Introduction
Sertoli cells (SCs) are located at the base of
seminiferous tubules and are important members of the wall
structure. Although SCs cannot differentiate directly into sperm,
they play an indispensable role in assisting and maintaining
spermatogenesis (1-3).
SCs perform their biological function mainly through two
mechanisms, by which they secrete a variety of growth factors to
establish a unique niche supporting spermatogonial stem cell
self-renewal and differentiation. SCs also construct the
blood-testis barrier (BTB) to separate the advanced germ cells
located in the adluminal compartment from immune cells and harmful
molecules in the blood, and to allow specific molecules to pass
through (1,4-7).
The dysfunction of SCs caused by imbalances in the expression of
specific genes, such as claudin-5 and JAM, is often the main factor
affecting spermatogenesis (8,9).
Therefore, the investigation of the molecular mechanisms that
influence SC function during spermiogenesis is of considerable
importance.
The BTB originates from adolescence and is composed
of SC bodies and cell junctions (including tight junctions,
adherens junctions, gap junctions and desmosome-like junctions)
between adjacent SCs. The tight junctions have been widely accepted
as the most important structures for the BTB (10). The maintenance of the normal
function of the BTB depends mainly on the expression levels of
adhesion proteins and tight junction proteins, such as occludin,
claudin-3, -5 and -11, zonula occludens (ZO) 1, 2 and 3, and JAM-A
and -B. Occludin and claudin-11 are responsible for the integrity
of the BTB. The absence of these proteins always leads to the lack
of integrity of the BTB and the increased autoimmune response of
the body to spermatogenic cells, which is accompanied by decreased
spermatogenesis (1,11). The expression levels of these
proteins are often regulated by specific transcription factors. For
example, transcription factor variant 5 (ETV5) can control the
expression of claudin-5. When ETV5 is knocked down, claudin-5
expression is inhibited, resulting in the destruction of the BTB
(9). In addition, the transcription
factor Krüppel-like factor 6, which is expressed in SCs,
contributes to the maintenance of the normal function of the BTB by
regulating the expression of the target genes ZO-1 and
claudin-3(12). It may be suggested
that, to some extent, the activity of the BTB is determined by
specific transcription factors.
As a heterogeneous nuclear protein, polypyrimidine
tract-binding protein 1 (PTBP1) exerts its functions mainly by
regulating precursor mRNA splicing and alternative splicing events
(13,14). PTBP1 is highly expressed in tumors
of various organs, including brain (15,16),
colorectal (17), ovarian (18), gastric (19), breast (20) and renal cancer (21). PTBP1 plays an important role in
accelerating cancer cell proliferation and migration. The
permeability of the blood-tumor barrier has been reported to be
enhanced following knockdown of PTBP1 in endothelial glioma cells,
as demonstrated in a recent study (22). This function may be closely
associated with the expression of tight junction proteins, which is
regulated by the transcription factor ETV1(22). Since the function of the BTB is also
highly dependent on the expression of tight junction proteins, it
was hypothesized that it could be influenced by PTBP1 via
regulation of the expression levels of these proteins.
In the present study, a gradual decrease in PTBP1
expression was noted, following suppression of spermatogenesis, as
a result of aging. Subsequently, the expression of PTBP1 was
silenced in SCs in order to investigate whether it could affect the
integrity and permeability of the BTB by regulating the expression
of tight junction proteins. The current study confirmed that PTBP1
served an important role in maintaining the integrity of tight
junctions between adjacent SCs by promoting the expression of tight
junction proteins, providing a new insight into the role of PTBP1
in regulating spermatogenesis.
Materials and methods
Isolation of primary mouse SCs
A total of 20 BALB/c mice (10 female and 10 male;
6-week-old; 20 g) were obtained from Sipeifu (Beijing)
Biotechnology Co., Ltd., and were maintained in pathogen-free
conditions with 50-65% humidity at 24˚C under a 12-h light/dark
cycle. The mice had free access to food and water, and were used to
breed the next generation of mice. All animal experiments were
performed in accordance with the guidelines of the Chinese Council
on Animal Care and the present study was approved by the Guizhou
Medical University Committee (permit. no. 1800013). The testes of
20 male mice (bred from the aforementioned mice; 3-week-old; 8.5 g)
were collected to isolate the SCs according to a previous report
(23,24). Briefly, following anesthesia with
sodium pentobarbital (150 mg/kg) by intraperitoneal injection, the
mice were sacrificed by cervical dislocation and capsules were
removed from the collected testicles. The seminiferous tubules were
extracted with a 1-ml syringe needle and subsequently cut into
small fragments with ophthalmic scissors. The extract was filtered
by cell filtration and digested with DMEM/Ham F-12 (DMEMF12; Gibco;
Thermo Fisher Scientific, Inc.) containing collagenase IV (Gibco;
Thermo Fisher Scientific, Inc.), hyaluronidase (Beijing Solarbio
Science & Technology Co., Ltd.), trypsin (Beijing Solarbio
Science & Technology Co., Ltd.) (all used at 1 mg/ml) and DNase
I (0.5 mg/ml; Corning, Inc.). The samples were oscillated for 20
min in a 37˚C water bath. Cell pellets were collected by
centrifugation (500 x g for 5 min at 24˚C) and resuspended in
DMEMF12 containing 15% FBS (cat. no. 04-001-1ACS; Biological
Industries) and 1% penicillin-streptomycin (Thermo Fisher
Scientific, Inc.). The cells were finally cultured in a 5%
CO2 incubator at 37˚C. After culture for 48 h, the
morphology of primary SCs was observed using an inverted phase
contrast microscope (magnification x100; NIKON ST100; Nikon
Corporation).
Cell line culture and treatment
The mouse SC line (TM4 cells) was provided by
Procell Life Science & Technology Co., Ltd. The cells were
cultured in DMEM containing 10% FBS and 1% penicillin-streptomycin
at 37˚C in a 5% CO2 incubator. The cells were grown to
90% confluence for subsequent experiments. To silence the
expression of PTBP1, TM4 cells were infected with lentivirus
(Lv)-short hairpin RNA (sh)PTBP1-EGFP (Cyagen Biosciences, Inc.) at
a multiplicity of infection (MOI) of 50 at 37˚C for 24 h, and then
cultured with fresh medium for another 48 h at 37˚C in a 5%
CO2 incubator before subsequent experiments. The cells
infected with Lv-EGFP (Cyagen Biosciences, Inc.; MOI, 50) were used
as negative control (NC). EGFP expression was analyzed using
fluorescence microscopy (magnification x100; NIKON ST100; Nikon
Corporation) following transfection.
Immunostaining analysis
In a previous study (25), mice were classified into the
following age groups: i) Postnatal (day 1-7); ii) Weaning (4
weeks); iii) Pubertal (6 weeks); iv) Reproductively active (15
weeks); and (v) Senescence or aged (>65 weeks). Another study
also reported that the rate of spermatogenesis declined at 16
months in male mice (26). Thus, in
the present study, 8-week-old mice (n=5) and 18-month-old mice
(n=5) were purchased from Sipeifu (Beijing) Biotechnology Co., Ltd.
The 8-week-old mice (between the pubertal and the reproductively
active age groups) were used as the young mice, and the
18-month-old mice were used as the aged mice. Following anesthesia
with sodium pentobarbital (150 mg/kg) by intraperitoneal injection,
the mice were sacrificed by cervical dislocation for collection of
testes. The testes collected from male mice were fixed with 4%
paraformaldehyde at 24˚C for 36 h, dehydrated, embedded with
paraffin and then sliced into 5-µm tissue sections. Following
incubation of the samples with 10% goat serum (cat. no. C0265;
Beyotime Institute of Biotechnology) at 24˚C for 30 min, the
sections were further incubated overnight at 4˚C with the PTBP1
primary antibodies (1:500; cat. no. ab133734; Abcam). A second
incubation was performed with FITC-tagged secondary antibodies
(1:200; cat. no. A0562; Beyotime Institute of Biotechnology) at
24˚C for 2 h. The nuclei were stained with 300 nM DAPI at 24˚C for
10 min. Similarly, the collected SCs (primary SCs) were fixed with
4% paraformaldehyde for 1 h, incubated with 10% goat serum for 30
min and permeabilized with 0.15% Triton X-100 for 20 min. These
cells were incubated overnight with the primary antibodies,
including PTBP1 (1:500; cat. no. ab133734; Abcam) or sex
determining region Y-box 9 (SOX-9; 1:500; cat. no. ab185966; Abcam)
at 4˚C for 16 h and further incubated with FITC-tagged secondary
antibodies (1:200; cat. no A0562; Beyotime Institute of
Biotechnology) or HRP-tagged secondary antibodies (1:200; cat. no.
STAR208P; Neobioscience Technology Co., Ltd.) at 24˚C for 2 h. The
nuclei were stained with 300 nM DAPI for 10 min. The immunostaining
images for SOX-9 were visualized and captured using an inverted
phase contrast microscope (magnification x100; NIKON ST100; Nikon
Corporation), while those for PTBP1 were visualized and captured
using an inverted fluorescence microscope (magnification x100;
NIKON Ti-u-dsri2; Nikon Corporation).
Western blot analysis
The total proteins were extracted from the testes
and from TM4 SCs using RIPA Lysis Buffer (Beyotime Institute of
Biotechnology) and the protein concentration was measured using a
BCA Protein Assay kit (Beyotime Institute of Biotechnology).
Following electrophoresis of the proteins (30 µg/lane) on 10%
sodium dodecyl sulfate-polyacrylamide gels, they were transferred
to PVDF membranes (EMD Millipore). The PVDF membranes were
incubated with 5% BSA (cat. no. 9048-46-8; Merck KGaA) at 24˚C for
2 h, primary antibodies at 4˚C for 16 h and HRP-conjugated IgG
antibody (1:3,500; cat. no. STAR208P; Neobioscience Technology Co.,
Ltd.) at 24˚C for 2 h. The primary antibodies used were as follows:
GAPDH (1:1,500; cat. no. ab181602; Abcam), PTBP1 (1:1,500; cat. no.
ab133734; Abcam), ZO-1 (1:1,500; cat. no. ab216880; Abcam),
occludin (1:1,500; cat. no. ab216327; Abcam) and claudin-5
(1:1,500; cat. no. ab131259; Abcam). The protein expression levels
were detected using the enhanced chemiluminescence kit assay system
(cat. no. CLINX-5600; Clinx Science Instruments Co., Ltd.), and the
band densities were analyzed using Image Lab 4.1 (Media
Cybernetics, Inc.) and standardized according to the expression
levels of GAPDH.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from testes and SCs using
TRNzol Universal Total RNA extraction kit (cat. no. DP424; Tiangen
Biotech Co., Ltd.) and cDNA was synthesized by FastKing-RT SuperMix
(cat. no. KR118; Tiangen Biotech Co., Ltd.) according to the
manufacturer's protocol. qPCR was performed using the SuperReal
PreMix Plus (SYBR Green) (cat. no. SYBR FP205; Tiangen Biotech Co.,
Ltd.) on ABI Prism 7900 system (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The qPCR thermocycling conditions were as
follows: 1 min at 95˚C, followed by 40 cycles of 5 sec at 95˚C, 10
sec at 55˚C and 15 sec at 72˚C. The aforementioned experiments were
conducted according to the manufacturer's instructions. GAPDH was
used as an endogenous control to normalize the gene expression
data. The 2-ΔΔCq method was used to quantify the
relative gene expression (27). The
following primer sequences were used: GAPDH (mouse), sense
(S)-5'-TGTTTCCTCGTCCCGTAG-3' and antisense
(A)-5'-CAATCTCCACTTTGCCACT-3'; PTBP1 (mouse),
S-5'-TCCACCCTCAGCTACCCT-3' and A-5'-CATCTTGCGGTCCTTCTG-3'.
Cell proliferation analysis
Cell proliferation was assessed using the WST-8 Cell
Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc.).
According to the manufacturer's instructions, 4x103 TM4
cells infected with or without shPTBP1 were transferred in a
96-well plate and cultured for 12, 24, 36 or 48 h. Subsequently,
the cells were washed with PBS and incubated with 100 µl serum-free
medium containing 10 µl CCK-8 solution. Following incubation at
37˚C for 2 h, the absorbance was measured at 450 nm using an Epoch
2 Microplate spectrophotometer (Biotek Epoch2).
Analysis of SC tight junction
permeability in vitro
The permeability of SC tight junctions was detected
using the Millicell Electrical Resistance system (MERS00002
Millicell-ERS; EMD Millipore) (12). Initially, 2.5x105 SCs
were plated in the Transwell upper chamber and cultured for 12, 24,
36 and 48 h. The resistance was quantified as the trans-epithelial
electrical resistance (TEER) in ohms (Ω) by placing two electrodes
across the SC epithelium, one in the apical and one in the basal
compartment of the bicameral units. Each sample was randomly tested
three times and the average value was multiplied by the Transwell
membrane area to yield the TEER value (Ω cm2). The final
valid value was defined as the TEER value minus the TEER of the
blank control.
Statistical analysis
Data were analyzed using GraphPad Prism v5.01
(GraphPad Software, Inc.) and presented as the mean ± SEM of ≥3
independent experiments. The differences between two groups were
analyzed using an unpaired Student's t-test and the differences
among multiple groups were determined by one-way ANOVA with Tukey's
post hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Laser scanning confocal microscopy and
electron microcopy
The methods of laser scanning confocal microscopy
and electron microcopy are described in Data S1.
Results
Expression levels of PTBP1 in mouse
testicles during spermatogenesis
To determine PTBP1 expression in the testes of young
and aged mice, the corresponding testicular samples were collected
from 8-week-old and 18-month-old mice. The PTBP1 expression
analysis was performed using immunofluorescence assays and the BTB
structures were visualized using an electron microscope. Western
blotting and RT-qPCR were used to confirm the results derived from
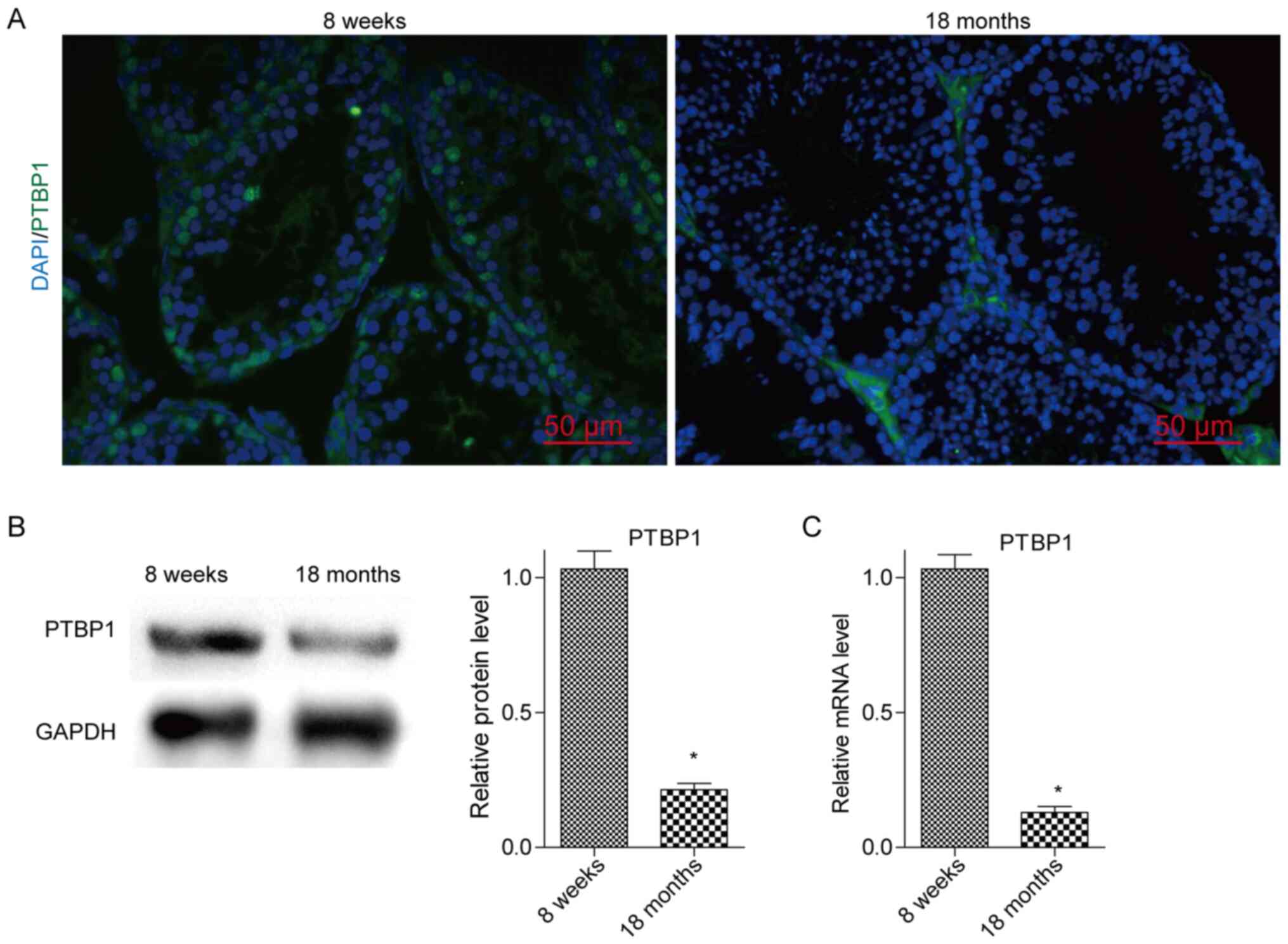
the immunofluorescence experiments. It was revealed that PTBP1 was
mainly expressed in SCs of the testes, whereas the expression
levels of PTBP1 were gradually decreased when the spermatogenic
ability was decreased following aging of the mice (Fig. 1A-C). In addition, the formation of
BTB structures was impaired in the testes from 18-month-old mice
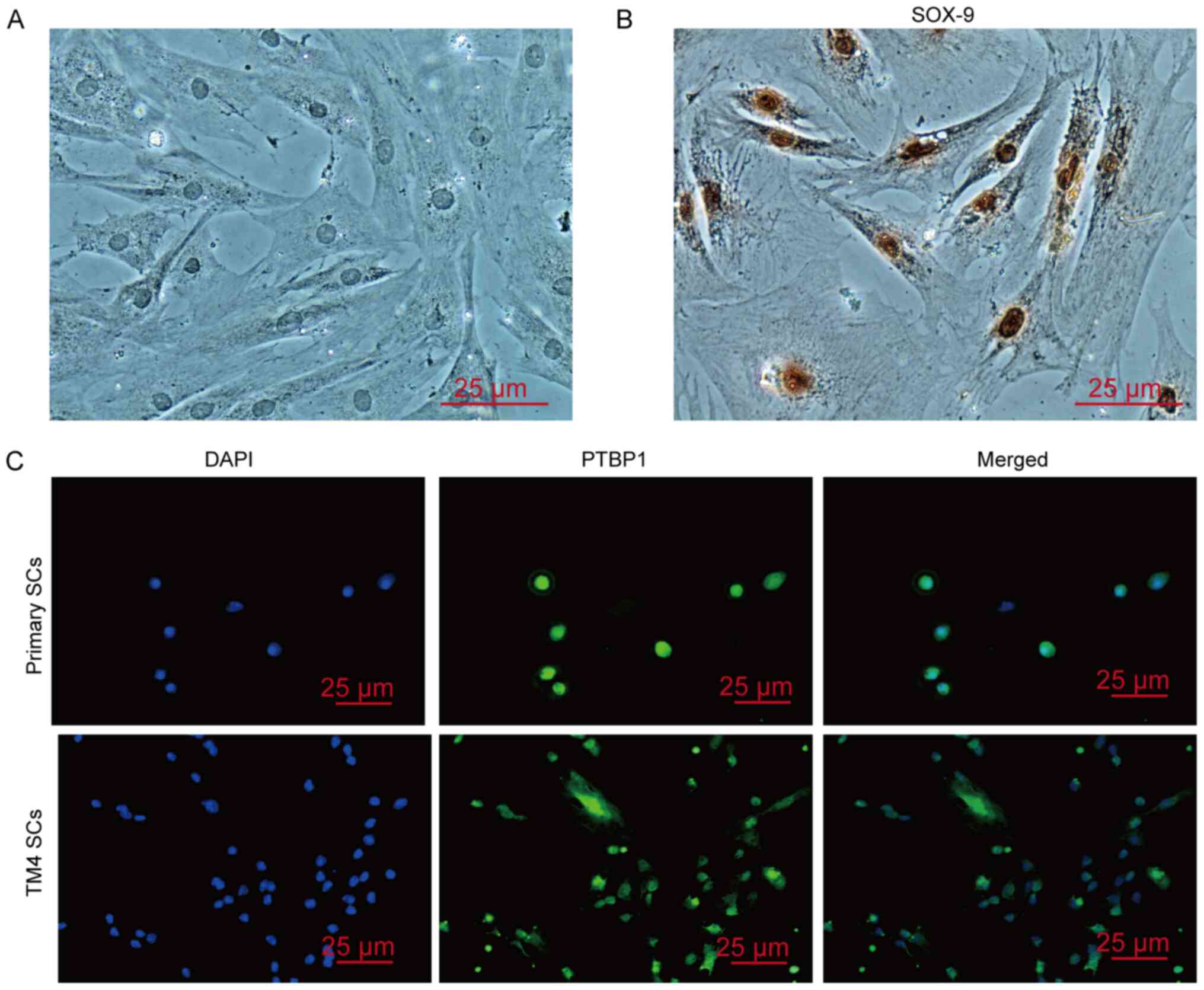
(Fig. S1). Furthermore, primary
mouse SCs isolated from 3-week-old mice were successfully isolated
and cultured; these cells were characterized by expression of the
SC-specific molecule SOX-9 and Wilms tumor 1 (Wt1) (Figs. 2A and B, and S2). The expression of PTBP1 was confirmed
in the nucleus of mouse primary SCs and TM4 cells (Fig. 2C). The aforementioned results
demonstrated that SCs were the main cells expressing PTBP1 in the
testes. Due to the important role of SCs in spermatogenesis, the
present study further investigated whether PTBP1 was involved in
regulating spermatogenesis by affecting the biological function of
SCs.
PTBP1 regulates the proliferation of
SCs
In previous studies, PTBP1 was shown to promote the
proliferation of various cell types (16,18).
SC proliferation is an important prerequisite for ensuring
appropriate spermatogenesis; therefore, the present study
investigated the role of PTBP1 in regulating SC proliferation. In
the present study, the expression levels of PTBP1 were inhibited by
infecting TM4 cells with Lv-shPTBP1-EGFP; infection of cells with
Lv-EGFP was used as NC. Following transfection for 3 days, the
expression levels of EGFP were assessed using fluorescence
microscopy (Fig. 3A).
Concomitantly, the expression levels of PTBP1 were knocked down
(Fig. 3B). Cell proliferation was
assessed with the CCK-8 assay and the results indicated that
suppression of PTBP1 expression resulted in decreased cell
proliferation (Fig. 3C). These
findings suggested that PTBP1 may serve an important role in
regulating SC proliferation.
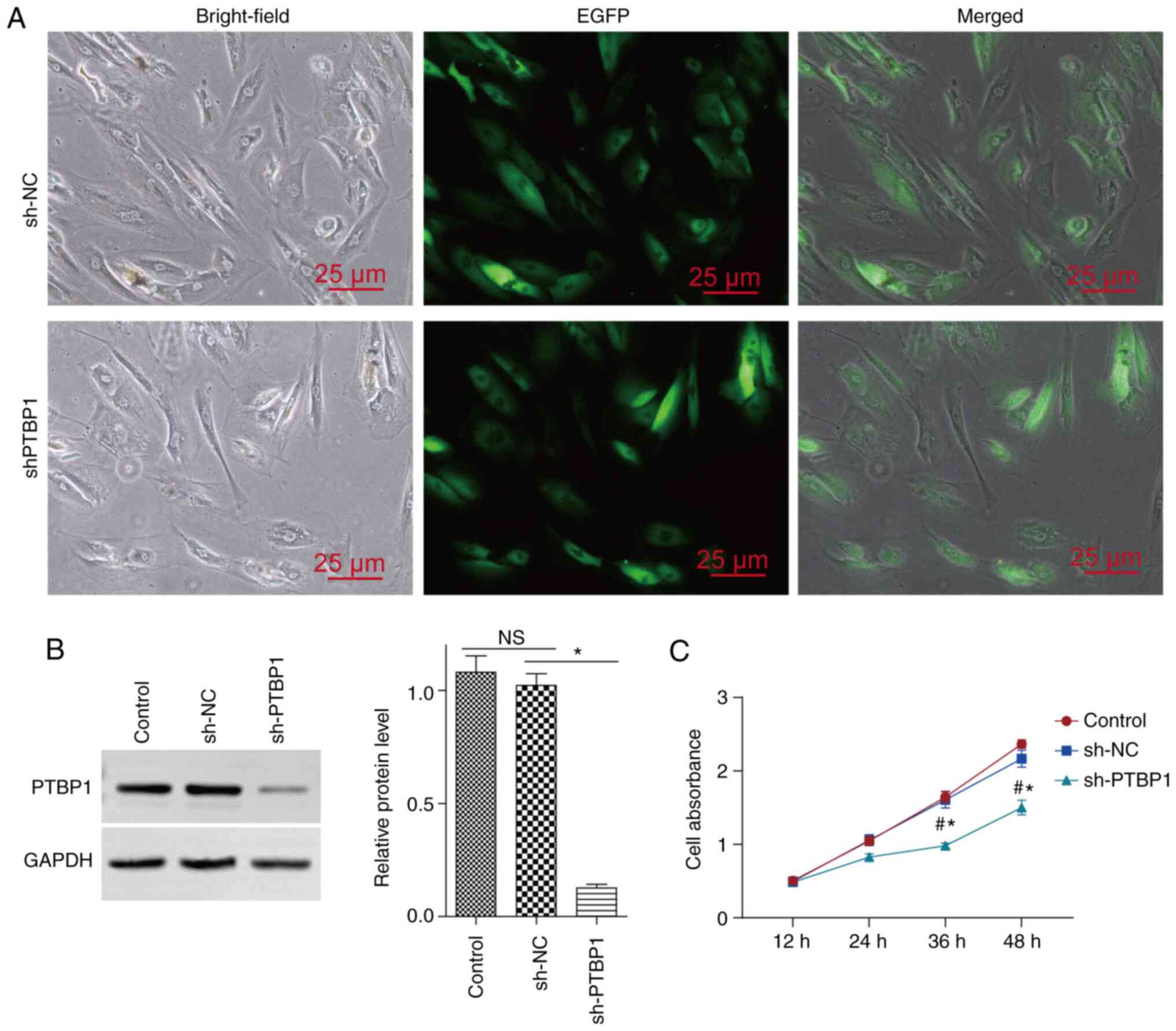 | Figure 3Effects of PTBP1 on the proliferation
of SCs. The expression levels of PTBP1 were knocked down in TM4 SCs
by infection of the cells with Lv-sh PTBP1-EGFP (sh-PTBP1). Cells
infected with Lv-EGFP (sh-NC) were used as NC cells. (A) After
infection, the cells were incubated for 3 days and the expression
levels of EGFP were observed under a fluorescence microscope. (B)
After infection with Lv-sh PTBP1-EGFP or Lv-EGFP, the cells were
cultured for 3 days and PTBP1 expression was observed by western
blotting. (C) Stable PTBP1-knockdown TM4 SCs or the corresponding
control cells were seeded in a 96-well plate and cell viability was
detected following culture for 12, 24, 36 and 48 h. Scale bar, 25
µm. Data are presented as the mean ± SEM (n=3).
#P<0.05 vs. the blank group (control);
*P<0.05 vs. the sh-NC group. PTBP1, polypyrimidine
tract-binding protein 1; SCs, Sertoli cells; NC, negative control;
NS, not significant; sh, short hairpin RNA; Lv, lentivirus. |
PTBP1 is necessary to maintain the
integrity and regulate the permeability of the BTB
The integrity and permeability of the BTB are very
important to maintain the appropriate environment required for
spermatogenesis (4,6,8).
Therefore, the present study explored the regulatory effect of
PTBP1 on the integrity and permeability of the BTB using an in
vitro cell model. Following infection of TM4 cells with
Lv-shPTBP1-EGFP or Lv-EGFP, changes in the integrity and
permeability of the tight connections between adjacent SCs were
detected using the Millicell Electrical Resistance system. The
results indicated that the resistance across the epithelium was
significantly decreased following the inhibition of PTBP1
expression (Fig. 4A). These results
indicated that PTBP1 could maintain the tight junctions of SCs, and
may have an important role in preserving the integrity of the BTB
and inhibiting its permeability.
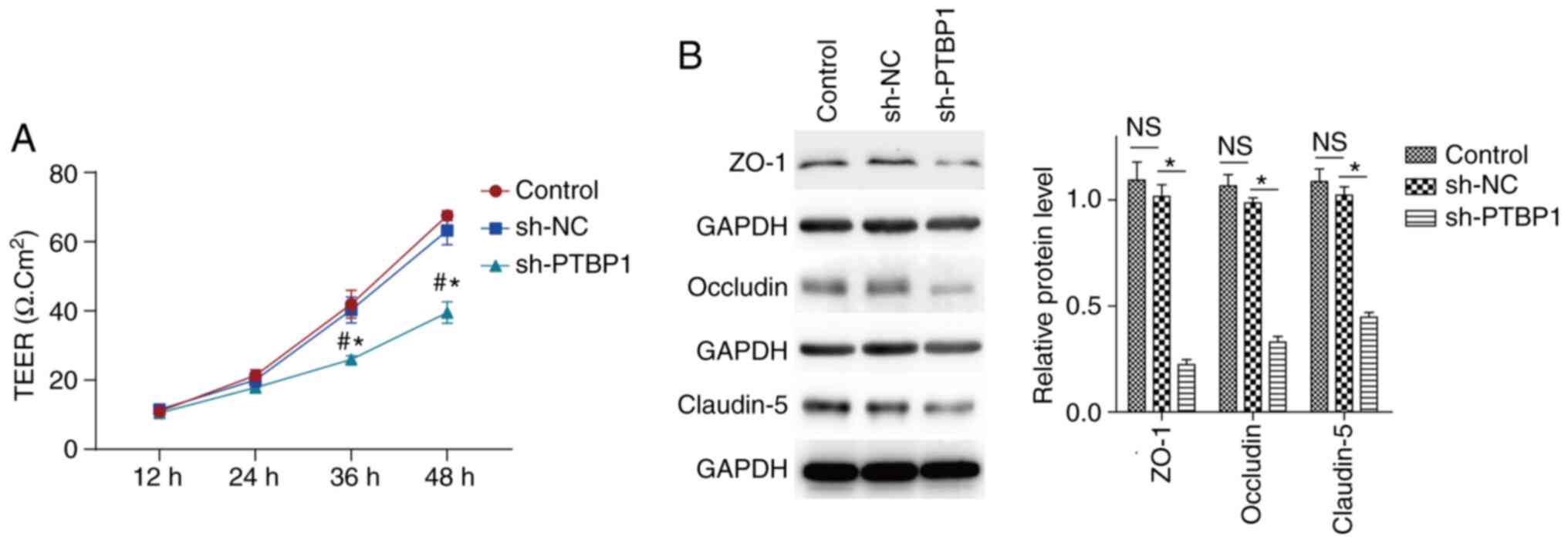 | Figure 4Knockdown of PTBP1 increases BTB
permeability in vitro and decreases the expression levels of
tight junction-associated proteins. (A) Following stable
PTBP1-knockdown in TM4 SCs, the infected cells and their
corresponding controls were seeded in a 96-well plate for 12, 24,
36 and 48 h. The permeability of the tight junction was measured by
a Millicell Electrical Resistance system. Non-infected TM4 SCs were
used as a blank control. (B) Protein expression levels of ZO-1,
occludin and claudin-5 were detected by western blot analysis
following infection of TM4 SCs with Lv-sh PTBP1-EGFP or Lv-EGFP for
3 days. Non-infected TM4 SCs were used as a blank control. Data are
presented as the mean ± SEM (n=3). #P<0.05 vs. the
blank group (control); *P<0.05 vs. the sh-NC group.
PTBP1, polypyrimidine tract-binding protein 1; BTB,
blood-testicular barrier; SCs, Sertoli cells; NC, negative control;
NS, not significant; sh, short hairpin RNA; TEER, trans-epithelial
electrical resistance; ZO-1, zona occludens 1. |
PTBP1 regulates the expression of
BTB-associated proteins in SCs
SCs are the main structural components of the BTB,
whose function mainly depends on the expression of various
BTB-associated proteins, including adhesive proteins and tight
connection molecules (ZO-1, occludin and claudin-5) (9-11).
In order to assess the effects of PTBP1 on the expression of
BTB-associated molecules, TM4 cells were infected with
Lv-shPTBP1-EGFP or Lv-shPTBP1-EGFP and cultured for 3 days. The
proteins were extracted to assess the expression levels of ZO-1,
occludin and claudin-5 by western blot analysis. As shown in
Fig. 4B, PTBP1 silencing resulted
in a significant decrease in the protein expression levels of ZO-1,
occludin and claudin-5. These results suggested that PTBP1 may
maintain the integrity and permeability of the BTB by controlling
the expression of tight connection-associated molecules.
Discussion
Spermatogonial stem cells (SSCs) in spermatogenic
tubules undergo proliferation, differentiation and morphological
changes to form sperm. During spermatogenesis, a series of changes
in SSCs are mainly dependent on the tissue microenvironment, which
is predominantly composed of SC-secreted growth factors, basal
membrane and the components exuded from blood vessels (1,28-31).
Due to the specific histological association between SCs and SSCs,
the former type of cells may be the most important contributor to
the tissue microenvironment of spermatogenic cells. SCs not only
provide autocrine/paracrine signals, but also secrete basement
membrane components. Moreover, adjacent SCs form the physical
barrier, the BTB, which inhibits autoimmune responses to the germ
cells by the formation of tight junctions (1). Spermatogenesis is accompanied by
dynamic changes in the wall structure of spermatogenic tubules and
notably in the relevant molecules of the BTB that support
spermatogenesis. These changes are closely associated with the
spatial and temporal expression of various genes (10). Therefore, it is of considerable
importance to investigate the molecular mechanism that regulates
the BTB in order to fully understand the regulatory mechanism of
spermatogenesis.
PTBP1 is an RNA-binding protein that exerts its
function by affecting mRNA splicing stability and translation
(13). PTBP1 regulates a series of
biological processes, including maintenance of cellular structure
and movement, immunity, protein metabolism and the cell cycle
(14,32,33).
Previous studies examined the ability of PTBP1 to regulate cancer
development and immune system function, and revealed that it could
directly accelerate proliferation and metastasis of tumor cells,
and promote the development of multiple tumors and the enhancement
of drug resistance by affecting the tumor inflammatory
microenvironment and the permeability of the blood-tumor barrier
(22). In addition, the mechanism
of action of PTBP1 was assessed with regard to glioma formation and
the results indicated that increased expression of PTBP1 inhibited
the permeability of the blood-tumor barrier of glioma, leading to
the decreased effectiveness of anti-tumor drug therapy. These
effects were closely associated with the expression of the tight
junction protein regulated by the circular
RNA_001160/microRNA-195-5p/ETV1 signaling cascade, which was in
turn mediated by PTBP1(22).
However, whether PTBP1 is involved in the regulation of the BTB for
maintaining spermatogenesis remains unclear.
In the present study, the data indicated that the
expression of PTBP1 was decreased with the reduction in
spermatogenic activity following aging. The results further
confirmed that PTBP1 was expressed in the nuclei of SCs. Testicular
maturation is accompanied by the proliferation of SCs and by
formation of the BTB. In response to aging, the degeneration of SC
function and the loss of BTB integrity may be important factors
leading to the weakening of spermatogenesis. Based on this
evidence, the present study hypothesized that the dynamic changes
in PTBP1 expression may be an important regulatory factor affecting
the biological function of SCs. To assess this hypothesis, gene
editing was used to silence the expression of the PTBP1 gene in TM4
cells and to further detect the changes in cell viability and
integrity of the BTB. The results indicated that PTBP1 played an
important role in maintaining the proliferation of SCs, whereas
PTBP1 silencing caused a significant increase in the permeability
of the BTB. In addition, the effects of PTBP1 silencing were
investigated on the expression of BTB-associated proteins in SCs.
PTBP1 silencing resulted in decreased expression levels of ZO-1,
occludin and claudin-5 in SCs. These results further supported the
conclusion that PTBP1 may maintain the integrity of the BTB by
regulating the expression of ZO-1, occludin and claudin-5.
Despite the significant findings, several issues
remain unresolved. Firstly, the signaling mechanisms responsible
for inducing the dynamic changes in PTBP1 expression during
spermatogenesis were not fully clarified. It is suspected that the
changes in the proportion and function of spermatogenic cells in
the testicular tissues during the flourishing and declining stages
of spermatogenesis may be the cause of the dynamic changes in PTBP1
expression. Secondly, the exact mechanism by which PTBP1 affects
the expression of BTB-associated proteins was not fully clarified.
A previous study (22) has also
demonstrated the direct or indirect regulatory effect of PTBP1 on
the expression of specific genes. For example, in glioma vascular
endothelial cells, PTBP1 and the transcription factor ETV5 were
revealed to cooperate to regulate the expression of tight junction
proteins (ZO-1, occludin, and claudin-5) (22). Therefore, it is possible that such
biological effects may also exist in SCs, but the association
between PTBP1 and ETV1 requires further elucidation. Whether
pharmacological intervention of PTBP1 activity can restore the
dysfunction of SCs to ultimately improve spermiogenesis remains
unknown; these topics will be further addressed in future
studies.
Supplementary Material
Supplementary materials and
methods
Electron microscopy of BTB structures
in the testes from 8-week-old and 18-month-old mice. The BTB
structures in the testes from 8-week-old and 18-month-old mice were
observed by a transmission electron microscope. Red boundary lines
indicate the BTB. Scale bar, 1 μm. BTB, blood.testis barrier.
Expression of Wt1 in primary SCs. The
expression of Wt1 in primary SCs was detected by
immunofluorescentstaining, and the images were captured by laser
scanning confocal fluorescence microscope. Scale bar, 25 μm. SCs,
Sertoli cells; Wt1, Wilms tumor 1.
Acknowledgements
The authors would like to thank the Research Center
for Basic Sciences of Medicine of Guizhou Medical University for
providing experimental equipment, such as the fluorescence
microscope (NIKON Ti-u-dsri2; Nikon Corporation) and the laser
scanning confocal fluorescence microscope (Olympus FV1000; Olympus
Corporation).
Funding
Funding: The present study was financially supported by grants
from the Guizhou Provincial Education Department Youth Science and
Technology talent Growth Project [grant no. KY (2018)172] and the
Science and Technology Joint Fund Project in Guizhou Province
[grant. no. LH (2016)7386].
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZY and XG conceived and designed the experiments.
ZY, ZL, YY and YD performed the experiments. ZY and ZL analyzed the
data. ZY and XG prepared the manuscript. All contributing authors
have participated in the study, and concur with the submission and
subsequent revisions submitted. XG and ZY confirm the authenticity
of all the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All animal experiments were performed in accordance
with the guidelines of the Chinese Council on Animal Care and the
present study was approved by the Guizhou Medical University
Committee (permit. no. 1800013).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
França LR, Hess RA, Dufour JM, Hofmann MC
and Griswold MD: The sertoli cell: One hundred fifty years of
beauty and plasticity. Andrology. 4:189–212. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Meroni SB, Galardo MN, Rindone G, Gorga A,
Riera MF and Cigorraga SB: Molecular mechanisms and signaling
pathways involved in sertoli cell proliferation. Front Endocrinol
(Lausanne). 10(224)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Griswold MD: 50 years of spermatogenesis:
Sertoli cells and their interactions with germ cells. Biol Reprod.
99:87–100. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Dym M and Fawcett DW: The blood-testis
barrier in the rat and the physiological compartmentation of the
seminiferous epithelium. Biol Reprod. 3:308–326. 1970.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Russell LD and Clermont Y: Degeneration of
germ cells in normal, hypophysectomized and hormone treated
hypophysectomized rats. Anat Rec. 187:347–366. 1977.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Mruk DD and Cheng CY: Tight junctions in
the testis: New perspectives. Philos Trans R Soc Lond B Biol Sci.
365:1621–1635. 2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Doyle TJ, Kaur G, Putrevu SM, Dyson EL,
Dyson M, McCunniff WT, Pasham MR, Kim KH and Dufour JM:
Immunoprotective properties of primary Sertoli cells in mice:
Potential functional pathways that confer immune privilege. Biol
Reprod. 86:1–14. 2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Shao M, Ghosh A, Cooke VG, Naik UP and
Martin-DeLeon PA: JAM-A is present in mammalian spermatozoa where
it is essential for normal motility. Dev Biol. 313:246–255.
2008.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Morrow CM, Tyagi G, Simon L, Carnes K,
Murphy KM, Cooke PS, Hofmann MCC and Hess RA: Claudin 5 expression
in mouse seminiferous epithelium is dependent upon the
transcription factor ets variant 5 and contributes to blood-testis
barrier function. Biol Reprod. 81:871–879. 2009.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mital P, Hinton BT and Dufour JM: The
blood-testis and blood-epididymis barriers are more than just their
tight junctions. Biol Reprod. 84:851–858. 2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gow A, Southwood CM, Li JS, Pariali M,
Riordan GP, Brodie SE, Danias J, Bronstein JM, Kachar B and
Lazzarini RA: CNS myelin and sertoli cell tight junction strands
are absent in Osp/claudin-11 null mice. Cell. 99:649–659.
1999.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wang XX, Zhang Y, Li XY, Li J, Tang JX, Li
YY, Deng SL, Cheng CY and Liu YX: Kruppel-like factor 6 regulates
Sertoli cell blood-testis barrier. Front Biosci (Landmark Ed).
24:1316–1329. 2019.PubMed/NCBI
|
|
13
|
Zhang L, Yang Z, Huang W and Wu J: H19
potentiates let-7 family expression through reducing PTBP1 binding
to their precursors in cholestasis. Cell Death Dis.
10(168)2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Glisovic T, Bachorik JL, Yong J and
Dreyfuss G: RNA-binding proteins and post-transcriptional gene
regulation. FEBS Lett. 582:1977–1986. 2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
McCutcheon IE, Hentschel SJ, Fuller GN,
Jin W and Cote GJ: Expression of the splicing regulator
polypyrimidine tract-binding protein in normal and neoplastic
brain. Neuro Oncol. 6:9–14. 2004.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Cheung HC, Hai T, Zhu W, Baggerly KA,
Tsavachidis S, Krahe R and Cote GJ: Splicing factors PTBP1 and
PTBP2 promote proliferation and migration of glioma cell lines.
Brain. 132:2277–2288. 2009.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Takahashi H, Nishimura J, Kagawa Y, Kano
Y, Takahashi Y, Wu X, Hiraki M, Hamabe A, Konno M, Haraguchi N, et
al: Significance of polypyrimidine tract-binding protein 1
expression in colorectal cancer. Mol Cancer Ther. 14:1705–1716.
2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
He X, Pool M, Darcy KM, Lim SB, Auersperg
N, Coon JS and Beck WT: Knockdown of polypyrimidine tract-binding
protein suppresses ovarian tumor cell growth and invasiveness in
vitro. Oncogene. 26:4961–4968. 2007.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sugiyama T, Taniguchi K, Matsuhashi N,
Tajirika T, Futamura M, Takai T, Akao Y and Yoshida K: miR-133b
inhibits growth of human gastric cancer cells by silencing pyruvate
kinase muscle-splicer polypyrimidine tract-binding protein 1.
Cancer Sci. 107:1767–1775. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
He X, Arslan AD, Ho TT, Yuan C, Stampfer
MR and Beck WT: Involvement of polypyrimidine tract-binding protein
(PTBP1) in maintaining breast cancer cell growth and malignant
properties. Oncogenesis. 3(e84)2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Shan H, Hou P, Zhang M, Li L, Pan Y, Chen
F and Jiang T: PTBP1 knockdown in renal cell carcinoma inhibits
cell migration, invasion and angiogenesis in vitro and
metastasis in vivo via the hypoxia inducible factor-1α
pathway. Int J Oncol. 52:1613–1622. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Li H, Shen S, Ruan X, Liu X, Zheng J, Liu
Y, Yang C, Wang D, Liu L, Ma J, et al: Biosynthetic CircRNA_001160
induced by PTBP1 regulates the permeability of BTB via the
CircRNA_001160/miR-195-5p/ETV1 axis. Cell Death Dis.
10(960)2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Cai H, Ren Y, Li XX, Yang JL, Zhang CP,
Chen M, Fan CH, Hu XQ, Hu ZY, Gao F and Liu YX: Scrotal heat stress
causes a transient alteration in tight junctions and induction of
TGF-β expression. Int J Androl. 34:352–362. 2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Li XX, Chen SR, Shen B, Yang JL, Ji SY,
Wen Q, Zheng QS, Li L, Zhang J, Hu ZY, et al: The heat-induced
reversible change in the blood-testis barrier (BTB) is regulated by
the androgen receptor (AR) via the partitioning-defective protein
(Par) polarity complex in the mouse. Biol Reprod.
89(12)2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Choubey M, Ranjan A, Bora PS, Baltazar F,
Martin LJ and Krishna A: Role of adiponectin as a modulator of
testicular function during aging in mice. Biochim Biophys Acta Mol
Basis Dis. 1865:413–427. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hacker-Klom UB: Age dependence of murine
spermatogenesis. Z Naturforsch C J Biosci. 50:303–310.
1995.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Chiarini-Garcia H, Hornick JR, Griswold MD
and Russell LD: Distribution of type A spermatogonia in the mouse
is not random. Biol Reprod. 65:1179–1185. 2001.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Chiarini-Garcia H, Raymer AM and Russell
LD: Non-random distribution of spermatogonia in rats: Evidence of
niches in the seminiferous tubules. Reproduction. 126:669–680.
2003.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kanatsu-Shinohara M, Inoue K, Ogonuki N,
Miki H, Yoshida S, Toyokuni S, Lee J, Ogura A and Shinohara T:
Leukemia inhibitory factor enhances formation of germ cell colonies
in neonatal mouse testis culture. Biol Reprod. 76:55–62.
2007.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Oatley JM and Brinster RL: The germline
stem cell niche unit in mammalian testes. Physiol Rev. 92:577–595.
2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Georgilis A, Klotz S, Hanley CJ, Herranz
N, Weirich B, Morancho B, Leote AC, D'Artista L, Gallage S and
Seehawer M: PTBP1-mediated alternative splicing regulates the
inflammatory secretome and the pro-tumorigenic effects of senescent
cells. Cancer Cell. 34:85–102. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wu P, Gao Y, Shen S, Xue Y, Liu X, Ruan X,
Shao L, Liu Y and Wang P: KHDRBS3 regulates the permeability of
blood-tumor barrier via cDENND4C/miR-577 axis. Cell Death Dis.
10(536)2019.PubMed/NCBI View Article : Google Scholar
|