Introduction
Burn wound healing can be characterized as
overheating, manifested by hypertrophic scar formation after burn
injury (1). The treatment of deep
burn wounds is a great challenge due to scar formation and poor
functioning after wound healing (2). The proliferation of human dermis
fibroblasts (HDFs) induced in the dermis exerts a crucial effect on
burn wound repair (2,3) and maintains the extracellular matrix
(ECM) synthesis to elevate scar quality (2,4). When
skin is injured, HDFs are activated to restore tissue integrity.
ECM metabolism is the major factor of burn wound repair quality and
outcomes (5). However, the
regulatory mechanism in the burn wound healing process has not been
specifically reported.
Long non-coding RNAs (lncRNAs) are classified as a
group of important regulators involved in multiple biological
processes (6-8).
As reported, lncRNAs exert vital effects on wound healing after
thermal injury. For instance, the lncRNA LET induces cell
proliferative ability and suppresses fibroblast apoptosis during
burn wound healing (9).
Additionally, the lncRNA AC067945.2 represses collagen levels and
promotes ECM synthesis by fibroblasts in the wound healing process
(10). According to the literature,
a microarray analysis illustrated that TPT1-AS1 is downregulated in
denatured dermal tissues (3).
However, there has been no report on how TPT1-AS1 exerts its
regulatory function in burn wound healing. Thus, the aim of the
present study was to investigate the biological role of TPT1-AS1 in
HDFs after thermal injury.
MicroRNAs (miRNAs) are small endogenous RNAs that
regulate gene expression post-transcriptionally (11,12).
In addition, lncRNAs exert their influences by serving as competing
endogenous RNAs (ceRNAs) for miRNAs to derepress messenger RNAs
(mRNAs) (13). For example, the
lncRNA ZFAS1 regulates proliferation, apoptosis and the
inflammatory response via the miR-2682-5p/ADAMTS9 axis in
rheumatoid arthritis (14).
Furthermore, the MEG3/miR-93/Nrf2 axis represses high
glucose-induced apoptosis in retinal pigment epithelium cells
(15). Hence, whether TPT1-AS1
exerts its regulatory function via the ceRNA pattern in the burn
wound healing process was investigated in the present study.
The present study primarily focused on the
biological function of TPT1-AS1 in HDFs after burn injury. It was
hypothesized that TPT1-AS1 may exert its regulatory effect as a
ceRNA in HDFs by interacting with miRNA. The findings revealed that
TPT1-AS1 mitigated cell injury and promoted ECM synthesis by
interacting with microRNA 324-5p (miR-324-5p) and targeting
cyclin-dependent kinase 16 (CDK16) in HDFs after thermal injury.
The present study findings may provide a potential novel insight
for clinical burn wound treatment.
Materials and methods
Cell culture and treatment
Human dermal fibroblasts (ATCC®,
PCS-201-012™) were provided by American Type Culture
Collection. and cultured in RPMI-1640 medium (Gibco; Thermo Fisher
Scientific, Inc.) containing 10% fetal bovine serum (FBS; Gibco;
Thermo Fisher Scientific, Inc.) in an incubator with a 5%
CO2 atmosphere at 37˚C. The use of HDFs was approved by
the ethics committee of The First Affiliated Hospital of Nanchang
University before the study. To establish a cellular heat-injury
model, HDFs were challenged in 52˚C water for 30 sec (2,9,16),
while cells in the control group were maintained in 37˚C water for
the same duration. In succession, cells were cultivated in the
incubator at normal temperatures.
Cell transfection
For cell transfection, short harpin RNA (shRNA)
against CDK16 (sh-CDK16#1, CCACUGAGGACAUCAACAA; and sh-CDK16#2,
GGAGAUCAGACUGGAACAU), a negative control (sh-NC; scrambled;
AGCCUAACCUAGAACACAG), the TPT1-AS1 overexpression vector
(pcDNA3.1-TPT1-AS1), an empty vector (pcDNA3.1-empty), miR-324-5p
mimics (miR-324-5p), and miRNA negative control (NC mimics,
scrambled) were synthesized by Shanghai GenePharma Co., Ltd. Using
LipoFiter™ liposomal transfection reagent (Hanbio
Biotechnology Co., Ltd.), HDFs were transfected with 40 nM
sh-CDK16#1/2, 10 nM miR-324-5p mimic or 2 µg pcDNA3.1 vector upon
reaching 60-70% confluence in 6-well plates. Twenty-four hours
after transfection, the cells were collected for thermal injury or
further analysis.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted with TRIzol®
reagent (Thermo Fisher Scientific, Inc.), and the concentration and
purity were assessed with a NanoDrop ND-2000 spectrophotometer
(Thermo Fisher Scientific, Inc.). The RNA was reverse transcribed
to cDNA with Taqman™ miRNA or a mRNA reverse
transcription kit (Thermo Fisher Scientific, Inc.). The reaction
conditions were as follows: 37˚C for 25 min, followed by 85˚C for 5
min. SYBR ® Premix ExTaq™ II kit (Takara Bio, Inc.) was
used to perform the PCR analysis on an ABI 7500 Real-Time PCR
System. The thermocycling conditions were: Pre-denaturation at 90˚C
for 3 min, denaturation at 90˚C for 20 sec, annealing at 60˚C for
20 sec, and extension at 72˚C for 40 sec, for a total of 40 cycles.
The samples were prepared in duplicate. GAPDH or U6 was used as the
internal reference. The relative expression levels were calculated
with the 2-ΔΔCq method (17). The following primer sequences were
used: TPT1-AS1 forward, 5'-CTATCCTTGCCCATCTTCCT-3'; TPT1-AS1
reverse, 5'-TCTACCGGAGCAATTGGAG-3'; miR-324-5p forward,
5'-TCGGCAGGCGCAUCCCCUAG-3'; miR-324-5p reverse,
5'-CACTCAACTGGTGTCGTGGA-3'; CDK16 forward,
5'-CTCTGCACCAGAGATTGTG-3', CDK16 reverse,
5'-CATACGCACTCTCACTGGA-3'; GAPDH forward,
5'-TCATTTCCTGGTATGACAACGA-3'; GAPDH reverse,
5'-GGTCTTACTCCTTGGAGGC-3'; U6 forward,
5'-CAATACAGAGAAAGTTAGCACG-3', and U6 reverse,
5'-AATGCTTCAAAGAGTTGTGC-3'.
3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide
(MTT) assay
Primary HDFs were seeded at a density of
1x104 cells/well in 96-well plates and maintained for 24
h for cell transfection. The viability of the transfected cells and
non-transfected cells was measured by MTT assay 24, 48 and 72 h
post-transfection. The optical density at 490 nm was measured and
utilized to plot a proliferation curve.
Flow cytometric analysis
For the cell cycle analysis, trypsin-digested
primary HDFs were seeded into 6-well plates at a density of
5x104 cells/well and cultured for 24 h. The HDFs were
then harvested, centrifuged for 5 min (4˚C; 1,000 x g), and fixed
with 70% ethanol at 4˚C for 24 h. The fixed HDFs were treated with
propidium iodide (PI; Clontech Laboratories, Inc.) at room
temperature for 30 min. Finally, the cell cycle was evaluated with
a FACSCanto flow cytometer and CellQuest software version 5.1 (both
obtained from BD Biosciences). The assays were performed in three
triplicates. For the cell apoptosis analysis, HDFs
(1x105) were seeded in 24-well plates and cultivated for
24 h. Annexin V-fluorescein isothiocyanate (FITC) and PI (Clontech
Laboratories, Inc.) were added to the cell cultures and incubated
at room temperature for 10 min in the dark. Finally, the FACSCanto
flow cytometer was used to assess the cell apoptosis rate. Annexin
V-positive and PI-negative cells were considered as apoptotic
cells.
Western blot analysis
Total protein was extracted with RIPA lysis buffer
(cat. no. R0020; Beijing Solarbio Science & Technology Co.,
Ltd.) after the HDFs were washed with PBS. After high-speed
centrifugation at 1,000 x g for 10 min at 4˚C, the bicinchoninic
acid (BCA) method was used to quantify the protein in the
supernatant using a BCA protein quantification kit (Shanghai Yeasen
Biotechnology Co., Ltd.). An equal amount of protein (20 µg) was
prepared for 10% SDS-PAGE, after which the proteins were
transferred to a nitrocellulose membrane (EMD Millipore) with
Western transfer buffer (Beyotime Institute of Biotechnology). Each
sample was prepared in triplicate. The membranes were blocked with
QuickBlock™ blocking buffer (Beyotime Institute of
Biotechnology) at room temperature for 15 min, allowed to interact
overnight with primary antibodies against cyclin A1 (cat. no.
ab53699; 1:500), cyclin D1 (cat. no. ab16663; 1:200), cyclin E1
(cat. no. ab33911; 1:1,000), CDK4 (cat. no. ab108357; 1:1,000),
Bcl-2 (cat. no. ab32124; 1:1,000), Bax (cat. no. ab32503; 1:1,000),
cleaved caspase-3 (cat. no. ab13847; 1:500), collagen I (cat. no.
ab34710; 1:1,000), α-smooth muscle actin (α-SMA; cat. no. ab7817;
1:3,000), CDK16 (cat. no. ab181208; 1:1,000) or GAPDH (cat. no.
ab8245; 1:500) (all from Abcam) at 4˚C and then cultured with IgG
H&L (HRP) secondary antibody (cat. no. ab205718; 1:2,000) for 2
h. The antibodies were all purchased from Abcam. BeyoECL Plus
(Beyotime Institute of Biotechnology) and film (Carestream Health,
Inc.) were applied to visualize the western blots in the dark.
Quantum One software version 4.6.8 (Bio-Rad Laboratories, Inc.) was
used to analyze the relative protein levels using GAPDH as an
internal control.
Bioinformatics analysis
The subcellular localization of the lncRNA TPT1-AS1
was predicted by the lncLocator website (http://www.csbio.sjtu.edu.cn/bioinf/lncLocator/).
Additionally, the binding site between TPT1-AS1 and miR-324-5p was
acquired through ‘StarBase’ (http://starbase.sysu.edu.cn). The binding site of
miR-324-5p in the 3' untranslated region (3'UTR) of CDK16 was
obtained from TargetScan (http://www.targetscan.org/vert_72/).
Subcellular fractionation assay
Cytoplasmic and nuclear extracts were obtained from
HDFs by Invitrogen PARIS nuclear and cytoplasmic extraction kit
(cat. no. AM1921; Invitrogen; Thermo Fisher Scientific, Inc.). RNA
isolated from the nucleus or cytoplasm using the Invitrogen PARIS
kit (cat. no. AM1921; Invitrogen; Thermo Fisher Scientific, Inc.)
was subjected to RT-qPCR analysis. The levels of U6 (nuclear
control), GAPDH (cytoplasmic control), and TPT1-AS1 were
determined.
Luciferase reporter assay
For the luciferase reporter assay, wild-type (Wt) or
mutant (Mut) luciferase reporter vectors TPT1-AS1 (or the 3'UTR of
CDK16) were established in firefly luciferase-expressing pmirGLO
vector (Promega Corporation), termed TPT1-AS1-Wt, TPT1-AS1-Mut,
CDK16-Wt and CDK16-Mut, respectively. HDFs were co-transfected with
the 50 nM of miR-324-5p mimics or NC mimics, 200 ng of Wt or Mut
luciferase reporter constructs and a Renilla vector using
LipoFiter™ liposomal transfection reagent. Forty-eight
hours after transfection, the luciferase activity was measured with
a luciferase reporter assay kit (Promega Corporation).
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 7 software (GraphPad Software, Inc.), and the data are
expressed as the means ± standard deviation (S.D.). All experiments
were repeated three times. Differences between two groups were
assessed for significance by Student's t test, and differences of
multiple groups were assessed by ANOVA followed by Tukey's post hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
TPT1-AS1 relieves cell injury and
induces ECM synthesis in thermally injured HDFs
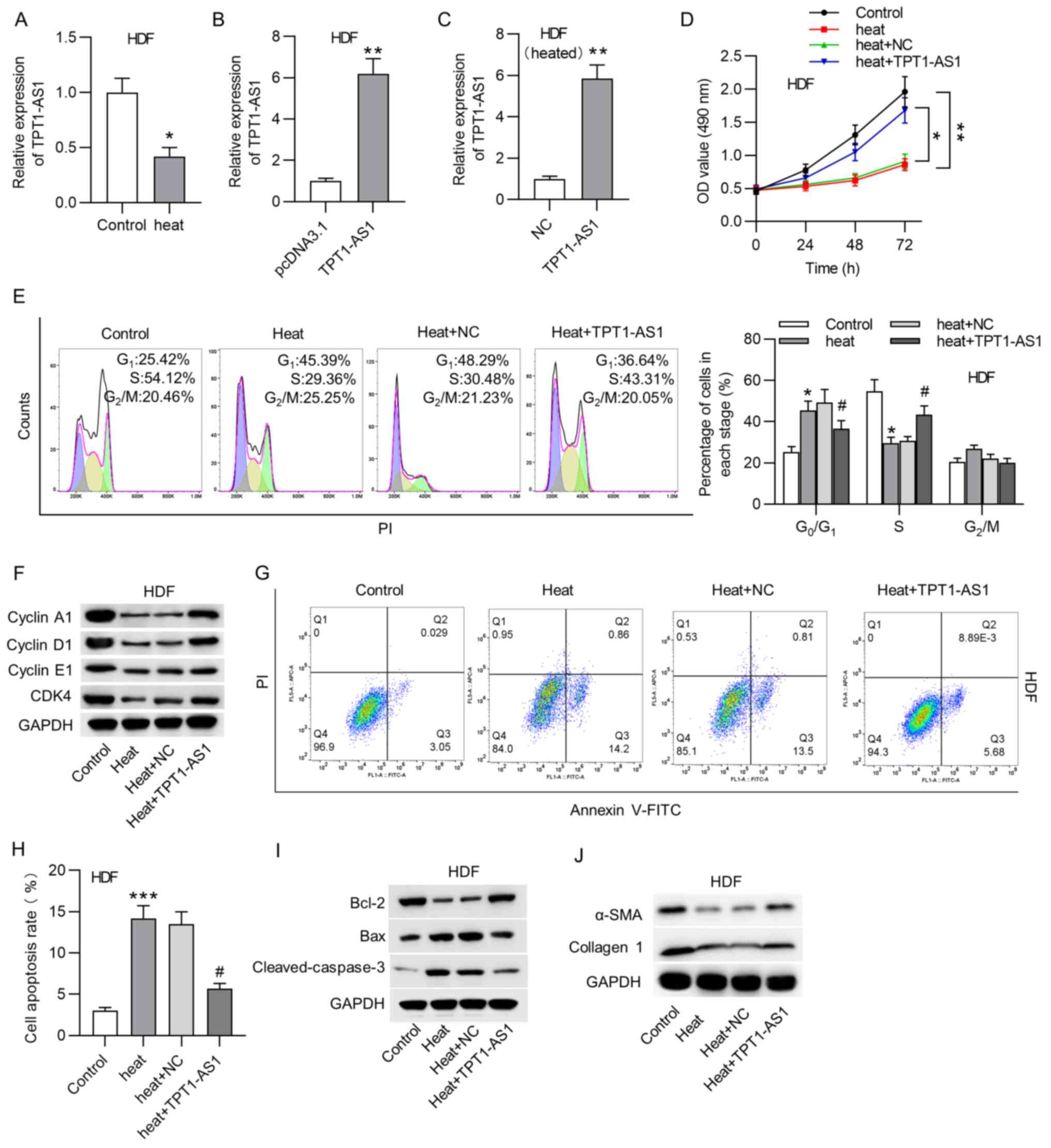
Firstly, TPT1-AS1 expression level was measured in
HDFs under heat stimulation. According to the RT-qPCR results, the
levels of TPT1-AS1 were decreased after thermal injury in HDFs
(Fig. 1A). Subsequently, the
transfection efficiency of TPT1-AS1 in HDFs was confirmed using
RT-qPCR analysis (Fig. 1B). In
order to investigate the biological role of TPT1-AS1 in HDFs after
thermal injury, the efficacy of the TPT1-AS1 transfection and
overexpression in HDFs was verified (Fig. 1C). MTT assays showed that TPT1-AS1
overexpression reversed the thermal injury-induced decrease in HDF
viability (Fig. 1D). The flow
cytometry results suggested that thermal injury caused a
significant increase in the proportion of cells at the
G0/G1 stage and a decrease in the proportion
of cells at the S stage, which indicated that thermal injury
induced cell cycle arrest at the G0/G1 stage.
The upregulation of TPT1-AS1 evidently attenuated cycle arrest at
the G0/G1 phase (Fig. 1E). The western blot analysis showed
that the levels of cyclin A1, cyclin D1, cyclin E1 and CDK4 were
significantly decreased in the heated HDFs, while TPT1-AS1
overexpression increased the levels of cell cycle-associated
factors (Fig. 1F). The flow
cytometry analysis suggested that the apoptosis rate of the HDFs
was increased by thermal injury and then significantly suppressed
by TPT1-AS1 overexpression (Fig.
1G-H). Furthermore, the western blot analysis demonstrated that
the protein levels of Bax and cleaved caspase-3 were significantly
higher, whereas the level of Bcl-2 was decreased in the heated
HDFs. However, TPT1-AS1 overexpression reversed the changes in the
levels of apoptosis-associated proteins in thermally injured HDFs
(Fig. 1I). Additionally, the
western blot analysis revealed that, in terms of ECM synthesis, the
heat stimulation-induced decrease in the protein levels of α-SMA
and collagen I were recovered by the overexpression of TPT1-AS1 in
the HDFs (Fig. 1J).
TPT1-AS1 is a sponge of
miR-324-5p
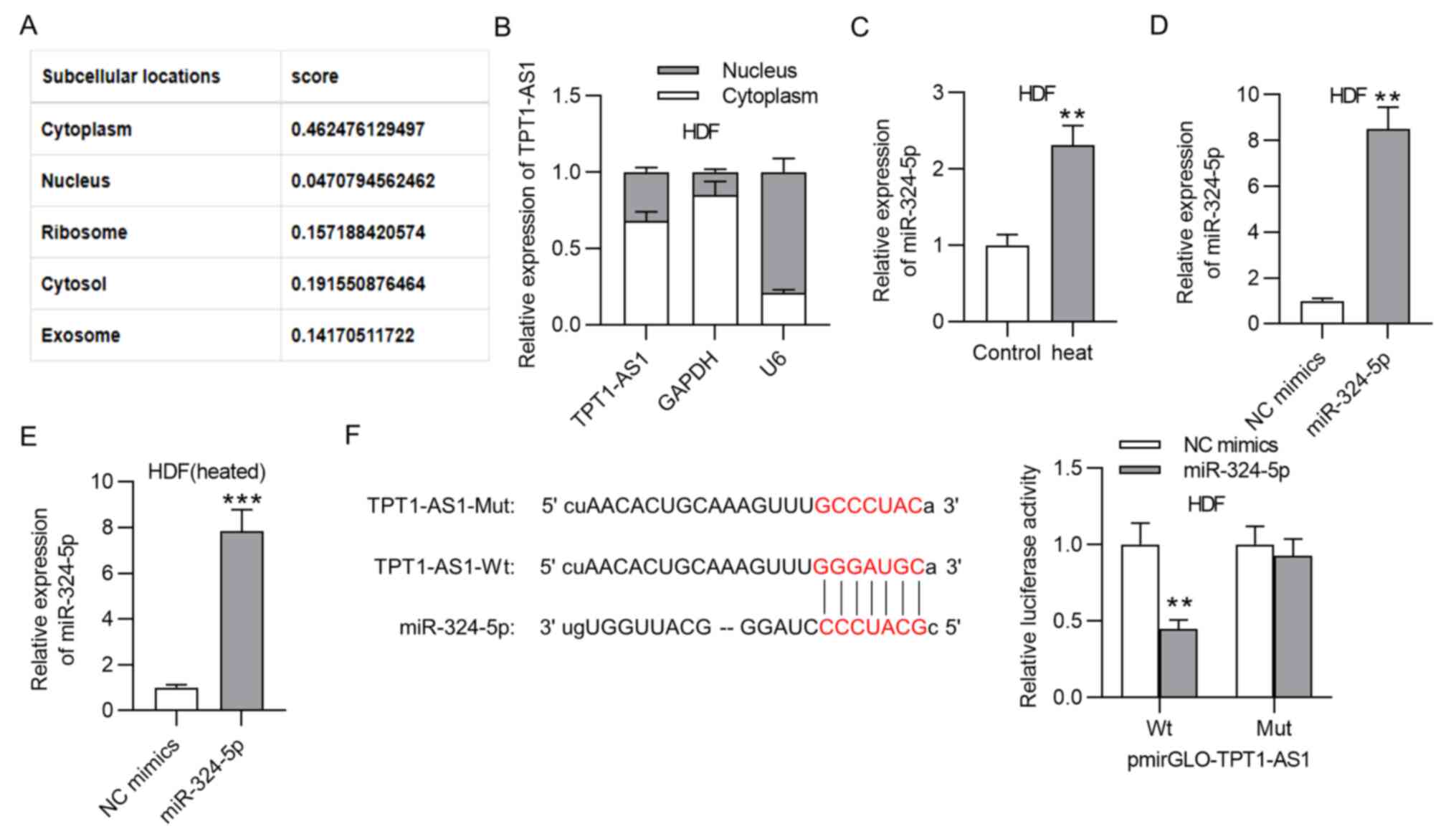
In order to further probe the regulatory mechanism
of TPT1-AS1, the subcellular localization of TPT1-A1 was predicted
with lncLocator. The results showed the cytoplasmic localization of
TPT1-AS1 (Fig. 2A). A subcellular
fractionation assay also validated the cytoplasm as the primary
location of TPTI-AS1, which indicated TPT1-AS1 as a
post-transcriptional regulator of gene expression (Fig. 2B). Then, miR-324-5p was screened as
a potential downstream target of TPT1-AS1 with the ‘StarBase’
database (conditions; Low clip and low degradome) and verified its
high expression in thermally injured HDFs via RT-qPCR (Fig. 2C). Subsequently, the miR-324-5p
overexpression efficiency was verified in the HDFs (Fig. 2D). Moreover, the transfection
efficiency of miR-324-5p mimics in heated HDFs was also confirmed
by RT-qPCR analysis (Fig. 2E). The
binding sequence of miR-324-5p in TPT1-AS1 was predicted. A
luciferase reporter assay was performed to validate the binding
capacity of miR-324-5p and TPT1-AS1. miR-324-5p upregulation
decreased the luciferase activity of TPT1-AS1-Wt in the HDFs, while
no evident change was observed for the TPT1-AS1-Mut group (Fig. 2F).
CDK16 is directly targeted by
miR-324-5p
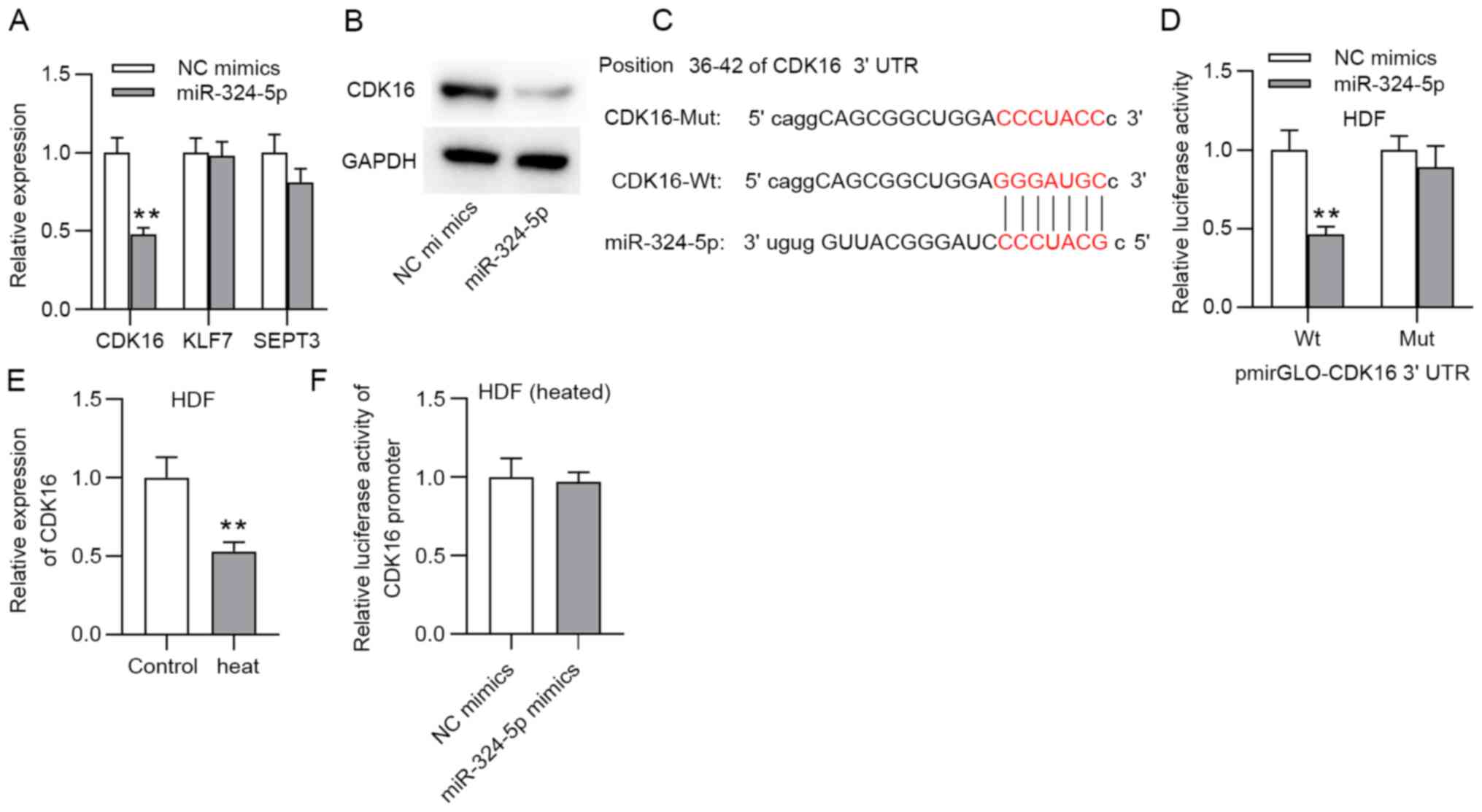
In order to further verify the hypothesis of the
ceRNA regulatory pattern, 3 mRNAs (CDK16, KLF7 and SEPT3), were
screened through overlapping prediction results of the microT,
PicTar and TargetScan databases (Table
SI). The RT-qPCR results illustrated that only CDK16 was
significantly downregulated in the HDFs in response to miR-324-5p
overexpression (Fig. 3A). The
western blotting results showed that CDK16 protein levels were
decreased by upregulating miR-324-5p (Fig. 3B). The target fragment of miR-324-5p
at the 3'UTR of CDK16 is shown in Fig.
3C. A luciferase reporter experiment showed that the luciferase
activity of the wild-type CDK16 3'UTR vector was significantly
decreased in the presence of the miR-324-5p mimics, while no
evident change was shown in the luciferase activity of the mutant
CDK16 3'UTR (Fig. 3D). Furthermore,
CDK16 was expressed at low levels in the heat-stimulated HDFs
according to the RT-qPCR results (Fig.
3E). A luciferase reporter assay also demonstrated that no
evident change in the luciferase activity of the CDK16 promoter
after miR-324-5p was overexpressed (Fig. 3F).
TPT1-AS1 alleviates cell injury and
promotes ECM synthesis via the miR-324-5p/CDK16 axis in thermally
injured HDFs
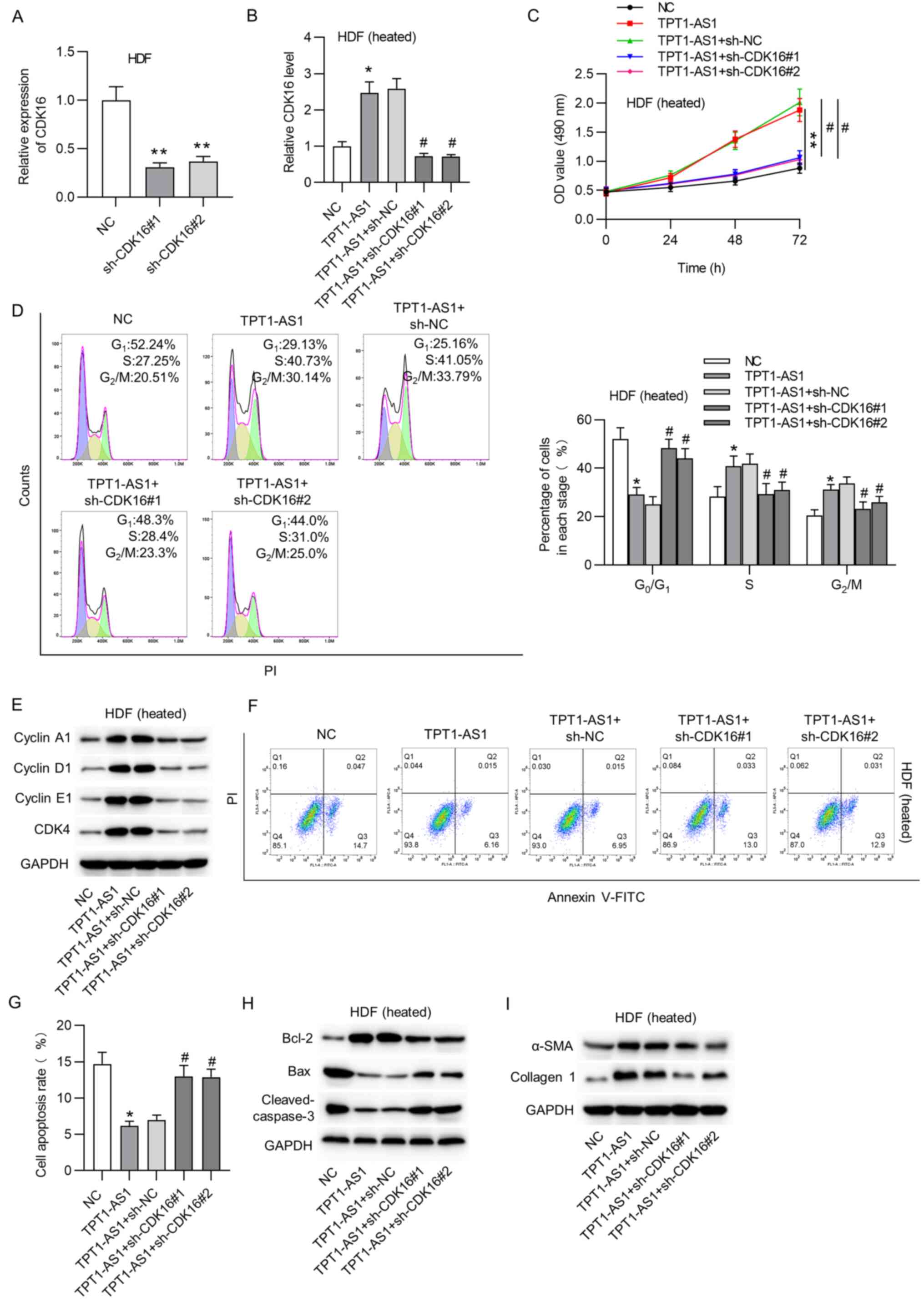
The knockdown efficiency of CDK16 in HDFs was
verified (Fig. 4A). The expression
of CDK16 was detected using RT-qPCR analysis. The results showed
that CDK16 expression was increased under TPT1-AS1 overexpression
and then knocked down by the co-transfection of sh-CDK16#1/#2
(Fig. 4B). Furthermore, CDK16
knockdown rescued the pcDNA3.1/TPT1-AS1-mediated increase in the
viability of the heat-treated HDFs (Fig. 4C). Flow cytometry revealed that
TPT1-AS1 attenuated cycle arrest at the G0/G1
phase and caused a decrease in the proportion of cells at the S
stage, which were reversed by CDK16 silencing (Fig. 4D). Moreover, knocking down CDK16
reversed the inhibition of TPT1-AS1 overexpression on cell
cycle-associated proteins in the thermally injured HDFs (Fig. 4E). CDK16 silencing counteracted the
suppressive effect of TPT1-AS1 overexpression on the apoptosis of
HDFs upon heat stimulation (Fig. 4F
and G). In addition, the protein
levels of Bax and cleaved caspase-3 were decreased, while the level
of Bcl-2 was elevated by TPT1-AS1 overexpression in the heated
HDFs; these outcomes were reversed by CDK16 knockdown (Fig. 4H). The promotive effect of
upregulated TPT1-AS1 on ECM synthesis in the heat-stimulated HDFs
was recovered after CDK16 downregulation (Fig. 4I).
Discussion
HDF cell function and ECM synthesis exert crucial
impacts on wound healing after thermal injury. Collagen
biosynthesis after injury is one of the major indicators for
predicting the outcome of healing (18). Fibroblasts express and secrete α-SMA
protein during wound healing, which supports contraction. Collagen
I and α-SMA are two major indicators of ECM synthesis (19). In addition, HDF activation
facilitates the restoration of tissue integrity (18). As previously reported, microarray
analysis showed that TPT1-AS1 expression is downregulated in
denatured dermal tissues (3). The
present study initially demonstrated that TPT1-AS1 was
significantly downregulated in the heat-stimulated HDFs.
Gain-of-function assays showed that TPT1-AS1 promoted cell
viability in the thermally injured HDFs. Moreover, the percentage
of cells at each cell cycle stage indicated that TPT1-AS1 inhibited
cell cycle arrest at the G0/G1 phase of
heated HDFs. The levels of cell cycle-associated proteins (cyclin
A1, cyclin D1, cyclin E1 and CDK4) were significantly decreased in
the heated HDFs, an outcome that was reversed by TPT1-AS1
overexpression. TPT1-AS1 also decreased the cell apoptosis rate of
the heated HDFs. The decrease in the protein levels of Bcl-2 or the
increase in the levels of Bax and cleaved caspase-3 caused by
thermal injury was reversed by the overexpression of TPT1-AS1.
Furthermore, the increase in the protein levels of collagen I and
α-SMA induced by heat stimulation was rescued by TPT1-AS1
upregulation.
Moreover, the cytoplasmic localization of TPT1-AS1
defined its posttranscriptional regulation of gene expression. As
previously reported, lncRNAs can serve as ceRNAs that
post-transcriptionally regulate gene expression by competitively
binding to miRNAs (20). Previous
research has revealed that TPT1-AS1 serves as a decoy of certain
miRNAs, such as miR-23a-5p (21)
and miRNA-770-5p. Thus, it is proposed that the lncRNA TPT1-AS1,
acting as a ceRNA, has a regulatory function by sponging miRNA and
targeting mRNA in HDFs. Through a bioinformatics analysis,
miR-324-5p was predicted to bind with TPT1-AS1. According to
previous reports, miR-324-5p is upregulated in osteoarthritis
(22) and hyperglycemia or
hyperlipidemia (23). In the
present study, the RT-qPCR analysis indicated that miR-324-5p was
highly expressed in the heat-treated HDFs. Furthermore, a
luciferase reporter assay revealed that TPT1-AS1 bound abundantly
to miR-324-5p.
The evidence suggests that lncRNAs act as ‘sponges’
by competitively binding to common miRNAs, attenuating the
repression of miRNAs on mRNAs (24-26).
In order to verify the ceRNA regulatory network, the targets of
miR-324-5p were identified. CDK16 was predicted as a putative
target of miR-324-5p in the bioinformatics analysis. According to
the literature, CDK16 has not been previously reported in diseases,
including burn wounds. The present study found that CDK16 was
expressed at low levels in HDFs after thermal injury.
Mechanistically, CDK16 was targeted by miR-324-5p at positions
36-42 of the 3'UTR, and TPT1-AS1 upregulated CDK16 expression by
sponging miR-324-5p. The mRNA and protein levels of CDK16 were also
negatively regulated by miR-324-5p in the HDFs. Functional
interactions in ceRNA networks aid in coordinating multiple
biological processes and, when perturbed, facilitate disease
pathogenesis (27). Thus, a series
of rescue assays were performed. The results indicated that CDK16
silencing reversed the promotion of TPT1-AS1 overexpression and
cell viability and ECM synthesis, the suppression of cell cycle
arrest at the G0/G1 phase and attenuated cell
apoptosis. Thus, TPT1-AS1 contributes to cell viability, increases
ECM synthesis, and inhibits cell cycle arrest and apoptosis to
relieve the thermal injury of HDFs by upregulating CDK16
expression.
In summary, the present study is the first to
demonstrate that TPT1-AS1 and CDK16 were upregulated in the HDFs
after thermal injury and that miR-324-5p led to their significant
downregulation in HDFs after thermal injury. TPT1-AS1 alleviated
cell injury and induced ECM synthesis after thermal injury via the
miR-324-5p/CDK16 axis. The present study suggested that TPT1-AS1
exerts a protective effect in the burn wound healing process. These
findings may provide a novel target for burn wound therapy
clinically.
Supplementary Material
Potential targets of
hsa-miR-324-5p
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by Science and Technology
Program of Jiangxi, China (grant nos. 20171BBG70061 and
S2020ZPYFB0615).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JL and HY performed the experiments and analyzed the
data. GG, XC, and GZ helped to perform the experiments and analyzed
the data. JL, HY, and JZ designed the study, wrote the manuscript
and provided material support. JL, HY, and JZ confirmed the
authenticity of all the raw data. All the authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The use of HDFs was approved by the ethics committee
of The First Affiliated Hospital of Nanchang University before the
study (ethics approval no. 2020-016).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zielins ER, Brett EA, Luan A, Hu MS,
Walmsley GG, Paik K, Senarath-Yapa K, Atashroo DA, Wearda T, Lorenz
HP, et al: Emerging drugs for the treatment of wound healing.
Expert Opin Emerg Drugs. 20:235–246. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Cao W and Feng Y: LncRNA XIST promotes
extracellular matrix synthesis, proliferation and migration by
targeting miR-29b-3p/COL1A1 in human skin fibroblasts after thermal
injury. Biol Res. 52(52)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Yu W, Guo Z, Liang P, Jiang B, Guo L, Duan
M, Huang X, Zhang P, Zhang M, Ren L, et al: Expression changes in
protein-coding genes and long non-coding RNAs in denatured dermis
following thermal injury. Burns. 46:1128–1135. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Takeo M, Lee W and Ito M: Wound healing
and skin regeneration. Cold Spring Harb Perspect Med.
5(a023267)2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Xue M and Jackson CJ: Extracellular matrix
reorganization during wound healing and its impact on abnormal
scarring. Adv Wound Care (New Rochelle). 4:119–136. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Xu J, Bai J, Zhang X, Lv Y, Gong Y, Liu L,
Zhao H, Yu F, Ping Y, Zhang G, et al: A comprehensive overview of
lncRNA annotation resources. Brief Bioinform. 18:236–249.
2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhang J, Cui X, Shen Y, Pang L, Zhang A,
Fu Z, Chen J, Guo X, Gan W and Ji C: Distinct expression profiles
of LncRNAs between brown adipose tissue and skeletal muscle.
Biochem Biophys Res Commun. 443:1028–1034. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Li J, Long W, Li Q, Zhou Q, Wang Y, Wang
H, Zhou B and Li J: Distinct expression profiles of lncRNAs between
regressive and mature scars. Cell Physiol Biochem. 35:663–675.
2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zheng W and Yu A: EZH2-mediated
suppression of lncRNA-LET promotes cell apoptosis and inhibits the
proliferation of post-burn skin fibroblasts. Int J Mol Med.
41:1949–1957. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chen L and Li J, Li Q, Li X, Gao Y, Hua X,
Zhou B and Li J: Overexpression of LncRNA AC067945.2 down-regulates
collagen expression in skin fibroblasts and possibly correlates
with the VEGF and Wnt signalling pathways. Cell Physiol Biochem.
45:761–771. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Correia de Sousa M, Gjorgjieva M, Dolicka
D, Sobolewski C and Foti M: Deciphering miRNAs' Action through
miRNA Editing. Int J Mol Sci. 20(6249)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lu TX and Rothenberg ME: MicroRNA. J
Allergy Clin Immunol. 141:1202–1207. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Paraskevopoulou MD and Hatzigeorgiou AG:
Analyzing miRNA-lncRNA interactions. Methods Mol Biol.
1402:271–286. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yang S, Yin W, Ding Y and Liu F: Lnc RNA
ZFAS1 regulates the proliferation, apoptosis, inflammatory response
and autophagy of fibroblast-like synoviocytes via
miR-2682-5p/ADAMTS9 axis in rheumatoid arthritis. Biosci Rep.
40(BSR20201273)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Luo R, Jin H, Li L, Hu YX and Xiao F: Long
noncoding RNA MEG3 inhibits apoptosis of retinal pigment epithelium
cells induced by high glucose via the miR-93/Nrf2 Axis. Am J
Pathol. 190:1813–1822. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhou S, Liang P, Zhang P, Zhang M and
Huang X: The long noncoding RNA PDK1-AS/miR-125b-5p/VEGFA axis
modulates human dermal microvascular endothelial cell and human
umbilical vein endothelial cell angiogenesis after thermal injury.
J Cell Physiol. 236:3129–3142. 2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hinz B: Myofibroblasts. Exp Eye Res.
142:56–70. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Guo L and Huang X, Liang P, Zhang P, Zhang
M, Ren L, Zeng J, Cui X and Huang X: Role of XIST/miR-29a/LIN28A
pathway in denatured dermis and human skin fibroblasts (HSFs) after
thermal injury. J Cell Biochem. 119:1463–1474. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Fu Z, Li G, Li Z, Wang Y, Zhao Y, Zheng S,
Ye H, Luo Y, Zhao X, Wei L, et al: Endogenous miRNA Sponge
LincRNA-ROR promotes proliferation, invasion and stem cell-like
phenotype of pancreatic cancer cells. Cell Death Discov.
3(17004)2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Gao X, Cao Y, Li J, Wang C and He H:
LncRNA TPT1-AS1 Sponges miR-23a-5p in glioblastoma to promote
cancer cell proliferation. Cancer Biother Radiopharm 2020 (Epub
ahead of print).
|
|
22
|
Woods S, Barter MJ, Elliott HR,
McGillivray CM, Birch MA, Clark IM and Young DA: miR-324-5p is up
regulated in end-stage osteoarthritis and regulates Indian Hedgehog
signalling by differing mechanisms in human and mouse. Matrix Biol.
77:87–100. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Guo J, Yang C, Lin Y, Hu G, Wei J, Zhang
X, Chen X and Li J: Enhanced peripheral blood miR-324-5p is
associated with the risk of metabolic syndrome by suppressing
ROCK1. Biochim Biophys Acta Mol Cell Biol Lipids.
1865(158727)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
An Y, Furber KL and Ji S: Pseudogenes
regulate parental gene expression via ceRNA network. J Cell Mol
Med. 21:185–192. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Liu XH, Sun M, Nie FQ, Ge YB, Zhang EB,
Yin DD, Kong R, Xia R, Lu KH, Li JH, et al: Lnc RNA HOTAIR
functions as a competing endogenous RNA to regulate HER2 expression
by sponging miR-331-3p in gastric cancer. Mol Cancer.
13(92)2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lei K, Liang X, Gao Y, Xu B, Xu Y, Li Y,
Tao Y, Shi W and Liu J: Lnc-ATB contributes to gastric cancer
growth through a miR-141-3p/TGFβ2 feedback loop. Biochem Biophys
Res Commun. 484:514–521. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Karreth FA and Pandolfi PP: ceRNA
cross-talk in cancer: When ce-bling rivalries go awry. Cancer
Discov. 3:1113–1121. 2013.PubMed/NCBI View Article : Google Scholar
|