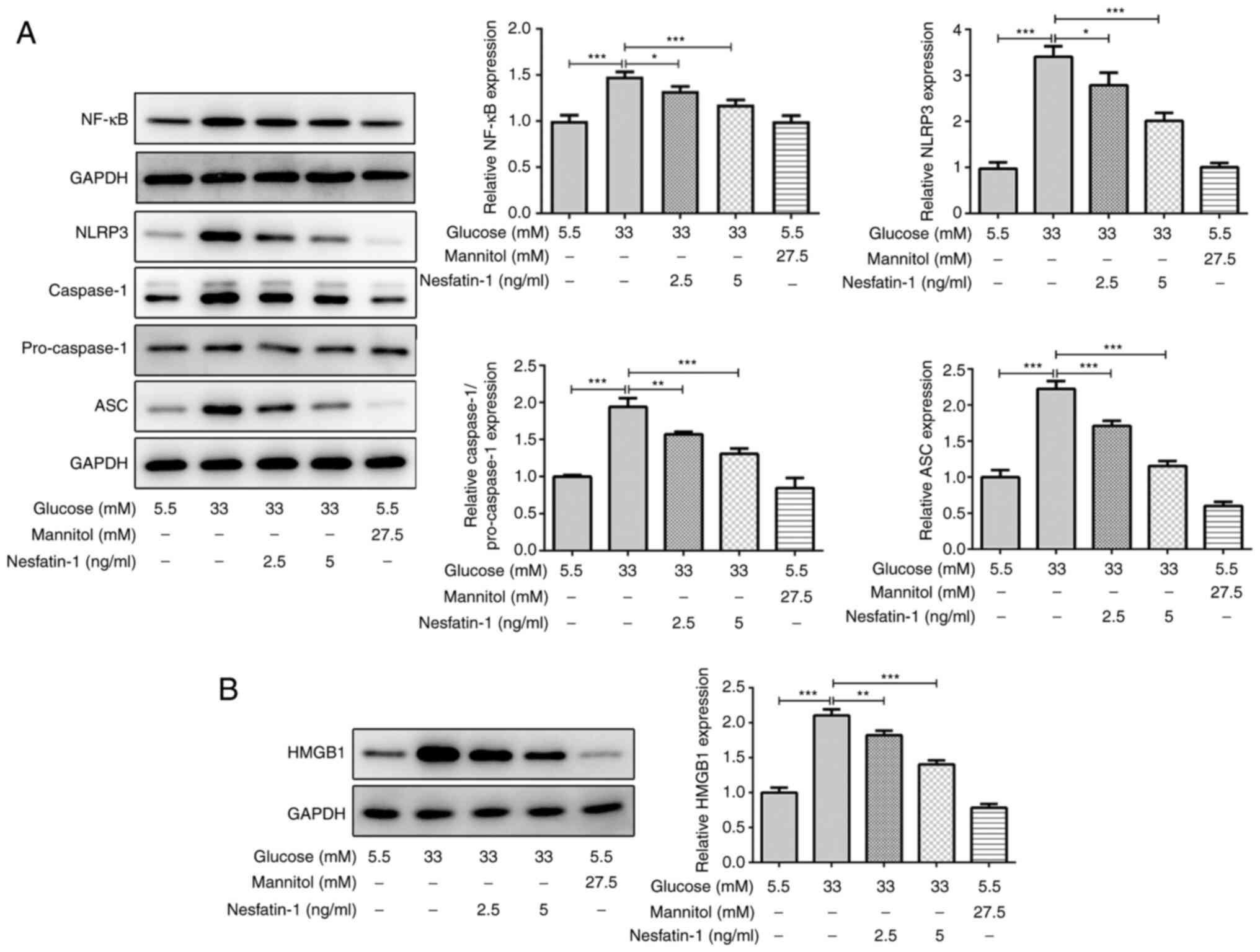
Nesfatin-1 inhibits the activation of
NF-κB/NLRP3 inflammasome signaling and HMGB1 expression in high
glucose-stimulated ARPE-19 cells
High glucose concentration stimulates the activation
of the NF-κB/NLRP3 inflammasome pathway, which has been indicated
to be associated with the dysfunction of high glucose-treated human
retinal endothelial cells (21,29).
There was no significant difference in the expression of NF-κB,
NLRP3, caspase-1 and ASC between the mannitol group and the normal
glucose group. In high glucose-stimulated ARPE-19 cells, the
protein expression levels of NF-κB, NLRP3, caspase-1 and ASC were
increased, whereas the levels of these proteins were markedly
reduced by nesfatin-1 treatment (Fig.
3A), indicating that nesfatin-1 inhibited the NF-κB/NLRP3
inflammasome signaling activation induced by high glucose exposure.
Furthermore, osmotic pressure exhibited no significant effect on
the expression of HMGB1, but the expression level of HMGB1 was
markedly increased in high glucose-treated ARPE-19 cells, and it
was reduced following nesfatin-1 treatment (Fig. 3B). Overall, these data suggested
that nesfatin-1 suppressed the NF-κB/NLRP3 inflammasome pathway
activation and HMGB1 expression in high glucose-stimulated ARPE-19
cells.
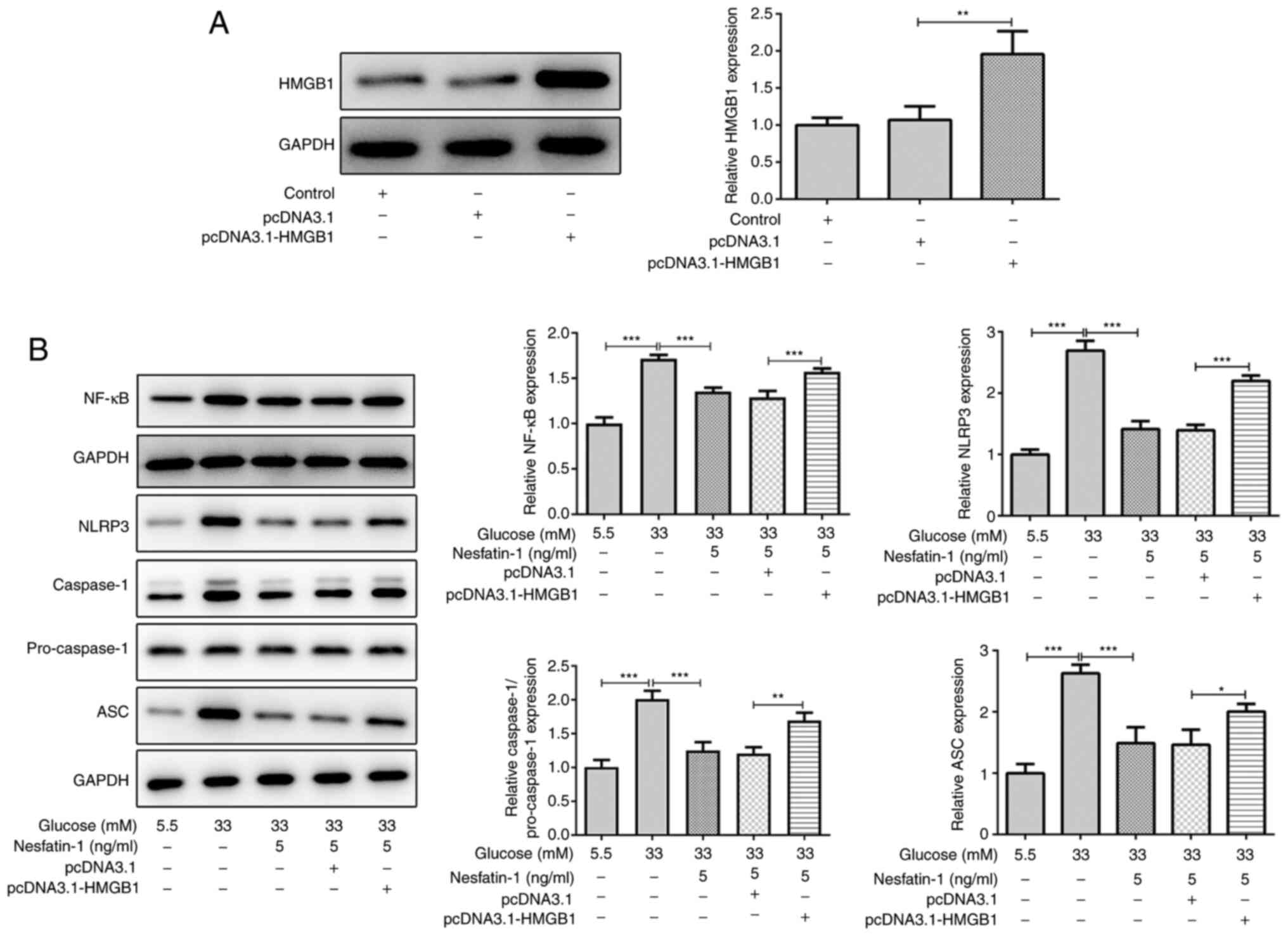
Nesfatin-1 prevents the activation of
NF-κB/NLRP3 inflammasome signaling via regulating HMGB1
Subsequently, the mechanism via which nesfatin-1
affected the activation of the NF-κB/NLRP3 inflammasome pathway was
further investigated. ARPE-19 cells were transfected with
pcDNA3.1-HMGB1. As demonstrated in Fig.
4A, pcDNA3.1-HMGB1 increased the expression level of HMGB1
compared with the empty vector, suggesting the efficiency of
pcDNA3.1-HMGB1 transfection. Of note, under high glucose
conditions, nesfatin-1 inhibited NF-κB/NLRP3 inflammasome
signaling, and this effect could be reversed via upregulation of
HMGB1 (Fig. 4B), indicating that
nesfatin-1 prevented the activation of NF-κB/NLRP3 inflammasome
signaling via the regulation of HMGB1.
Discussion
Nesfatin-1 is considered to have various functions
in different systems and metabolic processes, including the
endocrine and nervous system, blood glucose concentration,
cardiovascular system and lipid metabolism (30,31).
It has been reported that the concentration of nesfatin-1 in the
serum and vitreous humor was negatively correlated with DR
(14). However, the role of
nesfatin-1 in DR has not been extensively investigated to date.
Retinal pigment epithelial cells are the main cells
involved in DR (32). In the
present study, ARPE-19 human retinal epithelial cells were cultured
with high glucose in vitro to mimic hyperglycemia in
vivo. It has been previously reported that ROS-induced
oxidative stress and low-grade inflammation triggered by chronic
hyperglycemia contribute to the progression of DR (33). In the current study, it was observed
that high glucose concentration reduced cell viability, induced the
expression of inflammatory cytokines and increased the ROS and MDA
content following high glucose stimulation. Nesfatin-1 treatment
enhanced cell viability and suppressed the levels of TNF-α, IL-1β,
IL-6, ROS and MDA in high glucose-treated cells, suggesting that
nesfatin-1 may protect ARPE-19 cells against high glucose-induced
inflammation and oxidative stress. In addition, nesfatin-1
decreased cell apoptosis under high glucose conditions.
NLRP3 inflammasome is a protein complex in the
innate immune system that recognizes pathogen- and
danger-associated molecular patterns, which is composed of NLRP3,
ASC and caspase-1(34). Activation
of the NF-κB/NLRP3 inflammasome signaling serves a key role in the
progression of DR (19,20,35-38).
In the present study, it was observed that nesfatin-1 reduced the
protein levels of NF-κB, NLRP3, ASC and caspase-1, and inhibited
the activation of the NF-κB/NLRP3 inflammasome signaling following
high glucose stimulation. HMGB1 is a danger-associated molecular
pattern receptor that can sense high glucose as a stressor
(39). Elevated HMGB1 levels were
observed in patients with advanced DR compared with healthy
subjects (39). A recent study
reported that HMGB1 was revealed to be highly expressed in high
glucose-treated human retinal endothelial cells, and it may be
associated with the pathogenesis of DR (40). Emerging evidence supports that
nesfatin-1 can downregulate the expression of HMGB1 to alleviate
lipopolysaccharide-induced acute lung injury (26). The present study demonstrated that
high glucose concentration increased the expression level of HMGB1
in ARPE-19 cells, which was reduced by nesfatin-1 treatment. Of
note, overexpression of HMGB1 partially reversed the inhibitory
effect of nesfatin-1 on NF-κB/NLRP3 inflammasome signaling
activation, suggesting that nesfatin-1 may prevent the NF-κB/NLRP3
inflammasome pathway activation via inhibiting HMGB1. Consistently,
previous studies have reported that HMGB1 increased the expression
level of NF-κB and NLRP3 inflammasome proteins, including NLRP3,
ASC, caspase-1 or IL-1β (25,41,42).
In summary, the present study demonstrated the
beneficial effect of nesfatin-1 in high glucose-treated ARPE-19
cells via inhibiting inflammation, oxidative stress and apoptosis.
To the best of our knowledge, the present study was the first to
demonstrate that nesfatin-1 prevented the NF-κB/NLRP3 inflammasome
signaling activation via the inhibition of HMGB1. The role of
nesfatin-1 in animal experiments, the mechanisms mediating the
reduction of HMGB1 by nesfatin-1 in and the effective dose of
nesfatin-1 for clinical applications, which are limitations to the
present study, should be investigated in future studies.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of materials and data
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HS, HZ and ZY searched the literature, designed and
performed the experiments. XL and PY analyzed the data and wrote
the manuscript. JZ analyzed the data and revised the manuscript. HS
and JZ confirm the authenticity of the raw data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Toniolo A, Cassani G, Puggioni A, Rossi A,
Colombo A, Onodera T and Ferrannini E: The diabetes pandemic and
associated infections: Suggestions for clinical microbiology. Rev
Med Microbiol. 30:1–17. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Vieira-Potter VJ, Karamichos D and Lee DJ:
Ocular complications of diabetes and therapeutic approaches. Biomed
Res Int. 2016(3801570)2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Yau JW, Rogers SL, Kawasaki R, Lamoureux
EL, Kowalski JW, Bek T, Chen SJ, Dekker JM, Fletcher A, Grauslund
J, et al: Global prevalence and major risk factors of diabetic
retinopathy. Diabetes Care. 35:556–564. 2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Duh EJ, Sun JK and Stitt AW: Diabetic
retinopathy: Current understanding, mechanisms, and treatment
strategies. JCI insight. 2(e93751)2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ahmadieh H, Behbahani S and Safi S:
Continuous wavelet transform analysis of ERG in patients with
diabetic retinopathy. Doc Ophthalmol. 142:305–314. 2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Saaddine JB, Honeycutt AA, Narayan KM,
Zhang X, Klein R and Boyle JP: Projection of diabetic retinopathy
and other major eye diseases among people with diabetes mellitus:
United States, 2005-2050. Arch Ophthalmol. 126:1740–1747.
2008.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Rübsam A, Parikh S and Fort PE: Role of
inflammation in diabetic retinopathy. Int J Mol Sci.
19(942)2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Noda K, Nakao S, Ishida S and Ishibashi T:
Leukocyte adhesion molecules in diabetic retinopathy. J Ophthalmol.
2012(279037)2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kern TS: Contributions of inflammatory
processes to the development of the early stages of diabetic
retinopathy. Exp Diabetes Res. 2007(95103)2007.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Adamis AP: Is diabetic retinopathy an
inflammatory disease? Br J Ophthalmol. 86:363–365. 2002.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Maier R, Weger M, Haller-Schober EM,
El-Shabrawi Y, Wedrich A, Theisl A, Aigner R, Barth A and Haas A:
Multiplex bead analysis of vitreous and serum concentrations of
inflammatory and proangiogenic factors in diabetic patients. Mol
Vis. 14:637–643. 2008.PubMed/NCBI
|
|
12
|
Dai Y, Wu Z, Wang F, Zhang Z and Yu M:
Identification of chemokines and growth factors in proliferative
diabetic retinopathy vitreous. BioMed Res Int.
2014(486386)2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yoshimura T, Sonoda KH, Sugahara M,
Mochizuki Y, Enaida H, Oshima Y, Ueno A, Hata Y, Yoshida H and
Ishibashi T: Comprehensive analysis of inflammatory immune
mediators in vitreoretinal diseases. PLoS One.
4(e8158)2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Dai R, Deng G, Sun Z, Liu Z, Qian Y and
Han Y: Relation of serum and vitreous nesfatin-1 concentrations
with diabetic retinopathy. J Clin Lab Anal.
31(e22105)2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Shimizu H, Oh IS, Hashimoto K, Nakata M,
Yamamoto S, Yoshida N, Eguchi H, Kato I, Inoue K, Satoh T, et al:
Peripheral administration of nesfatin-1 reduces food intake in
mice: The leptin-independent mechanism. Endocrinology. 150:662–671.
2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Nazarnezhad S, Rahmati M, Shayannia A,
Abbasi Z, Salehi M and Khaksari M: Nesfatin-1 protects PC12 cells
against high glucose-induced cytotoxicity via inhibiting oxidative
stress, autophagy and apoptosis. Neurotoxicology. 74:196–202.
2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Tang CH, Fu XJ, Xu XL, Wei XJ and Pan HS:
The anti-inflammatory and anti-apoptotic effects of nesfatin-1 in
the traumatic rat brain. Peptides. 36:39–45. 2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chen H, Zhang X, Liao N, Mi L, Peng Y, Liu
B, Zhang S and Wen F: Enhanced expression of NLRP3
inflammasome-related inflammation in diabetic retinopathy. Invest
Ophthalmol Vis Sci. 59:978–985. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yin Y, Chen F, Wang W, Wang H and Zhang X:
Resolvin D1 inhibits inflammatory response in STZ-induced diabetic
retinopathy rats: Possible involvement of NLRP3 inflammasome and
NF-κB signaling pathway. Mol Vis. 23:242–250. 2017.PubMed/NCBI
|
|
20
|
Yang Q, Li S, Zhou Z, Fu M, Yang X, Hao K
and Liu Y: HDAC6 inhibitor Cay10603 inhibits high glucose-induced
oxidative stress, inflammation and apoptosis in retinal pigment
epithelial cells via regulating NF-κB and NLRP3 inflammasome
pathway. Gen Physiol Biophys. 39:169–177. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhang Y, Lv X, Hu Z, Ye X, Zheng X, Ding
Y, Xie P and Liu Q: Protection of Mcc950 against
high-glucose-induced human retinal endothelial cell dysfunction.
Cell Death Dis. 8(e2941)2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Chen XL, Zhang XD, Li YY, Chen XM, Tang DR
and Ran RJ: Involvement of HMGB1 mediated signalling pathway in
diabetic retinopathy: Evidence from type 2 diabetic rats and
ARPE-19 cells under diabetic condition. Br J Ophthalmol.
97:1598–1603. 2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yu Y, Yang L, Lv J, Huang X, Yi J, Pei C
and Shao Y: The role of high mobility group box 1 (HMGB-1) in the
diabetic retinopathy inflammation and apoptosis. Int J Clin Exp
Pathol. 8:6807–6813. 2015.PubMed/NCBI
|
|
24
|
Chi W, Chen H, Li F, Zhu Y, Yin W and Zhuo
Y: HMGB1 promotes the activation of NLRP3 and caspase-8
inflammasomes via NF-κB pathway in acute glaucoma. J
Neuroinflammation. 12(137)2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kim EJ, Park SY, Baek SE, Jang MA, Lee WS,
Bae SS, Kim K and Kim CD: HMGB1 Increases IL-1β production in
vascular smooth muscle cells via NLRP3 Inflammasome. Front Physiol.
9(313)2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wang ZZ, Chen SC, Zou XB, Tian LL, Sui SH
and Liu NZ: Nesfatin-1 alleviates acute lung injury through
reducing inflammation and oxidative stress via the regulation of
HMGB1. Eur Rev Med Pharmacol Sci. 24:5071–5081. 2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chen X, Shen WB and Yang P, Dong D, Sun W
and Yang P: High glucose inhibits neural stem cell differentiation
through oxidative stress and endoplasmic reticulum stress. Stem
Cells Dev. 27:745–755. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Farnoodian M, Halbach C, Slinger C,
Pattnaik BR, Sorenson CM and Sheibani N: High glucose promotes the
migration of retinal pigment epithelial cells through increased
oxidative stress and PEDF expression. Am J Physiol Cell Physiol.
311:C418–C436. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Hu YJ, Lin HJ, Dib B, Atik A, Bouzika P,
Lin C, Yan Y, Tang S, Miller JW and Vavvas DG: Cholesterol crystals
induce inflammatory cytokines expression in a human retinal pigment
epithelium cell line by activating the NF-κB pathway. Discov Med.
18:7–14. 2014.PubMed/NCBI
|
|
30
|
Tekin T, Cicek B and Konyaligil N:
Regulatory peptide nesfatin-1 and its relationship with metabolic
syndrome. Eurasian J Med. 51:280–284. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ayada C, Toru Ü and Korkut Y: Nesfatin-1
and its effects on different systems. Hippokratia. 19:4–10.
2015.PubMed/NCBI
|
|
32
|
Shivarudrappa AH and Ponesakki G: Lutein
reverses hyperglycemia-mediated blockage of Nrf2 translocation by
modulating the activation of intracellular protein kinases in
retinal pigment epithelial (ARPE-19) cells. J Cell Commun Signal.
14:207–221. 2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Singh LP: Thioredoxin interacting protein
(TXNIP) and pathogenesis of diabetic retinopathy. J Clin Exp
Ophthalmol. 4(287)2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ratsimandresy RA, Dorfleutner A and
Stehlik C: An update on PYRIN domain-containing pattern recognition
receptors: From immunity to pathology. Front Immunol.
4(440)2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Li W, Liu X, Tu Y, Ding D, Yi Q, Sun X,
Wang Y, Wang K, Zhu M and Mao J: Dysfunctional Nurr1 promotes high
glucose-induced Müller cell activation by up-regulating the
NF-κB/NLRP3 inflammasome axis. Neuropeptides.
82(102057)2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Lu L, Lu Q, Chen W, Li J, Li C and Zheng
Z: Vitamin D3 protects against diabetic retinopathy by
inhibiting high-glucose-induced activation of the ROS/TXNIP/NLRP3
inflammasome pathway. J Diabetes Res. 2018(8193523)2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Li S, Yang H and Chen X: Protective
effects of sulforaphane on diabetic retinopathy: Activation of the
Nrf2 pathway and inhibition of NLRP3 inflammasome formation. Exp
Animals. 68:221–231. 2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Loukovaara S, Piippo N, Kinnunen K, Hytti
M, Kaarniranta K and Kauppinen A: NLRP3 inflammasome activation is
associated with proliferative diabetic retinopathy. Acta
Ophthalmol. 95:803–808. 2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Steinle JJ: Role of HMGB1 signaling in the
inflammatory process in diabetic retinopathy. Cell Signal.
73(4)2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Liang WJ, Yang HW, Liu HN, Qian W and Chen
XL: HMGB1 upregulates NF-kB by inhibiting IKB-α and associates with
diabetic retinopathy. Life Sci. 241(117146)2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Duan J, Zhang Q, Hu X, Lu D, Yu W and Bai
H: N4-acetylcytidine is required for sustained NLRP3
inflammasome activation via HMGB1 pathway in microglia. Cell
Signal. 58:44–52. 2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Song E, Jahng JW, Chong LP, Sung HK, Han
M, Luo C, Wu D, Boo S, Hinz B, Cooper MA, et al: Lipocalin-2
induces NLRP3 inflammasome activation via HMGB1 induced TLR4
signaling in heart tissue of mice under pressure overload
challenge. Am J Transl Res. 9:2723–2735. 2017.PubMed/NCBI
|